H-Dot Mediated Nanotherapeutics Mitigate Systemic Toxicity of Platinum-Based Anticancer Drugs
Abstract
1. Introduction
2. Results
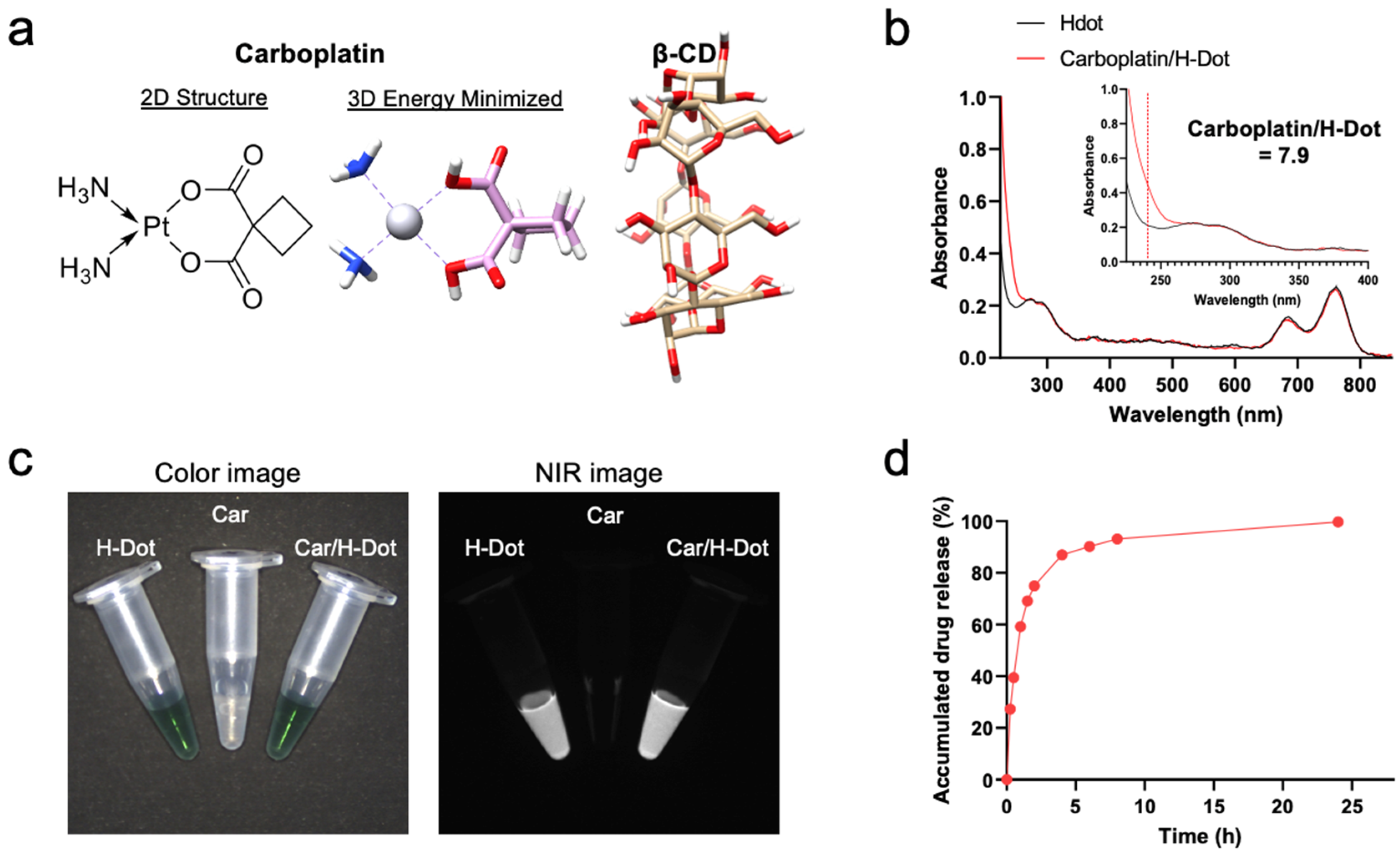
2.1. Preparation of Car/H-Dot Nanocomplex
2.2. Drug Release Test
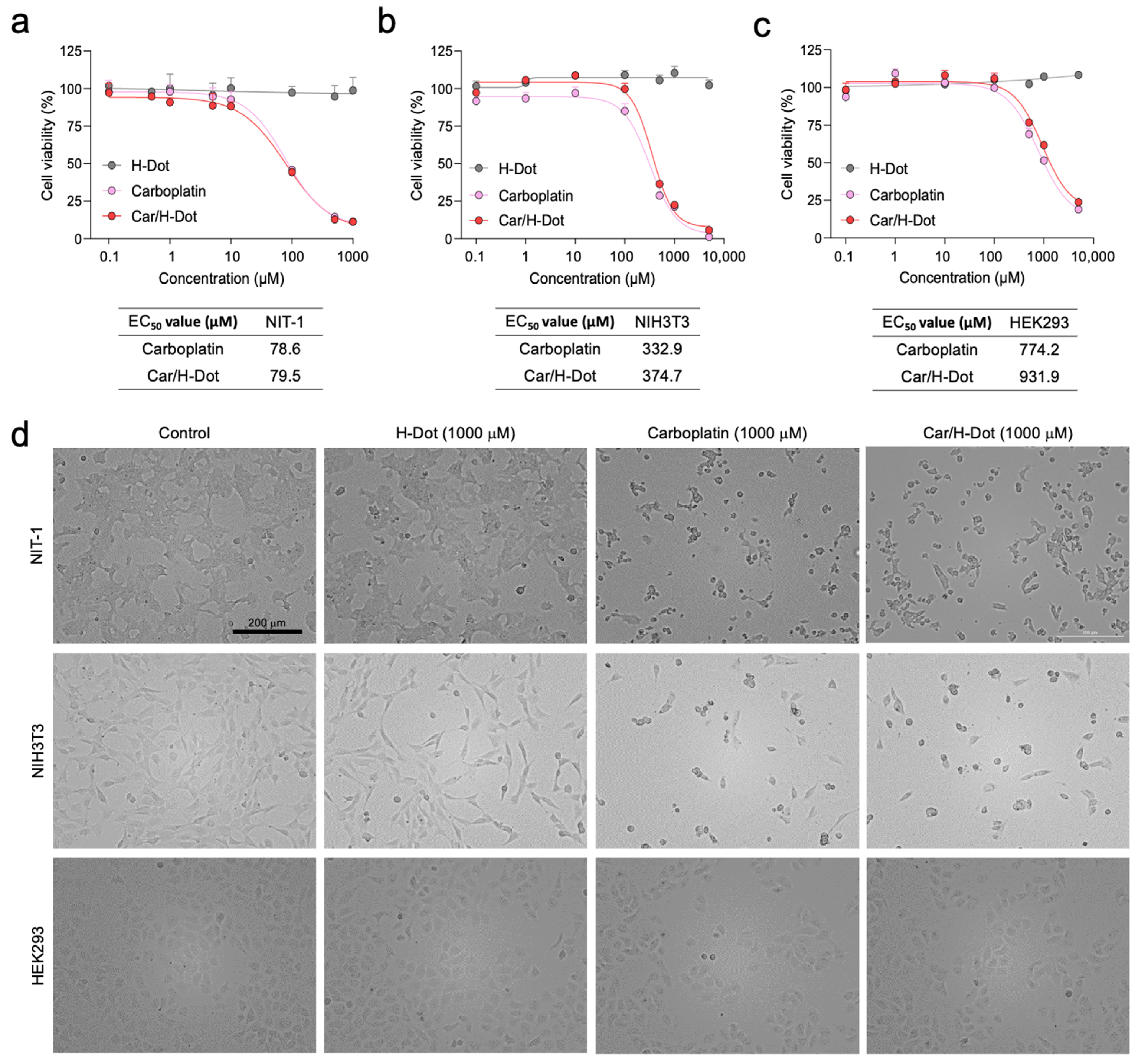
2.3. In Vitro Therapeutic Efficacy and Cytotoxicity Evaluation
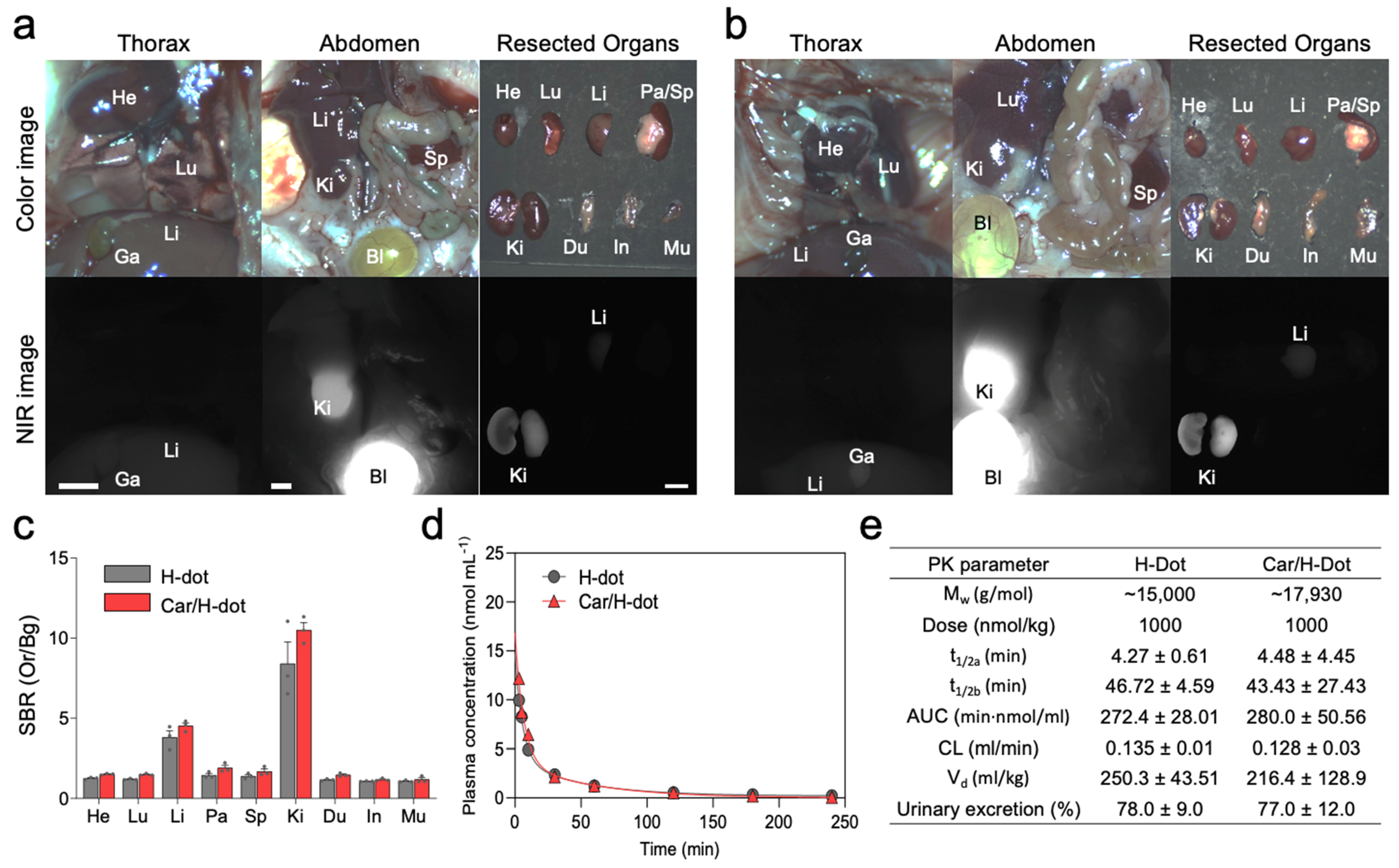
2.4. In Vivo Biodistribution and Pharmacokinetics
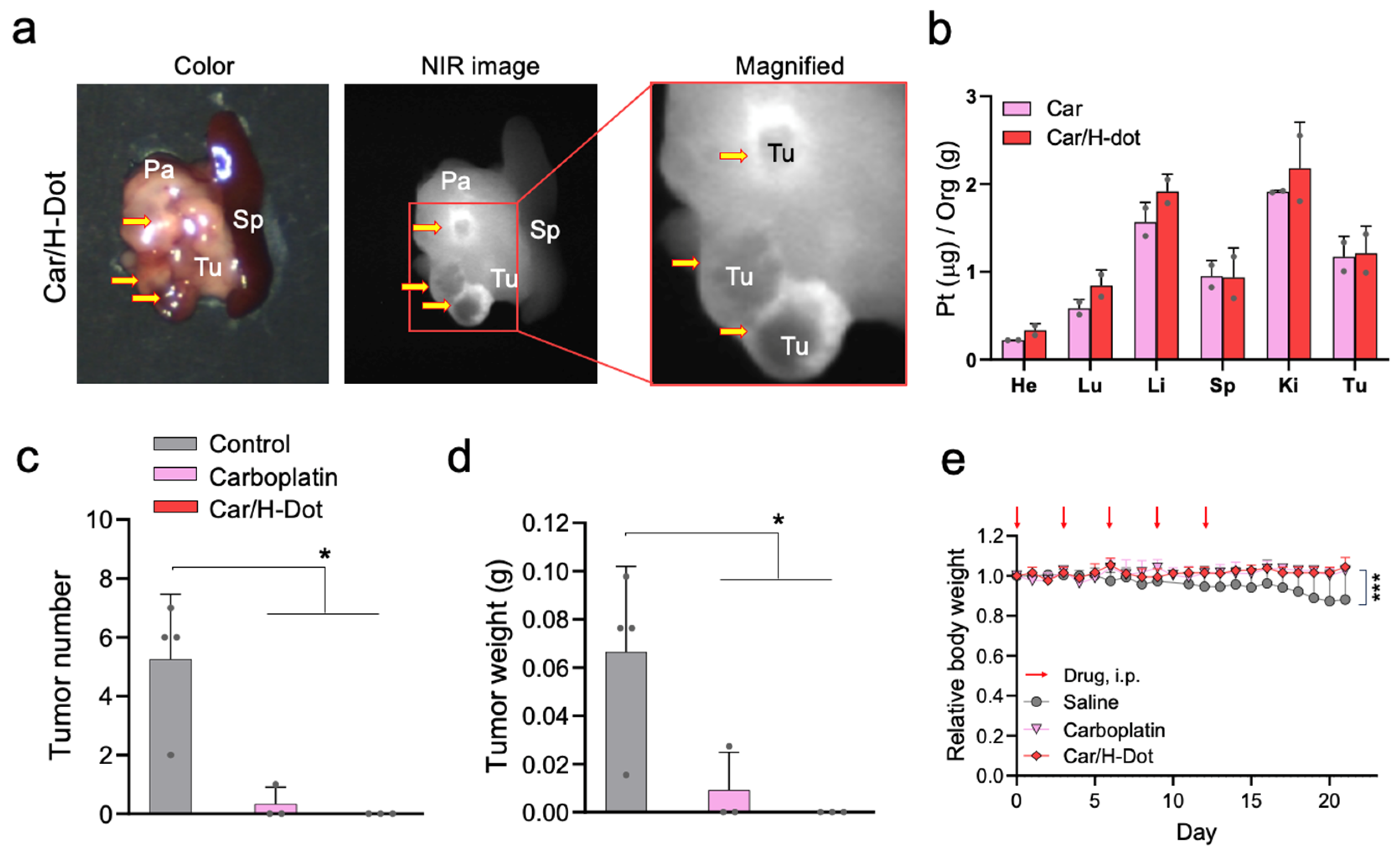
2.5. In Vivo Tumor Targeting and Therapeutic Efficacy of the Car/H-Dot
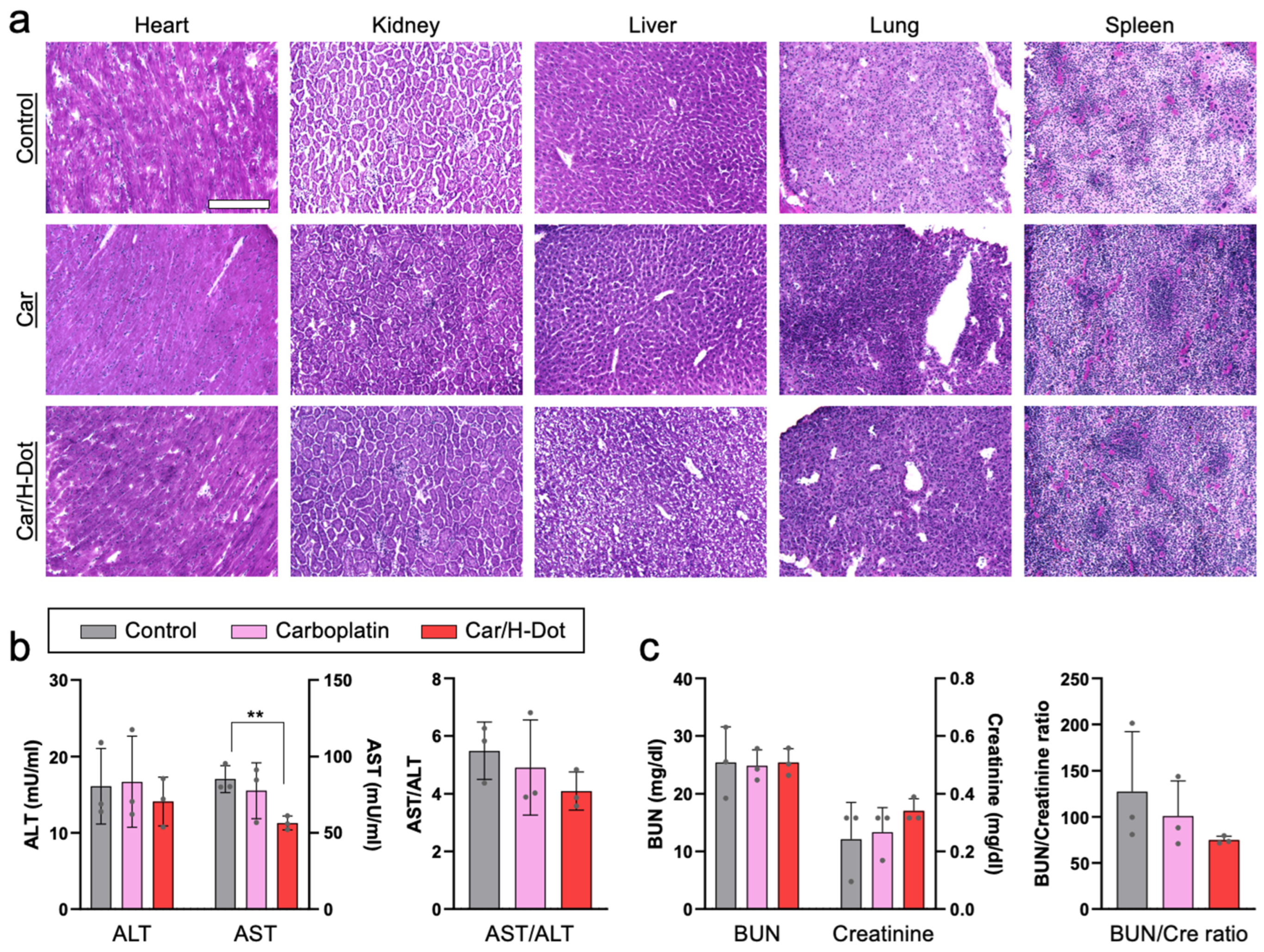
2.6. Systemic Toxicity Evaluation of Car and Car/H-Dot Complex
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis of H-Dot
4.3. Preparation of Car/H-Dot Inclusion Complexes
4.4. Measurement of Hydrodynamic Diameter of H-Dots
4.5. Drug Release Tests of Car/H-Dot Complexes
4.6. In Vitro Therapeutic Efficacy Test of Car/H-Dot Complexes
4.7. In Vivo Biodistribution and Pharmacokinetics of H-Dot and Car/H-Dot
4.8. In Vivo Antitumor Efficacy Test
4.9. Tissue Histopathology Evaluation
4.10. Toxicity Study
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, J. New clues for platinum antitumor chemistry: Kinetically controlled metal binding to DNA. Proc. Natl. Acad. Sci. USA 2003, 100, 3611–3616. [Google Scholar] [CrossRef]
- He, P.J.; Ge, R.F.; Mao, W.J.; Chung, P.S.; Ahn, J.C.; Wu, H.T. Oxidative stress induced by carboplatin promotes apoptosis and inhibits migration of HN-3 cells. Oncol. Lett. 2018, 16, 7131–7138. [Google Scholar] [CrossRef]
- Wlodarczyk, M.T.; Dragulska, S.A.; Camacho-Vanegas, O.; Dottino, P.R.; Jarzecki, A.A.; Martignetti, J.A.; Mieszawska, A.J. Platinum (II) complex-nuclear localization sequence peptide hybrid for overcoming platinum resistance in cancer therapy. ACS Biomater. Sci. Eng. 2018, 4, 463–467. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Megdanova-Chipeva, V.G.; Lamarca, A.; Backen, A.; McNamara, M.G.; Barriuso, J.; Sergieva, S.; Gocheva, L.; Mansoor, W.; Manoharan, P.; Valle, J.W. Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs). Cancers 2020, 12, 1988. [Google Scholar] [CrossRef]
- Turner, N.C.; Strauss, S.J.; Sarker, D.; Gillmore, R.; Kirkwood, A.; Hackshaw, A.; Papadopoulou, A.; Bell, J.; Kayani, I.; Toumpanakis, C.; et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br. J. Cancer 2010, 102, 1106–1112. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhang, T.; Li, H.; Yang, Z.; Wang, C. Effect of Micelle-Incorporated Cisplatin with Sizes Ranging from 8 to 40 nm for the Therapy of Lewis Lung Carcinoma. Front. Pharmacol. 2021, 12, 632877. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Lin, X.; Ma, W.; Chen, G.; Li, W.; Wang, X.; Yu, Z. Platinum(IV) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem. Commun. 2018, 54, 5369–5372. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Cisplatin-Loaded Polymer-Metal Complex Micelle with Time-Modulated Decaying Property as a Novel Drug Delivery System. Pharm. Res. 2001, 18, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z.; et al. Cisplatin Prodrug-Conjugated Gold Nanocluster for Fluorescence Imaging and Targeted Therapy of the Breast Cancer. Theranostics 2016, 6, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhury, S.; Xu, Y.; Rushdi, A.; Bao, Y. DNA Interaction of Pt-Attached Iron Oxide Nanoparticles. IEEE Trans. Magn. 2013, 49, 373–376. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef]
- Kang, H.; Gravier, J.; Bao, K.; Wada, H.; Lee, J.H.; Baek, Y.; El Fakhri, G.; Gioux, S.; Rubin, B.P.; Coll, J.L.; et al. Renal clearable organic nanocarriers for bioimaging and drug delivery. Adv. Mater. 2016, 28, 8162–8168. [Google Scholar] [CrossRef]
- Yin, X.; Cui, Y.; Kim, R.S.; Stiles, W.R.; Park, S.H.; Wang, H.; Ma, L.; Chen, L.; Baek, Y.; Kashiwagi, S.; et al. Image-guided drug delivery of nanotheranostics for targeted lung cancer therapy. Theranostics 2022, 12, 4147–4162. [Google Scholar] [CrossRef]
- Kang, H.; Stiles, W.R.; Baek, Y.; Nomura, S.; Bao, K.; Hu, S.; Park, G.K.; Jo, M.J.; Hoseok, I.; Coll, J.L.; et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv. Mater. 2020, 32, e1905899. [Google Scholar] [CrossRef]
- Hyun, H.; Owens, E.A.; Narayana, L.; Wada, H.; Gravier, J.; Bao, K.; Frangioni, J.V.; Choi, H.S.; Henary, M. Central C-C bonding increases optical and chemical stability of NIR fluorophores. RSC Adv. 2014, 4, 58762–58768. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, H.; Yokomizo, S.; Hickey, M.; Chang, H.; Kang, H.; Fukuda, T.; Song, M.Y.; Lee, S.Y.; Park, J.W.; et al. ZW800-PEG: A Renal Clearable Zwitterionic Near-Infrared Fluorophore for Potential Clinical Translation. Angew. Chem. Int. Ed. 2021, 60, 13847–13852. [Google Scholar] [CrossRef] [PubMed]
- Sisin, N.N.T.; Abdul Razak, K.; Zainal Abidin, S.; Che Mat, N.F.; Abdullah, R.; Ab Rashid, R.; Khairil Anuar, M.A.; Mohd Zainudin, N.H.; Tagiling, N.; Mat Nawi, N.; et al. Radiosensitization Effects by Bismuth Oxide Nanoparticles in Combination with Cisplatin for High Dose Rate Brachytherapy. Int. J. Nanomed. 2019, 14, 9941–9954. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Yuan, L.-Q.; Wang, C.; Lu, D.-F.; Zhao, X.-D.; Tan, L.-H.; Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging 2020, 12, 3662–3681. [Google Scholar] [CrossRef]
- Kleih, M.; Bopple, K.; Dong, M.; Gaissler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Bergers, G.; Javaherian, K.; Lo, K.-M.; Folkman, J.; Hanahan, D. Effects of Angiogenesis Inhibitors on Multistage Carcinogenesis in Mice. Science 1999, 284, 808–812. [Google Scholar] [CrossRef]
- Huang, J.; Ding, W.; Zhu, X.; Li, B.; Zeng, F.; Wu, K.; Wu, X.; Wang, F. Ligand Evolution in the Photoactivatable Platinum(IV) Anticancer Prodrugs. Front. Chem. 2022, 10, 876410. [Google Scholar] [CrossRef] [PubMed]
- Park, G.K.; Lee, J.H.; Soriano, E.; Choi, M.; Bao, K.; Katagiri, W.; Kim, D.Y.; Paik, J.H.; Yun, S.H.; Frangioni, J.V.; et al. Rapid and selective targeting of heterogeneous pancreatic neuroendocrine tumors. iScience 2020, 23, 101006. [Google Scholar] [CrossRef] [PubMed]
- Akerblom, B.; Zang, G.; Zhuang, Z.W.; Calounova, G.; Simons, M.; Welsh, M. Heterogeneity among RIP-Tag2 insulinomas allows vascular endothelial growth factor-A independent tumor expansion as revealed by studies in Shb mutant mice: Implications for tumor angiogenesis. Mol. Oncol. 2012, 6, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Ito, S.; Ogawa, Y.; Kobayashi, M.; Hanazaki, K. Diagnosis and management of insulinoma. World J. Gastroenterol. 2013, 19, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Bochner, F.; Mohan, V.; Zinger, A.; Golani, O.; Schroeder, A.; Sagi, I.; Neeman, M. Intravital imaging of vascular anomalies and extracellular matrix remodeling in orthotopic pancreatic tumors. Int. J. Cancer 2020, 146, 2209–2217. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Wu, R.; Cheng, M.-L.; Du, J.; Hu, X.-W.; Yu, L.; Zhao, X.-K.; Yao, Y.-M.; Long, Q.-Z.; Zhu, L.-L.; et al. Carboplatin-induced hematotoxicity among patients with non-small cell lung cancer: Analysis on clinical adverse events and drug-gene interactions. Oncotarget 2017, 8, 32228–32236. [Google Scholar] [CrossRef][Green Version]
- Dillard, L.K.; Lopez-Perez, L.; Martinez, R.X.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. Global burden of ototoxic hearing loss associated with platinum-based cancer treatment: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 79, 102203. [Google Scholar] [CrossRef]
- Cheng, C.F.; Juan, S.H.; Chen, J.J.; Chao, Y.C.; Chen, H.H.; Lian, W.S.; Lu, C.Y.; Chang, C.I.; Chiu, T.H.; Lin, H. Pravastatin attenuates carboplatin-induced cardiotoxicity via inhibition of oxidative stress associated apoptosis. Apoptosis 2008, 13, 883–894. [Google Scholar] [CrossRef]
- Csapo, M.; Lazar, L. Chemotherapy-Induced Cardiotoxicity: Pathophysiology and Prevention. Clujul Med. 2014, 87, 135–142. [Google Scholar] [CrossRef]
- Kanar, B.G.; Ozturk, A.; Kepez, A.; Akaslan, D.; Kavas, M.; Ogur, E.; Gulsen, K.; Kup, A.; Dalkilic, B.; Tigen, K.; et al. The effect of paclitaxel plus carboplatin chemotherapy on subclinical cardiotoxicity in patients with non-small cell lung cancer: A speckle tracking echocardiography-based study. Rev. Port. Cardiol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Jandial, D.D.; Messer, K.; Farshchi-Heydari, S.; Pu, M.; Howell, S.B. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol. Oncol. 2009, 115, 362–366. [Google Scholar] [CrossRef][Green Version]
- Wong, E.; Giandomenico, C.M. Current Status of Platinum-Based Antitumor Drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Contractor, T.; Vosburgh, E.; Du, Y.N.; Tang, L.H.; Clausen, R.; Harris, C.R. Alleles of Insm1 determine whether RIP1-Tag2 mice produce insulinomas or nonfunctioning pancreatic neuroendocrine tumors. Oncogenesis 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Djakpo, D.K.; Wang, Z.Q.; Shrestha, M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e279–e283. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Lam, K.Y.; Kung, A.W.; Lam, K.S.; Tung, P.H.; Fan, S.T. Pancreatin insulinomas. A 15-year experience. ARC Surg. 1997, 132, 926–930. [Google Scholar] [CrossRef]
- Mabrut, J.Y.; Lifante, J.C.; Cherki, S.; Sin, S.; Berger, N.; Peix, J.L. Is preoperative localization of insulinomas necessary? Ann. Chir. 2001, 126, 850–856. [Google Scholar] [CrossRef]
- Ravi, K.; Britton, B.J. Surgical approach to insulinomas: Are pre-operative localization tests necessary? Ann. R. Coll. Surg. Eng. 2007, 89, 212–217. [Google Scholar] [CrossRef]
- Mayo, S.C.; De Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef]
- Wong, K.P.; Tsang, J.S.; Lang, B.H. Role of surgery in pancreatic neuroendocrine tumor. Gland Surg. 2018, 7, 36–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, A.; Park, S.H.; Zeng, L.; Stiles, W.R.; Ahn, S.; Bao, K.; Kim, J.; Kang, H.; Choi, H.S. H-Dot Mediated Nanotherapeutics Mitigate Systemic Toxicity of Platinum-Based Anticancer Drugs. Int. J. Mol. Sci. 2023, 24, 15466. https://doi.org/10.3390/ijms242015466
Yamashita A, Park SH, Zeng L, Stiles WR, Ahn S, Bao K, Kim J, Kang H, Choi HS. H-Dot Mediated Nanotherapeutics Mitigate Systemic Toxicity of Platinum-Based Anticancer Drugs. International Journal of Molecular Sciences. 2023; 24(20):15466. https://doi.org/10.3390/ijms242015466
Chicago/Turabian StyleYamashita, Atsushi, Seung Hun Park, Lingxue Zeng, Wesley R. Stiles, Sung Ahn, Kai Bao, Jonghan Kim, Homan Kang, and Hak Soo Choi. 2023. "H-Dot Mediated Nanotherapeutics Mitigate Systemic Toxicity of Platinum-Based Anticancer Drugs" International Journal of Molecular Sciences 24, no. 20: 15466. https://doi.org/10.3390/ijms242015466
APA StyleYamashita, A., Park, S. H., Zeng, L., Stiles, W. R., Ahn, S., Bao, K., Kim, J., Kang, H., & Choi, H. S. (2023). H-Dot Mediated Nanotherapeutics Mitigate Systemic Toxicity of Platinum-Based Anticancer Drugs. International Journal of Molecular Sciences, 24(20), 15466. https://doi.org/10.3390/ijms242015466






