Inhibition of Polyamine Catabolism Reduces Cellular Senescence
Abstract
:1. Introduction
2. Results
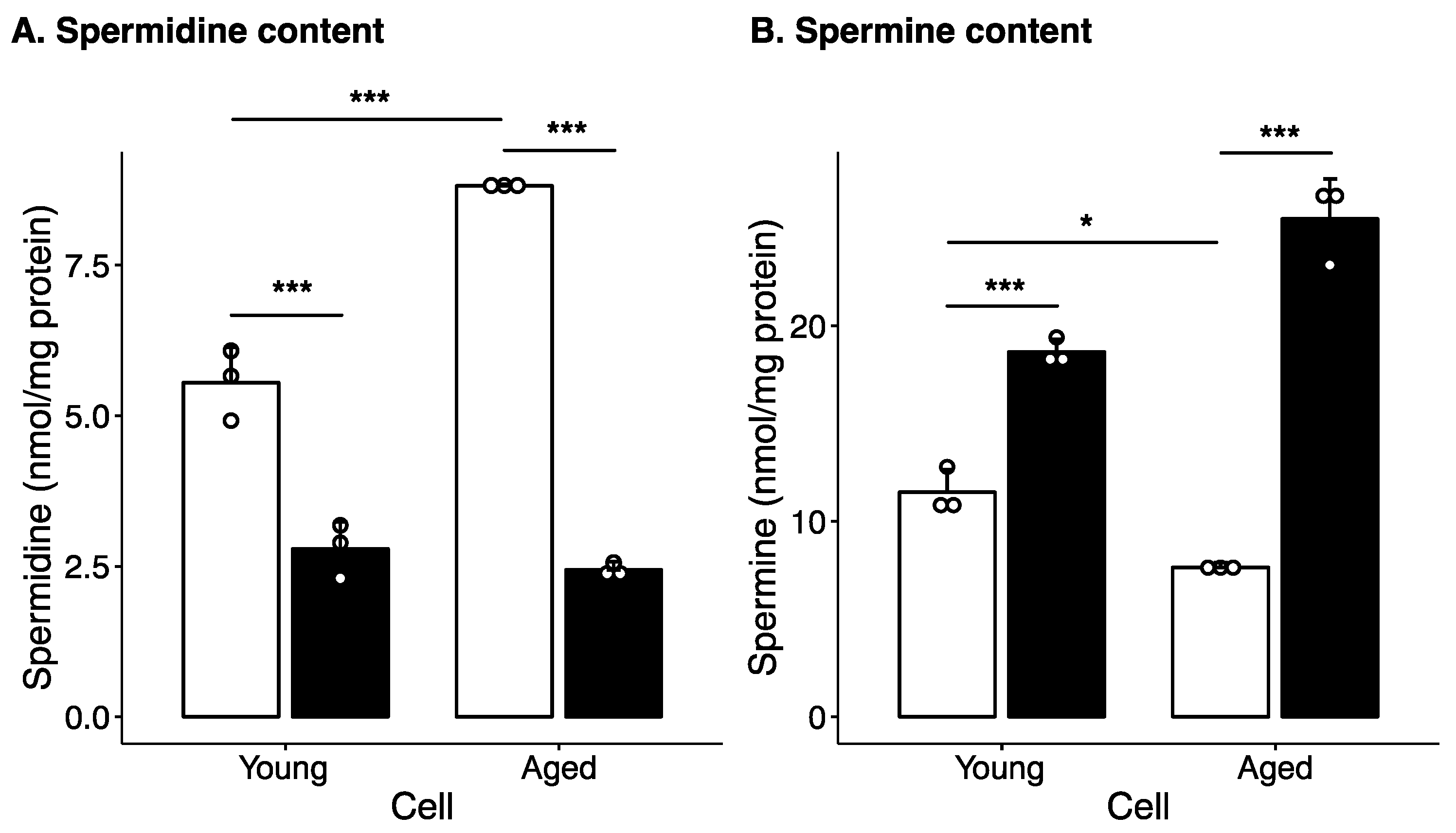
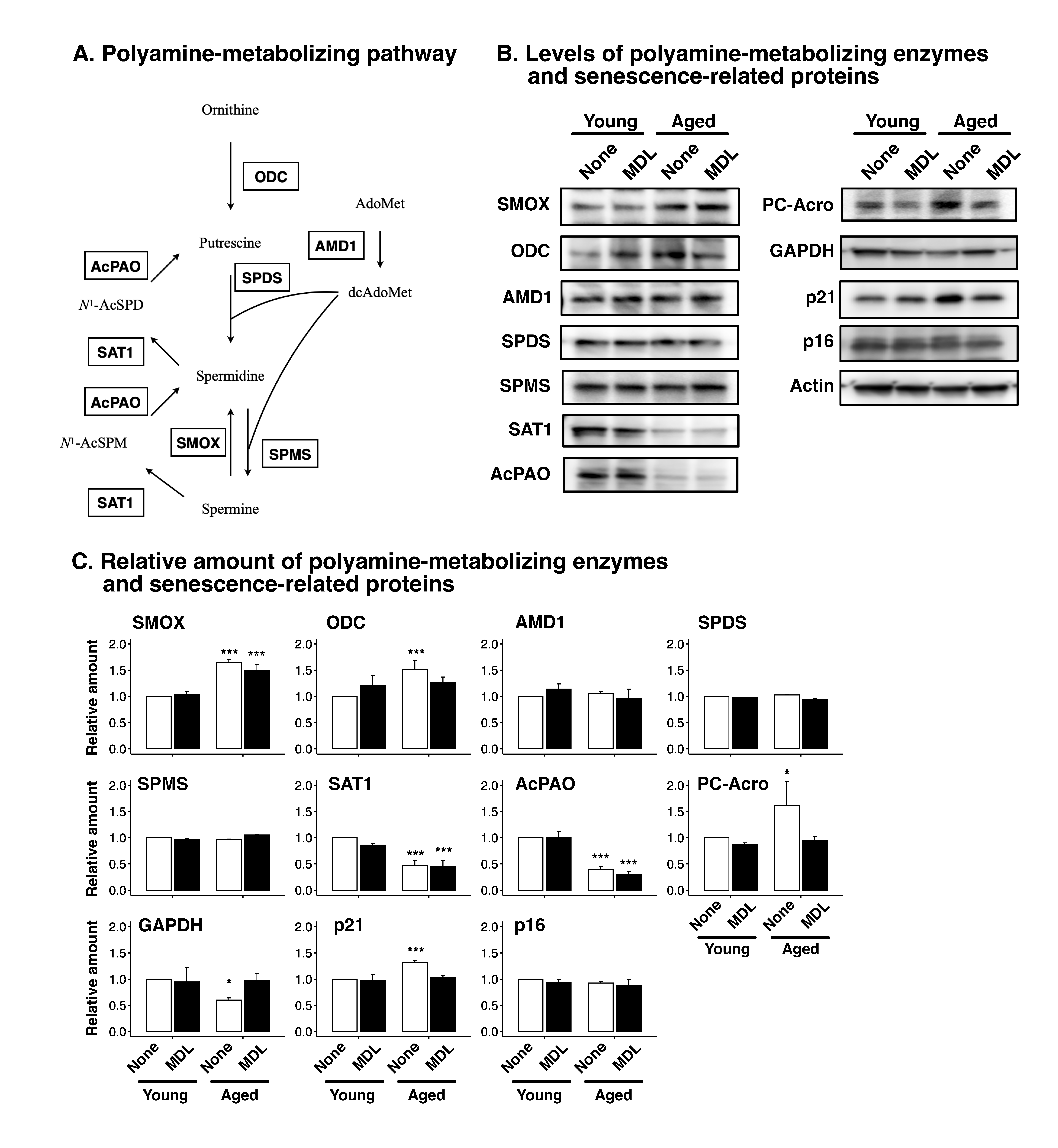
2.1. Effect of Long-Term Culture on Cellular Polyamine Levels and Polyamine Metabolism Enzyme Levels
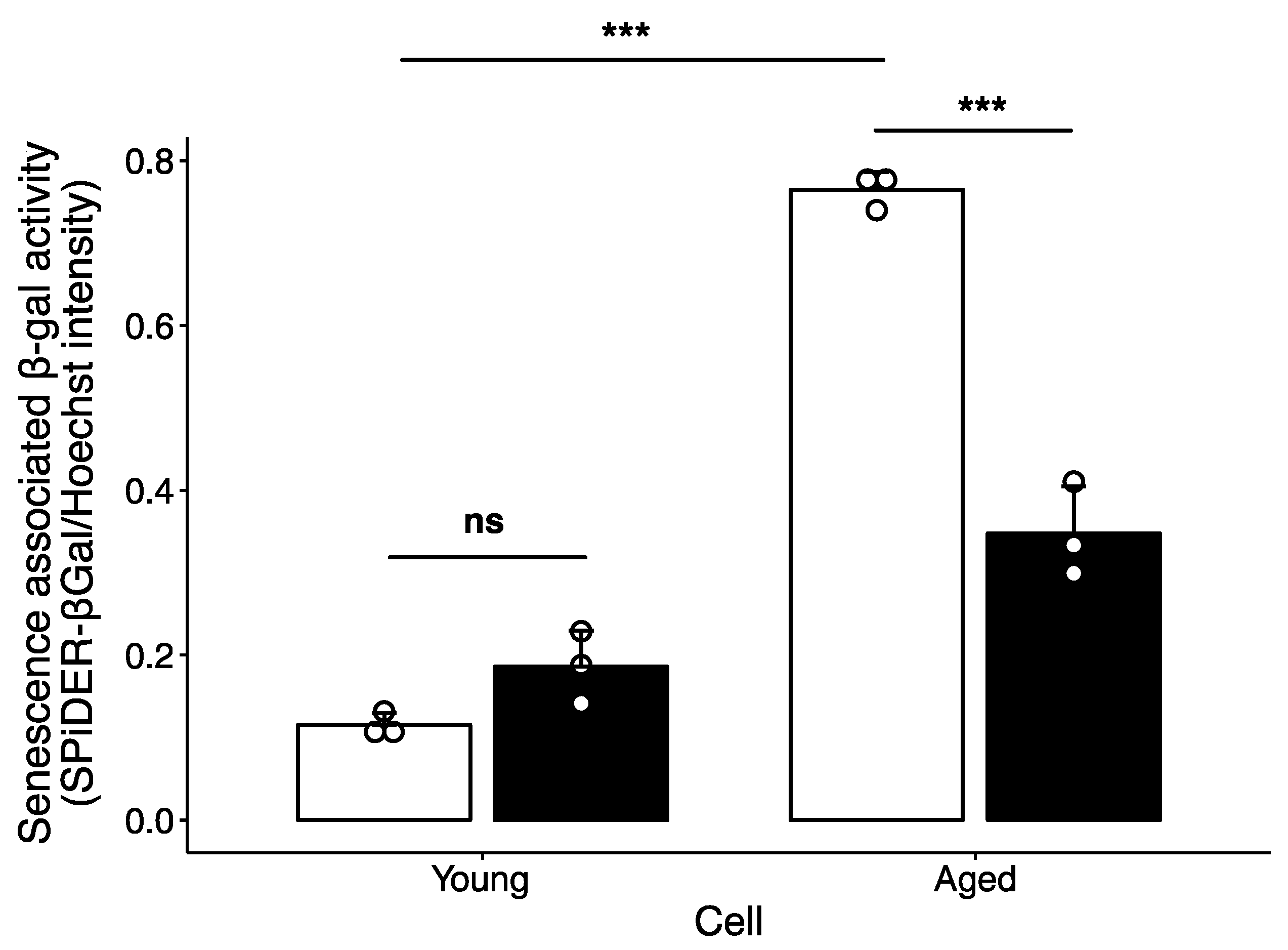
2.2. Effect of Polyamine-Catabolizing Enzyme Inhibitor on Cellular Senescence
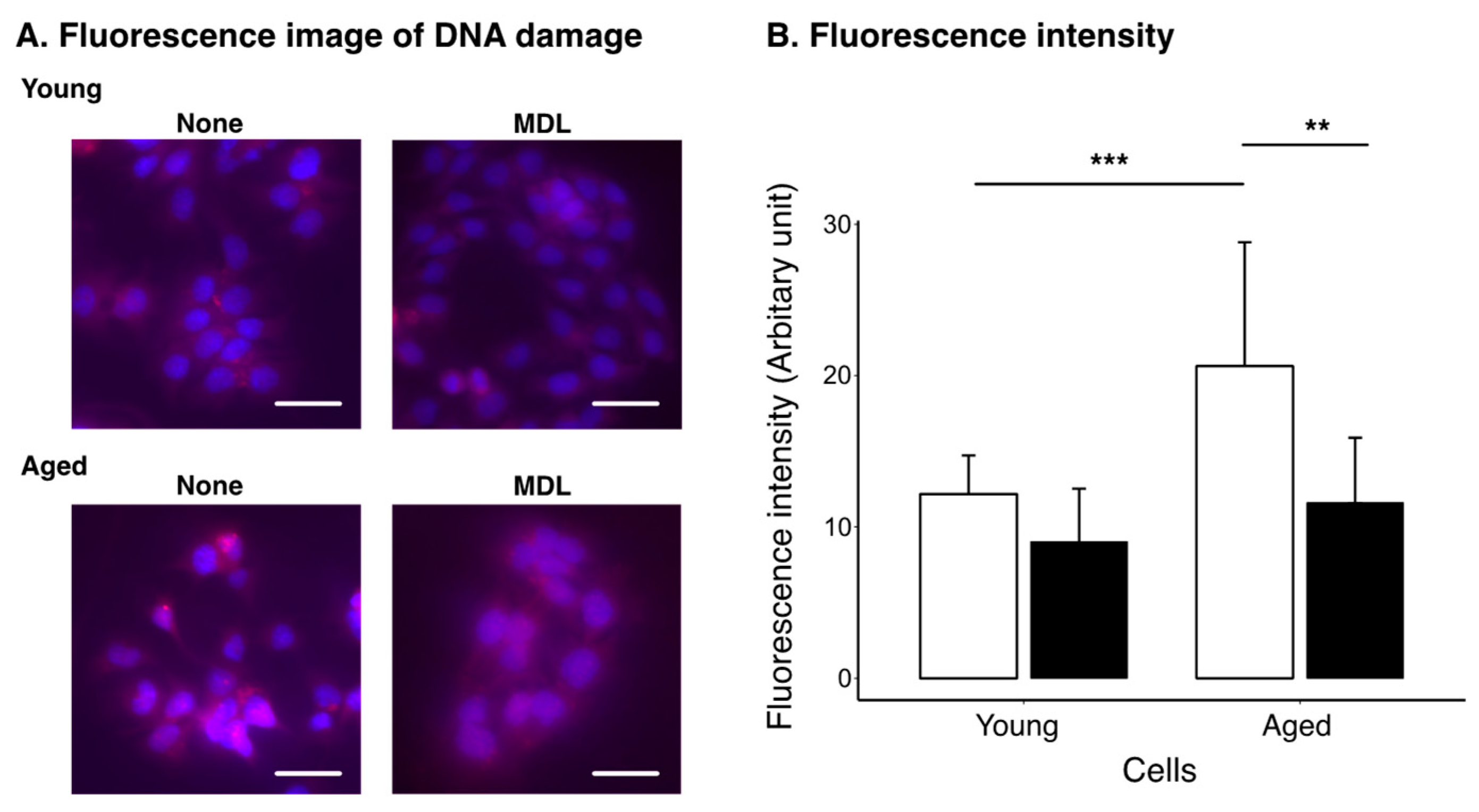
2.3. Effect of Polyamine Metabolism Inhibitor on DNA Damage Induced by Cellular Aging
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Measurement of Polyamine Content
4.4. Western Blotting
4.5. Measurement of Senescence-Associated β-gal Activity
4.6. Detection of DNA Damage
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging hallmarks and the role of oxidative stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Stites, S.D.; Harkins, K.; Rubright, J.D.; Karlawish, J. Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild alzheimer disease dementia, and normal cognition. Alzheimer Dis. Assoc. Discord 2018, 32, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ocana, P.D.; Darabseh, M.Z.; Ishihara, K.; Aburub, A.; Zambolin, F.; Montgomery, G.; Mills, R.; Scorcelletti, M.; Cameron, J.; Ganse, B.; et al. Age-related declines in muscle and respiratory function are proportionate to declines in performance in master track cyclists. Eur. J. Appl. Physiol. 2021, 121, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Uemura, T.; Kashiwagi, K. Acrolein toxicity at advanced age: Present and future. Amino Acids 2018, 50, 217–228. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634–634A. [Google Scholar] [CrossRef]
- Gil, L.; Nino, S.A.; Capdeville, G.; Jimenez-Capdeville, M.E. Aging and alzheimer’s disease connection: Nuclear tau and lamin a. Neurosci. Lett. 2021, 749, 135741. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Mchugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Guvatova, Z.; Lopes, I.A.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting aging mechanisms: Pharmacological perspectives. Trends Endocrinol. Metab. 2022, 33, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Sakamoto, A.; Terui, Y.; Uemura, T.; Igarashi, K.; Kashiwagi, K. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J. Biol. Chem. 2020, 295, 8736–8745. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Polyamine modulon in Escherichia coli: Genes involved in the stimulation of cell growth by polyamines. J. Biochem. 2006, 139, 11–16. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef]
- Liang, Y.; Piao, C.; Beuschel, C.B.; Toppe, D.; Kollipara, L.; Bogdanow, B.; Maglione, M.; Lützkendorf, J.; See, J.C.K.; Huang, S.; et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 2021, 35, 108941. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef]
- Sigrist, S.J.; Carmona-Gutierrez, D.; Gupta, V.K.; Bhukel, A.; Mertel, S.; Eisenberg, T.; Madeo, F. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 2014, 10, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.; Benson, G.; Schwarz, C.; Kobe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Simon, A.K. Polyamines reverse immune senescence via the translational control of autophagy. Autophagy 2020, 16, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef]
- Casero, A.R.; Pegg, E.A. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef]
- Qu, N.; Ignatenko, N.A.; Yamauchi, P.; Stringer, D.E.; Levenson, C.; Shannon, P.; Perrin, S.; Gerner, E.W. Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine. Biochem. J. 2003, 375, 465–470. [Google Scholar] [CrossRef]
- Bachrach, U. The early history of polyamine research. Plant Physiol. Biochem. 2010, 48, 490–495. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine oxidase: Ten years after. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. Polyamine homeostasis in development and disease. Med. Sci. 2021, 9, 28. [Google Scholar] [CrossRef]
- Uemura, T.; Akasaka, Y.; Ikegaya, H. Correlation of polyamines, acrolein-conjugated lysine and polyamine metabolic enzyme levels with age in human liver. Heliyon 2020, 6, e05031. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Suzuki, T.; Ko, K.; Nakamura, M.; Dohmae, N.; Sakamoto, A.; Terui, Y.; Toida, T.; Kashiwagi, K.; Igarashi, K. Structural change and degradation of cytoskeleton due to the acrolein conjugation with vimentin and actin during brain infarction. Cytoskeleton 2020, 77, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Suzuki, T.; Ko, K.; Watanabe, K.; Dohmae, N.; Sakamoto, A.; Terui, Y.; Toida, T.; Kashiwagi, K.; Igarashi, K. Inhibition of dendritic spine extension through acrolein conjugation with alpha-, beta-tubulin proteins. Int. J. Biochem. Cell Biol. 2019, 113, 58–66. [Google Scholar] [CrossRef]
- Uemura, T.; Tanaka, Y.; Higashi, K.; Miyamori, D.; Takasaka, T.; Nagano, T.; Toida, T.; Yoshimoto, K.; Igarashi, K.; Ikegaya, H. Acetaldehyde-induced cytotoxicity involves induction of spermine oxidase at the transcriptional level. Toxicology 2013, 310, 1–7. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef]
- Ma, J.; Liu, M.; Wang, Y.; Xin, C.; Zhang, H.; Chen, S.; Zheng, X.; Zhang, X.; Xiao, F.; Yang, S. Quantitative proteomics analysis of young and elderly skin with DIA mass spectrometry reveals new skin aging-related proteins. Aging 2020, 12, 13529–13554. [Google Scholar] [CrossRef]
- Ritschka, B.; Knauer-Meyer, T.; Gonçalves, D.S.; Mas, A.; Plassat, J.-L.; Durik, M.; Jacobs, H.; Pedone, E.; Di Vicino, U.; Cosma, M.P.; et al. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev. 2020, 34, 489–494. [Google Scholar] [CrossRef]
- Brenner, A.J.; Stampfer, M.R.; Aldaz, C.M. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 1998, 17, 199–205. [Google Scholar] [CrossRef]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulić, V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Takasaka, T.; Igarashi, K.; Ikegaya, H. Spermine oxidase promotes bile canalicular lumen formation through acrolein production. Sci. Rep. 2017, 7, 14841. [Google Scholar] [CrossRef]
- Wu, D.; Noda, K.; Murata, M.; Liu, Y.; Kanda, A.; Ishida, S. Regulation of spermine oxidase through hypoxia-inducible factor-1alpha signaling in retinal glial cells under hypoxic conditions. Investig. Ophthalmol. Vis. Sci. 2020, 61, 52. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Tanel, A.; Averill-Bates, D.A. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim. Biophys. Acta 2005, 1743, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Tomitori, H.; Suzuki, T.; Sakamoto, A.; Terui, Y.; Saiki, R.; Dohmae, N.; Igarashi, K.; Kashiwagi, K. Inactivation of GAPDH as one mechanism of acrolein toxicity. Biochem. Biophys. Res. Commun. 2013, 430, 1265–1271. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, L.; Feng, J.; Bao, L.; Wang, J.; Song, Z.; Mao, Z.; Li, J.; Hu, Z. Characterization of cellular senescence in doxorubicin-induced aging mice. Exp. Gerontol. 2022, 163, 111800. [Google Scholar] [CrossRef]
- Bauer, M.A.; Carmona-Gutierrez, D.; Ruckenstuhl, C.; Reisenbichler, A.; Megalou, E.V.; Eisenberg, T.; Magnes, C.; Jungwirth, H.; Sinner, F.M.; Pieber, T.R.; et al. Spermidine promotes mating and fertilization efficiency in model organisms. Cell Cycle 2013, 12, 346–352. [Google Scholar] [CrossRef]
- Gupta, V.K.; Scheunemann, L.; Eisenberg, T.; Mertel, S.; Bhukel, A.; Koemans, T.S.; Kramer, J.M.; Liu, K.S.; Schroeder, S.; Stunnenberg, H.G.; et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 2013, 16, 1453–1460. [Google Scholar] [CrossRef]
- Hofer, S.J.; Liang, Y.; Zimmermann, A.; Schroeder, S.; Dengjel, J.; Kroemer, G.; Eisenberg, T.; Sigrist, S.J.; Madeo, F. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy 2021, 17, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Takao, K.; Samejima, K.; Shirahata, A.; Igarashi, K.; Casero, R.A., Jr.; Kizawa, Y.; Sugita, Y. N(1)-Nonyl-1,4-diaminobutane ameliorates brain infarction size in photochemically induced thrombosis model mice. Neurosci. Lett. 2018, 672, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Carou, M.C.; Lahoz-Portoles, F.; Bover-Cid, S.; Marine-Font, A. Ion-pair high-performance liquid chromatographic determination of biogenic amines and polyamines in wine and other alcoholic beverages. J. Chromatogr. A 2003, 998, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2, Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, T.; Matsunaga, M.; Yokota, Y.; Takao, K.; Furuchi, T. Inhibition of Polyamine Catabolism Reduces Cellular Senescence. Int. J. Mol. Sci. 2023, 24, 13397. https://doi.org/10.3390/ijms241713397
Uemura T, Matsunaga M, Yokota Y, Takao K, Furuchi T. Inhibition of Polyamine Catabolism Reduces Cellular Senescence. International Journal of Molecular Sciences. 2023; 24(17):13397. https://doi.org/10.3390/ijms241713397
Chicago/Turabian StyleUemura, Takeshi, Miki Matsunaga, Yuka Yokota, Koichi Takao, and Takemitsu Furuchi. 2023. "Inhibition of Polyamine Catabolism Reduces Cellular Senescence" International Journal of Molecular Sciences 24, no. 17: 13397. https://doi.org/10.3390/ijms241713397
APA StyleUemura, T., Matsunaga, M., Yokota, Y., Takao, K., & Furuchi, T. (2023). Inhibition of Polyamine Catabolism Reduces Cellular Senescence. International Journal of Molecular Sciences, 24(17), 13397. https://doi.org/10.3390/ijms241713397





