A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses
Abstract
1. Introduction
2. Results
2.1. Production and Identification of Ad-E7 Adenovirus Vaccine
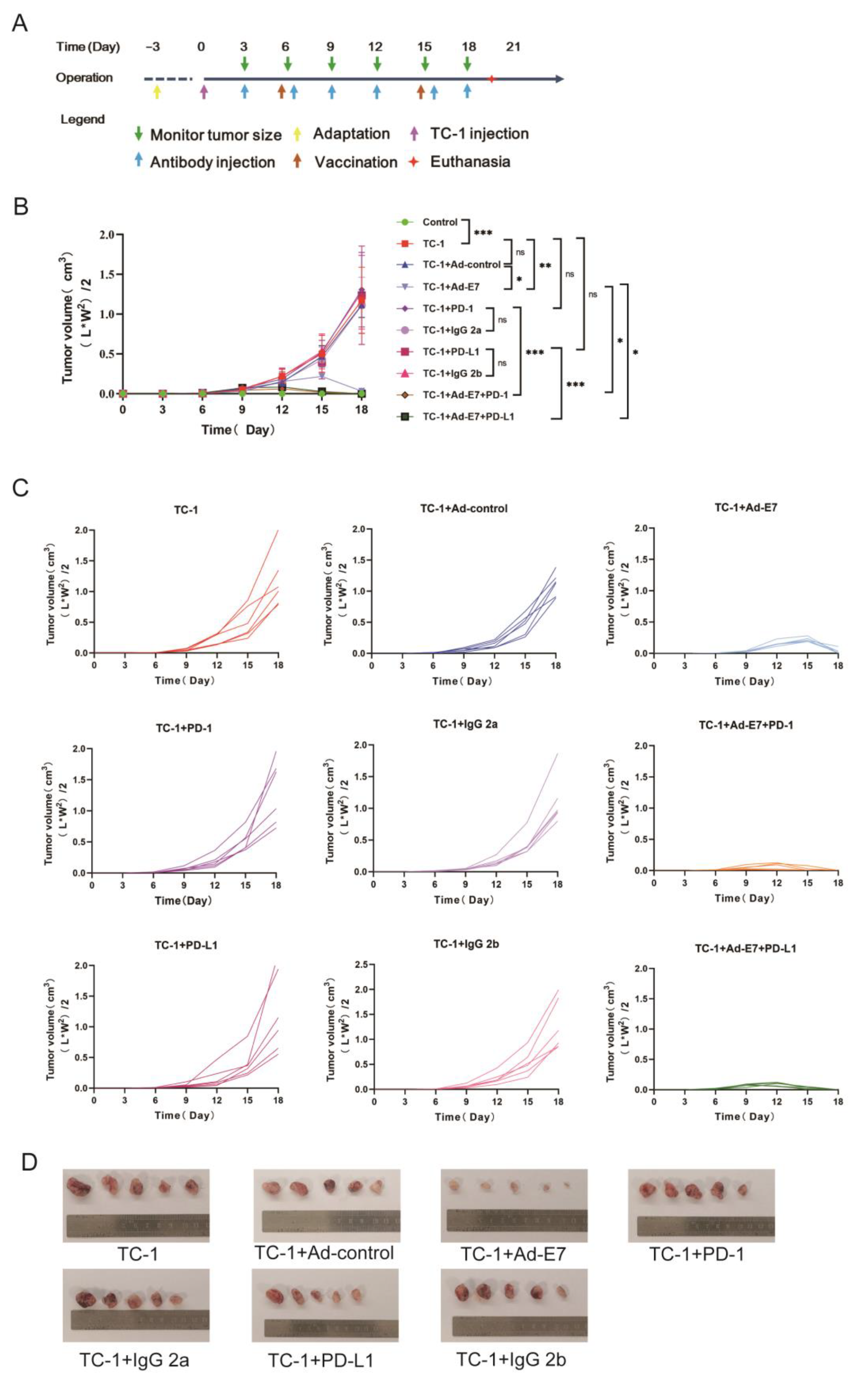
2.2. Ad-E7 Combined with PD-1/PD-L1 Antibody Inhibits Tumor Growth in Mice
2.3. Ad-E7 Combined with PD-1/PD-L1 Antibody Induces Strong CTL Responses in Spleen
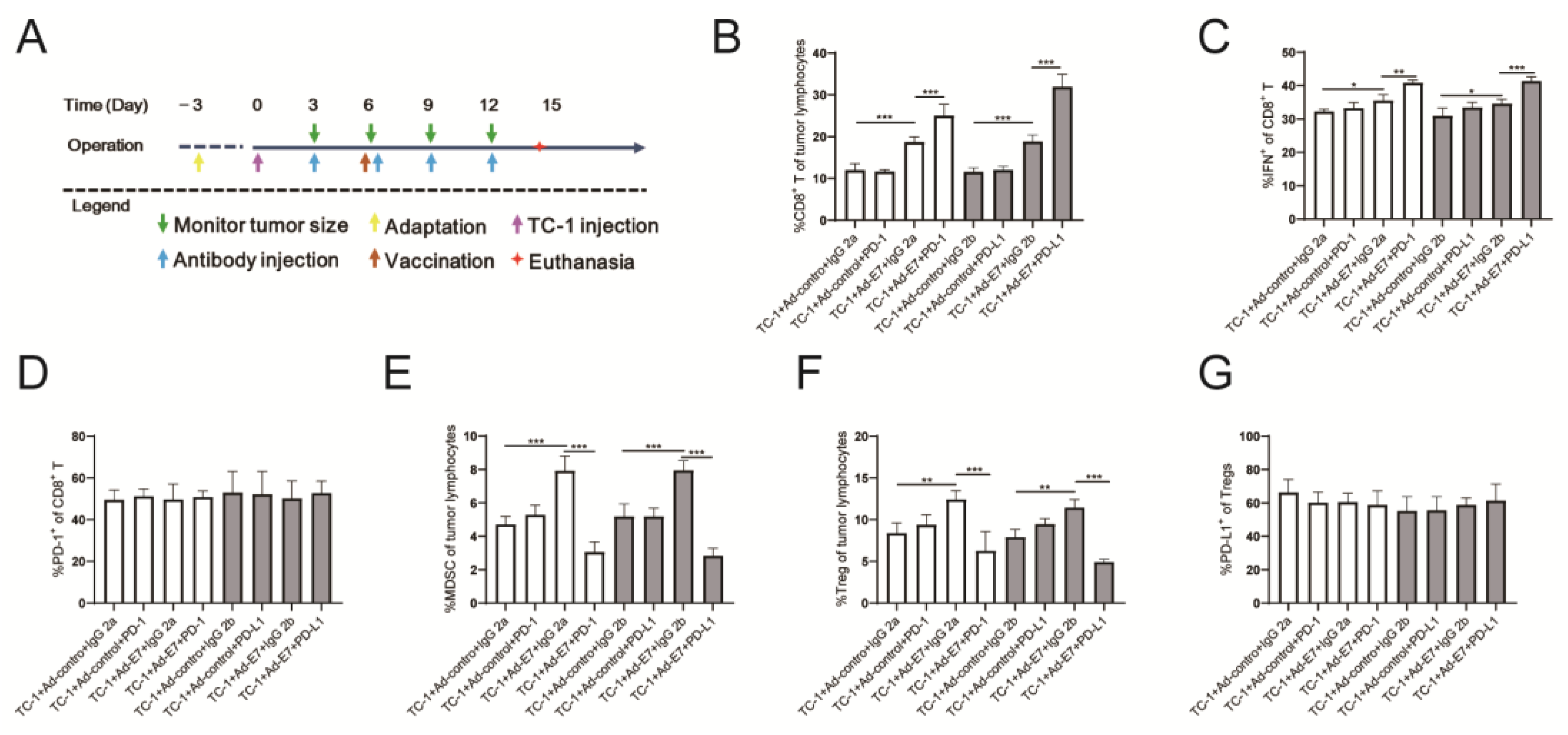
2.4. Ad-E7 Combined with PD-1/PD-L1 Antibody Induces Strong CTL Responses in Tumors
2.5. Ad-E7 Combined with PD-1/PD-L1 Antibody Inhibits the Secretion of Cytokines in Tumors
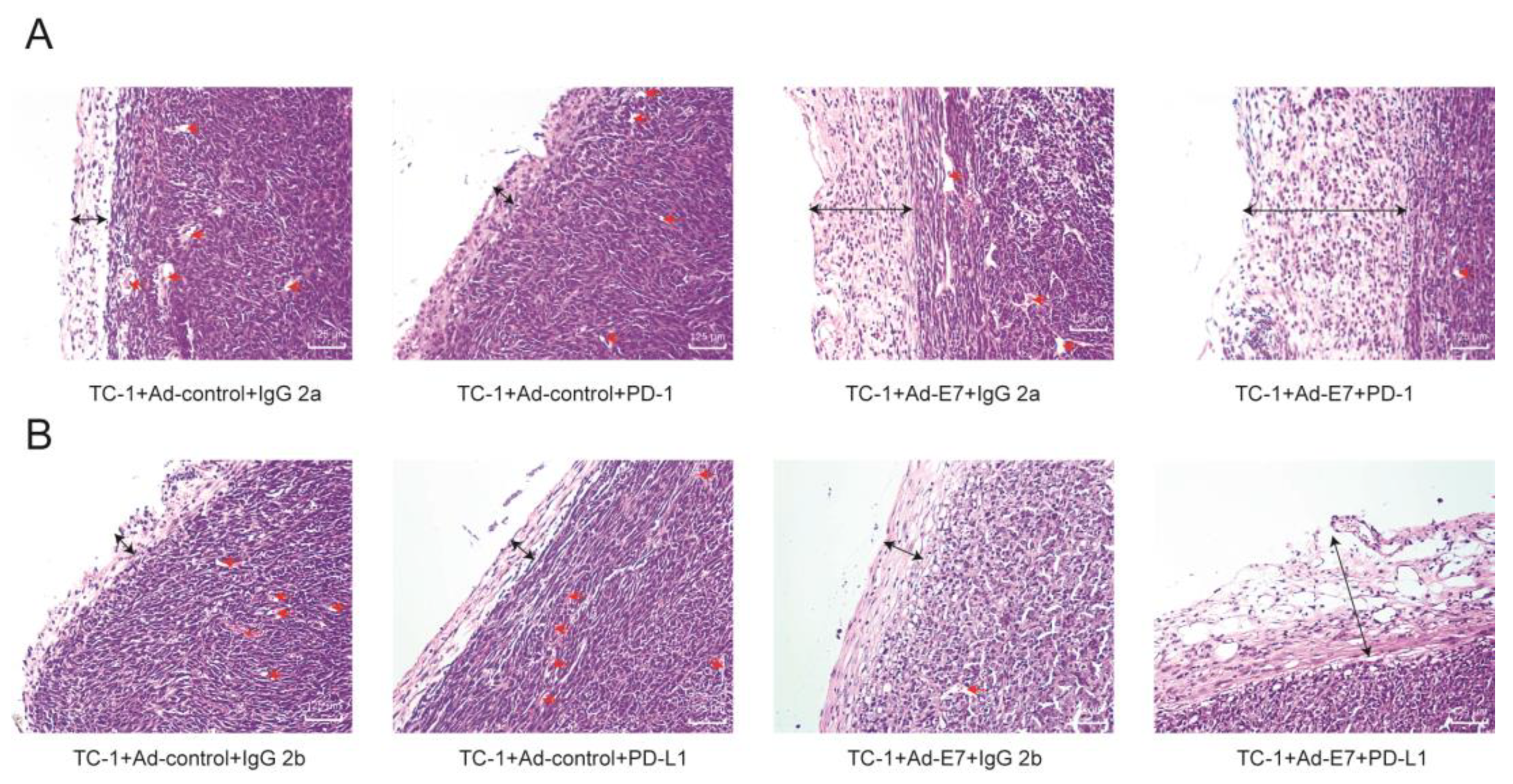
2.6. Ad-E7 Combined with PD1/PD-L1 Antibody Decreases Tumor Angiogenesis and Increases Capsule Thickness
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Mice
4.3. Western Blotting
4.4. Quantitative Reverse-Transcription PCR
- GAPDH-R: GCCTTCTCCATGGTGGTGAA.
- GAPDH-F: GCACCGTCAAGGCTGAGAAC.
- CCL2-R: GCATTAGCTTCAGATTTACGGGT.
- CCL2-F: TAAAAACCTGGATCGGAACCAAA.
- CCL3-R: CAACGATGAATTGGCGTGGAA.
- CCL3-F: TGTACCATGACACTCTGCAAC.
- CCL5-R: CGACTGCAAGATTGGAGCACT.
- CCL5-F: TTTGCCTACCTCTCCCTCG.
- CCL12-R: ATCCAGTATGGTCCTGAAGATCA.
- CCL12-F: ATTTCCACACTTCTATGCCTCCT.
- CCL19-R: TAGTGTGGTGAACACAACAGC.
- CCL19-F: CCTGGGAACATCGTGAAAGC.
- CCL20-R: GAGGAGGTTCACAGCCCTTTT.
- CCL20-F: ACTGTTGCCTCTCGTACATACA.
- CCL21-R: GGGATGGGACAGCCTAAACT.
- CCL21-F: GTGATGGAGGGGGTCAGGA.
- CXCL10-R: GGCTCGCAGGGATGATTTCAA.
- CXCL10-F: CCAAGTGCTGCCGTCATTTTC.
- IL-2-R: GTCCAAGTTCATCTTCTAGGCAC.
- IL-2-F: TGAGCAGGATGGAGAATTACAGG.
- IL-4-R: GCCGATGATCTCTCTCAAGTGAT.
- IL-4-F: GGTCTCAACCCCCAGCTAGT.
- IL-10-R: GCAGCTCTAGGAGCATGTGG.
- IL-10-F: CTTACTGACTGGCATGAGGATCA.
- MMP2-R: CTTCCGCATGGTCTCGATG.
- MMP2-F: ACCTGAACACTTTCTATGGCTG.
- MMP3-R: TGTCCATCGTTCATCATCGTCA.
- MMP3-F: GGCCTGGAACAGTCTTGGC.
- TNF-α-R: CGATCACCCCGAAGTTCAGTAG.
- TNF-α-F: CAGGCGGTGCCTATGTCTC.
- TGF-β-R: AATGGTACCGTCAGTGCTGGAAATA.
- TGF-β-F: TGGCTCATGTTGCAGAGGCTA.
4.5. Construction of the Adenovirus Vector
4.6. Flow Cytometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedeta, E.; Sung, H.; Laversanne, M.; Bray, F.; Jemal, A. Recent Mortality Patterns and Time Trends for the Major Cancers in 47 Countries Worldwide. Cancer Epidemiol. Biomark. Prev. 2023, 32, 894–905. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Albertini, S.; Gariglio, M. Subversion of Host Innate Immunity by Human Papillomavirus Oncoproteins. Pathogens 2020, 9, 292. [Google Scholar] [CrossRef]
- Hildesheim, A.; Gonzalez, P.; Kreimer, A.R.; Wacholder, S.; Schussler, J.; Rodriguez, A.C.; Porras, C.; Schiffman, M.; Sidawy, M.; Schiller, J.T.; et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am. J. Obstet. Gynecol. 2016, 215, 212.e1–212.e15. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2019, 10, 3116. [Google Scholar] [CrossRef]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Dyson, N.; Howley, P.M.; Münger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef]
- Morrow, M.P.; Yan, J.; Sardesai, N.Y. Human papillomavirus therapeutic vaccines: Targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev. Vaccines 2013, 12, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yang, A.; Wu, T.C.; Hung, C.F. Immunotherapy for human papillomavirus-associated disease and cervical cancer: Review of clinical and translational research. J. Gynecol. Oncol. 2016, 27, e51. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Franconi, R.; Brandi, R.; Muller, A.; Mett, V.; Yusibov, V.; Venuti, A. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine 2007, 25, 3018–3021. [Google Scholar] [CrossRef]
- Venuti, A.; Massa, S.; Mett, V.; Vedova, L.D.; Paolini, F.; Franconi, R.; Yusibov, V. An E7-based therapeutic vaccine protects mice against HPV16 associated cancer. Vaccine 2009, 27, 3395–3397. [Google Scholar] [CrossRef]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front. Oncol. 2014, 4, 385. [Google Scholar] [CrossRef]
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Chen, Z.; Pang, N.; Du, R.; Zhu, Y.; Fan, L.; Cai, D.; Ding, Y.; Ding, J. Elevated Expression of Programmed Death-1 and Programmed Death Ligand-1 Negatively Regulates Immune Response against Cervical Cancer Cells. Mediat. Inflamm. 2016, 2016, 6891482. [Google Scholar] [CrossRef]
- Abigail Robles-Rodríguez, O.; Pérez Trujillo, J.J.; Barrón Cantú, J.A.; Torres Cerda, A.; Gutiérrez Puente, Y.; García García, A.; Rodríguez Rocha, H.; Villanueva Olivo, A.; Zavala Flores, L.M.; Saucedo Cárdenas, O.; et al. Antitumor effect of adenoviruses expressing mutant non-oncogenic E7 versions from HPV-16 fused to calreticulin. J. BUON Off. J. Balk. Union Oncol. 2020, 25, 543–548. [Google Scholar]
- Bahrami, A.A.; Ghaemi, A.; Tabarraei, A.; Sajadian, A.; Gorji, A.; Soleimanjahi, H. DNA vaccine encoding HPV-16 E7 with mutation in L-Y-C-Y-E pRb-binding motif induces potent anti-tumor responses in mice. J. Virol. Methods 2014, 206, 12–18. [Google Scholar] [CrossRef]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.J.; Hill, A.V.; Dorrell, L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef]
- Jones, D.L.; Thompson, D.A.; Münger, K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 1997, 239, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Feltkamp, M.C.; Vreugdenhil, G.R.; Vierboom, M.P.; Ras, E.; van der Burg, S.H.; ter Schegget, J.; Melief, C.J.; Kast, W.M. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur. J. Immunol. 1995, 25, 2638–2642. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chang, M.C.; Chen, C.A.; Lin, H.W.; Cheng, W.F.; Chien, C.L. Depletion of regulatory T lymphocytes reverses the imbalance between pro- and anti-tumor immunities via enhancing antigen-specific T cell immune responses. PLoS ONE 2012, 7, e47190. [Google Scholar] [CrossRef][Green Version]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5(+) Myeloid-Derived Suppressor Cells Are Enriched and Activated in Melanoma Lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Huynh, L.K.; Hipolito, C.J.; Ten Dijke, P. A Perspective on the Development of TGF-β Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef]
- Han, L.; Sheng, B.; Zeng, Q.; Yao, W.; Jiang, Q. Correlation between MMP2 expression in lung cancer tissues and clinical parameters: A retrospective clinical analysis. BMC Pulm. Med. 2020, 20, 283. [Google Scholar] [CrossRef]
- Frieling, J.S.; Li, T.; Tauro, M.; Lynch, C.C. Prostate cancer-derived MMP-3 controls intrinsic cell growth and extrinsic angiogenesis. Neoplasia 2020, 22, 511–521. [Google Scholar] [CrossRef]
- Rahmanzade, R. Redefinition of tumor capsule: Rho-dependent clustering of cancer-associated fibroblasts in favor of tensional homeostasis. Med. Hypotheses 2020, 135, 109425. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Roosinovich, E.; Ma, B.; Hung, C.F.; Wu, T.C. Therapeutic HPV DNA vaccines. Immunol. Res. 2010, 47, 86–112. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Ji, H.; Trimble, C.; He, L.; Tsai, Y.C.; Yeatermeyer, J.; Boyd, D.A.; Hung, C.F.; Wu, T.C. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J. Virol. 2004, 78, 8468–8476. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Perales-Puchalt, A.; Stoltz, R.; Hiranjith, G.H.; Mandloi, N.; Barlow, J.; Chaudhuri, A.; Sardesai, N.Y.; Weiner, D.B. A Synthetic DNA, Multi-Neoantigen Vaccine Drives Predominately MHC Class I CD8(+) T-cell Responses, Impacting Tumor Challenge. Cancer Immunol. Res. 2019, 7, 174–182. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Münger, K. Oncogenic activities of human papillomaviruses. Virus Res. 2009, 143, 195–208. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Tang, S.; Ning, Q.; Yang, L.; Mo, Z.; Tang, S. Mechanisms of immune escape in the cancer immune cycle. Int. Immunopharmacol. 2020, 86, 106700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, Y.; Dong, W.; Lin, M.; He, J.; Zhang, X.; Tian, T.; Yang, Y.; Chen, K.; Lei, Q.Y.; et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc. Natl. Acad. Sci. USA 2022, 119, e2114851119. [Google Scholar] [CrossRef]
- Dorta-Estremera, S.; Hegde, V.L.; Slay, R.B.; Sun, R.; Yanamandra, A.V.; Nicholas, C.; Nookala, S.; Sierra, G.; Curran, M.A.; Sastry, K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV(+) oral cancer. J. Immunother. Cancer 2019, 7, 252. [Google Scholar] [CrossRef]
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.Y.; Huang, C.H.; Roden, R.B.S.; Wu, T.C.; Chang, Y.N.; Hung, C.F. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio 2021, 12, e03224-20. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Xie, F.; Tao, D.; Xiao, X.; Huang, C.; Wang, M.; Gu, C.; Zhang, X.; Jiang, G. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene 2020, 39, 1696–1709. [Google Scholar] [CrossRef]
- Liu, H.; Hu, G.; Wang, Z.; Liu, Q.; Zhang, J.; Chen, Y.; Huang, Y.; Xue, W.; Xu, Y.; Zhai, W. circPTCH1 promotes invasion and metastasis in renal cell carcinoma via regulating miR-485-5p/MMP14 axis. Theranostics 2020, 10, 10791–10807. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Gao, Z.; Cheng, Y.; Wu, S.; Chen, J.; Zhang, W. A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses. Int. J. Mol. Sci. 2023, 24, 15469. https://doi.org/10.3390/ijms242015469
Han X, Gao Z, Cheng Y, Wu S, Chen J, Zhang W. A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses. International Journal of Molecular Sciences. 2023; 24(20):15469. https://doi.org/10.3390/ijms242015469
Chicago/Turabian StyleHan, Xuechao, Zhixiao Gao, Yeping Cheng, Shuoshuo Wu, Jianxing Chen, and Weifang Zhang. 2023. "A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses" International Journal of Molecular Sciences 24, no. 20: 15469. https://doi.org/10.3390/ijms242015469
APA StyleHan, X., Gao, Z., Cheng, Y., Wu, S., Chen, J., & Zhang, W. (2023). A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses. International Journal of Molecular Sciences, 24(20), 15469. https://doi.org/10.3390/ijms242015469




