Overview of Recent Advances in Nano-Based Ocular Drug Delivery
Abstract
1. Introduction
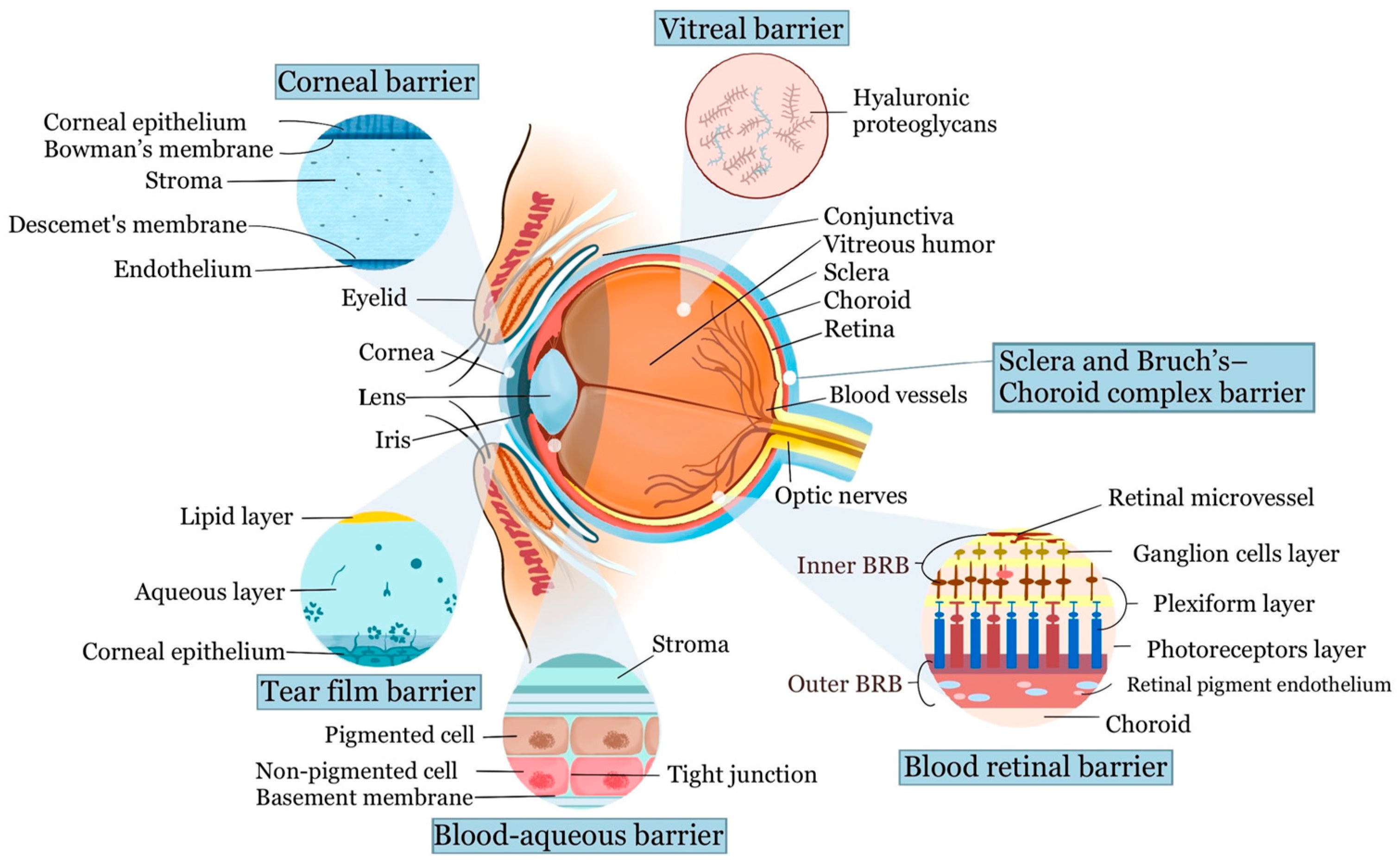
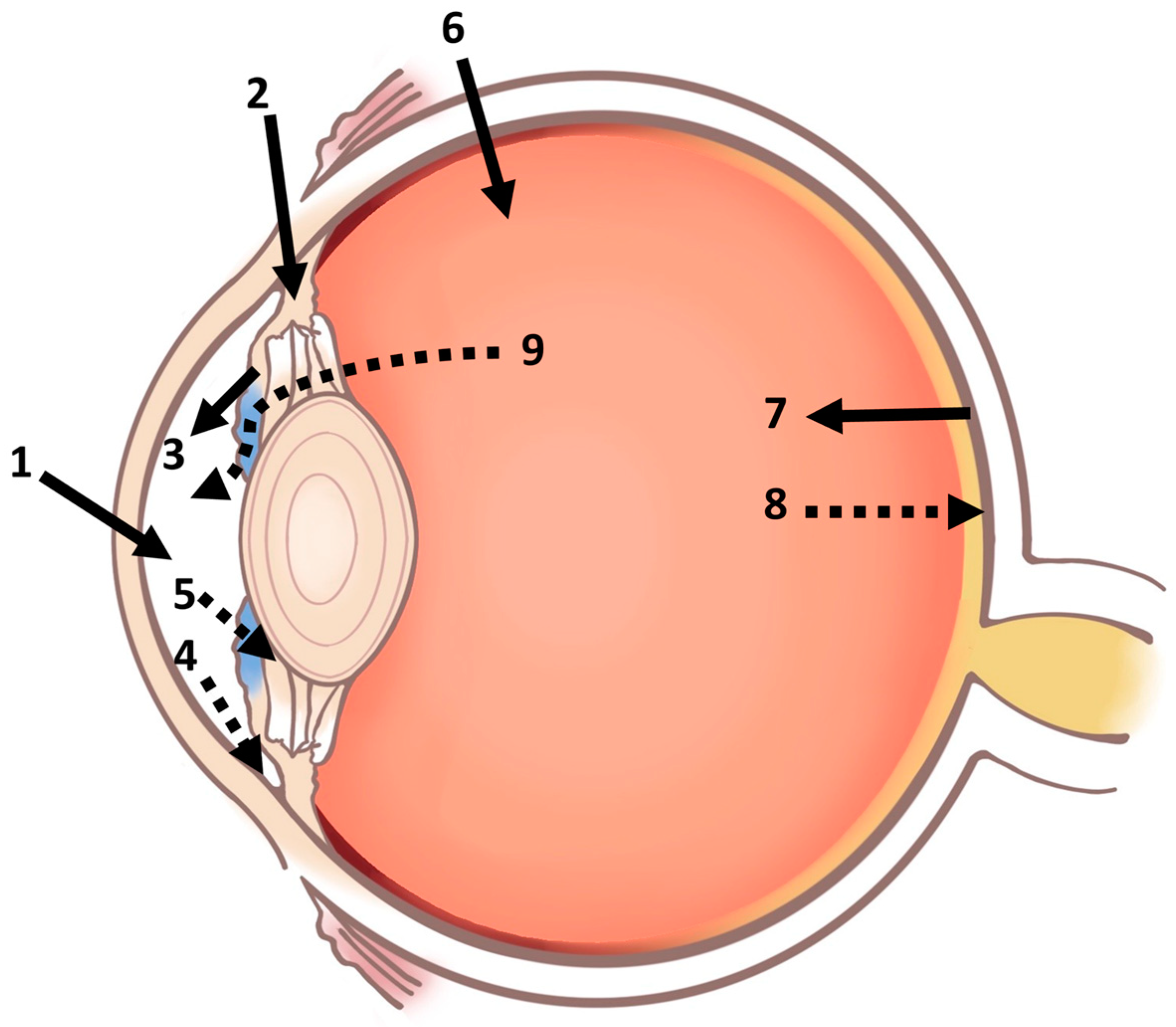
2. Anatomy and Barriers of Ocular Drug Delivery
2.1. Barriers of the Anterior Segment
2.1.1. Tear Film Barrier
2.1.2. Cornea and Conjunctival Barrier
2.1.3. Blood–Aqueous Barrier
2.2. Barriers of the Posterior Segment
2.2.1. Vitreal Barrier
2.2.2. Blood–Retinal Barrier
2.2.3. Sclera and Bruch’s–Choroid Complex Barrier
3. The Conventional Routes for Ocular Drug Administration
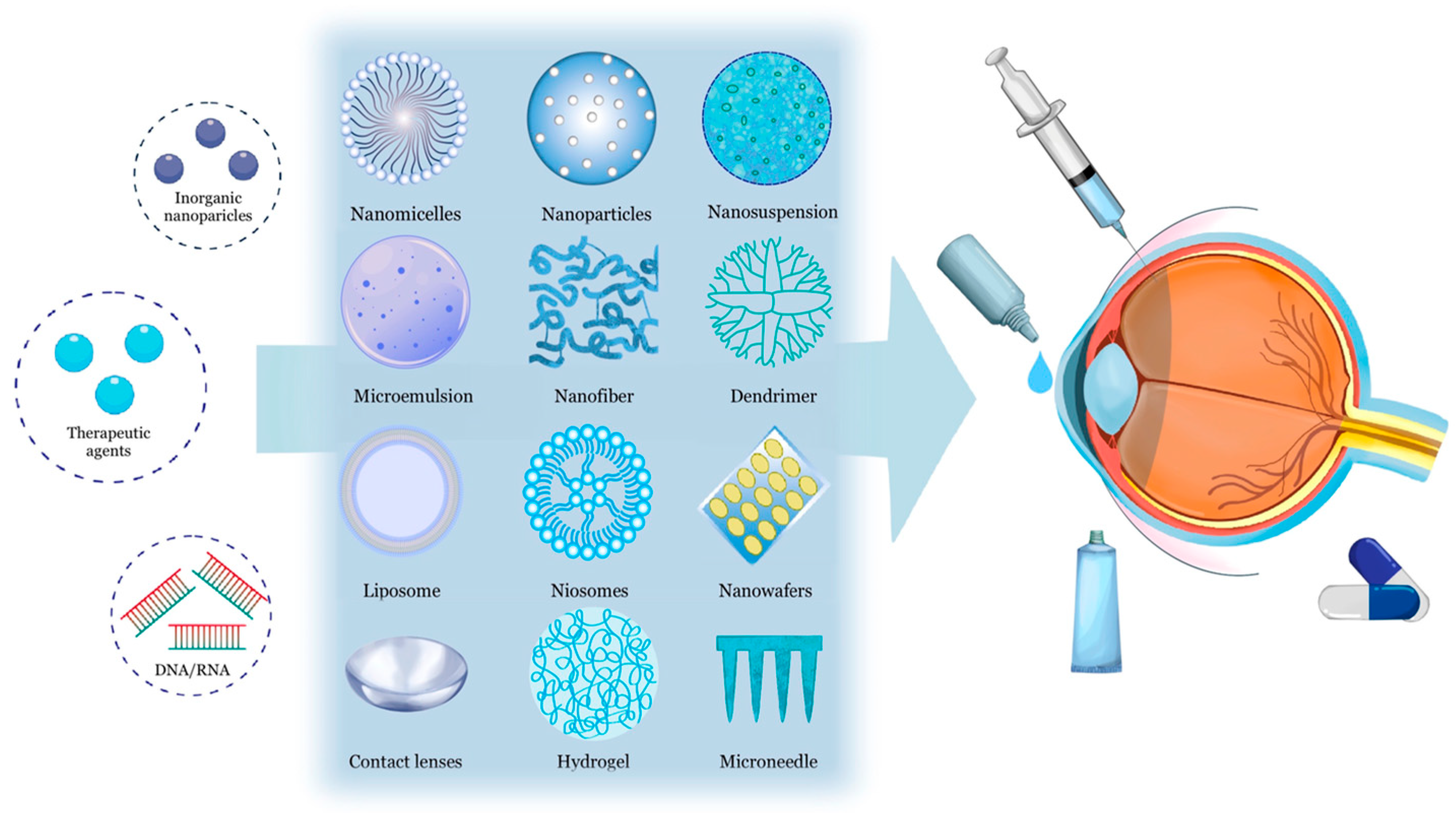
4. Nanotechnology-Based Ocular Drug Delivery Systems
4.1. Nanomicelles
4.1.1. Main Characteristics
4.1.2. Clinical Applications
4.2. Nanoparticles
4.2.1. Main Characteristics
4.2.2. Clinical Applications
4.3. Nanosuspension
4.3.1. Main Characteristics
4.3.2. Clinical Applications
4.4. Nanoemulsions and Microemulsions
4.4.1. Main Characteristics
4.4.2. Clinical Applications
4.5. Nanofibers
4.5.1. Main Characteristics
4.5.2. Clinical Applications
4.6. Dendrimers
4.6.1. Main Characteristics
4.6.2. Clinical Applications
4.7. Liposomes and Niosomes
4.7.1. Main Characteristics
4.7.2. Clinical Applications
4.8. Nanowafers
4.8.1. Main Characteristics
4.8.2. Clinical Applications
4.9. Contact Lenses
4.9.1. Main Characteristics
4.9.2. Clinical Applications
4.10. Hydrogels
4.10.1. Main Characteristics
4.10.2. Clinical Applications
4.11. Microneedles
4.11.1. Main Characteristics
4.11.2. Clinical Applications
4.12. Novel Gene Therapy with Nanotechnology-Based Ocular Delivery Techniques
5. Controlled Drug Delivery Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Report on Vision; WHO: Geneva, Switzerland, 8 October 2019. [Google Scholar]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.S.; Goostrey, T.C.; Ziolkowska, M.; Czerny-Holownia, S.; Hoare, T.; Sheardown, H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J. Control. Release 2021, 336, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. Aaps J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Adrianto, M.F.; Annuryanti, F.; Wilson, C.G.; Sheshala, R.; Thakur, R.R.S. In vitro dissolution testing models of ocular implants for posterior segment drug delivery. Drug Deliv. Transl. Res. 2022, 12, 1355–1375. [Google Scholar] [CrossRef]
- Kaushal, U.; Kaur, M.; Nagpal, M.; Bhuyan, M.; Gounder, K.P. Nanocarriers Based Ocular Therapeutics: Updates, Challenges and Future Prospectives. Curr. Drug Res. Rev. 2023, 15, 15–28. [Google Scholar]
- Qamar, Z.; Qizilbash, F.F.; Iqubal, M.K.; Ali, A.; Narang, J.K.; Ali, J.; Baboota, S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent. Pat. Drug Deliv. Formul. 2019, 13, 246–254. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano-Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef]
- Kels, B.D.; Grzybowski, A.; Grant-Kels, J.M. Human ocular anatomy. Clin. Dermatol. 2015, 33, 140–146. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Subrizi, A.; Del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar]
- Geroski, D.H.; Edelhauser, H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef]
- Coca-Prados, M. The blood-aqueous barrier in health and disease. J. Glaucoma 2014, 23, S36–S38. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; De Smedt, S.C.; Remaut, K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 44–57. [Google Scholar] [CrossRef]
- Tavakoli, S.; Peynshaert, K.; Lajunen, T.; Devoldere, J.; Del Amo, E.M.; Ruponen, M.; De Smedt, S.C.; Remaut, K.; Urtti, A. Ocular barriers to retinal delivery of intravitreal liposomes: Impact of vitreoretinal interface. J. Control. Release 2020, 328, 952–961. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Starita, C.; Hodgetts, A.; Marshall, J. Macromolecular diffusion characteristics of ageing human Bruch’s membrane: Implications for age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Cheruvu, N.P.; Amrite, A.C.; Kompella, U.B. Effect of eye pigmentation on transscleral drug delivery. Investig. Ophthalmol. Vis. Sci. 2008, 49, 333–341. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef]
- Sen, H.N.; Vitale, S.; Gangaputra, S.S.; Nussenblatt, R.B.; Liesegang, T.L.; Levy-Clarke, G.A.; Rosenbaum, J.T.; Suhler, E.B.; Thorne, J.E.; Foster, C.S.; et al. Periocular corticosteroid injections in uveitis: Effects and complications. Ophthalmology 2014, 121, 2275–2286. [Google Scholar] [CrossRef]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T., Jr. Risks of intravitreous injection: A comprehensive review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, C.; Amenabar Alonso, A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.; Barakat, M. Suprachoroidal Injection Update-A novel drug delivery method with potential applications in gene therapy and ocular oncology. Retin. Physician Symp. 2019, 2019, E1–E6. [Google Scholar]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: State of the art. Stem Cell Res. Ther. 2021, 12, 60. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotechnol. 2021, 15, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.I. Recent Advances in Nanomicelles Delivery Systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, L.; Chi, H. Recent development of polymer nanomicelles in the treatment of eye diseases. Front. Bioeng. Biotechnol. 2023, 11, 1246974. [Google Scholar] [CrossRef]
- Tsung, T.H.; Tsai, Y.C.; Lee, H.P.; Chen, Y.H.; Lu, D.W. Biodegradable Polymer-Based Drug-Delivery Systems for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Edman, M.C.; Reddy Janga, S.; Yarber, F.; Meng, Z.; Klinngam, W.; Bushman, J.; Ma, T.; Liu, S.; Louie, S.; et al. Rapamycin Eye Drops Suppress Lacrimal Gland Inflammation in a Murine Model of Sjögren’s Syndrome. Invest. Ophthalmol. Vis. Sci. 2017, 58, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, L.; Xia, H.; He, J.; Liu, S.; He, S.; Wang, L.; Zhang, J. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: In vitro characterization and in vivo permeation. Eur. J. Pharm. Sci. 2017, 109, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Kamaleddin, M.A. Nano-ophthalmology: Applications and considerations. Nanomedicine 2017, 13, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-Kinani, A.A.; Alany, R.G.; Monti, D. Assembling Surfactants-Mucoadhesive Polymer Nanomicelles (ASMP-Nano) for Ocular Delivery of Cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, Y.; Chaurasiya, B.; Li, X.; Xu, Y.; Webster, T.J.; Tu, J.; Sun, R. Stability, safety, and transcorneal mechanistic studies of ophthalmic lyophilized cyclosporine-loaded polymeric micelles. Int. J. Nanomed. 2018, 13, 8281–8296. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Sun, X.; Sheng, Y.; Li, K.; Sai, S.; Feng, J.; Li, Y.; Zhang, J.; Han, J.; Tian, B. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022, 138, 193–207. [Google Scholar] [CrossRef]
- Osouli, M.; Abdollahizad, E.; Alavi, S.; Mahboubi, A.; Abbasian, Z.; Haeri, A.; Dadashzadeh, S. Biocompatible phospholipid-based mixed micelles for posaconazole ocular delivery: Development, characterization, and in vitro antifungal activity. J. Biomater. Appl. 2023, 37, 969–978. [Google Scholar] [CrossRef]
- Li, X.; Fang, J.; Xin, M.; Li, Q.; Wang, J.; Yang, H.; Wu, X. Rebaudioside A/TPGS mixed nanomicelles as promising nanocarriers for nimodipine ocular delivery. Drug Deliv. Transl. Res. 2021, 11, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Chang, P.C. Novel pluronic-chitosan micelle as an ocular delivery system. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Aqil, M.; Sultana, Y. A grafted copolymer-based nanomicelles for topical ocular delivery of everolimus: Formulation, characterization, ex-vivo permeation, in-vitro ocular toxicity, and stability study. Eur. J. Pharm. Sci. 2021, 159, 105735. [Google Scholar] [CrossRef]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers 2021, 13, 3038. [Google Scholar] [CrossRef]
- Gote, V.; Mandal, A.; Alshamrani, M.; Pal, D. Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery. Pharmaceutics 2020, 12, 1072. [Google Scholar] [CrossRef]
- Zhao, X.; Seah, I.; Xue, K.; Wong, W.; Tan, Q.S.W.; Ma, X.; Lin, Q.; Lim, J.Y.C.; Liu, Z.; Parikh, B.H.; et al. Antiangiogenic Nanomicelles for the Topical Delivery of Aflibercept to Treat Retinal Neovascular Disease. Adv. Mater. 2022, 34, e2108360. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Tsung, T.H.; Chen, Y.H.; Lu, D.W. Updates on Biodegradable Formulations for Ocular Drug Delivery. Pharmaceutics 2023, 15, 734. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Qian, W.; Li, Y.; Liu, B.; Aaberg, M.; Henry, J.; Zhang, W.; Wang, X.; Paulus, Y.M. Chain-like gold nanoparticle clusters for multimodal photoacoustic microscopy and optical coherence tomography enhanced molecular imaging. Nat. Commun. 2021, 12, 34. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Li, Y.; Qian, W.; Liu, B.; Tian, C.; Zhang, W.; Huang, Z.; Ponduri, A.; Tarnowski, M.; Wang, X.; et al. Contrast Agent Enhanced Multimodal Photoacoustic Microscopy and Optical Coherence Tomography for Imaging of Rabbit Choroidal and Retinal Vessels in vivo. Sci. Rep. 2019, 9, 5945. [Google Scholar] [CrossRef]
- Afarid, M.; Mahmoodi, S.; Baghban, R. Recent achievements in nano-based technologies for ocular disease diagnosis and treatment, review and update. J. Nanobiotechnol. 2022, 20, 361. [Google Scholar] [CrossRef]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Asadi Amoli, F. Increasing the efficiency of the retinoblastoma brachytherapy protocol with ultrasonic hyperthermia and gold nanoparticles: A rabbit model. Int. J. Radiat. Biol. 2020, 96, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.; Kara, D.D.; Roychowdhury, R.; Tanvi, K.; Rathnanand, M. Polymersomes Based Versatile Nanoplatforms for Controlled Drug Delivery and Imaging. Adv. Pharm. Bull. 2023, 13, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. A 2015, 103, 3148–3156. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef]
- Arafa, M.G.; Girgis, G.N.S.; El-Dahan, M.S. Chitosan-Coated PLGA Nanoparticles for Enhanced Ocular Anti-Inflammatory Efficacy of Atorvastatin Calcium. Int. J. Nanomed. 2020, 15, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized hyaluronic acid coated spanlastics for cyclosporine A ocular delivery: Prolonged ocular retention, enhanced corneal permeation and improved tear production. Int. J. Pharm. 2019, 565, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef]
- Khames, A.; Khaleel, M.A.; El-Badawy, M.F.; El-Nezhawy, A.O.H. Natamycin solid lipid nanoparticles—sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515–2531. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Kumar, B.; Chitranshi, N.; Gupta, V.K.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; Vishwas, S.; et al. Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: A new dimension to an anticancer drug. Int. J. Biol. Macromol. 2023, 224, 810–830. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, W.; Gu, D.; Wang, T.; Li, J.; Pan, H. Chondroitin sulfate and L-Cysteine conjugate modified cationic nanostructured lipid carriers: Pre-corneal retention, permeability, and related studies for dry eye treatment. Int. J. Biol. Macromol. 2023, 228, 624–637. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Khanuja, H.K.; Awasthi, R.; Mehta, M.; Satija, S.; Aljabali, A.A.A.; Tambuwala, M.M.; Chellappan, D.K.; Dua, K.; Dureja, H. Nanosuspensions—An Update on Recent Patents, Methods of Preparation, and Evaluation Parameters. Recent. Pat. Nanotechnol. 2021, 15, 351–366. [Google Scholar] [CrossRef]
- Manca, M.L.; Lai, F.; Pireddu, R.; Valenti, D.; Schlich, M.; Pini, E.; Ailuno, G.; Fadda, A.M.; Sinico, C. Impact of nanosizing on dermal delivery and antioxidant activity of quercetin nanocrystals. J. Drug Deliv. Sci. Technol. 2020, 55, 101482. [Google Scholar] [CrossRef]
- Ma, Y.; Cong, Z.; Gao, P.; Wang, Y. Nanosuspensions technology as a master key for nature products drug delivery and In vivo fate. Eur. J. Pharm. Sci. 2023, 185, 106425. [Google Scholar] [CrossRef] [PubMed]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, S.W.; Han, S.D.; Hwang, H.D.; Choi, H.G. Development of a novel ophthalmic ciclosporin A-loaded nanosuspension using top-down media milling methods. Pharmazie 2011, 66, 491–495. [Google Scholar]
- Soltani, S.; Zakeri-Milani, P.; Barzegar-Jalali, M.; Jelvehgari, M. Comparison of Different Nanosuspensions as Potential Ophthalmic Delivery Systems for Ketotifen Fumarate. Adv. Pharm. Bull. 2016, 6, 345–352. [Google Scholar] [CrossRef]
- Qin, T.; Dai, Z.; Xu, X.; Zhang, Z.; You, X.; Sun, H.; Liu, M.; Zhu, H. Nanosuspension as an Efficient Carrier for Improved Ocular Permeation of Voriconazole. Curr. Pharm. Biotechnol. 2021, 22, 245–253. [Google Scholar] [CrossRef]
- Ali, H.S.; York, P.; Ali, A.M.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef]
- Jadhav, P.A.; Yadav, A.V. Design, development and characterization of ketorolac tromethamine polymeric nanosuspension. Ther. Deliv. 2019, 10, 585–597. [Google Scholar] [CrossRef]
- Donia, M.; Osman, R.; Awad, G.A.S.; Mortada, N. Polypeptide and glycosaminoglycan polysaccharide as stabilizing polymers in nanocrystals for a safe ocular hypotensive effect. Int. J. Biol. Macromol. 2020, 162, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Hegde, R.R.; Verma, A.; Ghosh, A. Microemulsion: New insights into the ocular drug delivery. ISRN Pharm. 2013, 2013, 826798. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Henostroza, M.A.B.; Melo, K.J.C.; Yukuyama, M.N.; Löbenberg, R.; Bou-Chacra, N.A. Cationic rifampicin nanoemulsion for the treatment of ocular tuberculosis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124755. [Google Scholar] [CrossRef]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef]
- Gupta, A. Chapter 21—Nanoemulsions. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar]
- Fardous, J.; Inoue, Y.; Yoshida, K.; Ono, F.; Higuchi, A.; Ijima, H. Delivery of Hydrophobic Drugs to the Posterior Ocular Region by Gel-in-Water Nanoemulsion. Transl. Vis. Sci. Technol. 2022, 11, 16. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; Al-Anazy, M.M.; Ragupathy, D. Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Uzel, E.; Durgun, M.E.; Esentürk-Güzel, İ.; Güngör, S.; Özsoy, Y. Nanofibers in Ocular Drug Targeting and Tissue Engineering: Their Importance, Advantages, Advances, and Future Perspectives. Pharmaceutics 2023, 15, 1062. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, E.A.; Alshamsan, A.; Abul Kalam, M.; Raish, M.; Alkholief, M.; Stapleton, P.; Harvey, K.; Craig, D.Q.M.; Barker, S.A. In vitro and in vivo biological assessment of dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm. 2021, 604, 120732. [Google Scholar] [CrossRef]
- Göttel, B.; Lucas, H.; Syrowatka, F.; Knolle, W.; Kuntsche, J.; Heinzelmann, J.; Viestenz, A.; Mäder, K. In situ Gelling Amphotericin B Nanofibers: A New Option for the Treatment of Keratomycosis. Front. Bioeng. Biotechnol. 2020, 8, 600384. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.R.; Lima, T.H.; Fernandes-Cunha, G.M.; Oréfice, R.L.; Da Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. Ocular biocompatibility of dexamethasone acetate loaded poly(ɛ-caprolactone) nanofibers. Eur. J. Pharm. Biopharm. 2019, 142, 20–30. [Google Scholar] [CrossRef]
- Taka, E.; Karavasili, C.; Bouropoulos, N.; Moschakis, T.; Andreadis, D.D.D.; Zacharis, C.K.K.; Fatouros, D.G.G. Ocular co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2020, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Cegielska, O.; Sierakowski, M.; Sajkiewicz, P.; Lorenz, K.; Kogermann, K. Mucoadhesive brinzolamide-loaded nanofibers for alternative glaucoma treatment. Eur. J. Pharm. Biopharm. 2022, 180, 48–62. [Google Scholar] [CrossRef]
- Andreadis, I.I.; Karavasili, C.; Thomas, A.; Komnenou, A.; Tzimtzimis, M.; Tzetzis, D.; Andreadis, D.; Bouropoulos, N.; Fatouros, D.G. In Situ Gelling Electrospun Ocular Films Sustain the Intraocular Pressure-Lowering Effect of Timolol Maleate: In Vitro, Ex Vivo, and Pharmacodynamic Assessment. Mol. Pharm. 2022, 19, 274–286. [Google Scholar] [CrossRef]
- de Souza, S.O.L.; Guerra, M.C.A.; Heneine, L.G.D.; de Oliveira, C.R.; Cunha Junior, A.D.S.; Fialho, S.L.; Oréfice, R.L. Biodegradable core-shell electrospun nanofibers containing bevacizumab to treat age-related macular degeneration. J. Mater. Sci. Mater. Med. 2018, 29, 173. [Google Scholar] [CrossRef]
- Romeo, A.; Kazsoki, A.; Omer, S.; Pinke, B.; Mészáros, L.; Musumeci, T.; Zelkó, R. Formulation and Characterization of Electrospun Nanofibers for Melatonin Ocular Delivery. Pharmaceutics 2023, 15, 1296. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G., 3rd; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wen, S.; Zhu, S.; Liu, D.; Chen, S.; Qie, J.; Chen, H.; Lin, Q. Are Poly(amidoamine) Dendrimers Safe for Ocular Applications? Toxicological Evaluation in Ocular Cells and Tissues. J. Ocul. Pharmacol. Ther. 2020, 36, 715–724. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Lancina III, M.G.; Wang, J.; Williamson, G.S.; Yang, H. DenTimol as a dendrimeric timolol analogue for glaucoma therapy: Synthesis and preliminary efficacy and safety assessment. Mol. Pharm. 2018, 15, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Orash Mahmoud Salehi, A.; Heidari-Keshel, S.; Poursamar, S.A.; Zarrabi, A.; Sefat, F.; Mamidi, N.; Behrouz, M.J.; Rafienia, M. Bioprinted Membranes for Corneal Tissue Engineering: A Review. Pharmaceutics 2022, 14, 2797. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Bhutto, I.A.; Wu, T.; Ho, K.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release 2021, 335, 527–540. [Google Scholar] [CrossRef]
- Kannan, R.M.; Pitha, I.; Parikh, K.S. A new era in posterior segment ocular drug delivery: Translation of systemic, cell-targeted, dendrimer-based therapies. Adv. Drug Deliv. Rev. 2023, 200, 115005. [Google Scholar] [CrossRef]
- Inoue, M.; Muta, K.; Mohammed, A.F.A.; Onodera, R.; Higashi, T.; Ouchi, K.; Ueda, M.; Ando, Y.; Arima, H.; Jono, H.; et al. Feasibility Study of Dendrimer-Based TTR-CRISPR pDNA Polyplex for Ocular Amyloidosis in Vitro. Biol. Pharm. Bull. 2022, 45, 1660–1668. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert. Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol. Pharm. 2018, 15, 4862–4871. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Teng, F.; Sun, Q.X.; Wang, S.Z.; Liu, C.; Zhao, G.Q. Rapamycin liposome gutta inhibiting fungal keratitis of rats. Int. J. Ophthalmol. 2019, 12, 536–541. [Google Scholar]
- El-Nabarawi, M.A.; Abd El Rehem, R.T.; Teaima, M.; Abary, M.; El-Mofty, H.M.; Khafagy, M.M.; Lotfy, N.M.; Salah, M. Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev. Ind. Pharm. 2019, 45, 922–936. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, R.; Liu, L.; Lu, Y.; Qi, J.; Wu, W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): In vitro characterization and improved corneal permeation. Int. J. Nanomed. 2013, 8, 1921–1933. [Google Scholar]
- Fathalla, D.; Fouad, E.A.; Soliman, G.M. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Drug Dev. Ind. Pharm. 2020, 46, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-De la Rosa, A.; Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Hernandez-Gamez, A.G.; Garcia-Bañuelos, J.J.; Armendariz-Borunda, J.; Santos, A. Novel Triamcinolone Acetonide-Loaded Liposomes Topical Formulation for the Treatment of Cystoid Macular Edema After Cataract Surgery: A Pilot Study. J. Ocul. Pharmacol. Ther. 2019, 35, 106–115. [Google Scholar] [CrossRef]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Hashmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: A potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol. 2020, 143, 483–491. [Google Scholar] [CrossRef]
- Kattar, A.; Quelle-Regaldie, A.; Sánchez, L.; Concheiro, A.; Alvarez-Lorenzo, C. Formulation and Characterization of Epalrestat-Loaded Polysorbate 60 Cationic Niosomes for Ocular Delivery. Pharmaceutics 2023, 15, 1247. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef]
- Marcano, D.C.; Shin, C.S.; Lee, B.; Isenhart, L.C.; Liu, X.; Li, F.; Jester, J.V.; Pflugfelder, S.C.; Simpson, J.; Acharya, G. Synergistic Cysteamine Delivery Nanowafer as an Efficacious Treatment Modality for Corneal Cystinosis. Mol. Pharm. 2016, 13, 3468–3477. [Google Scholar] [CrossRef]
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control. Release 2015, 213, 168–174. [Google Scholar] [CrossRef]
- Filipe, H.P.; Henriques, J.; Reis, P.; Silva, P.C.; Quadrado, M.J.; Serro, A.P. Contact lenses as drug controlled release systems: A narrative review. Rev. Bras. De Oftalmol. 2016, 75, 241–247. [Google Scholar] [CrossRef][Green Version]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef]
- Peral, A.; Martinez-Aguila, A.; Pastrana, C.; Huete-Toral, F.; Carpena-Torres, C.; Carracedo, G. Contact lenses as drug delivery system for glaucoma: A review. Appl. Sci. 2020, 10, 5151. [Google Scholar] [CrossRef]
- ElShaer, A.; Ghatora, B.; Mustafa, S.; Alany, R.G. Contact lenses as drug reservoirs & delivery systems: The successes & challenges. Ther. Deliv. 2014, 5, 1085–1100. [Google Scholar] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, C.; Sun, Z.; Zhang, X.; Liang, N.; Mao, S. Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 220–229. [Google Scholar] [CrossRef]
- Bajgrowicz, M.; Phan, C.M.; Subbaraman, L.N.; Jones, L. Release of Ciprofloxacin and Moxifloxacin from Daily Disposable Contact Lenses from an In Vitro Eye Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2234–2242. [Google Scholar] [CrossRef]
- Phan, C.M.; Bajgrowicz, M.; McCanna, D.J.; Subbaraman, L.N.; Jones, L. Effects of Antifungal Soaked Silicone Hydrogel Contact Lenses on Candida albicans in an Agar Eye Model. Eye Contact Lens 2016, 42, 313–317. [Google Scholar] [CrossRef]
- Rad, M.S.; Sajadi Tabassi, S.A.; Moghadam, M.H.; Mohajeri, S.A. Controlled release of betamethasone from vitamin E-loaded silicone-based soft contact lenses. Pharm. Dev. Technol. 2016, 21, 894–899. [Google Scholar] [CrossRef]
- Soeken, T.A.; Ross, A.E.; Kohane, D.S.; Kuang, L.; Legault, G.L.; Caldwell, M.C.; Brundridge, W.L.; Merkley, M.B.; Ciolino, J.B.; Townley, J.R. Dexamethasone-Eluting Contact Lens for the Prevention of Postphotorefractive Keratectomy Scar in a New Zealand White Rabbit Model. Cornea 2021, 40, 1175–1180. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D.P. Controlled bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–328. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, X.; Yao, F.; Luo, Z.; Jin, L.; Xie, B.; Shi, S.; Ma, H.; Li, X.; Chen, H. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. Int. J. Pharm. 2014, 470, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Swarup, A.; Grosskopf, A.K.; Stapleton, L.M.; Subramaniam, V.R.; Li, B.; Weissman, I.L.; Appel, E.A.; Wu, A.Y. PNP Hydrogel Prevents Formation of Symblephara in Mice After Ocular Alkali Injury. Transl. Vis. Sci. Technol. 2022, 11, 31. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Shah, R.; Raghurama, R.S.S.; Anwar, K.N.; Shen, X.; An, S.; Omidi, M.; Rosenblatt, M.I.; Shokuhfar, T.; Djalilian, A.R. In-situ porcine corneal matrix hydrogel as ocular surface bandage. Ocul. Surf. 2021, 21, 27–36. [Google Scholar] [CrossRef]
- Gao, H.; Chen, M.; Liu, Y.; Zhang, D.; Shen, J.; Ni, N.; Tang, Z.; Ju, Y.; Dai, X.; Zhuang, A.; et al. Injectable Anti-Inflammatory Supramolecular Nanofiber Hydrogel to Promote Anti-VEGF Therapy in Age-Related Macular Degeneration Treatment. Adv. Mater. 2023, 35, e2204994. [Google Scholar] [CrossRef] [PubMed]
- Cocarta, A.I.; Hobzova, R.; Sirc, J.; Cerna, T.; Hrabeta, J.; Svojgr, K.; Pochop, P.; Kodetova, M.; Jedelska, J.; Bakowsky, U.; et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109799. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Mishra, D.; Gade, S.; Vora, L.K.; Wu, Y.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Microneedles for advanced ocular drug delivery. Adv. Drug Deliv. Rev. 2023, 201, 115082. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak, J.; Osmałek, T. Microneedle-based ocular drug delivery systems—recent advances and challenges. Beilstein. J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Lutton, R.E.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R. Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Gupta, P.; Yadav, K.S. Applications of microneedles in delivering drugs for various ocular diseases. Life Sci 2019, 237, 116907. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, J.; Wang, Y.; Zhu, Y.; Lin, D.; Lei, L.; Vakal, S.; Wang, J.; Li, X. A Rapid Corneal Healing Microneedle for Efficient Ocular Drug Delivery. Small 2022, 18, e2104657. [Google Scholar] [CrossRef]
- Amer, M.; Chen, R.K. Self-Adhesive Microneedles with Interlocking Features for Sustained Ocular Drug Delivery. Macromol. Biosci. 2020, 20, e2000089. [Google Scholar] [CrossRef]
- Matadh, A.V.; Jakka, D.; Pragathi, S.G.; Poornima, K.; Shivakumar, H.N.; Murthy, R.N.; Rangappa, S.; Shivanna, M.; Murthy, S.N. Polymer coated polymeric microneedles for intravitreal delivery of dexamethasone. Exp. Eye Res. 2023, 231, 109467. [Google Scholar] [CrossRef]
- Tawfik, M.; Chen, F.; Goldberg, J.L.; Sabel, B.A. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 1477–1507. [Google Scholar] [CrossRef] [PubMed]
- Chapa González, C.; Martínez Saráoz, J.V.; Roacho Pérez, J.A.; Olivas Armendáriz, I. Lipid nanoparticles for gene therapy in ocular diseases. Daru 2023, 31, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.R.; Bhatia, S. Carbon Dot Nanoparticles: Exploring the Potential Use for Gene Delivery in Ophthalmic Diseases. Nanomaterials 2021, 11, 935. [Google Scholar] [CrossRef]
- Sahu, B.; Chug, I.; Khanna, H. The Ocular Gene Delivery Landscape. Biomolecules 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Kantor, A.; McClements, M.E.; Marfany, G.; Trigueros, S.; MacLaren, R.E. Non-Viral Delivery of CRISPR/Cas Cargo to the Retina Using Nanoparticles: Current Possibilities, Challenges, and Limitations. Pharmaceutics 2022, 14, 1842. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chau, Y. Enhanced Delivery of siRNA to Retinal Ganglion Cells by Intravitreal Lipid Nanoparticles of Positive Charge. Mol. Pharm. 2021, 18, 377–385. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Wu, Y.; Feng, L.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320, 100–117. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, e1903060. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Kontturi, L.; Viitala, L.; Yliperttula, M.; Murtomäki, L.; Urtti, A. Light activated liposomes: Functionality and prospects in ocular drug delivery. J. Control. Release 2016, 244, 157–166. [Google Scholar] [CrossRef]
- Giannos, S.A.; Kraft, E.R.; Zhao, Z.Y.; Merkley, K.H.; Cai, J. Photokinetic Drug Delivery: Near infrared (NIR) Induced Permeation Enhancement of Bevacizumab, Ranibizumab and Aflibercept through Human Sclera. Pharm. Res. 2018, 35, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Li, W.; Wei, W.; Qin, M.; Li, Q.; Liu, X.; Zhang, X.; Wang, X. A graphene-Ag based near-infrared defined accurate anti-scarring strategy for ocular glaucoma surgery. Biomater. Sci. 2022, 10, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Kari, O.K.; Tavakoli, S.; Parkkila, P.; Baan, S.; Savolainen, R.; Ruoslahti, T.; Johansson, N.G.; Ndika, J.; Alenius, H.; Viitala, T.; et al. Light-Activated Liposomes Coated with Hyaluronic Acid as a Potential Drug Delivery System. Pharmaceutics 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A Review of the Mechanism of Action of Cyclosporine A: The Role of Cyclosporine A in Dry Eye Disease and Recent Formulation Developments. Clin. Ophthalmol. 2020, 14, 4187–4200. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Colombo, A.P.; Briançon, S.; Lieto, J.; Fessi, H. Project, design, and use of a pilot plant for nanocapsule production. Drug Dev. Ind. Pharm. 2001, 27, 1063–1072. [Google Scholar] [CrossRef]
| Type | Method | Area | Clinical Applications | Benefits | Challenges |
|---|---|---|---|---|---|
| Systemic | Intravenous/Oral | – | Ocular infection, Ocular hypertension, Uveitis, Optic neuritis | High patient compliance | BOB, low bioavailability systemic toxicity caused by high dosing |
| Topical | – | On the surface of the cornea | Keratitis, uveitis, conjunctivitis, scleritis, episcleritis, blepharitis | High patient compliance, self-administration, non-invasiveness | Tear dilution/turnover tear film and cornea barriers, efflux pumps |
| Intraocular | Intracameral | Into the anterior chamber | Anesthesia, pupil dilation, endophthalmitis | Direct delivery to the target location, lower dosing, BRB avoidance, higher efficiency | Poor patient compliance, invasiveness, drug toxicity, puncture-related complications (pain, bleeding, vitreous hemorrhage, ocular hypertension, retinal detachment, endophthalmitis, lens and optic nerve damage) |
| Intravitreal | Into the vitreal body | AMD, RVO, DME, endophthalmitis, uveitis, CMV retinitis | |||
| Subretinal | Between neurosensory retina and RPE | AMD, DME, cell therapy for inherited retinal dystrophies [33] | |||
| Intrastromal | Into the corneal stroma | Keratitis | |||
| Suprachoroidal | Between the sclera and choroid | Uveitic macular edema and DME [34] | |||
| Subconjunctival | Beneath conjunctiva | Keratitis, corneal neovascularization [35] | |||
| Periocular | Posterior juxta scleral | Posterior to the supertemporal limbus down to the sclera | Anecortave acetate (Retaane®) for AMD, triamcinolone for DME | Selective delivery to both anterior and posterior segments, avoidance of corneal and conjunctival barriers, long duration of action | Poor patient compliance, invasiveness, drug deposition, puncture- related complications (pain, bleeding, infection), risk of globe rupture or scarring, nerve/muscle damage |
| Retrobulbar | Intraconal space | Anesthesia | |||
| Peribulbar | Outside the four rectus muscles and their intramuscular septum | Anesthesia | |||
| Sub-tenon | Beneath tenon capsule | Chronic uveitis, macular telangiectasia, anesthesia |
| Product | Nanocarrier Types | Constituents | Indications |
|---|---|---|---|
| Hylo® gel | Hydrogel | Hyaluronate, sorbitol | Dry eye disease |
| Dextenza® | Hydrogel | Dexamethasone | Ocular inflammation, allergic conjunctivitis |
| ReSure® | Hydrogel | Polyethylene glycol | Corneal incisions |
| Retisert® | Intravitreal implant | Fluocinolone acetonide | Uveitis and macular edema |
| Artelac Rebalance® | Liposome | Vitamin B12 | Dry eye disease |
| Lacrisek® | Liposome | Vitamin A, E | Dry eye disease |
| Visudyne® | Liposome | Verteporfin | Wet age macular degeneration |
| Clinitas Hydrate® | Liposome | Carbomer 980 | Dry eye disease |
| Cequa® | Micelles | Cyclosporine A | Dry eye disease |
| AzaSite® | Micelles | Azithromycin | Dry eye disease, keratitis, eye inflammation |
| Durezol® | Nanoemulsion | Difluprednate | Postoperative ocular inflammation |
| Restasis® | Nanoemulsion | Cyclosporine A | Dry eye disease |
| Durezol® | Nanoemulsion | Difluprednate | Eye infection and pain |
| Cationorm® | Nanoemulsion | Medical device | Dry eye disease |
| Ikervis® | Nanoemulsion | Cyclosporine A | Keratitis |
| Xelpros® | Nanoemulsion | Latanopros | Open-angle glaucoma |
| Verkazia® | Nanoemulsion | Cyclosporine | Vernal keratoconjunctivitis |
| Cyclokat® | Nanoemulsion | Cyclosporine A | Dry eye disease |
| Systane® | Nanoemulsion | Aminomethyl propanol | Relieve dryness of the eye |
| Trivaris™ | Nanoparticles | Triamcinolone acetonide | Uveitis |
| BromSite® | Nanoparticles | Bromfenac | Postoperative inflammation and pain |
| Besivance® | Nanosuspension | Besifloxacin | Ocular bacterial infection |
| Tobradex ST® | Nanosuspension | Tobramycin dexamethasone | Ocular inflammation and bacterial infection |
| Inveltys® | Nanosuspension | Loteprednol etabonate | Postoperative ocular inflammation and pain |
| Eysuvis® | Nanosuspension | Loteprednol etabonate | Dry eye disease |
| Lacrisert® | Ocular inserts | Hydroxypropyl cellulose | Dry eye disease |
| Biofinity® | Soft contact lens | Silicone hydrogel | Correction of ametropia |
| Ocular Disorders | Loaded Agents | Micelles | Description | Reference, Year |
|---|---|---|---|---|
| DED | Cyclosporine A | VitE-TPGS and OPEE | Increase residence time in tear fluid with a t1/2 value four times greater than Ikervis. | [47], 2020 |
| Cyclosporin A | mPEG–hexPLA | In vivo transcorneal permeability was improved and nanomicelle formulation was significantly efficacious in preventing corneal graft rejection. | [48], 2018 | |
| Keratitis | Acyclovir | PVCL-PVA-PEG, Soluplus® | Acyclovir-loaded Soluplus micelles showed homogeneous nanometric particle size and slightly negative Z-potential values, facilitating penetration through the cornea and sclera. | [49], 2018 |
| Voriconazole | PBA-CSVE | PBA-CSVE-loaded voriconazole polymeric micelles proved to have good therapeutic effects, water solubility, biodegradability, low toxicity, and robust mucosal adhesion. | [50], 2022 | |
| Posaconazole | EPC-TPGS | Desirable stability over a month, slow release without an initial burst, and a significantly higher in vitro antifungal activity in comparison with the drug suspension. | [51], 2023 | |
| Glaucoma | Nimodipine (NMD) | Rebaudioside A/TPGS | NMD micelles improved the in vivo permeation, intraocular pressure reduction, and miosis. | [52], 2021 |
| Metipranolol | Pluronic F127 with Chitosan (0.3–0.8%) | Pharmacological response significantly improved upon the incorporation of chitosan. | [53], 2013 | |
| Uveitis | Dexamethasone | CSO-VV-SA | Both CSO-VV-SA nanomicelles and HCO-40/OC-40 mixed nanomicelles showed good retention in rabbit tears and equal delivering efficiency. | [54], 2020 |
| Everolimus | PVCL-PVA-PEG, Soluplus® | Everolimus nanomicelles showed significantly higher permeation across goat cornea than everolimus suspension (p < 0.001). | [55], 2021 | |
| AMD | Artemisinin | PVP K90 and Poloxamer 407 | 96.0–99.0% artemisinin was released from the nanomicelles within 8 h in vitro. Artemisinin-loaded nanomicelles show superior anti-angiogenic activity compared to artemisinin suspension. | [56], 2021 |
| Tacrolimus | PEG- HCO-40 and OC-40 (Cequa®) | Tacrolimus nanomicellar formulation lowers the pro-inflammatory cytokines and ROS. | [57], 2020 | |
| CNV | Aflibercept | PEG, PPG, and PCL copolymer EPC (nEPC) | Aflibercept-loaded nEPCs can penetrate the cornea in ex vivo models and deliver a significant amount of aflibercept to the retina in laser-induced CNV murine models, causing CNV regression. | [58], 2022 |
| Ocular Disorders | Loaded Agents | Stabilizers | Description | Reference, Year |
|---|---|---|---|---|
| DED | Ciclosporin A | PVA, PVP, HPMC, HPC, HEC | NS was physically and chemically stable for at least two months and caused less irritation to the rabbits’ eyes compared to the commercial product noted by the Schirmer tear test. | [87], 2011 |
| Conjunctivitis | Ketotifen Fumarate | PLGA Eudragit RL100 | Both NSs provide a useful dosage form for ocular drug delivery which can enhance the permeability of ketotifen fumarate. | [88], 2016 |
| Keratitis | Voriconazole | Eudragit RS 100 | Voriconazole-loaded NS enhances permeability and antifungal activity, effectively inhibiting Candida albicans growth at a lower concentration (2.5 μg/mL, p < 0.05) compared to the commercial voriconazole injection. | [89], 2021 |
| Inflammation | Hydrocortisone | PVP, HPMC, Tween 80 | NS sustained drug action was maintained up to 9 h compared to 5 h for the drug solution and showed good stability in room-temperature storage. | [90], 2011 |
| Ketorolac | Eudragit RL-100 | NS increases viscosity and avoids drug loss from the precorneal surface and rapid drainage through nasolacrimal areas. | [91], 2019 | |
| Glaucoma | Acetazolamide | Anionic polypeptide, poly-γ-glutamic acid (PG), and the glycosaminoglycan, hyaluronic acid | Enhanced saturation solubility, higher reduction of IOP with a longer duration of spray-dried acetazolamide NS, and sustained drug release were confirmed. | [92], 2020 |
| Ocular Disorders | Loaded Agents | Polymers | Description | Reference, Year |
|---|---|---|---|---|
| Corneal abrasion | Moxifloxacin pirfenidone | PLGA and polyvinylpyrrolidone (PVP) | The antimicrobial activity of moxifloxacin remained effective when encapsulated in the nanofibers, with a sustained release over 24 h. This nanofiber system holds promise for once-daily dosing for the treatment of corneal abrasions | [104], 2021 |
| Keratomycosis | Amphotericin-B | PLGA/Eu-L/Gellan Gum/Pullulan | Amphotericin B complex retained the antifungal activity with sufficient stability against irradiation-sterilization-induced drug degradation and was less toxic to cornea cells in vitro. | [105], 2020 |
| Inflammation | Dexamethasone (DX) | poly(ε-caprolactone) (PCL) | DX PCL nanofibers exhibited ocular biocompatibility and safety by SD-OCT images and histological analysis of neuroretina and choroid in the rodent eye. The nanofiber could provide controlled DX release for 10 days. | [106], 2019 |
| Glaucoma | Timolol maleate (TM) Brimonidine (BR) | Self-assembling peptide ac-(RADA)4-CONH2 | A rapid and complete release of both drugs was achieved within 8 h, while a 2.8-fold and 5.4-fold higher corneal permeability was achieved for TM and BR, respectively | [107], 2020 |
| Brinzolamide | β-cyclodextrin, hydroxypropyl cellulose, and polycaprolactone | The nanofiber provides more precise dosing and permeation through sheep corneas was almost linear in time, achieving therapeutic concentrations in the receptor medium over 6 h. | [108], 2022 | |
| Timolol maleate (TM) | In situ gelling nanofiber films (PVA and Poloxamer 407) | In vivo administration of the ocular films in rabbits induced a faster onset and a sustained IOP-lowering effect up to 24 h. | [109], 2022 | |
| AMD | Bevacizumab | PCL and gelatin form the shell of the nanofibers, PVA in the core | Bevacizumab retained its antiangiogenic activity when loaded into the biodegradable core–shell electrospun nanofibers. These nanofibers have the potential to be an alternative treatment option to frequent intravitreal injections of antiangiogenic agents for AMD | [110], 2018 |
| AMD, DR, and glaucoma | Melatonin (MEL) | PVA, PLA | MEL release rates are based on the nature of the polymer. The fast and complete release was observed in PVA-based samples, while the PLA polymer showed slow, controlled MEL release. | [111], 2023 |
| Ocular Disorders | Loaded Agents | Nanocarriers | Description | Reference, Year |
|---|---|---|---|---|
| DED | Azithromycin (AZM) | Liposome | The corneal permeation of AZM-liposome is approximately 2-fold greater than that of the AZM solution, and AZM-liposome significantly improved symptoms of dry eye in rats compared to hyaluronic acid sodium eye drops | [130], 2018 |
| Fungal keratitis | Rapamycin | Liposome | The severity of corneal lesions in the rapamycin-liposome treatment group was reduced | [131], 2019 |
| Natamycin (NAT) | Niosome | NAT niosomal dispersion exhibited prolonged drug release (40.96–77.49% over 24 h) in vitro. Superiority in treatment of candida keratitis and better results on corneal infiltration and hypopyon level in vivo. | [132], 2019 | |
| Ocular infection | Vancomycin | Niosome | In rabbits infected with MRSA, vancomycin niosomal gels showed a 180-fold increase in antibacterial effectiveness compared to untreated animals and a 2.5-fold improvement compared to those treated with the free vancomycin solution. | [133], 2019 |
| GVHD | Tacrolimus | Liposomes containing bile salts | Liposomes containing bile salts increase the corneal transport of tacrolimus to 3–4-fold compared with conventional liposomes. | [134], 2013 |
| Glaucoma | Latanoprost | Niosome | Nonspecific interactions between latanoprost and different niosomal components vary drug encapsulation efficiency. Latanoprost niosomal Pluronic® F127 gel had the best ability to sustain drug release in rabbits’ eyes without toxic and irritant effects and significantly reduced IOP. | [135], 2020 |
| PCME | Triamcinolone acetonide (TA) | Liposome | Patients with refractory PCME under TA-liposome formulation therapy showed a significant improvement in BCVA and central foveal thickness without IOP increase. | [136], 2019 |
| AMD | Berberine hydrochloride chrysophanol | PAMAM G3.0-coated compound liposomes | PAMAM G3.0-coated compound liposomes exhibited good cellular permeability in human corneal epithelial cells and enhanced bio-adhesion to the corneal epithelium in a rabbit model. Liposomes were proven to possess protective effects in human retinal pigment epithelial cells. | [137], 2019 |
| CNV | Triamcinolone acetonide | Chitosan coated liposomes (CCL) | CCL showed a higher encapsulation efficiency with a highly positive surface charge (+41.1 Mv) that increased retention time, sustained release, and penetration via the corneal mucosal barrier to the vitreous body. | [138], 2020 |
| DR | Epalrestat | Cationic niosomes | Niosomal had higher permeation than an unencapsulated drug in the sclera and showed the capability to encapsulate and carry epalrestat through the ocular barrier to treat the diabetic eye. | [139], 2023 |
| Ocular Disorders | Loaded Agents | Method Used | Description | Reference, Year |
|---|---|---|---|---|
| Infection | Ciprofloxacin Moxifloxacin | Soaking commercial hydrogel and silicone hydrogel lenses | Release in the vial for both drugs was rapid, reaching a plateau between 15 min and 2 h while under physiological flow conditions; a constant and slow release was observed over 24 h. | [149], 2015 |
| Natamycin (NA) Fluconazole (FL) | Soaking commercial silicone hydrogel contact lenses | Limited yeast growth. | [150], 2016 | |
| Inflammation | Betamethasone | Commercial contact lenses soaked in vitamin E solutions | Vitamin E can be applied as a hydrophobic diffusion barrier for controlling and sustaining BMZ release from silicone-based soft contact lenses. | [151], 2016 |
| Dexamethasone | Drug-eluting contact lenses | In a rabbit model following photorefractive keratectomy, weekly use of dexamethasone- eluting contact lenses for 4 weeks proved safe and equally effective as applying 0.1% dexamethasone eye drops four times a day over the same duration in preventing corneal haze. | [152], 2021 | |
| Glaucoma | Bimatoprost | Graphene oxide-loaded silicone hydrogel contact lenses | Significant improvement in mean residence time and area under the curve with DL-GO-0.2 μg-BMT-100 contact lens was found in the rabbit tear fluid in comparison to the eye drop solution. | [153], 2021 |
| Timolol Latanoprost | Micelles-laden contact lenses (CLs-M) | Significant improvement of the mean residence time and bioavailability of CLs-M compared with eye drops. The relative pharmacological availability of CLs-M was 9.8 times as high as the eye drops. | [154], 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-C.; Chen, Y.-H.; Lu, D.-W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. https://doi.org/10.3390/ijms242015352
Liu L-C, Chen Y-H, Lu D-W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. International Journal of Molecular Sciences. 2023; 24(20):15352. https://doi.org/10.3390/ijms242015352
Chicago/Turabian StyleLiu, Li-Ching, Yi-Hao Chen, and Da-Wen Lu. 2023. "Overview of Recent Advances in Nano-Based Ocular Drug Delivery" International Journal of Molecular Sciences 24, no. 20: 15352. https://doi.org/10.3390/ijms242015352
APA StyleLiu, L.-C., Chen, Y.-H., & Lu, D.-W. (2023). Overview of Recent Advances in Nano-Based Ocular Drug Delivery. International Journal of Molecular Sciences, 24(20), 15352. https://doi.org/10.3390/ijms242015352





