Tiliroside Protects against Lipopolysaccharide-Induced Acute Kidney Injury via Intrarenal Renin–Angiotensin System in Mice
Abstract
:1. Introduction
2. Results
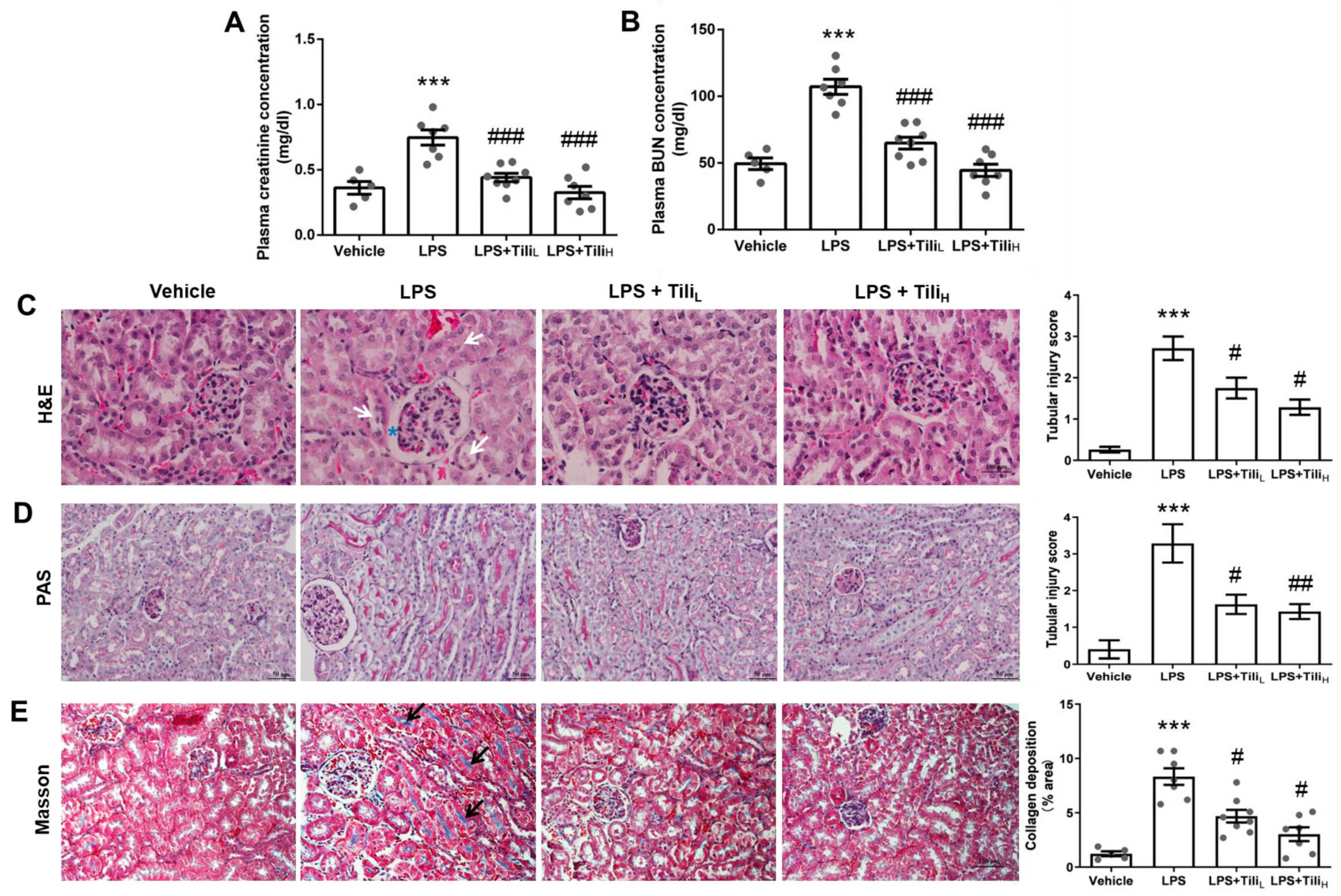
2.1. Tiliroside Ameliorated LPS-Induced Renal Dysfunction and Histological Abnormalities
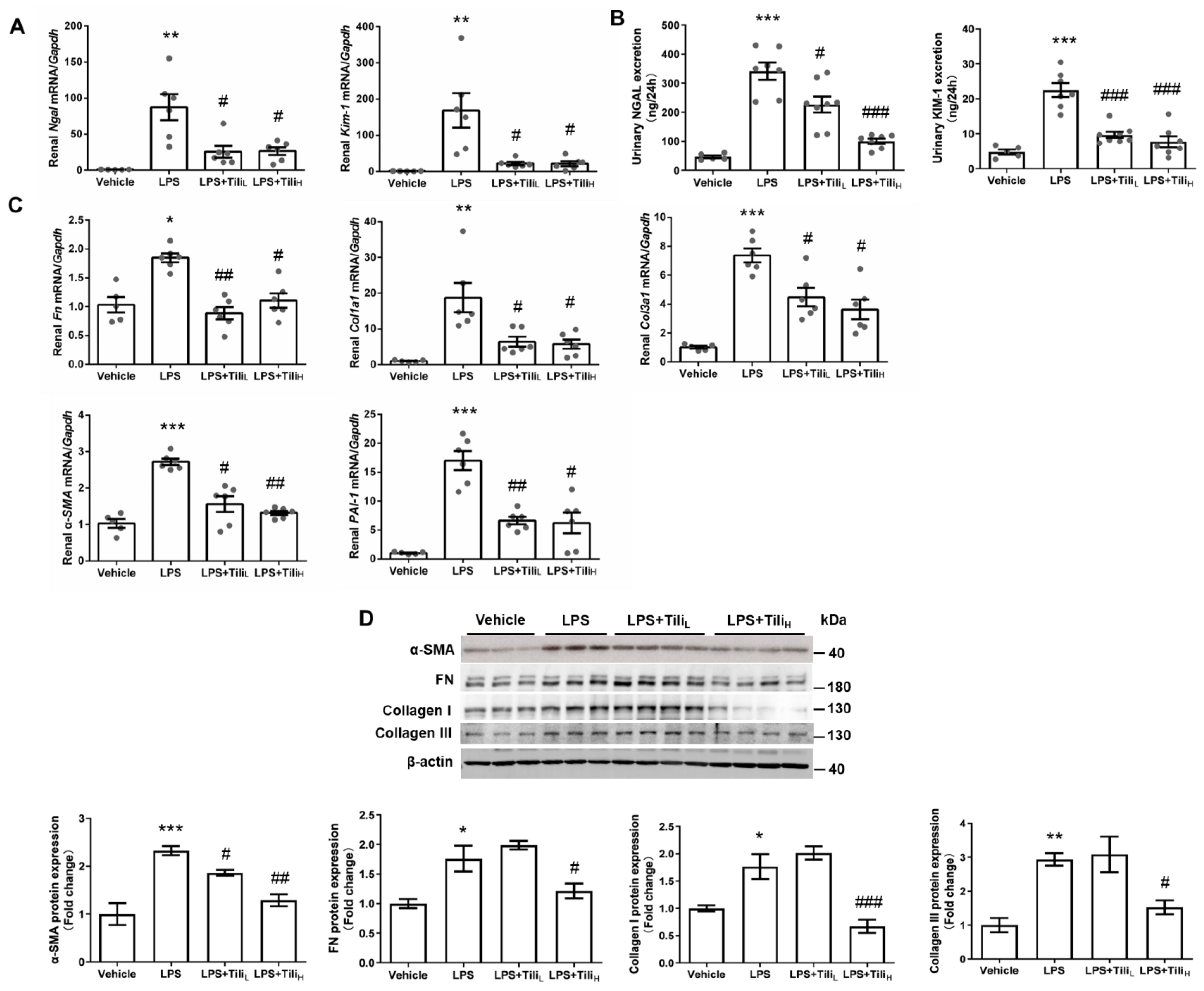
2.2. Tiliroside Suppressed LPS-Induced Renal fibrosis
2.3. Tiliroside Suppressed LPS-Induced Inflammation
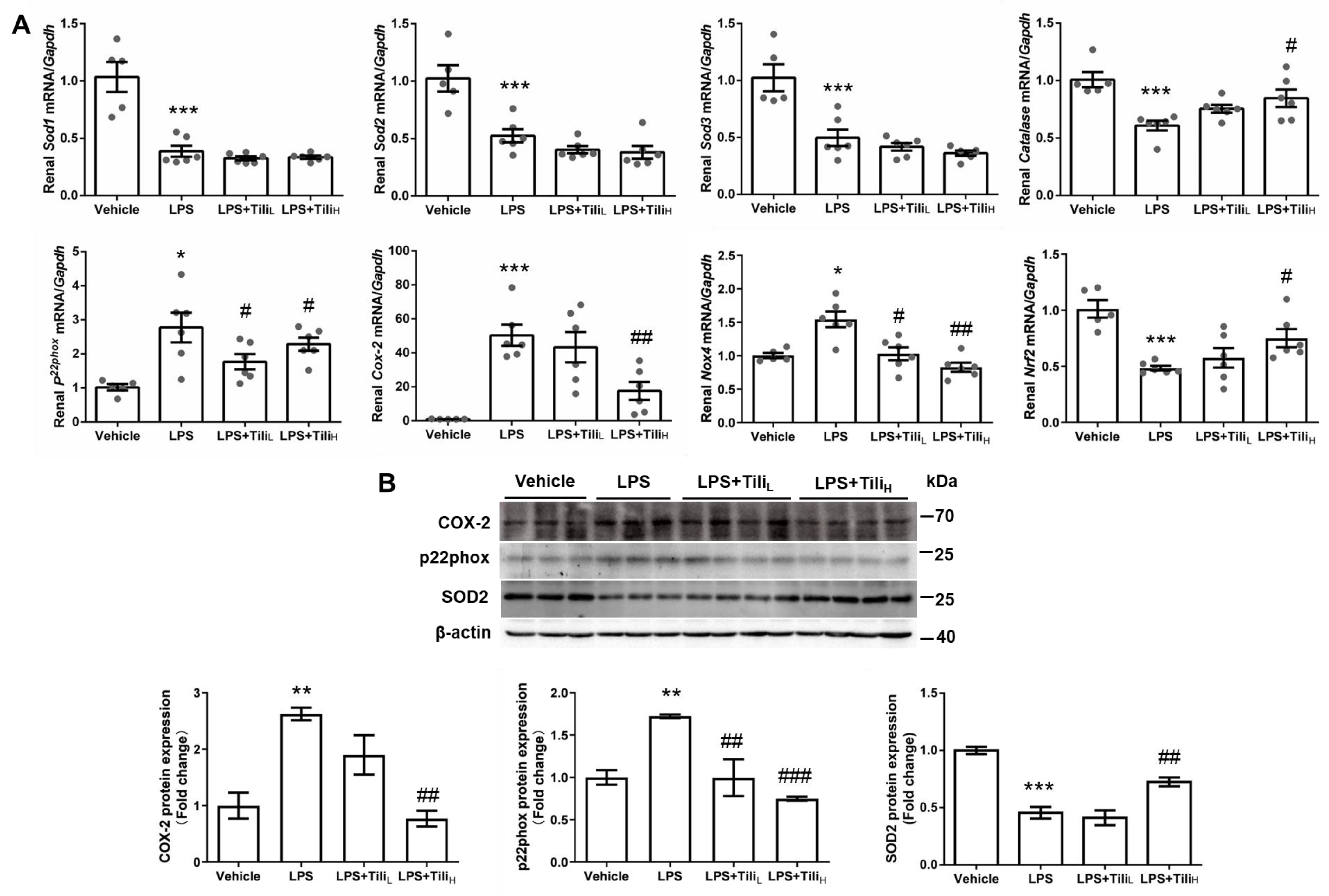
2.4. Tiliroside Inhibited LPS-Induced Oxidative Stress
2.5. Tiliroside Enhanced Autophagic Flux but Not the Formation of Autophagosomes
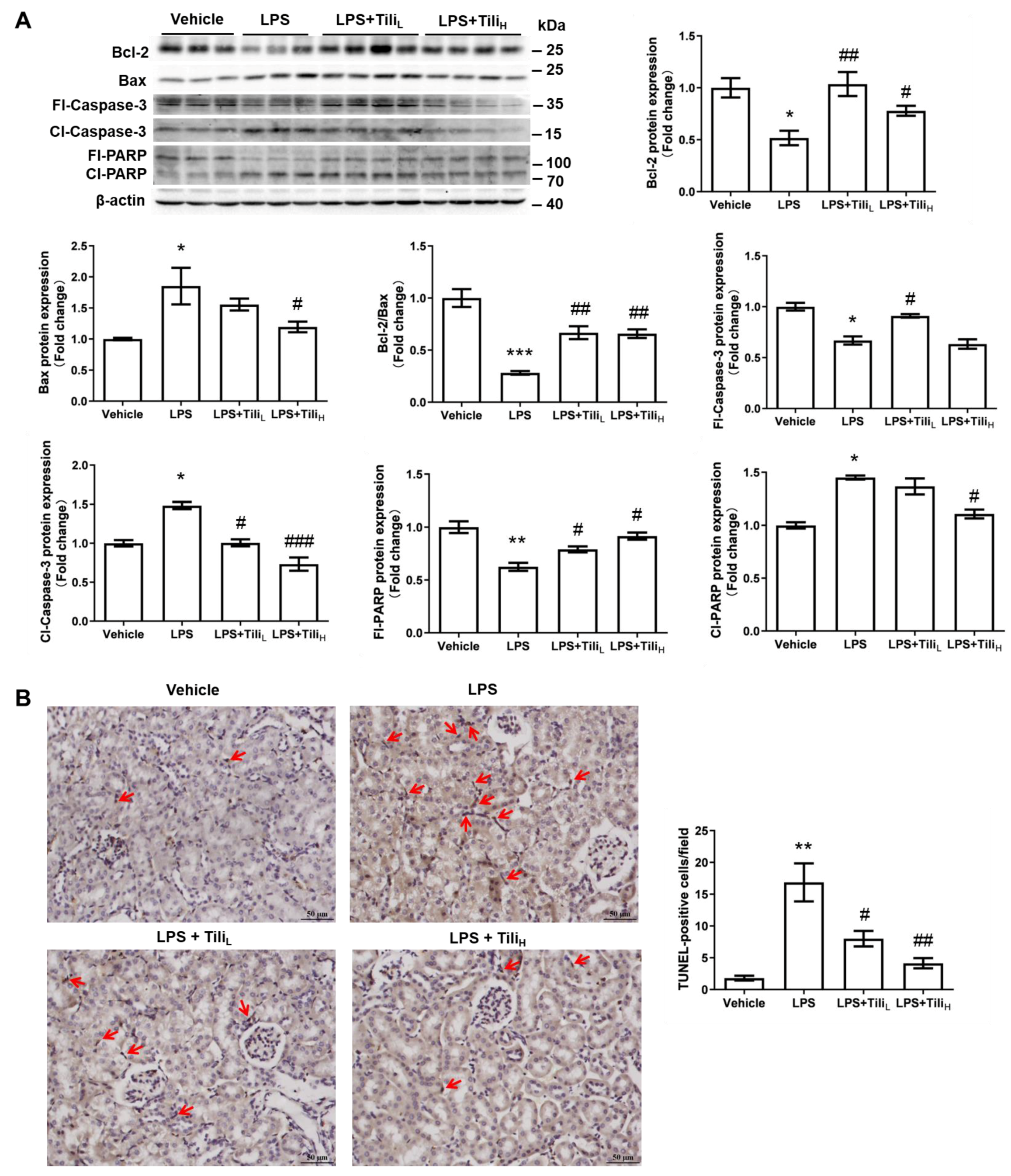
2.6. Tiliroside Abolished LPS-Induced Apoptosis
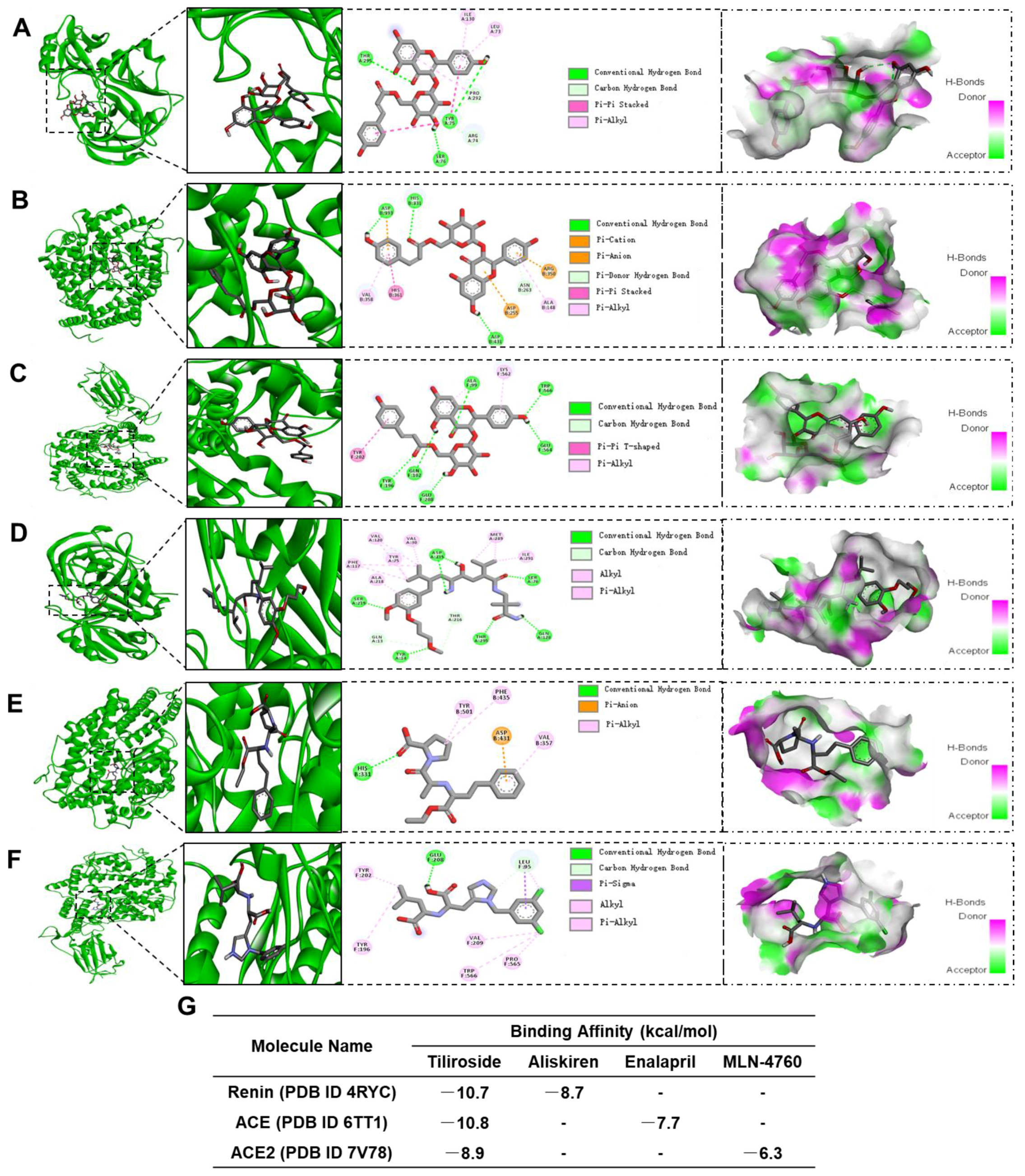
2.7. Tiliroside Regulated Intrarenal Renin-Angiotensin System
3. Discussion
4. Materials and Methods
4.1. Animal Care
4.2. Animal Treatment
4.3. Biochemical Analyses of Plasma and Kidney Tissues
4.4. Quantitative Reverse Transcriptase PCR (RT-qPCR)
4.5. Western Blotting (WB)
4.6. Histological Examination and Immunofluorescence Staining
4.7. Molecular Docking Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuura, R.; Doi, K.; Rabb, H. Acute kidney injury and distant organ dysfunction-network system analysis. Kidney Int. 2023, 103, 1041–1055. [Google Scholar] [PubMed]
- Yang, L.; Xing, G.; Wang, L.; Wu, Y.; Li, S.; Xu, G.; He, Q.; Chen, J.; Chen, M.; Liu, X.; et al. ISN AKF 0by25 China Consortiums. Acute kidney injury in China: A cross-sectional survey. Lancet 2015, 386, 1465–1471. [Google Scholar] [PubMed]
- Xu, C.; Chen, Y.; Yu, J. Foe and friend in the COVID-19-associated acute kidney injury: An insight on intrarenal renin-angiotensin system. Acta Biochim. Biophys. Sin. 2022, 54, 1–11. [Google Scholar] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar]
- Tigerstedt, R.; Bergman, P.G. Niere und kreislauf. Scand Arch. Physiol. 1898, 8, 223–271. [Google Scholar]
- Crowley, S.D.; Rudemiller, N.P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2017, 28, 1350–1361. [Google Scholar]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar]
- Kim, K.; Leem, J. Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice. Molecules 2022, 27, 2019. [Google Scholar]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar]
- Zhu, Y.; Fu, Y.; Lin, H. Baicalin Inhibits Renal Cell Apoptosis and Protects against Acute Kidney Injury in Pediatric Sepsis. Med. Sci. Monit. 2016, 22, 5109–5115. [Google Scholar] [PubMed]
- Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids-Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022, 23, 2176. [Google Scholar] [PubMed]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [PubMed]
- Chávez-Morales, Y.; Jiménez-Ferrer, E.; Martínez-Hernández, G.B.; Tortoriello, J.; Román-Ramos, R.; Zamilpa, A.; Herrera-Ruiz, M. Effect of Standardized Fractions and Tiliroside from Leaves of Tilia americana on Depression Tests in Mice. Iran J. Pharm. Res. 2019, 18, 1931–1946. [Google Scholar] [PubMed]
- Lagunas-Herrera, H.; Tortoriello, J.; Herrera-Ruiz, M.; Martínez-Henández, G.B.; Zamilpa, A.; Santamaría, L.A.; Lorenzana, M.G.; Lombardo-Earl, G.; Jiménez-Ferrer, E. Acute and Chronic Antihypertensive Effect of Fractions, Tiliroside and Scopoletin from Malva parviflora. Biol. Pharm. Bull. 2019, 42, 18–25. [Google Scholar]
- Silva, G.C.; Pereira, A.C.; Rezende, B.A.; da Silva, J.P.; Cruz, J.S.; de Souza Mde, F.; Gomes, R.A.; Teles, Y.C.; Cortes, S.F.; Lemos, V.S. Mechanism of the antihypertensive and vasorelaxant effects of the flavonoid tiliroside in resistance arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar]
- Han, R.; Yang, H.; Ling, C.; Lu, L. Tiliroside suppresses triple-negative breast cancer as a multifunctional CAXII inhibitor. Cancer Cell Int. 2022, 22, 368. [Google Scholar] [PubMed]
- Yang, C.; Lu, T.; Liu, M.; Yuan, X.; Li, D.; Zhang, J.; Zhou, L.; Xu, M. Tiliroside targets TBK1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine 2023, 111, 154668. [Google Scholar]
- Li, K.; Xiao, Y.; Wang, Z.; Fu, F.; Shao, S.; Song, F.; Zhao, J.; Lin, X.; Liu, Q.; Xu, J. Tiliroside is a new potential therapeutic drug for osteoporosis in mice. J. Cell Physiol. 2019, 234, 16263–16274. [Google Scholar]
- Zhuang, H.; Lv, Q.; Zhong, C.; Cui, Y.; He, L.; Zhang, C.; Yu, J. Tiliroside Ameliorates Ulcerative Colitis by Restoring the M1/M2 Macrophage Balance via the HIF-1α/glycolysis Pathway. Front. Immunol. 2021, 12, 649463. [Google Scholar] [PubMed]
- Goto, T.; Teraminami, A.; Lee, J.Y.; Ohyama, K.; Funakoshi, K.; Kim, Y.I.; Hirai, S.; Uemura, T.; Yu, R.; Takahashi, N.; et al. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese-diabetic mice. J. Nutr. Biochem. 2012, 23, 768–776. [Google Scholar] [PubMed]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [PubMed]
- Gwon, M.G.; Gu, H.; Leem, J.; Park, K.K. Protective Effects of 6-Shogaol, an Active Compound of Ginger, in a Murine Model of Cisplatin-Induced Acute Kidney Injury. Molecules 2021, 26, 5931. [Google Scholar]
- Pak, E.S.; Uddin, M.J.; Ha, H. Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice. Int. J. Mol. Sci. 2020, 21, 8246. [Google Scholar]
- Han, Y.P.; Liu, L.J.; Yan, J.L.; Chen, M.Y.; Meng, X.F.; Zhou, X.R.; Qian, L.B. Autophagy and its therapeutic potential in diabetic nephropathy. Front. Endocrinol. 2023, 14, 1139444. [Google Scholar]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese Herbal Medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar]
- Li, H.D.; Meng, X.M.; Huang, C.; Zhang, L.; Lv, X.W.; Li, J. Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 2019, 10, 376. [Google Scholar]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 Pathway Contributes to Neuroprotection by the Dietary Flavonoid Tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar]
- Kim, J.Y.; Leem, J.; Park, K.K. Antioxidative, Antiapoptotic, and Anti-Inflammatory Effects of Apamin in a Murine Model of Lipopolysaccharide-Induced Acute Kidney Injury. Molecules 2020, 25, 5717. [Google Scholar]
- Corrêa, W.R.; Serain, A.F.; Aranha Netto, L.; Marinho, J.V.N.; Arena, A.C.; Figueiredo de Santana Aquino, D.; Kuraoka-Oliveira, Â.M.; Júnior, A.J.; Bernal, L.P.T.; Kassuya, C.A.L.; et al. Anti-Inflammatory and Antioxidant Properties of the Extract, Tiliroside, and Patuletin 3-O-β-D-Glucopyranoside from Pfaffia townsendii (Amaranthaceae). Evid. Based Complement. Alternat. Med. 2018, 2018, 6057579. [Google Scholar] [PubMed]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim. Biophys. Acta. 2014, 1840, 3311–3319. [Google Scholar] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [PubMed]
- Kim, J.Y.; Jo, J.; Leem, J.; Park, K.K. Inhibition of p300 by Garcinol Protects against Cisplatin-Induced Acute Kidney Injury through Suppression of Oxidative Stress, Inflammation, and Tubular Cell Death in Mice. Antioxidants 2020, 9, 1271. [Google Scholar] [PubMed]
- Watson, A.M.; Olukman, M.; Koulis, C.; Tu, Y.; Samijono, D.; Yuen, D.; Lee, C.; Behm, D.J.; Cooper, M.E.; Jandeleit-Dahm, K.A.; et al. Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: Studies in humans and in a mouse model of diabetes. Diabetologia 2013, 56, 1155–1165. [Google Scholar]
- Ow, C.P.C.; Trask-Marino, A.; Betrie, A.H.; Evans, R.G.; May, C.N.; Lankadeva, Y.R. Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. J. Clin. Med. 2021, 10, 3798. [Google Scholar]
- Koçkara, A.; Kayataş, M. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren. Fail. 2013, 35, 291–294. [Google Scholar]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar]
- Zalba, G.; San José, G.; Moreno, M.U.; Fortuño, A.; Díez, J. NADPH oxidase-mediated oxidative stress: Genetic studies of the p22(phox) gene in hypertension. Antioxid. Redox Signal. 2005, 7, 1327–1336. [Google Scholar]
- Zhao, S.; Cheng, C.K.; Zhang, C.L.; Huang, Y. Interplay Between Oxidative Stress, Cyclooxygenases, and Prostanoids in Cardiovascular Diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar]
- Yoo, J.Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. LPS-Induced Acute Kidney Injury Is Mediated by Nox4-SH3YL. Cell Rep. 2020, 33, 108245. [Google Scholar] [PubMed]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vázquez-Carballo, C.; Palomino-Antolín, A.; García-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.L.; Herencia, C.; Mas, S.; Ortiz, A.; et al. Protective Role of Nrf2 in Renal Disease. Antioxidants 2020, 10, 39. [Google Scholar] [PubMed]
- Feng, L.X.; Zhao, F.; Liu, Q.; Peng, J.C.; Duan, X.J.; Yan, P.; Wu, X.; Wang, H.S.; Deng, Y.H.; Duan, S.B. Role of Nrf2 in Lipopolysaccharide-Induced Acute Kidney Injury: Protection by Human Umbilical Cord Blood Mononuclear Cells. Oxid. Med. Cell Longev. 2020, 2020, 6123459. [Google Scholar] [PubMed]
- Xu, C.; Yi, X.; Tang, L.; Wang, H.; Chu, S.; Yu, J. Differential Regulation of Autophagy on Urine-Concentrating Capability through Modulating the Renal AQP2 Expression and Renin-Angiotensin System in Mice. Am. J. Physiol. Renal. Physiol. 2023, 325, F503–F518. [Google Scholar] [PubMed]
- Cui, J.; Bai, X.; Chen, X. Autophagy and Acute Kidney Injury. Adv. Exp. Med. Biol. 2020, 1207, 469–480. [Google Scholar] [PubMed]
- Zhao, S.; Liao, J.; Shen, M.; Li, X.; Wu, M. Epigenetic dysregulation of autophagy in sepsis-induced acute kidney injury: The underlying mechanisms for renoprotection. Front. Immunol. 2023, 14, 1180866. [Google Scholar]
- Xu, G.; Mo, L.; Wu, C.; Shen, X.; Dong, H.; Yu, L.; Pan, P.; Pan, K. The miR-15a-5p-XIST-CUL3 regulatory axis is important for sepsis-induced acute kidney injury. Ren. Fail 2019, 41, 955–966. [Google Scholar]
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension 2017, 69, 339–348. [Google Scholar]
- Xu, C.; Wang, F.; Chen, Y.; Xie, S.; Sng, D.; Reversade, B.; Yang, T. ELABELA antagonizes intrarenal renin-angiotensin system to lower blood pressure and protects against renal injury. Am. J. Physiol. Renal. Physiol. 2020, 318, F1122–F1135. [Google Scholar]
- Cao, Y.; Liu, Y.; Shang, J.; Yuan, Z.; Ping, F.; Yao, S.; Guo, Y.; Li, Y. Ang-(1-7) treatment attenuates lipopolysaccharide-induced early pulmonary fibrosis. Lab. Investig. 2019, 99, 1770–1783. [Google Scholar]
- Hagiwara, S.; Iwasaka, H.; Hidaka, S.; Hasegawa, A.; Koga, H.; Noguchi, T. Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med. 2009, 35, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [PubMed]
- Yin, X.; Wang, M.; Xia, Z. In vitro evaluation of intestinal absorption of tiliroside from Edgeworthia gardneri (Wall.) Meisn. Xenobiotica 2021, 51, 728–736. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [PubMed]
- Xu, C.; Chen, Y.; Ramkumar, N.; Zou, C.J.; Sigmund, C.D.; Yang, T. Collecting duct renin regulates potassium homeostasis in mice. Acta Physiol. 2023, 237, e13899. [Google Scholar] [CrossRef]
- Xu, C.; Yang, G.; Fu, Z.; Chen, Y.; Xie, S.; Wang, F.; Yang, T. Na+-Retaining Action of COX-2 (Cyclooxygenase-2)/EP1 Pathway in the Collecting Duct via Activation of Intrarenal Renin-Angiotensin-Aldosterone System and Epithelial Sodium Channel. Hypertension 2022, 79, 1190–1202. [Google Scholar]
- Cao, W.; Xu, J.; Zhou, Z.M.; Wang, G.B.; Hou, F.F.; Nie, J. Advanced oxidation protein products activate intrarenal renin-angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxid. Redox Signal. 2013, 18, 19–35. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Y.; Song, K.; Huang, Y.; Zhang, L.; Zhang, C.; Yan, Q.; Gao, H. Pre-emptive pharmacological inhibition of fatty acid-binding protein 4 attenuates kidney fibrosis by reprogramming tubular lipid metabolism. Cell Death Dis. 2021, 12, 572. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided. Mol. Des. 2010, 24, 417–422. [Google Scholar]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [PubMed]





| Gene | Sequence (5′→3′) |
|---|---|
| Ngal | (F) GCAGGTGGTACGTTGTGGG |
| (R) CTCTTGTAGCTCATAGATGGTGC | |
| Fn | (F) ATGTGGACCCCTCCTGATAGT |
| (R) GCCCAGTGATTTCAGCAAAGG | |
| Col1a1 | (F) TAAGGGTCCCCAATGGTGAGA |
| (R) GGGTCCCTCGACTCCTACAT | |
| Col3a1 | (F) CTGTAACATGGAAACTGGGGAAA |
| (R) CCATAGCTGAACTGAAAACCACC | |
| α-SMA | (F) CCCAGACATCAGGGAGTAATGG |
| (R) TCTATCGGATACTTCAGCGTCA | |
| Kim-1 | (F) AGCAGTCGGTACAACTTAAAGG |
| (R) ACTCGACAACAATACAGACCAC | |
| PAI-1 | (F) TCTGGGAAAGGGTTCACTTTACC |
| (R) GACACGCCATAGGGAGAGAAG | |
| Tnf-α | (F) CCCTCACACTCAGATCATCTTCT |
| (R) GCTACGACGTGGGCTACAG | |
| Mcp-1 | (F) TTAAAAACCTGGATCGGAACCAA |
| (R) GCATTAGCTTCAGATTTACGGGT | |
| Tgf-β | (F) TACGCCTGAGTGGCTGTCTT |
| (R) CGTGGAGTTTGTTATCTTTGCT | |
| Il-6 | (F) TAGTCCTTCCTACCCCAATTTCC |
| (R) TTGGTCCTTAGCCACTCCTTC | |
| Il-17a | (F) TCAGCGTGTCCAAACACTGAG |
| (R) CGCCAAGGGAGTTAAAGACTT | |
| Il-18 | (F) GTGAACCCCAGACCAGACTG |
| (R) CCTGGAACACGTTTCTGAAAGA | |
| Il-1β | (F) GAAATGCCACCTTTTGACAGTG |
| (R) TGGATGCTCTCATCAGGACAG | |
| Il-23a | (F) ATGCTGGATTGCAGAGCAGTA |
| (R) ACGGGGCACATTATTTTTAGTCT | |
| Sod1 | (F) AACCAGTTGTGTTGTCAGGAC |
| (R) CCACCATGTTTCTTAGAGTGAGG | |
| Sod2 | (F) TGGACAAACCTGAGCCCTAAG |
| (R) CCCAAAGTCACGCTTGATAGC | |
| Sod3 | (F) CCTTCTTGTTCTACGGCTTGC |
| (R) GCGTGTCGCCTATCTTCTCAA | |
| Catalase | (F) AGCGACCAGATGAAGCAGTG |
| (R) TCCGCTCTCTGTCAAAGTGTG | |
| p22phox | (F) AGCGATGTGGACAGAAGTACC |
| (R) CAGCCCGGACGTAGTAATTCC | |
| Cox-2 | (F) TGCACTATGGTTACAAAAGCTGG |
| (R) TCAGGAAGCTCCTTATTTCCCTT | |
| Nrf2 | (F) GCCCACATTCCCAAACAA |
| (R) TGTCCTGCTCTATGCTGCT | |
| Nox4 | (F) TGTTGGGCCTAGGATTGTGTT |
| (R) AGGGACCTTCTGTGATCCTCG | |
| Agt | (F) TGTGACAGGGTGGAAGATGA |
| (R) AGATCATGGGCACAGACACC | |
| Renin | (F) GTGACTGTGGGTGGAATCACTGT |
| (R) GCCAGCATGAAAGGGATCAG | |
| Ace | (F) TTGCTATGGGCATGGAAGAG |
| (R) CAGGTCTTGCTCCAGGTTGT | |
| Ace2 | (F) ACTACAGGCCCTTCAGCAAA |
| (R) TGTCGCCATTATTTCATCCA | |
| At1ar | (F) AACTCACAGCAACCCTCCAA |
| (R) ATCACCACCAAGCTGTTTCC | |
| Gapdh | (F) AGGTCGGTGTGAACGGATTTG |
| (R) TGTAGACCATGTAGTTGAGGTCA |
| Antibodies | Identifier | Source | Dilution |
|---|---|---|---|
| Anti-β-Actin | TA811000 | ORIGENE | 1:2000 |
| Anti-α-Smooth Muscle Actin | 19245T | CST | 1:1000 |
| Anti-FN/Fibronectin | WL03677 | Wanaleibio | 1:500 |
| Anti-Collagen I | WL0088 | Wanaleibio | 1:500 |
| Anti-Collagen III | WL03186 | Wanaleibio | 1:500 |
| Anti-TGF β1 | ab179695 | Abcam | 1:1000 |
| Anti-COX2 | ab179800 | Abcam | 1:1000 |
| Anti-p22-phox | WL03514 | Wanaleibio | 1:500 |
| Anti-mTOR | T55306F | Abmart | 1:1000 |
| Anti-Phospho-mTOR(S2448) | T56571F | Abmart | 1:500 |
| Anti-ATG5 | T55766F | Abmart | 1:1000 |
| Anti-Beclin 1 | 11306-1-AP | Proteintech | 1:1000 |
| Anti-LC3b | 2775s | CST | 1:1000 |
| Anti-p62 | 18420-1-AP | Proteintech | 1:1000 |
| Anti-Bcl-2 | 15071S | CST | 1:1000 |
| Anti-Bax | 14796S | CST | 1:1000 |
| Anti-Caspase-3/Cleaved Caspase-3 | WL02117 | Wanaleibio | 1:500 |
| Anti-PARP/Cleaved-PARP | WL01932 | Wanaleibio | 1:300 |
| Anti-ACE | ab39172 | Abcam | 1:1000 |
| Anti-ACE2 | ab15348 | Abcam | 1:1000 |
| Anti-SOD2 | WL02506 | Wanaleibio | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Xu, C.; Yang, J.; Zhong, C.; Yang, H.; Tang, L.; Song, S.; Yu, J. Tiliroside Protects against Lipopolysaccharide-Induced Acute Kidney Injury via Intrarenal Renin–Angiotensin System in Mice. Int. J. Mol. Sci. 2023, 24, 15556. https://doi.org/10.3390/ijms242115556
Yi X, Xu C, Yang J, Zhong C, Yang H, Tang L, Song S, Yu J. Tiliroside Protects against Lipopolysaccharide-Induced Acute Kidney Injury via Intrarenal Renin–Angiotensin System in Mice. International Journal of Molecular Sciences. 2023; 24(21):15556. https://doi.org/10.3390/ijms242115556
Chicago/Turabian StyleYi, Xiaoli, Chuanming Xu, Jing Yang, Chao Zhong, Huiru Yang, Le Tang, Shanshan Song, and Jun Yu. 2023. "Tiliroside Protects against Lipopolysaccharide-Induced Acute Kidney Injury via Intrarenal Renin–Angiotensin System in Mice" International Journal of Molecular Sciences 24, no. 21: 15556. https://doi.org/10.3390/ijms242115556
APA StyleYi, X., Xu, C., Yang, J., Zhong, C., Yang, H., Tang, L., Song, S., & Yu, J. (2023). Tiliroside Protects against Lipopolysaccharide-Induced Acute Kidney Injury via Intrarenal Renin–Angiotensin System in Mice. International Journal of Molecular Sciences, 24(21), 15556. https://doi.org/10.3390/ijms242115556





