Polyamines in Ovarian Aging and Disease
Abstract
1. Introduction
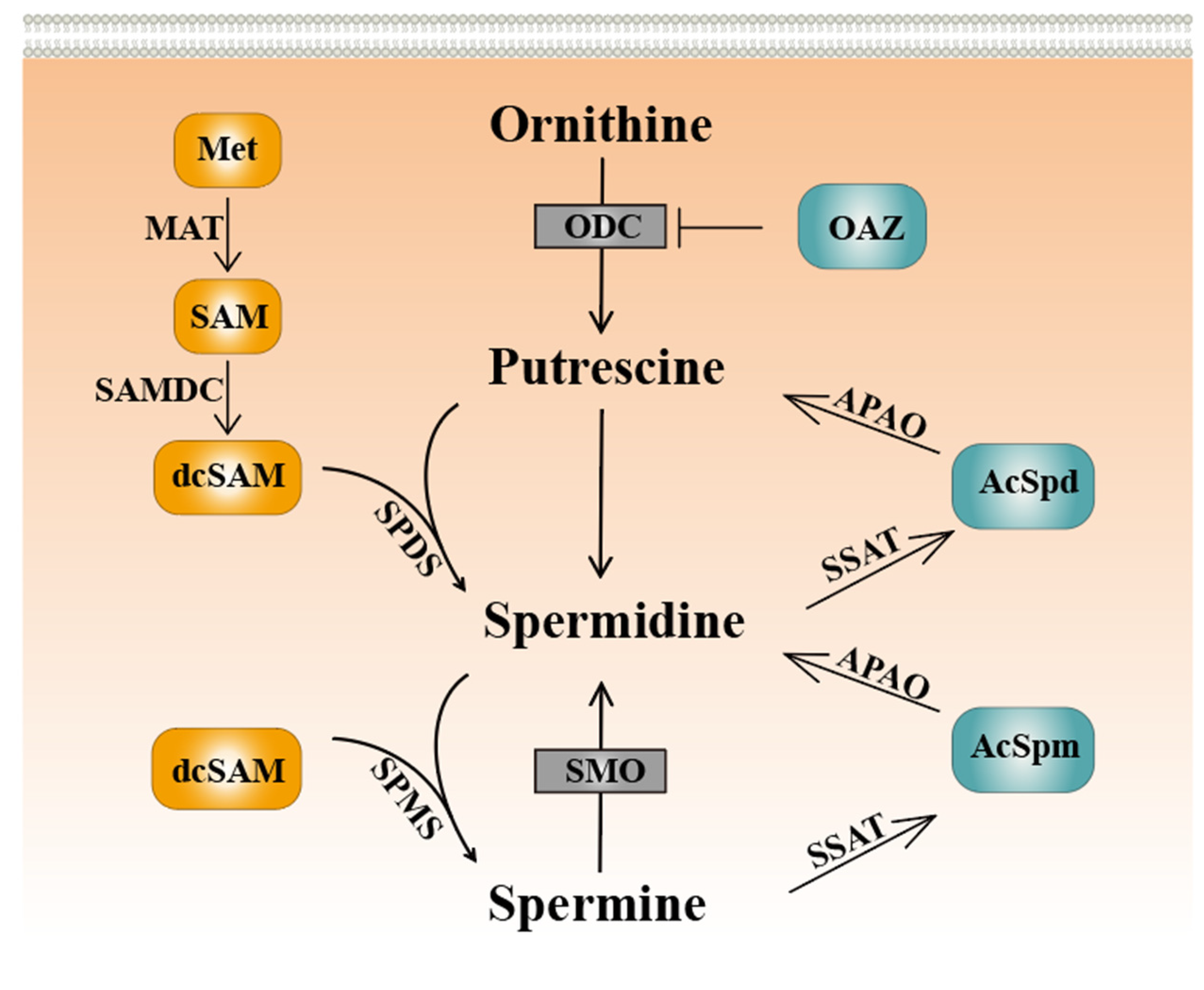
2. Polyamine Metabolism
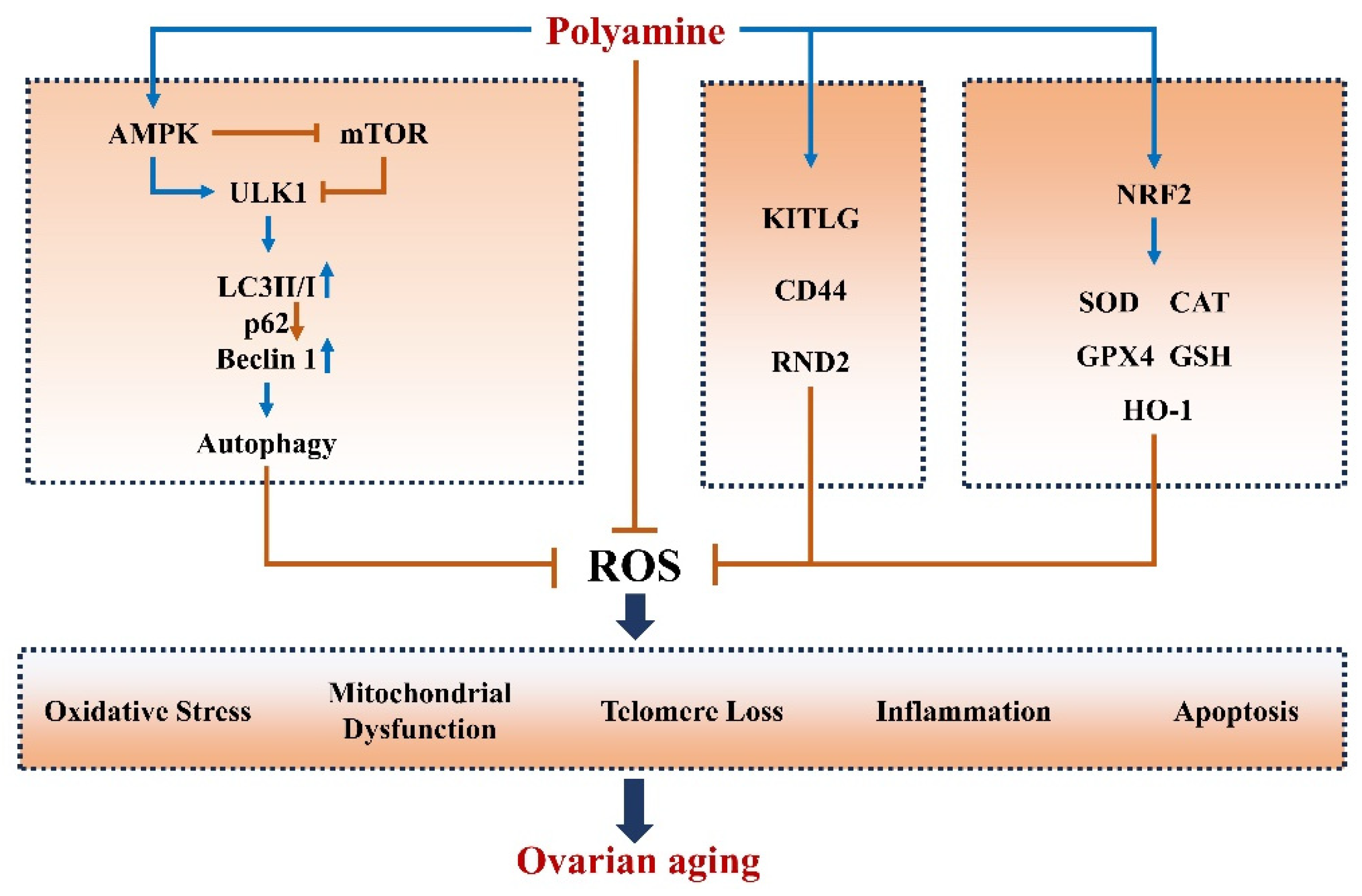
3. Polyamines Improve Ovarian Aging
3.1. Polyamines Mitigate Oxidative Stress and Apoptosis in Ovaries
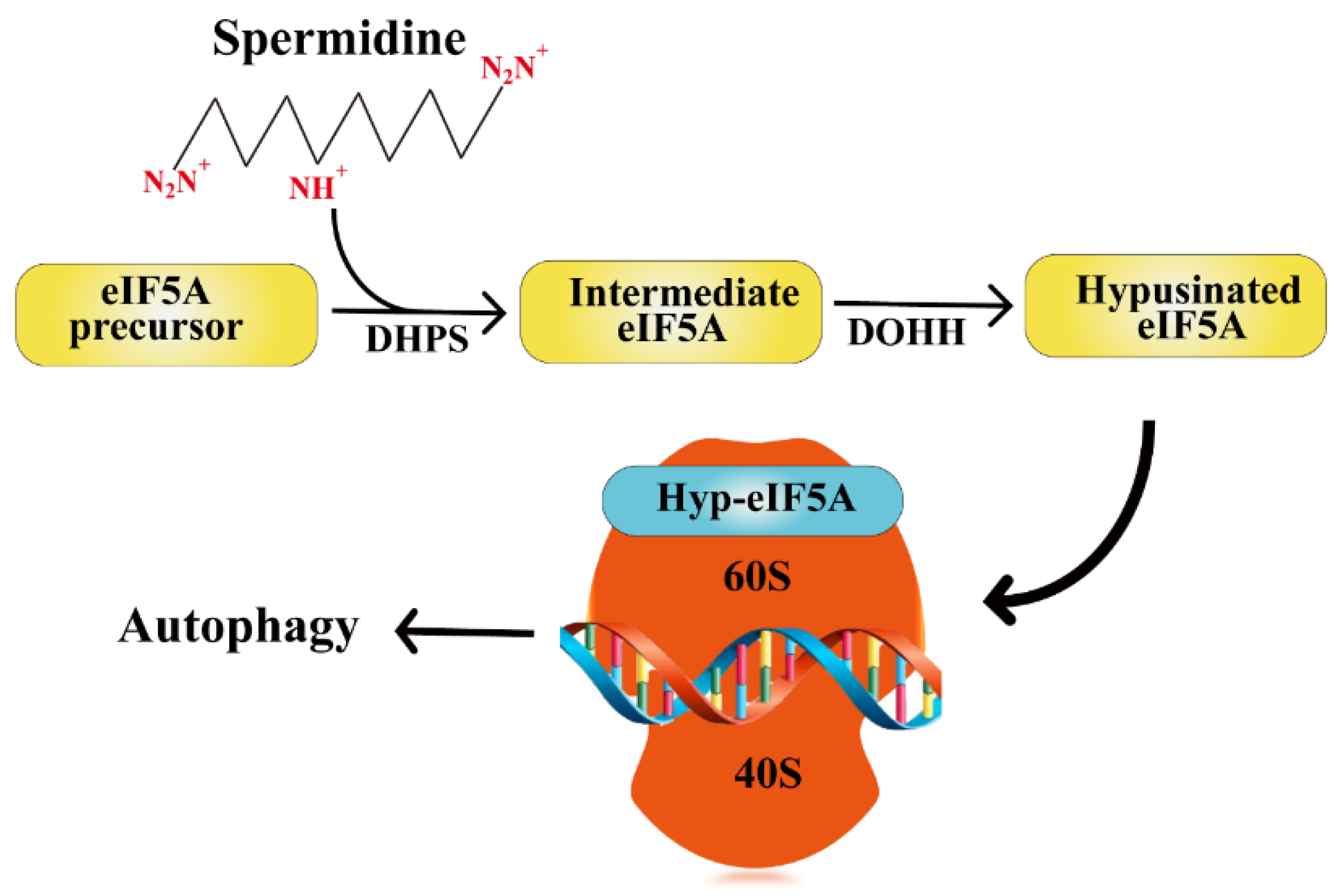
3.2. Polyamine Induces Autophagy in Ovaries
3.3. Polyamines Inhibit Inflammation in Ovaries
3.4. Polyamines Alleviate Telomere Damage in Ovaries
3.5. Polyamines Improve Mitochondrial Function
3.6. Polyamines Improve Oocyte Quality in Ovaries
4. Polyamines and Ovarian Disease
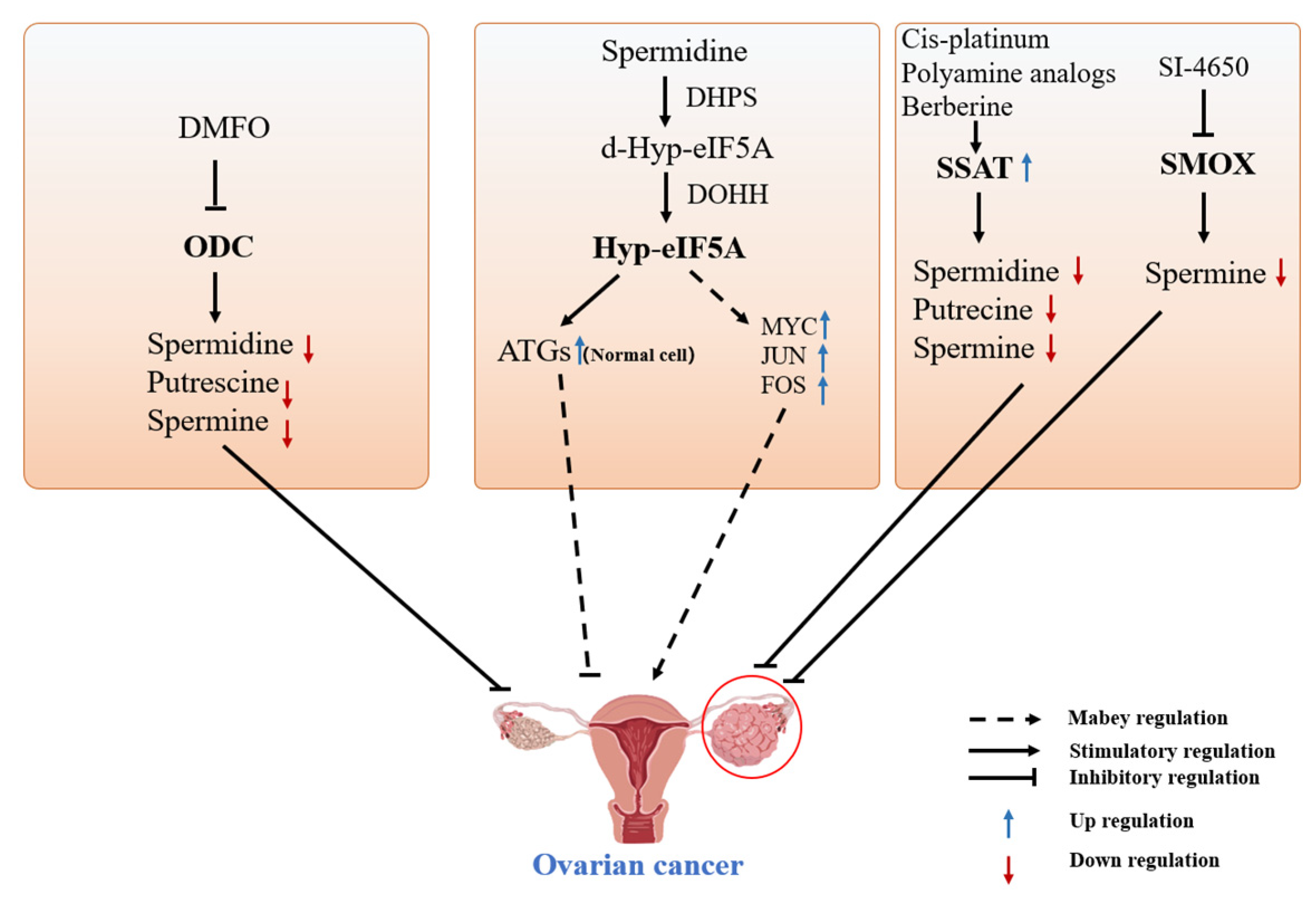
4.1. Polyamines and Ovarian Cancer
4.1.1. DFMO-Targeted Inhibition of ODC for the Treatment of Ovarian Cancer
4.1.2. Spermine Analogs Treat Cancer by Reducing Cellular Polyamine Concentrations
4.1.3. Targeted Modulation of SSAT Enzyme for Treating Ovarian Cancer
4.1.4. Other Treatments for Ovarian Cancer
4.2. Polyamines and Other Ovarian Diseases
4.2.1. Polyamines May Be a Potential Treatment for PCOS
4.2.2. Potential Roles of Spermidine in Primary Ovarian Insufficiency
4.2.3. Polyamines Are Involved in Luteal Phase Deficiency
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pegg, A.E.; Casero, R.A., Jr. Current status of the polyamine research field. Methods Mol. Biol. 2011, 720, 3–35. [Google Scholar] [PubMed]
- Seiler, N. Polyamine metabolism and function in brain. Neurochem. Int. 1981, 3, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.Y.; Zolp, A.; Miska, J. Polyamine Immunometabolism: Central Regulators of Inflammation, Cancer and Autoimmunity. Cells 2022, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Besora-Casals, L.; van Veen, S.; Zielich, J.; Van den Haute, C.; Hamouda, N.N.; Fischer, C.; Ghesquière, B.; Tournev, I.; Agostinis, P.; et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc. Natl. Acad. Sci. USA 2020, 117, 31198–31207. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef] [PubMed]
- Dviri, M.; Madjunkova, S.; Koziarz, A.; Antes, R.; Abramov, R.; Mashiach, J.; Moskovtsev, S.; Kuznyetsova, I.; Librach, C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3118 embryos derived from young egg donors. Fertil. Steril. 2020, 114, 293–300. [Google Scholar] [CrossRef]
- Dong, L.; Teh, D.B.L.; Kennedy, B.K.; Huang, Z. Unraveling female reproductive senescence to enhance healthy longevity. Cell Res. 2023, 33, 11–29. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Munoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Baste, O.; Toro-Funes, N.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, P.; Bauer, S.; Bauer, F.; Dicakova, Z. Contents of Polyamines and Biogenic Amines in Canned Pet (Dogs and Cats) Food on the Austrian Market. Foods 2021, 10, 2365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Chen, Z.; Li, S.; Che, B.; Wang, N.; Chen, J.; Xu, C.; Wei, C. Exogenous spermine attenuates diabetic kidney injury in rats by inhibiting AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2021, 47, 27. [Google Scholar] [CrossRef]
- Vujcic, S.; Halmekyto, M.; Diegelman, P.; Gan, G.; Kramer, D.L.; Janne, J.; Porter, C.W. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J. Biol. Chem. 2000, 275, 38319–38328. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, J.L.; Tolbert, W.D.; Xiong, H.; Pegg, A.E.; Ealick, S.E.J.B. Structure of a human S-adenosylmethionine decarboxylase self-processing ester intermediate and mechanism of putrescine stimulation of processing as revealed by the H243A mutant. Biochemistry 2001, 40, 9495–9504. [Google Scholar] [CrossRef] [PubMed]
- Raney, A.; Law, G.L.; Mize, G.J.; Morris, D.R. Regulated Translation Termination at the Upstream Open Reading Frame in S-Adenosylmethionine Decarboxylase mRNA. J. Biol. Chem. 2002, 277, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, Y.; Bewley, M.C.; Pegg, A.E. Aminopropyltransferases: Function, structure and genetics. J. Biochem. 2006, 139, 1–9. [Google Scholar] [CrossRef]
- Kang, B.; Xu, Q.; Chen, Z.; Wu, Y.; Yang, S.; Yang, X.; Zhang, Z.; Jiang, D. Characterization of goose SPMS: Molecular characterization and expression profiling of SPMS in the goose ovary. Reprod. Biol. 2018, 18, 60–65. [Google Scholar] [CrossRef]
- Zhang, M.; Pickart, C.M.; Coffino, P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003, 22, 1488–1496. [Google Scholar] [CrossRef]
- Kang, B.; Jiang, D.M.; Ma, R.; He, H.; Yi, Z.X.; Chen, Z.Y. OAZ1 knockdown enhances viability and inhibits ER and LHR transcriptions of granulosa cells in geese. PLoS ONE 2017, 12, e0175016. [Google Scholar] [CrossRef]
- Jiang, D.M.; Chen, Z.Y.; Yi, Z.X.; Kang, B. Characterization of the Ssat1 Gene and Its Expression Profiling in Various Tissues and Follicles in Geese. Ann. Anim. Sci. 2018, 18, 675–684. [Google Scholar] [CrossRef]
- Barba-Aliaga, M.; Alepuz, P. Role of eIF5A in Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 1284. [Google Scholar] [CrossRef] [PubMed]
- Klier, H.; Csonga, R.; Joao, H.C.; Eckerskorn, C.; Auer, M.; Lottspeich, F.; Eder, J. Isolation and structural characterization of different isoforms of the hypusine-containing protein eIF-5A from HeLa cells. Biochemistry 1995, 34, 14693–14702. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pang, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Cook, S.A.; Suzuki, A.; Diehl, A.M.; Petretto, E.; et al. Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression. Nat. Commun. 2022, 13, 5202. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef]
- Gupta, V.K.; Scheunemann, L.; Eisenberg, T.; Mertel, S.; Bhukel, A.; Koemans, T.S.; Kramer, J.M.; Liu, K.S.; Schroeder, S.; Stunnenberg, H.G.; et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 2013, 16, 1453–1460. [Google Scholar] [CrossRef]
- Chevalier, C.; Kieser, S.; Çolakoğlu, M.; Hadadi, N.; Brun, J.; Rigo, D.; Suárez-Zamorano, N.; Spiljar, M.; Fabbiano, S.; Busse, B.; et al. Warmth Prevents Bone Loss through the Gut Microbiota. Cell Metab. 2020, 32, 575–590.e7. [Google Scholar] [CrossRef]
- Puleston, D.J.; Simon, A.K. New roles for autophagy and spermidine in T cells. Microb. Cell 2015, 2, 91–93. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Y.; Cheng, J.; Yuan, S.; Zhou, S.; Yan, W.; Liu, J.; Luo, A.; Wang, S. CCL5 secreted by senescent theca-interstitial cells inhibits preantral follicular development via granulosa cellular apoptosis. J. Cell. Physiol. 2019, 234, 22554–22564. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging 2019, 11, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, D.; Liu, W.; Qin, Q.; Fang, Z.; Pan, Z. Hedgehog pathway inhibition causes primary follicle atresia and decreases female germline stem cell proliferation capacity or stemness. Stem Cell Res. Ther. 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Nottola, S.A.; Tunjung, W.A.; Kadowaki, A.; Bianchi, S.; Cecconi, S.; Sato, E.; Macchiarelli, G. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem. Biophys. Res. Commun. 2016, 481, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid Med. Cell Longev. 2017, 2017, 4371714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, Y.; Niu, C.; Long, S.; Jiang, Y.; Wang, Z.; Wang, X.; Sun, Q.; Ling, W.; An, X.; et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. Int. J. Mol. Sci. 2023, 24, 5793. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Jiang, D.; Guo, Y.; Wang, Z.; Sun, Q.; Wang, X.; Ling, W.; An, X.; Ji, C.; Li, S.; et al. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023, 332, 122109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, X.; Zhou, X.; Wang, Z.; Li, S.; Sun, Q.; Jiang, Y.; Ji, C.; Ling, W.; An, X.; et al. Spermidine alleviating oxidative stress and apoptosis by inducing autophagy of granulosa cells in Sichuan white geese. Poult. Sci. 2023, 102, 102879. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef]
- Sava, I.G.; Battaglia, V.; Rossi, C.A.; Salvi, M.; Toninello, A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic. Biol. Med. 2006, 41, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, S.; Mo, G.; Jiang, Y.; Li, L.; Xu, H.; Han, C.; Zhao, H.; Yan, Y.; Hu, S.; et al. Effects of ODC on polyamine metabolism, hormone levels, cell proliferation and apoptosis in goose ovarian granulosa cells. Poult. Sci. 2021, 100, 101226. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.Q.; Liu, Y.S. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021, 12, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pando, J.M.; Alcocer-Gómez, E.; Castejón-Vega, B.; Navarro-Villarán, E.; Condés-Hervás, M.; Mundi-Roldan, M.; Muntané, J.; Pérez-Pulido, A.J.; Bullon, P.; Wang, C.; et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci. Adv. 2021, 7, eabc7409. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Lubas, M.; Harder, L.M.; Kumsta, C.; Tiessen, I.; Hansen, M.; Andersen, J.S.; Lund, A.H.; Frankel, L.B. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 2018, 19, e46072. [Google Scholar] [CrossRef]
- Frankel, L.B. EIF5A mediates autophagy via translation of ATG3. Autophagy 2018, 14, 1288–1289. [Google Scholar] [CrossRef]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Liang, Y.; Zimmermann, A.; Schroeder, S.; Dengjel, J.; Kroemer, G.; Eisenberg, T.; Sigrist, S.J.; Madeo, F. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy 2021, 17, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Piao, C.; Beuschel, C.B.; Toppe, D.; Kollipara, L.; Bogdanow, B.; Maglione, M.; Lützkendorf, J.; See, J.C.K.; Huang, S.; et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 2021, 35, 108941. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shangguan, J.; Wang, Z.; Li, Y.; Fan, J.; Ren, A.; Zhao, M. Spermidine Regulates Mitochondrial Function by Enhancing eIF5A Hypusination and Contributes to Reactive Oxygen Species Production and Ganoderic Acid Biosynthesis in Ganoderma lucidum. Appl. Environ. Microbiol. 2022, 88, e0203721. [Google Scholar] [CrossRef] [PubMed]
- Gulappa, T.; Menon, B.; Menon, K.M. Hypusination of eukaryotic initiation factor 5A via cAMP-PKA-ERK1/2 pathway is required for ligand-induced downregulation of LH receptor mRNA expression in the ovary. Mol. Cell. Endocrinol. 2015, 413, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef]
- Timóteo-Ferreira, F.; Mendes, S.; Rocha, N.A.; Matos, L.; Rodrigues, A.R.; Almeida, H.; Silva, E. Apocynin Dietary Supplementation Delays Mouse Ovarian Ageing. Oxid Med. Cell Longev. 2019, 2019, 5316984. [Google Scholar] [CrossRef]
- Jeong, J.W.; Cha, H.J.; Han, M.H.; Hwang, S.J.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol. Ther. 2018, 26, 146–156. [Google Scholar] [CrossRef]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Wirth, A.; Wolf, B.; Huang, C.K.; Glage, S.; Hofer, S.J.; Bankstahl, M.; Bär, C.; Thum, T.; Kahl, K.G.; Sigrist, S.J.; et al. Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. GeroScience 2021, 43, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, C.; Li, C.; Huang, Z.; Zeng, J.; Han, L.; Wang, Q. SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging 2019, 11, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M.; et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum. Reprod. 2012, 27, 1411–1420. [Google Scholar] [CrossRef]
- Aihara, S.; Torisu, K.; Uchida, Y.; Imazu, N.; Nakano, T.; Kitazono, T. Spermidine from arginine metabolism activates Nrf2 and inhibits kidney fibrosis. Commun. Biol. 2023, 6, 676. [Google Scholar] [CrossRef] [PubMed]
- Alberico, H.C.; Woods, D.C. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front. Physiol. 2021, 12, 800739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Zhang, Y.; Zhang, J.; Xu, W.; Wu, C.; Zhou, P. Growth hormone protects against ovarian granulosa cell apoptosis: Alleviation oxidative stress and enhancement mitochondrial function. Reprod. Biol. 2021, 21, 100504. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.A.M.; Umehara, T.; Kawai, T.; Shimada, M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic. Biol. Med. 2021, 163, 344–355. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Wang, J.; Wu, F.; Chen, Y.; Zhang, H.; Guo, Y.; Lin, Y.; Li, L.; Yu, X.; et al. Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging 2020, 12, 650–671. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Ikeda, K.; Yamazaki, E.; Katayama, A.; Urata, R.; Matoba, S. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci. Rep. 2023, 13, 8338. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef]
- Messerer, J.; Wrede, C.; Schipke, J.; Brandenberger, C.; Abdellatif, M.; Eisenberg, T.; Madeo, F.; Sedej, S.; Mühlfeld, C. Spermidine supplementation influences mitochondrial number and morphology in the heart of aged mice. J. Anat. 2023, 242, 91–101. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, X.J. Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell 2013, 12, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Tartia, A.; Lawson, M.; Zelinski, M.B.; Wu, W.; Liu, J.Y.; Smitz, J.; Léveillé, M.C.; Leader, A.; Wang, H.; et al. Can peri-ovulatory putrescine supplementation improve egg quality in older infertile women? J. Assist. Reprod. Genet. 2019, 36, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res. 2016, 363, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mo, G.; Tao, Y.; Wang, H.; Liu, X.J. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod. Fertil. Dev. 2017, 29, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yan, Z.; Zhang, Y.; Qin, L.; Wu, W.; Gao, C.; Gao, L.; Liu, J.; Cui, Y. Effects of putrescine on the quality and epigenetic modification of mouse oocytes during in vitro maturation. Reprod. Fertil. Dev. 2022, 34, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Bedenk, J.; Vrtačnik-Bokal, E.; Virant-Klun, I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J. Assist. Reprod. Genet. 2020, 37, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V. Inhibitors of polyamine metabolism: Review article. Amino Acids 2004, 26, 353–365. [Google Scholar] [CrossRef]
- Wallace, H.M.; Niiranen, K. Polyamine analogues—An update. Amino Acids 2007, 33, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.S.; Geerts, D. Polyamine synthesis as a target of MYC oncogenes. J. Biol. Chem. 2018, 293, 18757–18769. [Google Scholar] [CrossRef] [PubMed]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A., Jr.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Rajput, R.; Gupta, G.; Ydyrys, A.; Kulbayeva, M.; Abdull Razis, A.F.; et al. Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms. Front. Chem. 2023, 11, 1164477. [Google Scholar] [CrossRef] [PubMed]
- Niemi, R.J.; Roine, A.N.; Häkkinen, M.R.; Kumpulainen, P.S.; Keinänen, T.A.; Vepsäläinen, J.J.; Lehtimäki, T.; Oksala, N.K.; Mäenpää, J.U. Urinary Polyamines as Biomarkers for Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Park, W.H.; Suh, D.H.; Kim, K.; Kim, Y.B.; No, J.H. Difluoromethylornithine Induces Apoptosis through Regulation of AP-1 Signaling via JNK Phosphorylation in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 10255. [Google Scholar] [CrossRef]
- Kun, E.; Kirsten, E.; Mendeleyev, J.; Ordahl, C.P. Regulation of the enzymatic catalysis of poly(ADP-ribose) polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3, and ATP. Biochemistry 2004, 43, 210–216. [Google Scholar] [CrossRef]
- El Naggar, O.; Doyle, B.; Mariner, K.; Gilmour, S.K. Difluoromethylornithine (DFMO) Enhances the Cytotoxicity of PARP Inhibition in Ovarian Cancer Cells. Med. Sci. 2022, 10, 28. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Woster, P.M. Terminally alkylated polyamine analogues as chemotherapeutic agents. J. Med. Chem. 2001, 44, 1–26. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.K.; Valasinas, A.; Sarkar, A.; Basu, H.S.; Marton, L.J.; Frydman, B. Conformationally restricted analogues of 1N,12N-bisethylspermine: Synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998, 41, 4723–4732. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Diegelman, P.; Hector, S.; Kramer, D.L.; Clark, K.; Zagst, P.; Fetterly, G.; Porter, C.W.; Pendyala, L. Combination effects of platinum drugs and N1, N11 diethylnorspermine on spermidine/spermine N1-acetyltransferase, polyamines and growth inhibition in A2780 human ovarian carcinoma cells and their oxaliplatin and cisplatin-resistant variants. Cancer Chemother. Pharmacol. 2011, 67, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.J.; Müller, R.; Huang, G.; McManis, J.S.; Algee, S.E.; Yao, H.; Weimar, W.R.; Wiegand, J. Synthesis and evaluation of hydroxylated polyamine analogues as antiproliferatives. J. Med. Chem. 2001, 44, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Foley, J.R.; Murray Stewart, T.; Casero, R.A., Jr. Expanded Potential of the Polyamine Analogue SBP-101 (Diethyl Dihydroxyhomospermine) as a Modulator of Polyamine Metabolism and Cancer Therapeutic. Int. J. Mol. Sci. 2022, 23, 6798. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Lei, X.M.; Wang, Y.L. Spermidine/spermine N1-acetyltransferase and cancers. Chem. Life 2013, 33, 638–643. [Google Scholar]
- Tse, R.T.; Ding, X.; Wong, C.Y.; Cheng, C.K.; Chiu, P.K.; Ng, C.F. The Association between Spermidine/Spermine N(1)-Acetyltransferase (SSAT) and Human Malignancies. Int. J. Mol. Sci. 2022, 23, 5926. [Google Scholar] [CrossRef] [PubMed]
- Marverti, G.; Ligabue, A.; Lombardi, P.; Ferrari, S.; Monti, M.G.; Frassineti, C.; Costi, M.P. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int. J. Oncol. 2013, 43, 1269–1280. [Google Scholar] [CrossRef]
- Marverti, G.; Ligabue, A.; Guerrieri, D.; Paglietti, G.; Piras, S.; Costi, M.P.; Farina, D.; Frassineti, C.; Monti, M.G.; Moruzzi, M.S. Spermidine/spermine N1-acetyltranferase modulation by novel folate cycle inhibitors in cisplatin-sensitive and -resistant human ovarian cancer cell lines. Gynecol. Oncol. 2010, 117, 202–210. [Google Scholar] [CrossRef]
- Marverti, G.; Giuseppina Monti, M.; Pegg, A.E.; McCloskey, D.E.; Bettuzzi, S.; Ligabue, A.; Caporali, A.; D’Arca, D.; Moruzzi, M.S. Spermidine/spermine N1-acetyltransferase transient overexpression restores sensitivity of resistant human ovarian cancer cells to N1,N12-bis(ethyl)spermine and to cisplatin. Carcinogenesis 2005, 26, 1677–1686. [Google Scholar] [CrossRef][Green Version]
- Yang, J.L.; Tian, J.J.; Zhang, H.; Chen, Q.Y.; Lyu, Y.F.; Cao, C.Y. Effects of a novel spermine oxidase inhibitor SI-4650 on proliferation and EMT of human ovarian cancer SKVO-3 cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2022, 38, 175–180. [Google Scholar] [PubMed]
- Stilgenbauer, M.; Jayawardhana, A.; Datta, P.; Yue, Z.; Gray, M.; Nielsen, F.; Bowers, D.J.; Xiao, H.; Zheng, Y.R. A spermine-conjugated lipophilic Pt(iv) prodrug designed to eliminate cancer stem cells in ovarian cancer. Chem. Commun. 2019, 55, 6106–6109. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nature reviews. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar]
- Coni, S.; Serrao, S.M.; Yurtsever, Z.N.; Di Magno, L.; Bordone, R.; Bertani, C.; Licursi, V.; Ianniello, Z.; Infante, P.; Moretti, M.; et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis. 2020, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Coni, S.; Bordone, R.; Ivy, D.M.; Yurtsever, Z.N.; Di Magno, L.; D’Amico, R.; Cesaro, B.; Fatica, A.; Belardinilli, F.; Bufalieri, F.; et al. Combined inhibition of polyamine metabolism and eIF5A hypusination suppresses colorectal cancer growth through a converging effect on MYC translation. Cancer Lett. 2023, 559, 216120. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Smith, T.M.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Williams, K.J.; McNamara, K.M.; Delgado, A.G.; Short, S.P.; et al. Hypusination Maintains Intestinal Homeostasis and Prevents Colitis and Carcinogenesis by Enhancing Aldehyde Detoxification. Gastroenterology 2023, 165, 656–669.e8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ji, C.; Kang, L.; Ling, W.; Wang, Z.; Wang, X.; Niu, C.; Guo, Y.; Sun, Q.; An, X.; et al. Correlation analysis of polyamine metabolism and reproductive hormone levels in goose ovarian follicles. Theriogenology 2023, 210, 244–250. [Google Scholar] [CrossRef]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Bañuls, C.; Rovira-Llopis, S.; Martinez de Marañon, A.; Veses, S.; Jover, A.; Gomez, M.; Rocha, M.; Hernandez-Mijares, A.; Victor, V.M. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Metabolism 2017, 71, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Alvarez, A.; de Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.S. Primary Ovarian Insufficiency. Semin. Reprod. Med. 2016, 34, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Mears, J.A.; Buchner, D.A. Mitochondrial Dysfunction in Primary Ovarian Insufficiency. Endocrinology 2019, 160, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Müller, M.; et al. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef] [PubMed]
- Boutzios, G.; Karalaki, M.; Zapanti, E. Common pathophysiological mechanisms involved in luteal phase deficiency and polycystic ovary syndrome. Impact on fertility. Endocrine 2013, 43, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Young, S. Diagnosis and treatment of luteal phase deficiency: A committee opinion. Fertil. Steril. 2021, 115, 1416–1423. [Google Scholar]
- Mäkitie, L.T.; Kanerva, K.; Sankila, A.; Andersson, L.C. High expression of antizyme inhibitor 2, an activator of ornithine decarboxylase in steroidogenic cells of human gonads. Histochem. Cell Biol. 2009, 132, 633–638. [Google Scholar] [CrossRef]
- Lefèvre, P.L.; Palin, M.F.; Murphy, B.D. Polyamines on the reproductive landscape. Endocr. Rev. 2011, 32, 694–712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Wang, X.; An, X.; Ji, C.; Ling, W.; Qi, Y.; Li, S.; Jiang, D. Polyamines in Ovarian Aging and Disease. Int. J. Mol. Sci. 2023, 24, 15330. https://doi.org/10.3390/ijms242015330
Kang B, Wang X, An X, Ji C, Ling W, Qi Y, Li S, Jiang D. Polyamines in Ovarian Aging and Disease. International Journal of Molecular Sciences. 2023; 24(20):15330. https://doi.org/10.3390/ijms242015330
Chicago/Turabian StyleKang, Bo, Xin Wang, Xiaoguang An, Chengweng Ji, Weikang Ling, Yuxin Qi, Shuo Li, and Dongmei Jiang. 2023. "Polyamines in Ovarian Aging and Disease" International Journal of Molecular Sciences 24, no. 20: 15330. https://doi.org/10.3390/ijms242015330
APA StyleKang, B., Wang, X., An, X., Ji, C., Ling, W., Qi, Y., Li, S., & Jiang, D. (2023). Polyamines in Ovarian Aging and Disease. International Journal of Molecular Sciences, 24(20), 15330. https://doi.org/10.3390/ijms242015330




