The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages
Abstract
:1. Introduction
2. Results
2.1. Topical Toxicity of Fluralaner against S. incivta
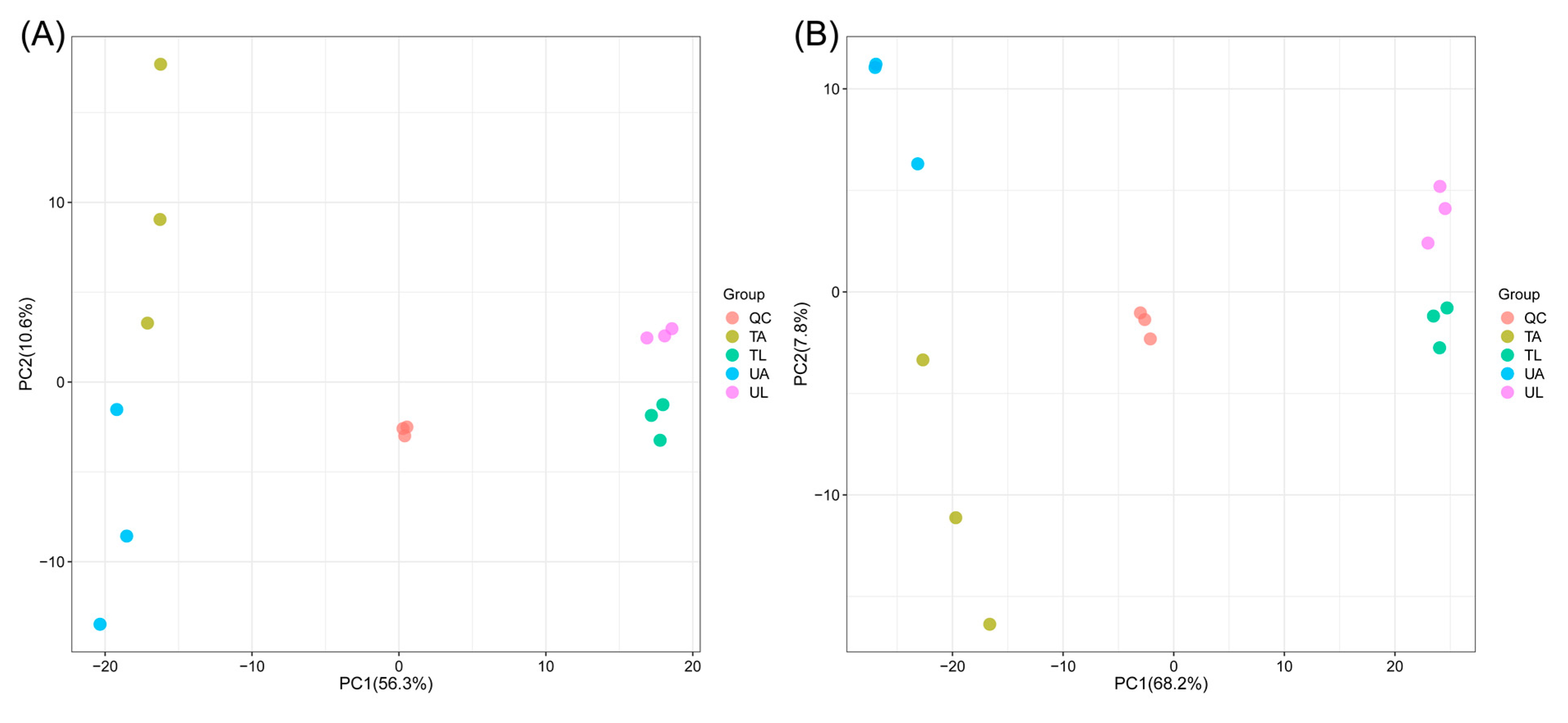
2.2. Effect of Fluralaner on the Metabolites of S. incivta at Different Reproductive Stages
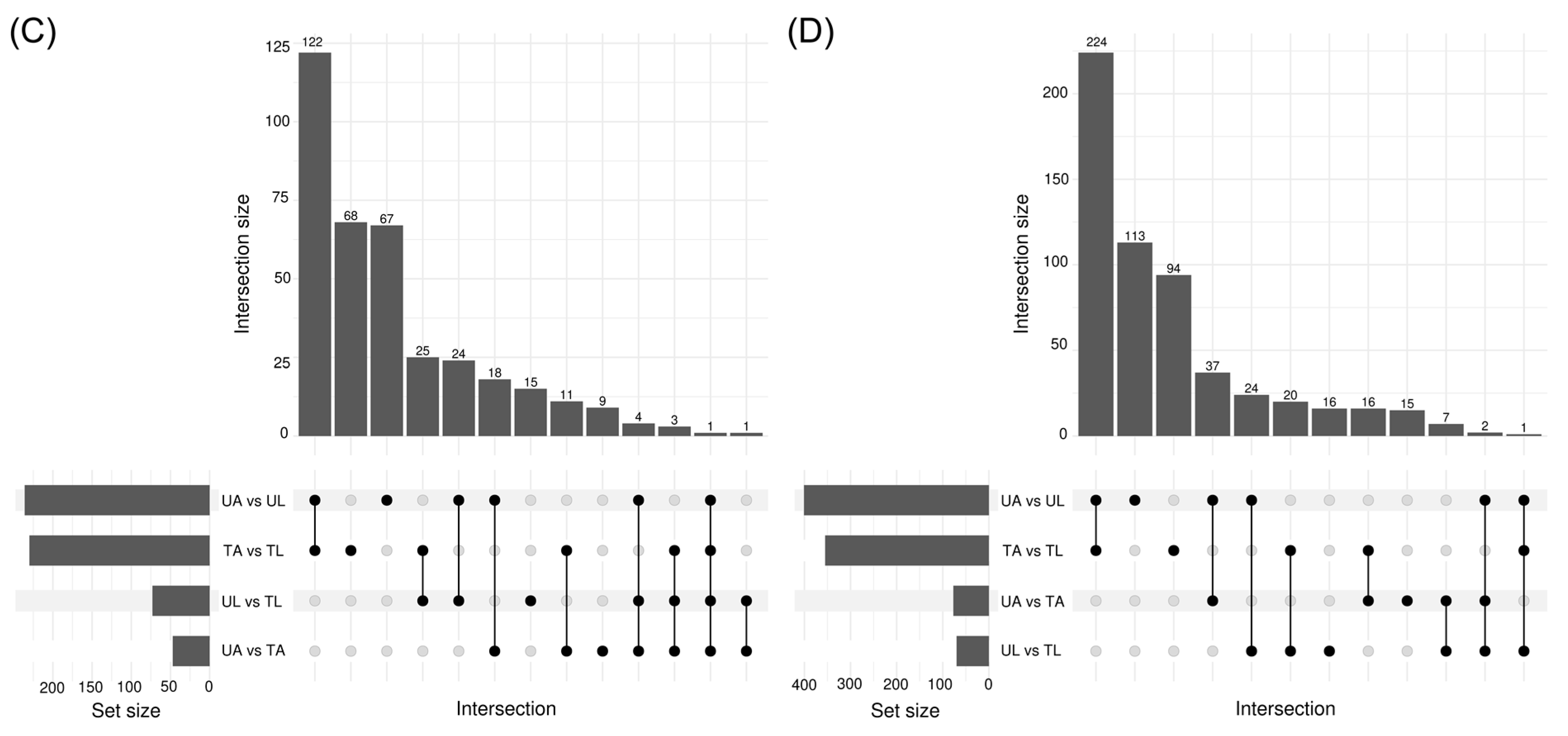
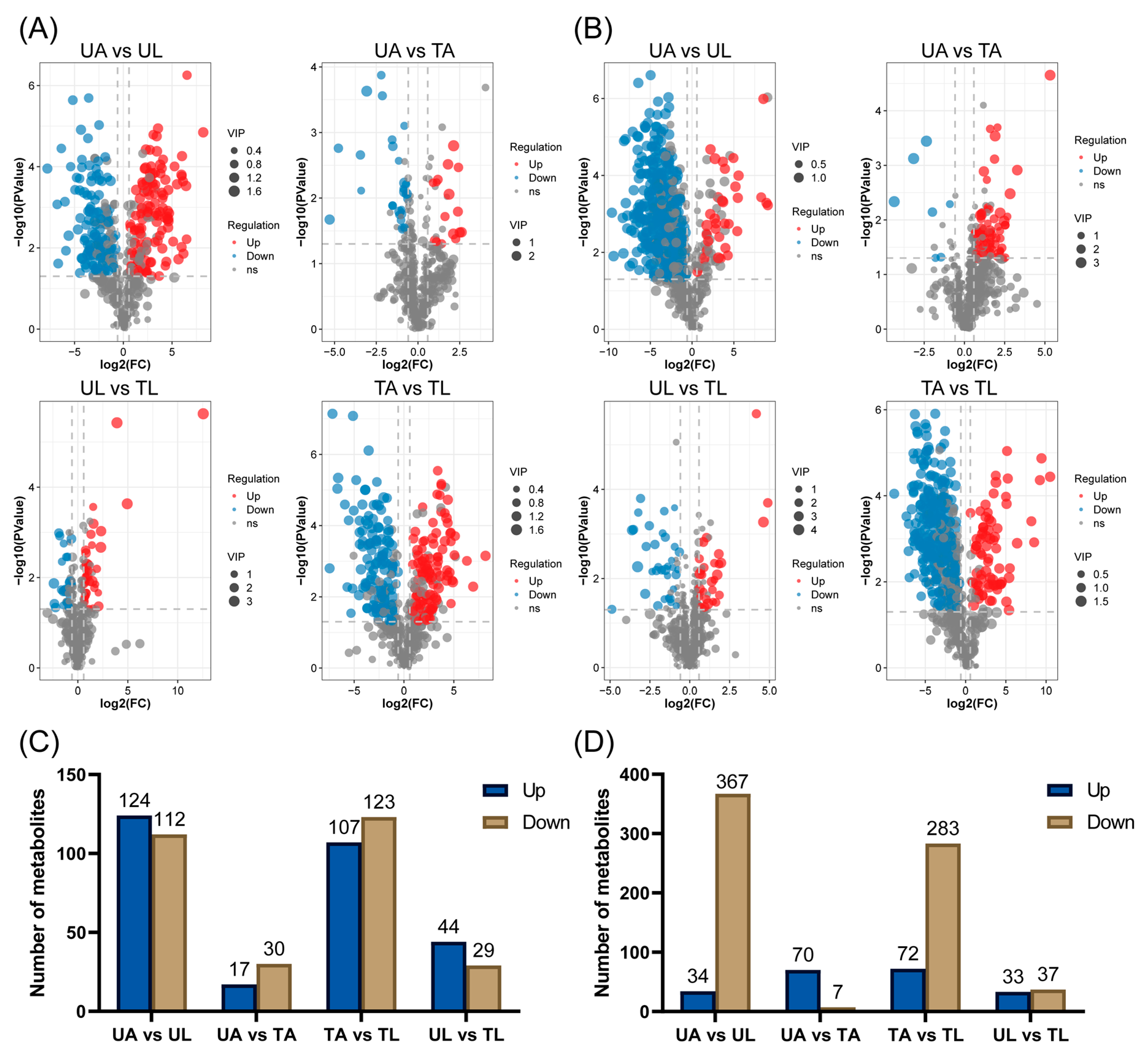
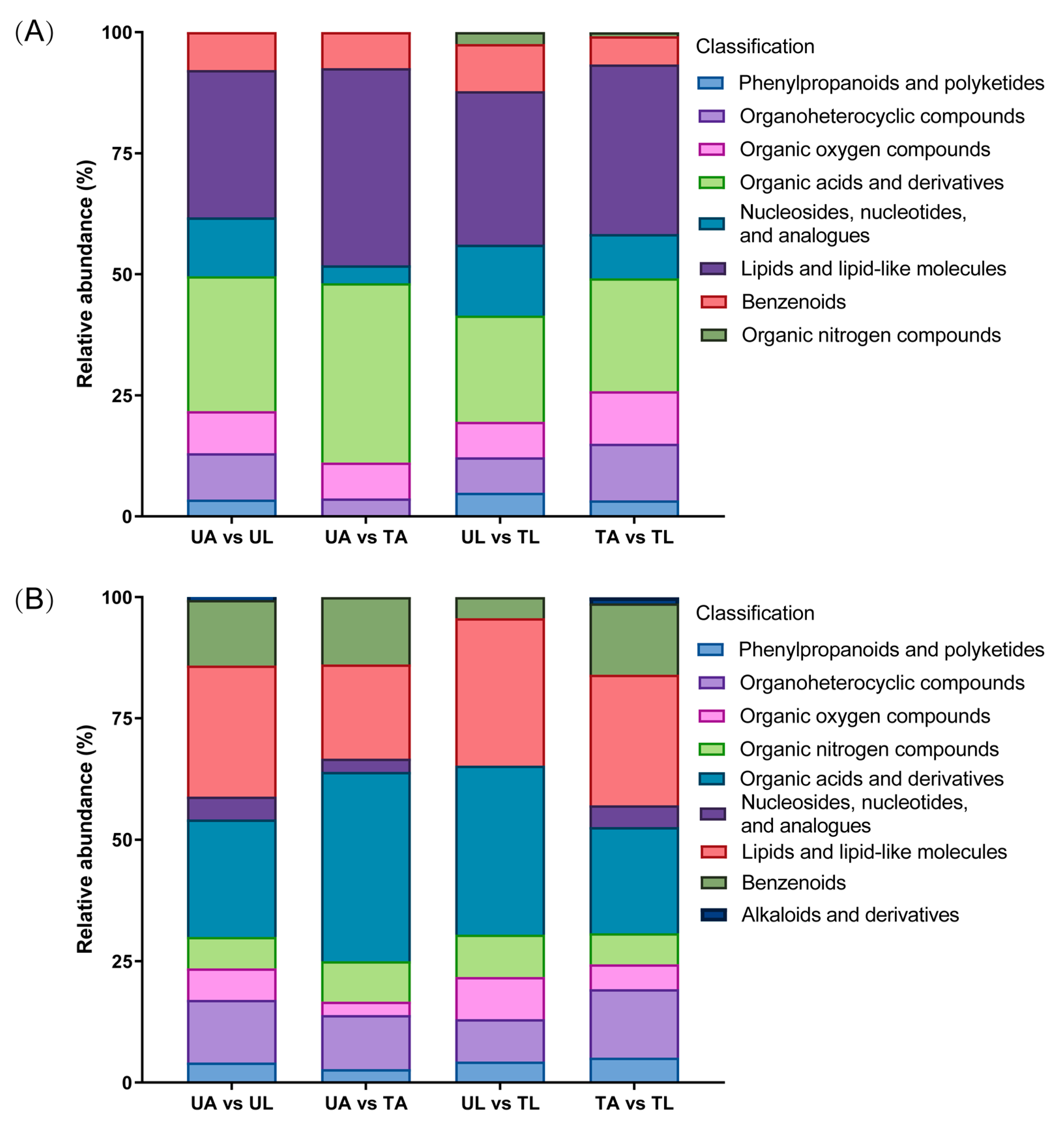
2.3. Classes of DEMs
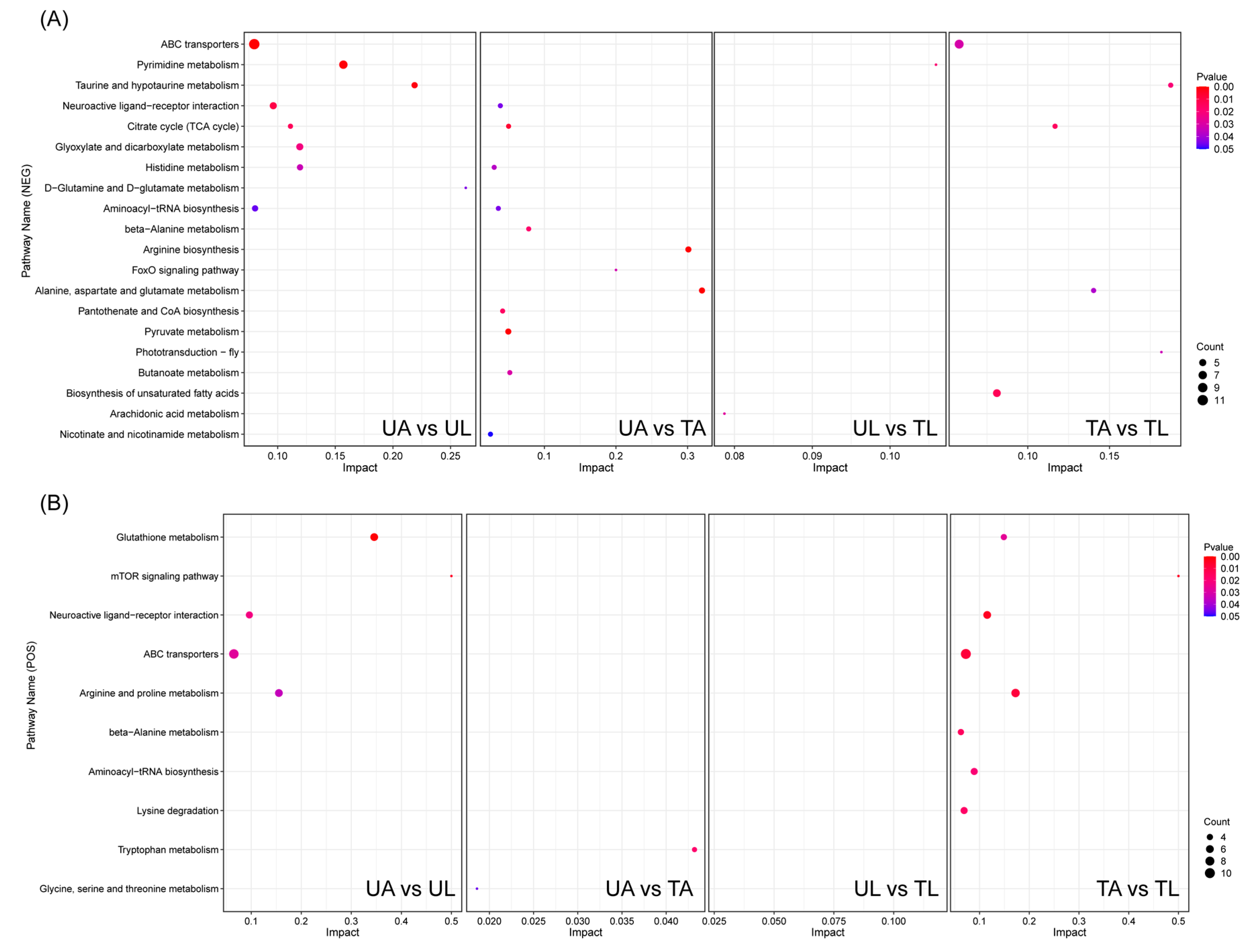
2.4. KEGG Pathway Enrichment Analysis
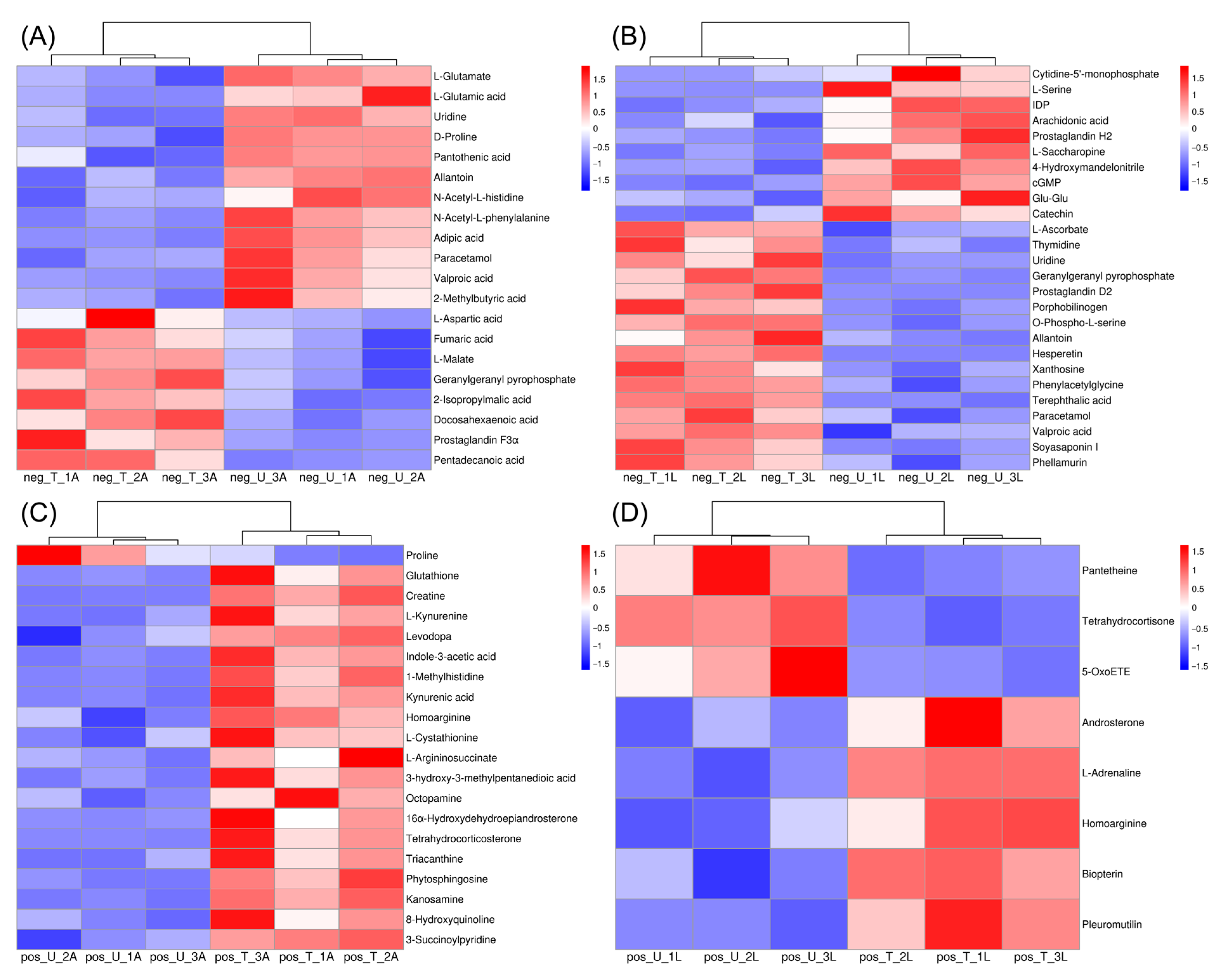
2.5. Expression of DEMs
3. Discussion
4. Material and Methods
4.1. Insects and Chemicals
4.2. Topical Toxicity
4.3. Metabolomic Data Acquisition and Processing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calcaterra, L.A.; Livore, J.P.; Delgado, A.; Briano, J.A. Ecological dominance of the red imported fire ant, Solenopsis invicta, in its native range. Oecologia 2008, 156, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Gotzek, D.; Ross, K.G. Experimental conversion of colony social organization in fire ants (Solenopsis invicta): Worker genotype manipulation in the absence of queen effects. J. Insect Behav. 2008, 21, 337–350. [Google Scholar] [CrossRef]
- Ross, K.G.; Keller, L. Genetic control of social organization in an ant. Proc. Natl. Acad. Sci. USA 1998, 95, 14232–14237. [Google Scholar] [CrossRef]
- Wanchoo, A.; Zhang, W.; Ortiz-Urquiza, A.; Boswell, J.; Xia, Y.X.; Keyhani, N.O. Red imported fire ant (Solenopsis invicta) chemosensory proteins are expressed in tissue, developmental, and caste-specific patterns. Front. Physiol. 2020, 11, 585883. [Google Scholar] [CrossRef] [PubMed]
- Dussutour, A.; Simpson, S.J. Communal nutrition in ants. Curr. Biol. 2009, 19, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Drees, B.M.; Calixto, A.A.; Nester, P.R. Integrated pest management concepts for red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). Insect Sci. 2013, 20, 429–438. [Google Scholar] [CrossRef]
- Dryden, M.W.; Smith, V.; Bennett, T.; Math, L.; Kallman, J.; Heaney, K.; Sun, F. Efficacy of fluralaner flavored chews (Bravecto®) administered to dogs against the adult cat flea, Ctenocephalides felis felis and egg production. Parasites Vectors 2015, 8, 364. [Google Scholar] [CrossRef]
- Burgio, F.; Meyer, L.; Armstrong, R. A comparative laboratory trial evaluating the immediate efficacy of fluralaner, afoxolaner, sarolaner and imidacloprid + permethrin against adult Rhipicephalus sanguineus (sensu lato) ticks attached to dogs. Parasites Vectors 2016, 9, 626. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Matsui, H.; Nakahira, K. Differential mechanisms of action of the novel γ-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil. Pest Manag. Sci. 2015, 71, 91–95. [Google Scholar] [CrossRef]
- Zhao, C.; Casida, J.E. Insect γ-aminobutyric acid receptors and isoxazoline insecticides: Toxicological profiles relative to the binding sites of [3H]fluralaner, [3H]-4′-ethynyl-4-n-propylbicycloorthobenzoate, and [3H]avermectin. J. Agric. Food Chem. 2014, 62, 1019–1024. [Google Scholar] [CrossRef]
- Sheng, C.W.; Jia, Z.Q.; Liu, D.; Wu, H.Z.; Luo, X.M.; Song, P.P.; Xu, L.; Peng, Y.C.; Han, Z.J.; Zhao, C.Q. Insecticidal spectrum of fluralaner to agricultural and sanitary pests. J. Asia-Pac. Entomol. 2017, 20, 1213–1218. [Google Scholar] [CrossRef]
- Liu, D.; Jia, Z.Q.; Peng, Y.C.; Sheng, C.W.; Tang, T.; Xu, L.; Han, Z.J.; Zhao, C.Q. Toxicity and sublethal effects of fluralaner on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2018, 152, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Khan, M.M.; Fajar, A.; Chen, S.; Guo, M.; Chen, Y.; Yang, C.; Wu, J.; Qiu, B.; Zhou, X.; et al. Toxicity of fluralaner against vegetable pests and its sublethal impact on a biocontrol predatory ladybeetle. Ecotoxicol. Environ. Saf. 2021, 225, 112743. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.Q.; Liu, D.; Sheng, C.W.; Casida, J.E.; Wang, C.; Song, P.P.; Chen, Y.M.; Han, Z.J.; Zhao, C.Q. Acute toxicity, bioconcentration, elimination and antioxidant effects of fluralaner in zebrafish, Danio rerio. Environ. Pollut. 2018, 232, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Qiu, X.H.; Ling, S.Q.; Liu, J.L.; Zeng, X.N. Interaction of fipronil and the red imported fire ant (Solenopsis invicta): Toxicity differences and detoxification responses. J. Insect Physiol. 2019, 115, 20–26. [Google Scholar] [CrossRef]
- Tingle, C.C.D.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 2003, 176, 1–66. [Google Scholar]
- Jiang, S.; Tsikolia, M.; Bernier, U.R.; Bloomquist, J.R. Mosquitocidal activity and mode of action of the Isoxazoline Fluralaner. Int. J. Environ. Res. Public Health 2017, 14, 154. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, T.; Feng, G.; Feng, Y.; Zhang, A.; Grodowitz, M.J. Insecticidal activity of methyl benzoate analogs against red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2018, 112, 691–698. [Google Scholar] [CrossRef]
- Xiong, T.; Ling, S.; Liu, J.; Zeng, X. Insecticidal and P450 mediate metabolism of fluralaner against red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Pestic. Biochem. Physiol. 2022, 187, 105184. [Google Scholar] [CrossRef]
- Du, M.; Yin, Z.; Xu, K.; Huang, Y.; Xu, Y.; Wen, W.; Zhang, Z.; Xu, H.; Wu, X. Integrated mass spectrometry imaging and metabolomics reveals sublethal effects of indoxacarb on the red fire ant Solenopsis invicta. Pest Manag. Sci. 2023, 79, 3122–3132. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Ogg, C.L. Prostaglandin biosynthesis by fat body from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 1994, 24, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Burton, S.; Wang, Y.; Xu, S.; Zhang, W.; Yu, L. Metabolomic analysis of honeybee, Apis mellifera L. response to thiacloprid. Pestic. Biochem. Phys. 2018, 152, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Liu, T.; Chai, Y.; Ji, D.; Chen, Y.; Hu, J.; Yao, H.; Lin, Q.; Wen, T.; Shi, B. Efficient detection of L-aspartic acid and L-glutamic acid by self-assembled fluorescent microparticles with AIE and FRET activities. Org. Biomol. Chem. 2023, 21, 4022. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Delgado-Coello, B.A. Influence of pipecolic acid on the release and uptake of [3H]GABA from brain slices of mouse cerebral cortex. Neurochem. Res. 1989, 14, 405–408. [Google Scholar] [CrossRef]
- Kawasaki, H.; Hori, T.; Nakajima, M.; Takeshita, K. Plasma levels of pipecolic acid in patients with chronic liver disease. Hepatology 1988, 8, 286–289. [Google Scholar] [CrossRef]
- Takahama, K.; Miyata, T.; Hashimoto, T.; Okano, Y.; Hitoshi, T.; Kase, Y. Pipecolic acid: A new type of α-amino acid possessing bicuculline-sensitive action in the mammalian brain. Brain Res. 1982, 239, 294–298. [Google Scholar] [CrossRef]
- Takahama, K.; Hashimoto, T.; Wang, M.W.; Akaike, N.; Hitoshi, T.; Okano, Y.; Kasé, Y.; Miyata, T. Pipecolic acid enhancement of GABA route in the rat brain. Neuropharmacology 1986, 25, 339–342. [Google Scholar] [CrossRef]
- Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Kruszelnicka, O.; Rycaj, J.; Chyrchel, B.; Surdacki, A.; Bode-Böger, S.M. Opposite associations of plasma homoarginine and ornithine with arginine in healthy children and adolescents. Int. J. Mol. Sci. 2013, 14, 21819–21832. [Google Scholar] [CrossRef]
- Popolo, A.; Adesso, S.; Pinto, A.; Autore, G.; Marzocco, S. L-Arginine and its metabolites in kidney and cardiovascular disease. Amino Acids 2014, 46, 2271–2286. [Google Scholar] [CrossRef]
- Patetsini, E.; Dimitriadis, V.; Kaloyianni, M. Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat. Toxicol. 2013, 126, 338–345. [Google Scholar] [CrossRef]
- Weihrauch, D.; O’Donnell, M.J. Mechanisms of nitrogen excretion in insects. Curr. Opin. Insect Sci. 2021, 47, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Holstein, S.A.; Hohl, R.J. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal. Biochem. 2005, 336, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Dunford, J.E.; Bunkoczi, G.; Russell, R.G.G.; Oppermann, U. The crystal structure of human geranylgeranyl pyrophosphate synthase reveals a novel hexameric arrangement and inhibitory product binding. J. Biol. Chem. 2006, 281, 22004–22012. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, K.S.; Aly, N.M.; Mahmoud, F.H.; Kenawy, A.; El-Sebae, A.K.H. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem. Toxicol. 2010, 48, 215–221. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- John, X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar]
- Hideg, E.; Jansen, M.A.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Stevens, R.; Page, D.; Gouble, B.; Garchery, C.; Zamir, D.; Causse, M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 2008, 31, 1086–1096. [Google Scholar] [CrossRef]
- Lv, N.; Ma, K.; Li, R.; Liang, P.; Liang, P.; Gao, X. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2021, 212, 111969. [Google Scholar] [CrossRef]
- Gao, Z.; Batool, R.; Xie, W.; Huang, X.; Wang, Z. Transcriptome and metabolome analysis reveals the importance of amino-acid metabolism in Spodoptera frugiperda exposed to spinetoram. Insects 2022, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.T.; Nusbaum, R.; Zack, J.A.; et al. Interferoninducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Lee, W.J. Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr. Opin. Insect Sci. 2018, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T. Development of selective immune tolerance towards the allogeneic fetus during pregnancy: Role of tryptophan catabolites. Int. J. Mol. Med. 2010, 25, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, Glycine: A review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar]
- Sceniak, M.P.; Spitsbergen, J.B.; Sabo, S.L.; Yuan, Y.; Atchison, W.D. Acute neurotoxicant exposure induces hyperexcitability in mouse lumbar spinal motor neurons. J. Neurophysiol. 2020, 123, 1448–1459. [Google Scholar] [CrossRef]
- Humphries, P.; Pretorius, E.; Naude, H. Direct and indirect cellular effects of aspartame on the brain. Eur. J. Clin. Nutr. 2008, 62, 451–462. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Kobayashi, T.; Narumiya, S. Function of prostanoid receptors: Studies on knockout mice. Prostaglandins Lipid Mediat. 2002, 68–69, 557–573. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: Biosynthesis and biological actions. Front. Physiol. 2018, 9, 1927. [Google Scholar] [CrossRef] [PubMed]
- Tunaz, H.; Putnam, S.M.; Stanley, D.W. Prostaglandin biosynthesis by fat body from larvae of the beetle Zophobas atratus. Arch. Insect Biochem. Physiol. 2002, 49, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Stanley, D.; Kim, Y. An insect prostaglandin E2 synthase acts in immunity and reproduction. Front. Physiol. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef]
- Sajjadian, S.M.; Ahmed, S.; Baki, M.A.A.; Kim, Y. Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect, Spodoptera exigua. Gen. Comp. Endocrinol. 2020, 287, 113352. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting pyrimidine metabolism in the era of precision cancer medicine. Front. Oncol. 2021, 11, 684961. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D. Identifcation of polygyne and monogyne fre ant colonies (Solenopsis invicta) by multiplex PCR of Gp-9 alleles. Insect. Soc. 2003, 50, 199–200. [Google Scholar] [CrossRef]
| Caste/Developmental Stage | Time (h) | LD50 (95% CL) (ng/Insect) | Weight (mg) | N | p-Value | |
|---|---|---|---|---|---|---|
| Adult worker | 24 | 8.62 (7.75, 9.64) | 1.06 | 540 | 28.08 (13) | 0.242 |
| Larva | 24 | 1744.23 (1488.07, 2060.67) | 1.10 | 540 | 21.71 (13) | 0.594 |
| Class | DEMs (Neg) | DEMs (Pos) | ||
|---|---|---|---|---|
| UA vs. TA | UL vs. TL | UA vs. TA | UL vs. TL | |
| Phenylpropanoids and polyketides | - | Hesperetin (↑); Catechin (↓) | - | - |
| Organoheterocyclic compounds | Allantoin (↓) | Allantoin (↑); l-Ascorbate (↑) | Kynurenic acid (↑); Indole-3-acetic acid (↑) | Biopterin (↑) |
| Organic oxygen compounds | Pantothenic acid (↓) | - | l-Kynurenine (↑) | - |
| Organic nitrogen compounds | - | Porphobilinogen (↑) | Phytosphingosine (↑) | - |
| Organic acids and derivatives | N-Acetyl-l-phenylalanine (↓); l-Glutamate (↓); N-Acetyl-l-histidine (↓); l-Glutamic acid (↓); D-Proline (↓); l-Aspartic acid (↑); Fumaric acid (↑); l-Malate (↑) | O-Phospho-l-serine (↑); l-Saccharopine (↓); l-Serine (↓); Phenylacetylglycine (↑); Glutamyl-glutamic acid (↓) | Homoarginine (↑); Creatine (↑); 1-Methylhistidine (↑); 3-Succinoylpyridine (↑); l-Argininosuccinate (↑); l-Cystathionine (↑); Glutathione (↑); Levodopa (↑); Proline (↓) | Homoarginine (↑); Pantetheine (↓) |
| Nucleosides, nucleotides, and analogues | Uridine (↓) | Uridine (↑); cGMP (↓); Xanthosine (↑); Thymidine (↑); Inosine diphosphate (↓); Cytidine-5’-monophosphate (↓) | - | - |
| Lipids and lipid-like molecules | Geranylgeranyl pyrophosphate (↑); Valproic acid (↓); Pentadecanoic acid (↑); Adipic acid (↓); Prostaglandin F3α (↑); Docosahexaenoic acid (↑); 2-Methylbutyric acid (↓); 2-Isopropylmalic acid (↑) | Geranylgeranyl pyrophosphate (↑); Valproic acid (↑); Soyasaponin I (↑); Prostaglandin D2 (↑); Phellamurin (↑); Prostaglandin H2 (↓); Arachidonic acid (↓) | 3-hydroxy-3-methylpentanedioic acid (↑); Tetrahydrocorticosterone (↑); 16α-Hydroxydehydroepiandrosterone (↑) | Tetrahydrocortisone (↓); Androsterone (↑); 5-OxoETE (↓) |
| Benzenoids | Paracetamol (↓) | Paracetamol (↑); Terephthalic acid (↑); 4-Hydroxymandelonitrile (↓) | Octopamine (↑) | l-Adrenaline (↑) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Wang, W.; Gong, X.; Yu, Y.; Xue, J.; Zeng, X.; Liu, J. The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages. Int. J. Mol. Sci. 2023, 24, 15627. https://doi.org/10.3390/ijms242115627
Shao L, Wang W, Gong X, Yu Y, Xue J, Zeng X, Liu J. The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages. International Journal of Molecular Sciences. 2023; 24(21):15627. https://doi.org/10.3390/ijms242115627
Chicago/Turabian StyleShao, Leyi, Wei Wang, Xin Gong, Yinghao Yu, Junao Xue, Xinnian Zeng, and Jiali Liu. 2023. "The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages" International Journal of Molecular Sciences 24, no. 21: 15627. https://doi.org/10.3390/ijms242115627
APA StyleShao, L., Wang, W., Gong, X., Yu, Y., Xue, J., Zeng, X., & Liu, J. (2023). The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages. International Journal of Molecular Sciences, 24(21), 15627. https://doi.org/10.3390/ijms242115627






