Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy
Abstract
:1. Introduction
2. Experimental Results
3. Discussion
4. Objects and Methods of Investigation
4.1. Design of the Study
4.2. Equipment Setup and Sample Scanning
4.3. Data Processing
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathee, M.; Sapra, A. Dental Caries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Golubnitschaja, O.; Topolcan, O.; Kucera, R.; Costigliola, V.; Akopyan, M.; Akulov, S.N.; Alexandrova, O.; Alonso, A.; Andrews, R.J.; Duarte, A.A.; et al. 10th Anniversary of the European Association for Predictive, Preventive and Personalised (3P) Medicine—EPMA World Congress Supplement 2020. EPMA J. 2020, 11, 1–133. [Google Scholar] [CrossRef]
- Medina-Solís, C.E.; Ávila-Burgos, L.; Borges-Yañez, S.A.; Irigoyen-Camacho, M.E.; Sánchez-Pérez, L.; Zepeda-Zepeda, M.A.; Lucas-Rincón, S.E.; Medina-Solís, J.J.; Márquez-Corona, M.d.L.D.; Islas-Granillo, H.; et al. Ecological Study on Needs and Cost of Treatment for Dental Caries in Schoolchildren Aged 6, 12, and 15 Years. Medicine 2020, 99, e19092. [Google Scholar] [CrossRef] [PubMed]
- Stoica, O.E.; Esian, D.; Bud, A.; Stoica, A.M.; Beresescu, L.; Bica, C.I. The Assessment of Early Server Childhood Caries Status in Abandoned Institutionalized Children. Int. J. Environ. Res. Public. Health 2022, 19, 8632. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-Implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kripal, K.; Bhavanam, S.; Anuroopa, P.; Kumar, P.; Chandrasekaran, K.; Paul, P. Comparision of the Microbial Count in Supragingival Plaque, Gingival Crevicular Blood and Saliva Samples Immediately after Diode Laser (970 ± 15 Nm) Application in Chronic Periodontitis Patients: A Randomized Controlled Split Mouth Clinical Trial. Dentistry 2018, 8, 4. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chu, C.-H.; Yu, O.Y. Oral Microbiome and Dental Caries Development. Dent. J. 2022, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.; Barão, V.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef]
- Portaccio, M.; d’Apuzzo, F.; Perillo, L.; Grassia, V.; Errico, S.; Lepore, M. Infrared Microspectroscopy Characterization of Gingival Crevicular Fluid during Orthodontic Treatment. J. Mol. Struct. 2019, 1176, 847–854. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y. Pimm Vongsvivut Pathology-Specific Molecular Profiles of Saliva in Patients with Multiple Dental Caries—Potential Application for Predictive, Preventive and Personalised Medical Services. EPMA J. 2018, 9, 195–203. [Google Scholar] [CrossRef]
- Lips, A.; Antunes, L.S.; Antunes, L.A.; Pintor, A.V.B.; dos Santos, D.A.B.; Bachinski, R.; Küchler, E.C.; Alves, G.G. Salivary Protein Polymorphisms and Risk of Dental Caries: A Systematic Review. Braz. Oral Res. 2017, 31, e41. [Google Scholar] [CrossRef]
- Nazar Majeed, Z.; Philip, K.; Alabsi, A.M.; Pushparajan, S.; Swaminathan, D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis. Markers 2016, 2016, 1804727. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological Factors in Dental Caries: Role of Saliva and Dental Plaque in the Dynamic Process of Demineralization and Remineralization (Part 1). J. Clin. Pediatr. Dent. 2004, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- García-Godoy, F.; Hicks, M.J. Maintaining the Integrity of the Enamel Surface: The Role of Dental Biofilm, Saliva and Preventive Agents in Enamel Demineralization and Remineralization. J. Am. Dent. Assoc. 2008, 139 (Suppl. 2), S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Alammari, M. Innovative Technology for Caries Detection and Validation Histologically to Support Restorative Dentists and Researchers’ Decision-Making in Vitro. Saudi J. Oral Sci. 2017, 4, 22. [Google Scholar] [CrossRef]
- Scherdin-Almhöjd, U. Identification of Esters in Carious Dentine Staining and Chemo-Mechanical Excavation. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2017. ISBN 978-91-629-0069-4. [Google Scholar]
- Seredin, P.V.; Goloshchapov, D.L.; Plotnikova, Y.A.; Ippolitov, Y.A.; Vongsvivut, J. Diagnostic Potential of the Oral Fluid for the Observation People with Multiple Dental Caries by Means of FTIR. J. Phys. Conf. Ser. 2018, 1124, 031007. [Google Scholar] [CrossRef]
- Kalburgi, C.V.; Naik, K.L.; Kokatnur, M.V.; Warad, S. Estimation and Correlation of Salivary Thiocyanate Levels in Healthy and Different Forms of Tobacco Users Having Chronic Periodontitis: A Cross-Sectional Biochemical Study. Contemp. Clin. Dent. 2014, 5, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Z.; Zong, S.; Cui, Y. Rapid and Reproducible Analysis of Thiocyanate in Real Human Serum and Saliva Using a Droplet SERS-Microfluidic Chip. Biosens. Bioelectron. 2014, 62, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Geraldeli, S.; Li, Y.; Hogan, M.M.B.; Tjaderhane, L.S.; Pashley, D.H.; Morgan, T.A.; Zimmerman, M.B.; Brogden, K.A. Inflammatory Mediators in Fluid Extracted from the Coronal Occlusal Dentine of Trimmed Teeth. Arch. Oral Biol. 2012, 57, 264–270. [Google Scholar] [CrossRef]
- Gupta, G. Gingival Crevicular Fluid as a Periodontal Diagnostic Indicator-I: Host Derived Enzymes and Tissue Breakdown Products. J. Med. Life 2012, 5, 390–397. [Google Scholar] [PubMed]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Spectroscopic Signature of the Pathological Processes of Carious Dentine Based on FTIR Investigations of the Oral Biological Fluids. Biomed. Opt. Express 2019, 10, 4050–4058. [Google Scholar] [CrossRef]
- Havsed, K.; Stensson, M.; Jansson, H.; Carda-Diéguez, M.; Pedersen, A.; Neilands, J.; Svensäter, G.; Mira, A. Bacterial Composition and Metabolomics of Dental Plaque from Adolescents. Front. Cell. Infect. Microbiol. 2021, 11, 716493. [Google Scholar] [CrossRef]
- Bottoni, U.; Tiriolo, R.; Pullano, S.A.; Dastoli, S.; Amoruso, G.F.; Nisticò, S.P.; Fiorillo, A.S. Infrared Saliva Analysis of Psoriatic and Diabetic Patients: Similarities in Protein Components. IEEE Trans. Biomed. Eng. 2016, 63, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.; Prasad, N. Dentine Phosphoproteins in Gingival Crevicular Fluid during Root Resorption. Eur. J. Orthod. 2004, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Nesterov, D.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. Effect of Exo/Endogenous Prophylaxis Dentifrice/Drug and Cariogenic Conditions of Patient on Molecular Property of Dental Biofilm: Synchrotron FTIR Spectroscopic Study. Pharmaceutics 2022, 14, 1355. [Google Scholar] [CrossRef] [PubMed]
- Chirman, D.; Pleshko, N. Characterization of Bacterial Biofilm Infections with Fourier Transform Infrared Spectroscopy: A Review. Appl. Spectrosc. Rev. 2021, 56, 673–701. [Google Scholar] [CrossRef]
- Seredin, P.; Kashkarov, V.; Lukin, A.; Ippolitov, Y.; Julian, R.; Doyle, S. Local Study of Fissure Caries by Fourier Transform Infrared Microscopy and X-Ray Diffraction Using Synchrotron Radiation. J. Synchrotron Radiat. 2013, 20, 705–710. [Google Scholar] [CrossRef]
- Titus, J.; Ghimire, H.; Viennois, E.; Merlin, D.; Perera, A.G.U. Protein Secondary Structure Analysis of Dried Blood Serum Using Infrared Spectroscopy to Identify Markers for Colitis Screening. J. Biophotonics 2018, 11, e201700057. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Evaluation of Protein Secondary Structure from FTIR Spectra Improved after Partial Deuteration. Eur. Biophys. J. 2021, 50, 613–628. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Yang, H.; Shi, H.; Dong, A.; Wang, L.; Yu, S. Progress in Infrared Spectroscopy as an Efficient Tool for Predicting Protein Secondary Structure. Int. J. Biol. Macromol. 2022, 206, 175–187. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Comparative Analysis of Dentine and Gingival Fluid Molecular Composition and Protein Conformations during Development of Dentine Caries: A Pilot Study. Vib. Spectrosc. 2020, 108, 103058. [Google Scholar] [CrossRef]
- Amenabar, I.; Poly, S.; Nuansing, W.; Hubrich, E.H.; Govyadinov, A.A.; Huth, F.; Krutokhvostov, R.; Zhang, L.; Knez, M.; Heberle, J.; et al. Structural Analysis and Mapping of Individual Protein Complexes by Infrared Nanospectroscopy. Nat. Commun. 2013, 4, 2890. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.; Li, C.; Eremina, N.; Goormaghtigh, E.; Barth, A.; Baldassarre, M.; Li, C.; Eremina, N.; Goormaghtigh, E.; Barth, A. Simultaneous Fitting of Absorption Spectra and Their Second Derivatives for an Improved Analysis of Protein Infrared Spectra. Molecules 2015, 20, 12599–12622. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Amino Acid Side Chain Contribution to Protein FTIR Spectra: Impact on Secondary Structure Evaluation. Eur. Biophys. J. 2021, 50, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Eliades, G.; Palaghias, G.; Vougiouklakis, G. Effect of Acidic Conditioners on Dentin Morphology, Molecular Composition and Collagen Conformation in Situ. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1997, 13, 24–33. [Google Scholar] [CrossRef]
- Besnard, C.; Marie, A.; Sasidharan, S.; Harper, R.A.; Shelton, R.M.; Landini, G.; Korsunsky, A.M. Synchrotron X-Ray Studies of the Structural and Functional Hierarchies in Mineralised Human Dental Enamel: A State-of-the-Art Review. Dent. J. 2023, 11, 98. [Google Scholar] [CrossRef]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Searching for a Better Match between Protein Secondary Structure Definitions and Protein FTIR Spectra. Anal. Chem. 2021, 93, 1561–1568. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Petibois, C.; Déléris, G. Chemical Mapping of Tumor Progression by FT-IR Imaging: Towards Molecular Histopathology. Trends Biotechnol. 2006, 24, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Reeb, J.; Rost, B. Secondary Structure Prediction. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 488–496. ISBN 978-0-12-811432-2. [Google Scholar]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Yamazaki, H.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Protein Phosphorylation and Mineral Binding Affect the Secondary Structure of the Leucine-Rich Amelogenin Peptide. Front. Physiol. 2017, 8, 450. [Google Scholar] [CrossRef]
- Gallagher, W. FTIR Analysis of Protein Structure. Course Man. Chem 2009, 455, 1–8. [Google Scholar]
- Bunaciu, A.A.; Fleschin, Ş.; Aboul-Enein, H.Y. Evaluation of the Protein Secondary Structures Using Fourier Transform Infrared Spectroscopy. Gazi Univ. J. Sci. 2014, 27, 637–644. [Google Scholar]
- Boddapati, S.; Rai, R.; Gummadi, S.N. Structural Analysis and Antioxidative Properties of Mutan (Water-Insoluble Glucan) and Carboxymethyl Mutan from Streptococcus Mutans. Process. Biochem. 2020, 97, 130–139. [Google Scholar] [CrossRef]
- Carrasquillo, K.G.; Sanchez, C.; Griebenow, K. Relationship between Conformational Stability and Lyophilization-Induced Structural Changes in Chymotrypsin. Biotechnol. Appl. Biochem. 2000, 31, 41–53. [Google Scholar] [CrossRef]
- Sharma, V.; Srinivasan, A.; Roychoudhury, A.; Rani, K.; Tyagi, M.; Dev, K.; Nikolajeff, F.; Kumar, S. Characterization of Protein Extracts from Different Types of Human Teeth and Insight in Biomineralization. Sci. Rep. 2019, 9, 9314. [Google Scholar] [CrossRef]
- Gurbanov, R.; Yıldız, F. Molecular Profile of Oral Probiotic Bacteria to Be Used with Functional Foods. J. Food Health Sci. 2017, 3, 117–131. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular Mechanisms of Inhibiting Glucosyltransferases for Biofilm Formation in Streptococcus Mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.M.; D’Antonio, J.; Manning, M.C.; Al-Azzam, W. Use of the Amide II Infrared Band of Proteins for Secondary Structure Determination and Comparability of Higher Order Structure. Curr. Pharm. Biotechnol. 2014, 15, 880–889. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Bambery, K. The Investigations of Changes in Mineral–Organic and Carbon–Phosphate Ratios in the Mixed Saliva by Synchrotron Infrared Spectroscopy. Results Phys. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Liao, H.-F.; Wang, S.-L.; Lin, S.-Y. Glycation and Secondary Conformational Changes of Human Serum Albumin: Study of the FTIR Spectroscopic Curve-Fitting Technique. AIMS Biophys. 2016, 3, 247–260. [Google Scholar] [CrossRef]
- Pitts, N.; Ekstrand, K.; The ICDAS Foundation. International Caries Detection and Assessment System (ICDAS) and Its International Caries Classification and Management System (ICCMS)—Methods for Staging of the Caries Process and Enabling Dentists to Manage Caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Rup, A.G.; Izquierdo, C.D.M.; Rios, F.S.; Costa, R.D.S.A.; Jardim, J.J.; Haas, A.N.; Alves, L.S.; Maltz, M. Classification of a Patient’s Caries Activity Based on Lesion Activity Assessment among Adults: Findings from a Prospective Cohort Study. Clin. Oral Investig. 2023, 27, 1123–1131. [Google Scholar] [CrossRef]
- Nalin, E.K.P.; Danelon, M.; da Silva, E.S.; Hosida, T.Y.; Pessan, J.P.; Delbem, A.C.B. Surface Free Energy, Interaction, and Adsorption of Calcium and Phosphate to Enamel Treated with Trimetaphosphate and Glycerophosphate. Caries Res. 2021, 55, 496–504. [Google Scholar] [CrossRef]
- Zaze, A.C.S.F.; Dias, A.P.; Sassaki, K.T.; Delbem, A.C.B. The Effects of Low-Fluoride Toothpaste Supplemented with Calcium Glycerophosphate on Enamel Demineralization. Clin. Oral Investig. 2014, 18, 1619–1624. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Ippolitov, Y.A.; Vongsvivut, J. A Spectroscopic Study of Changes in the Secondary Structure of Proteins of Biological Fluids of the Oral Cavity by Synchrotron Infrared Microscopy. Opt. Spectrosc. 2019, 127, 1002–1010. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR Investigation of the Secondary Structure of Type I Collagen: New Insight into the Amide III Band. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef]
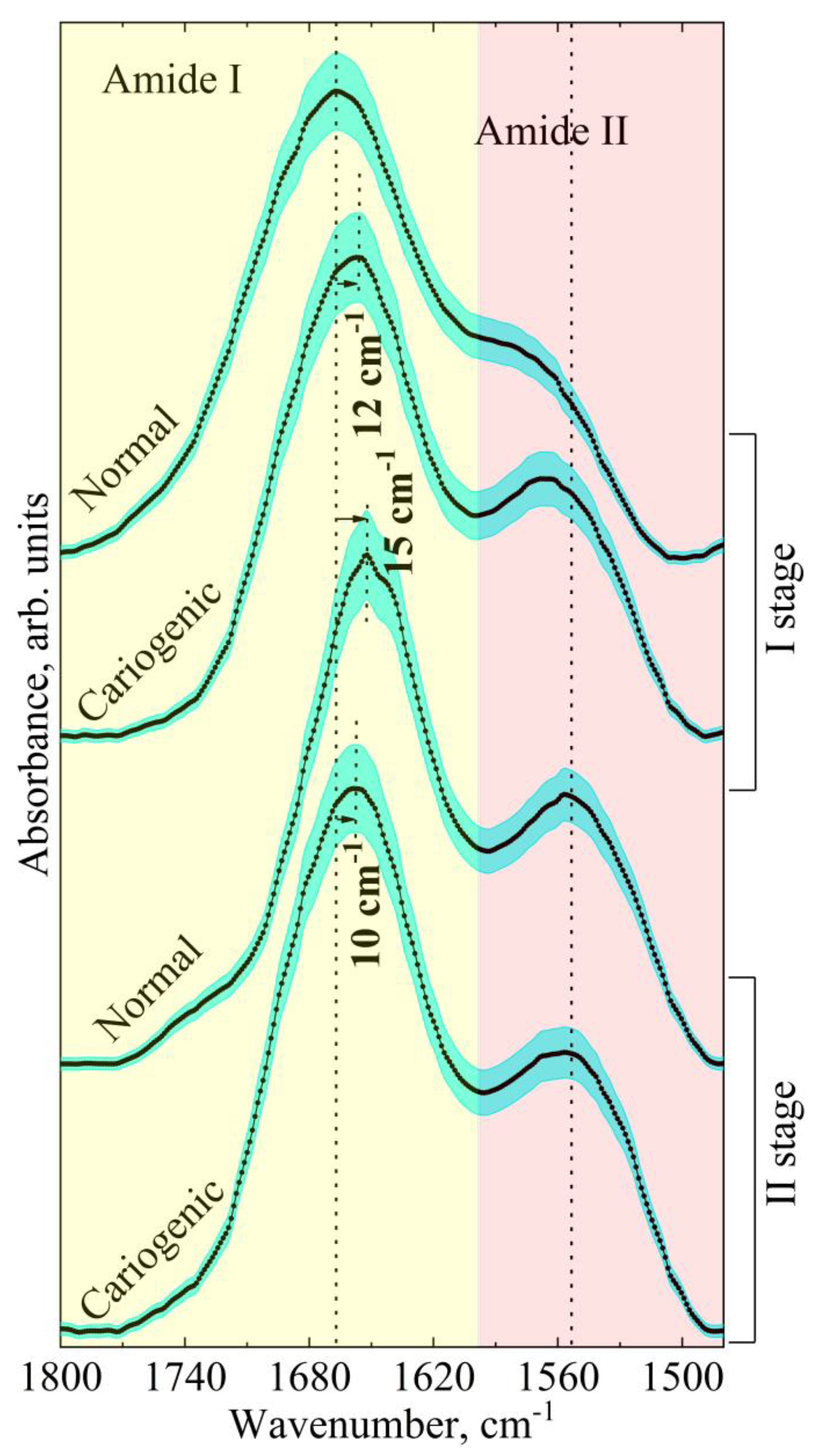

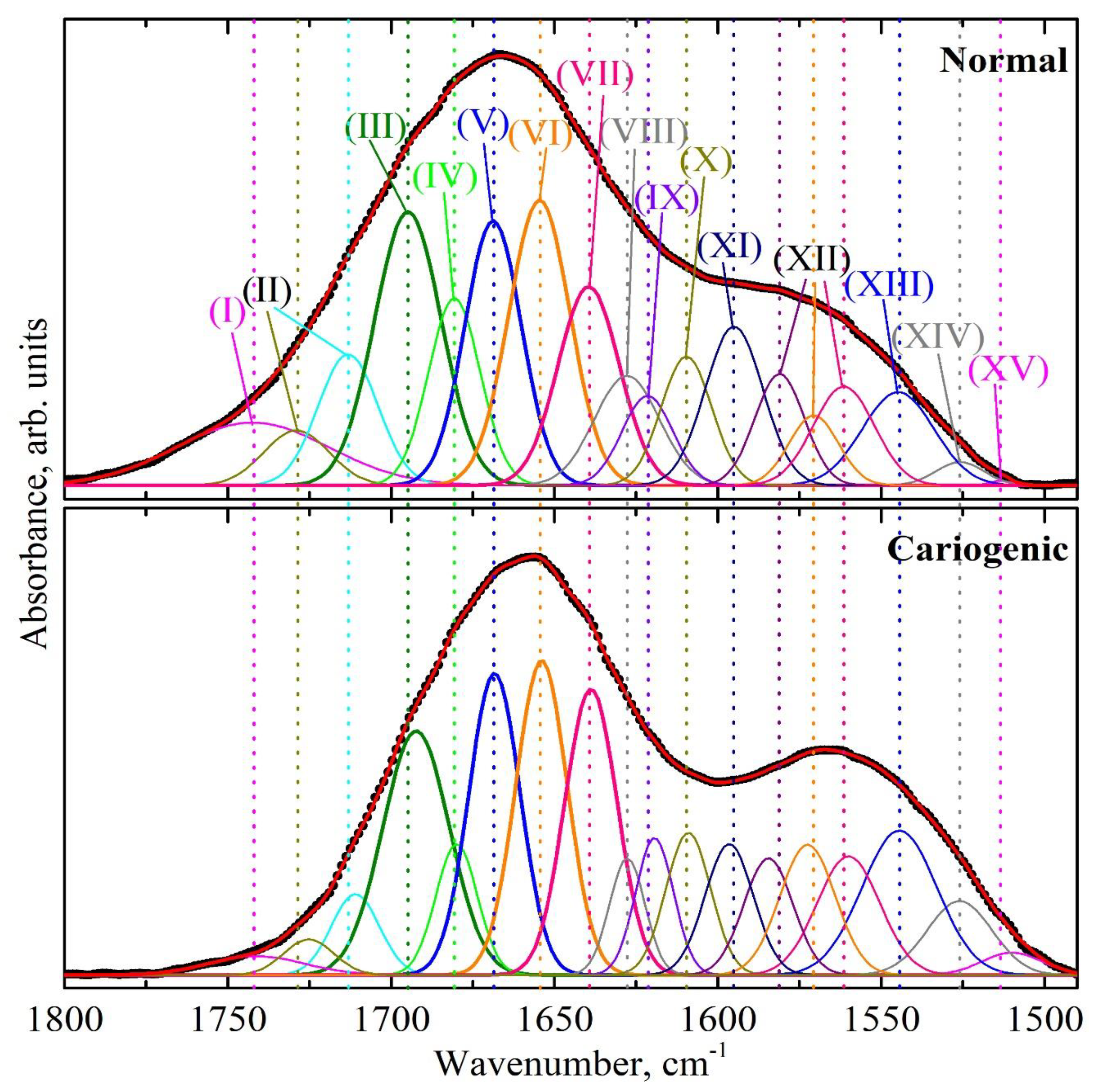
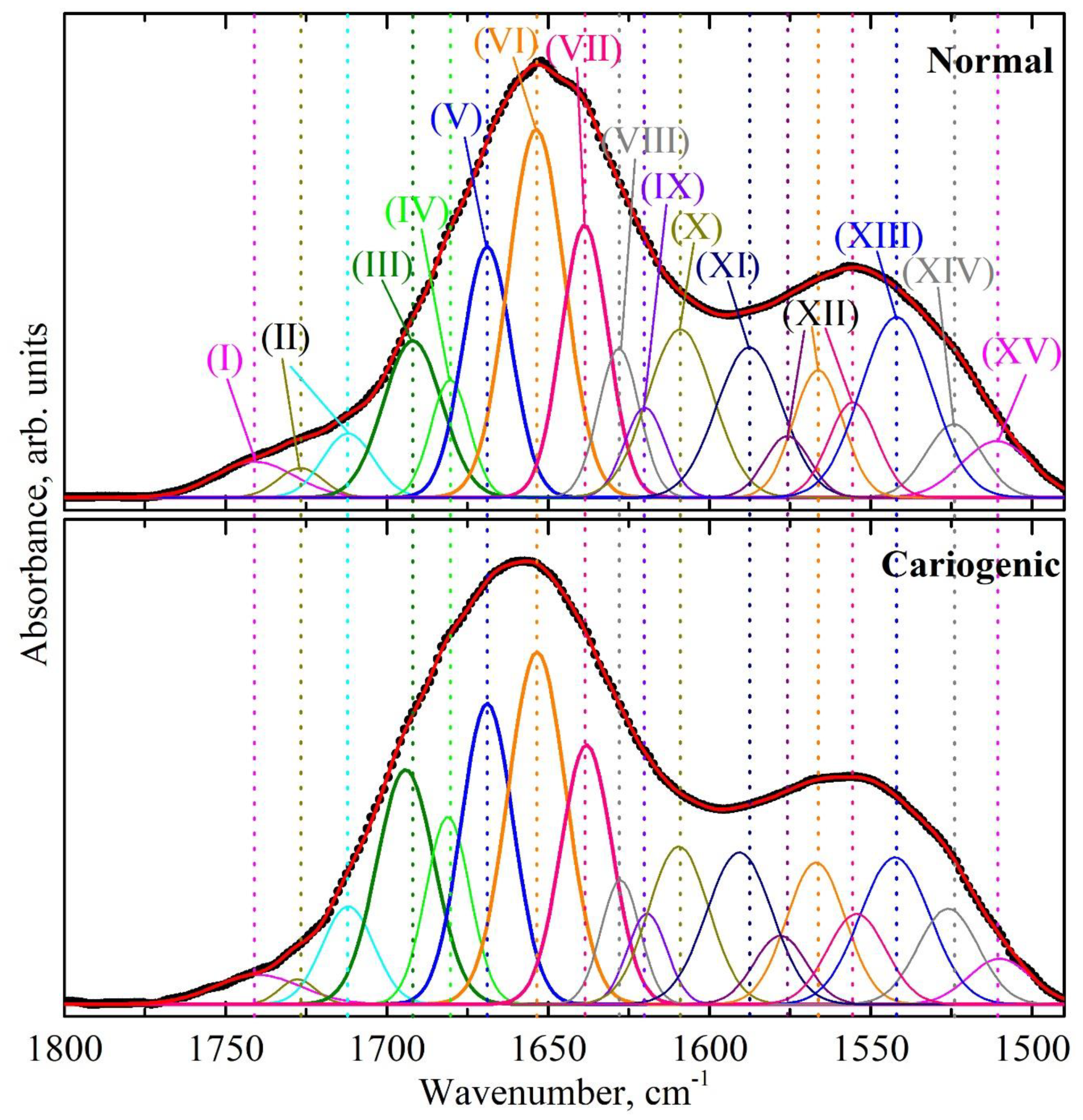
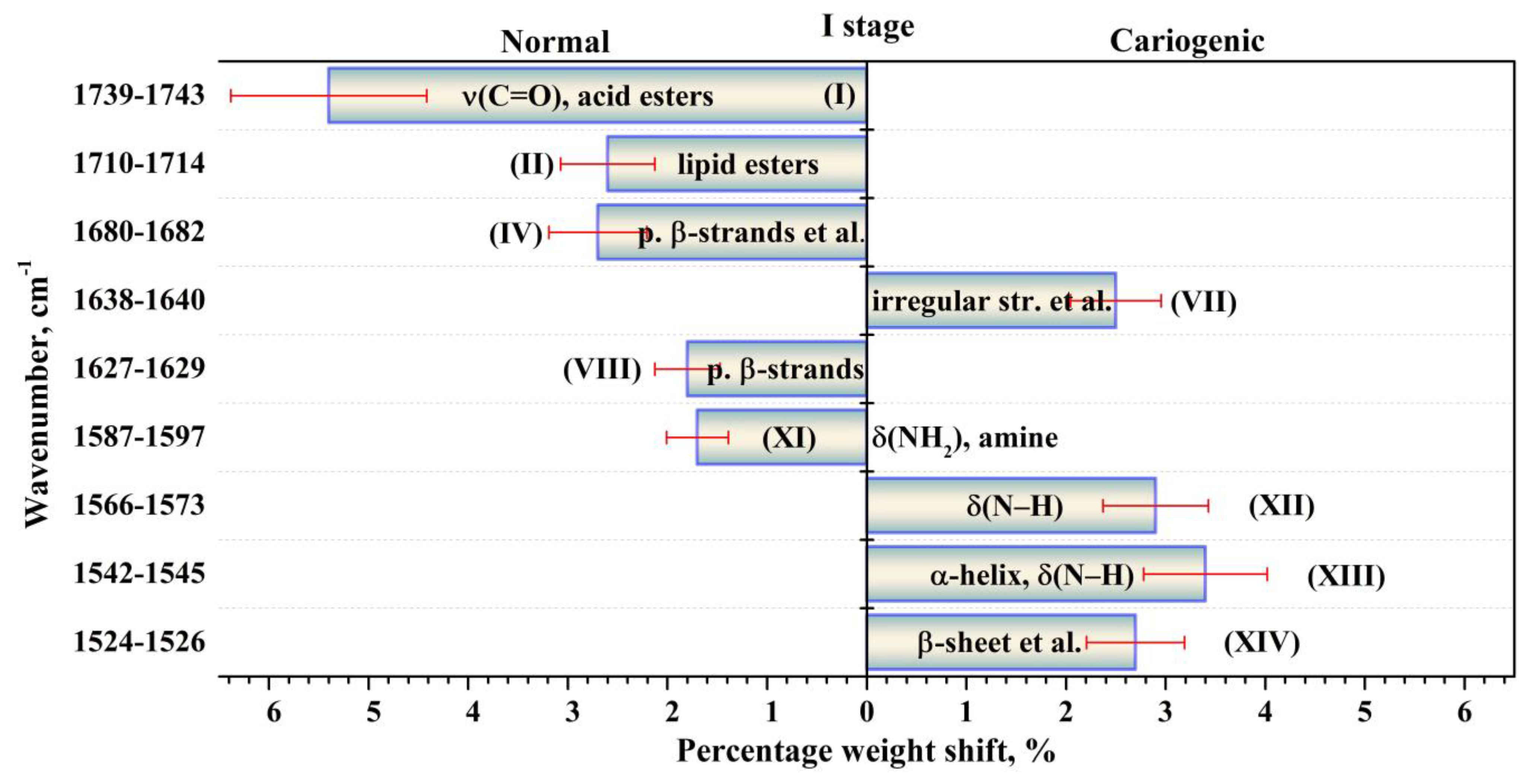

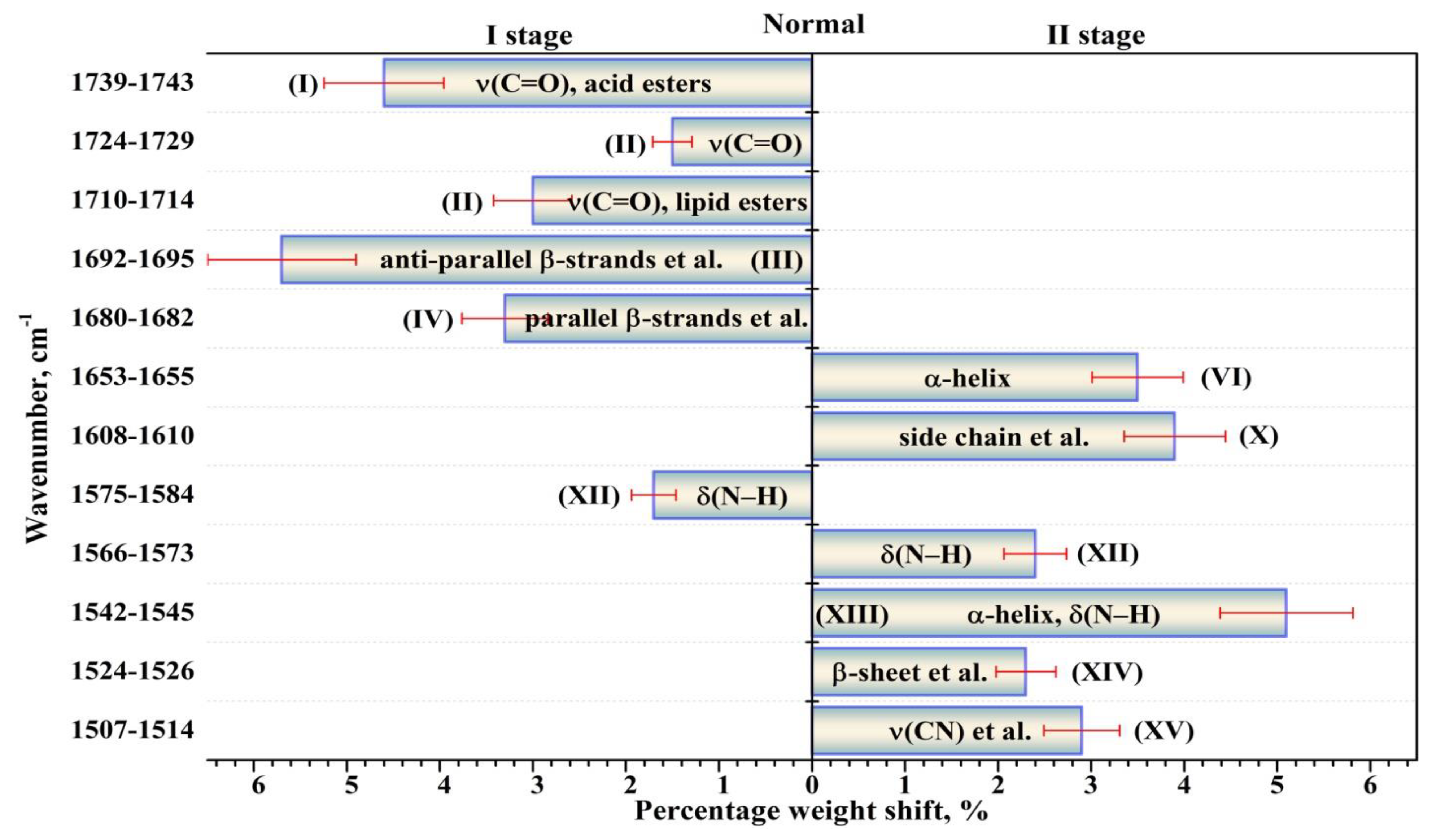
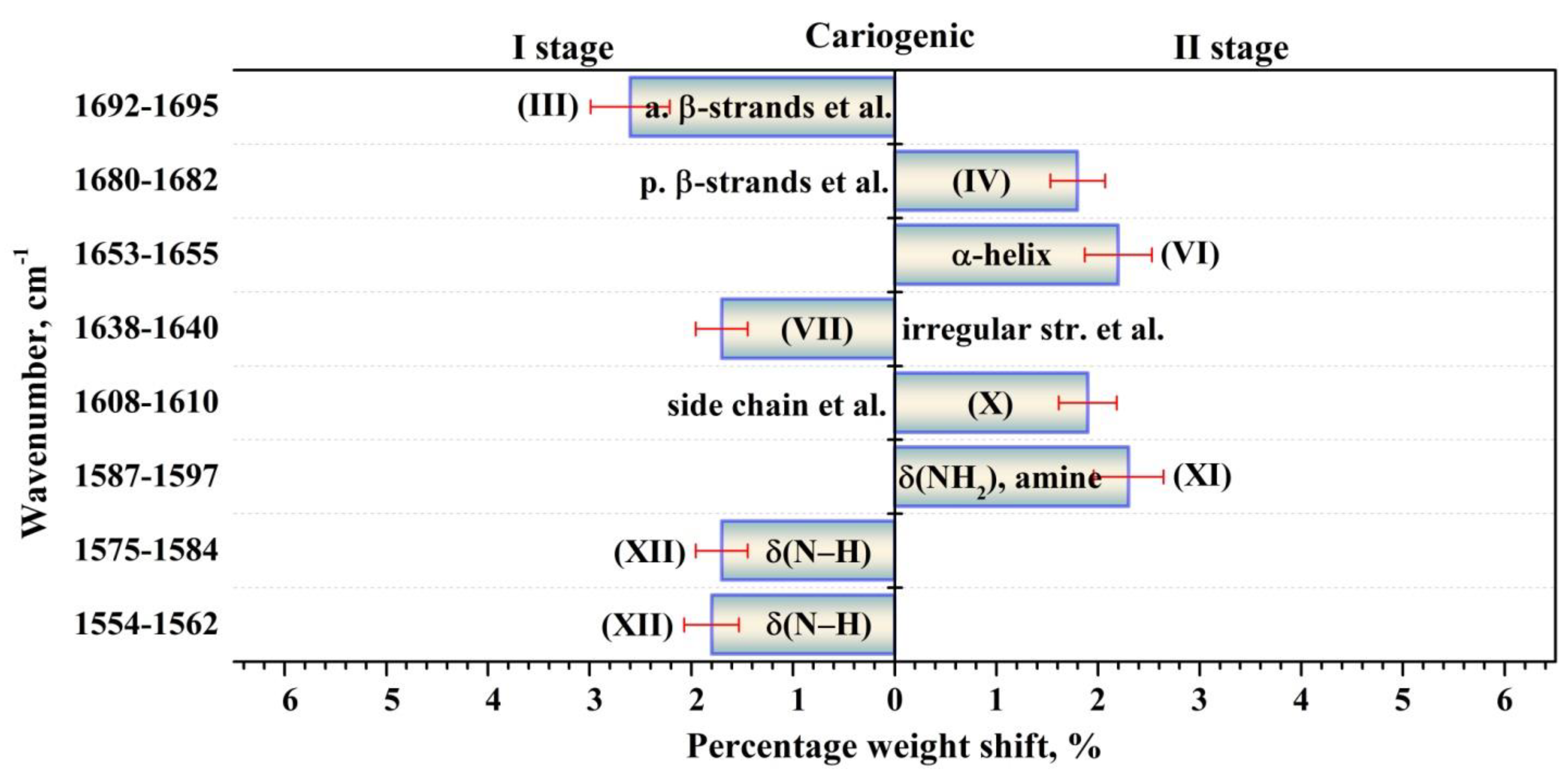
| Component | Assignment | I Stage | II Stage | ||
|---|---|---|---|---|---|
| Normal | Cariogenic | Normal | Cariogenic | ||
| Wavenumber, cm−1 FWHM, cm−1 Integ. Int., cm−2 | Wavenumber, cm−1 FWHM, cm−1 Integ. Int., cm−2 | Wavenumber, cm−1 FWHM, cm−1 Integ. Int., cm−2 | Wavenumber, cm−1 FWHM, cm−1 Integ. Int., cm−2 | ||
| XV | ν(CN), δ(CH), δ(NH) [39,46] | 1513.3 (±0.9) 7.8 (±0.1) 0.09 (±0.00(2)) | 1510.0 (±0.7) 21.5 (±0.4) 1.16 (±0.04) | 1510.9 (±0.8) 25.8 (±0.6) 3.33 (±0.15) | 1510.0 (±0.9) 22.6 (±0.5) 2.33 (±0.10) |
| XIV | β-sheet [30] δs(NH+3) [39,46] Mutans streptococci [39] | 1525.9 (±0.8) 17.4 (±0.3) 0.94 (±0.03) | 1526.0 (±0.8) 23.4 (±0.7) 4.13 (±0.24) | 1524.2 (±1.0) 20.3 (±0.7) 3.43 (±0.23) | 1526.0 (±1.1) 22.0 (±0.7) 4.74 (±0.30) |
| XIII | α-helix [30] δ(N–H) [39,43] | 1544.8 (±0.9) 25.8 (±0.6) 5.60 (±0.27) | 1544.4 (±0.9) 27.0 (±1.1) 9.32 (±0.74) | 1542.0 (±0.9) 26.0 (±1.1) 10.83 (±0.90) | 1542.4 (±0.9) 25.5 (±1.0) 8.44 (±0.68) |
| XII | δ(N–H) [39,43,46] | 1561.4 (±0.8) 22.0 (±0.7) 5.06 (±0.31) | 1560.0 (±0.8) 22.7 (±1.0) 6.41 (±0.56) | 1555.5 (±0.9) 17.7 (±0.8) 3.89 (±0.34) | 1554.3 (±0.9) 21.3 (±0.9) 4.34 (±0.37) |
| 1570.6 (±0.7) 17.7 (±0.6) 2.88 (±0.19) | 1572.5 (±0.7) 20.0 (±0.9) 6.22 (±0.56) | 1566.3 (±0.8) 18.0 (±0.8) 5.31 (±0.45) | 1567.0 (±0.8) 21.4 (±0.9) 6.86 (±0.58) | ||
| 1581.1 (±0.8) 17.8 (±0.6) 4.61 (±0.32) | 1584.5 (±0.6) 17.7 (±0.8) 4.94 (±0.42) | 1575.9 (±0.9) 16.5 (±0.6) 2.33 (±0.18) | 1578.0 (±0.8) 19.0 (±0.8) 2.94 (±0.23) | ||
| XI | δ(NH2), amine [39,43,46] | 1595.0 (±0.7) 20.8 (±0.7) 7.68 (±0.55) | 1596.5 (±0.8) 17.4 (±0.7) 5.45 (±0.44) | 1587.6 (±1.0) 24.0 (±0.9) 8.32 (±0.61) | 1590.7 (±0.9) 24.0 (±0.9) 8.22 (±0.62) |
| X | Amino acid side chain [36] Intermolecular β-sheet [30] | 1609.6 (±0.7) 18.2 (±0.7) 5.46 (±0.41) | 1609.0 (±0.6) 16.1 (±0.7) 5.48 (±0.46) | 1609.0 (±0.9) 24.0 (±1.0) 9.3 (±0.76) | 1609.5 (±0.6) 22.0 (±0.9) 7.8 (±0.63) |
| IX | β-sheet [31,47,48,49], β-turn [43] | 1621.5 (±0.6) 18.3 (±0.8) 3.81 (±0.39) | 1619.3 (±0.7) 13.7 (±0.7) 4.47 (±0.43) | 1620.0 (±0.7) 14.9 (±0.7) 3.10 (±0.30) | 1619.3 (±0.5) 14.3 (±0.7) 2.93 (±0.27) |
| VIII | Parallel β-strands [43,48] Mutans streptococci [39,50] | 1627.8 (±0.5) 23.4 (±1.1) 5.96 (±0.54) | 1627.7 (±0.4) 12.8 (±0.7) 3.57 (±0.39) | 1628.1 (±0.4) 14.8 (±0.8) 5.11 (±0.57) | 1627.6 (±0.3) 14.2 (±0.7) 3.97 (±0.42) |
| VII | Irregular structure [30,47,48,49,51,52] Triple helix [43] | 1639.5 (±0.3) 22.1 (±1.2) 10.21 (±1.08) | 1638.8 (±0.4) 18.8 (±1.2) 12.85 (±1.70) | 1638.7 (±0.3) 17.1 (±1.2) 10.71 (±1.48) | 1638.1 (±0.3) 18.7 (±1.2) 10.96 (±1.41) |
| VI | α-helix [30,43,44,47,51,53] | 1654.6 (±0.4) 22.1 (±1.4) 14.67 (±1.82) | 1654.0 (±0.5) 18.7 (±1.4) 13.98 (±2.10) | 1653.7 (±0.3) 20.6 (±1.5) 17.50 (±2.57) | 1653.5 (±0.5) 21.1 (±1.5) 16.79 (±2.46) |
| V | β-turn [30,43,44,47,48] | 1668.8 (±0.4) 20.4 (±1.3) 12.53 (±1.61) | 1668.4 (±0.4) 18.4 (±1.2) 13.26 (±1.66) | 1668.9 (±0.5) 17.8 (±1.3) 10.30 (±1.47) | 1668.9 (±0.4) 19.0 (±1.3) 12.90 (±1.82) |
| IV | Parallel β-strands [43,47] β-turn [30,44,48,49,53] | 1680.7 (±0.3) 18.7 (±1.1) 8.13 (±0.99) | 1680.0 (±0.3) 15.0 (±0.9) 4.68 (±0.59) | 1680.4 (±0.3) 14.1 (±0.7) 3.83 (±0.38) | 1681.1 (±0.3) 16.1 (±1.0) 6.82 (±0.86) |
| III | Anti-parallel β-strands [43,49,51,53], β-turn [30,47,49] | 1694.9 (±0.4) 24.0 (±1.3) 15.27 (±1.63) | 1692.4 (±0.3) 24.0 (±1.2) 13.97 (±1.35) | 1692.0 (±0.4) 21.4 (±0.7) 7.75 (±0.53) | 1694.2 (±0.3) 21.0 (±1.0) 11.13 (±1.10) |
| II | ν(C=O): lipid esters [43,53] | 1713.2 (±0.6) 21.6 (±0.8) 6.59 (±0.51) | 1711.0 (±0.6) 17.3 (±0.5) 3.34 (±0.18) | 1712.0 (±0.7) 18.6 (±0.4) 2.74 (±0.12) | 1712.0 (±0.4) 18.8 (±0.5) 4.17 (±0.23) |
| 1729.0 (±0.7) 23.4 (±0.7) 2.99 (±0.17) | 1725.3 (±0.7) 18.0 (±0.3) 1.53 (±0.05) | 1726.8 (±0.9) 16.5 (±0.3) 1.11 (±0.04) | 1727.7 (±0.6) 13.0 (±0.2) 0.73 (±0.03) | ||
| I | ν(C=O): acid esters [43,53] | 1742.4 (±1.1) 55.0 (±1.2) 8.04 (±0.34) | 1741.2 (±0.8) 34.7 (±0.5) 1.55 (±0.04) | 1741.0 (±0.9) 29.0 (±0.5) 2.37 (±0.07) | 1740.0 (±1.0) 31.3 (±0.4) 2.06 (±0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Lukin, A.; Peshkov, Y.; Ippolitov, I.; Ippolitov, Y.; Litvinova, T.; Vongsvivut, J.; Chae, B.; et al. Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy. Int. J. Mol. Sci. 2023, 24, 15324. https://doi.org/10.3390/ijms242015324
Seredin P, Goloshchapov D, Kashkarov V, Lukin A, Peshkov Y, Ippolitov I, Ippolitov Y, Litvinova T, Vongsvivut J, Chae B, et al. Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy. International Journal of Molecular Sciences. 2023; 24(20):15324. https://doi.org/10.3390/ijms242015324
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Anatoly Lukin, Yaroslav Peshkov, Ivan Ippolitov, Yuri Ippolitov, Tatiana Litvinova, Jitraporn Vongsvivut, Boknam Chae, and et al. 2023. "Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy" International Journal of Molecular Sciences 24, no. 20: 15324. https://doi.org/10.3390/ijms242015324
APA StyleSeredin, P., Goloshchapov, D., Kashkarov, V., Lukin, A., Peshkov, Y., Ippolitov, I., Ippolitov, Y., Litvinova, T., Vongsvivut, J., Chae, B., & Freitas, R. O. (2023). Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy. International Journal of Molecular Sciences, 24(20), 15324. https://doi.org/10.3390/ijms242015324









