Comprehensive Analysis of the Role of Gene Variants in Matrix Metalloproteinases and Their Tissue Inhibitors in Retinopathy of Prematurity: A Study in the Polish Population
Abstract
:1. Introduction
2. Results
2.1. Clinical Data
2.2. Association Studies
2.2.1. Single Variant Tests of Association
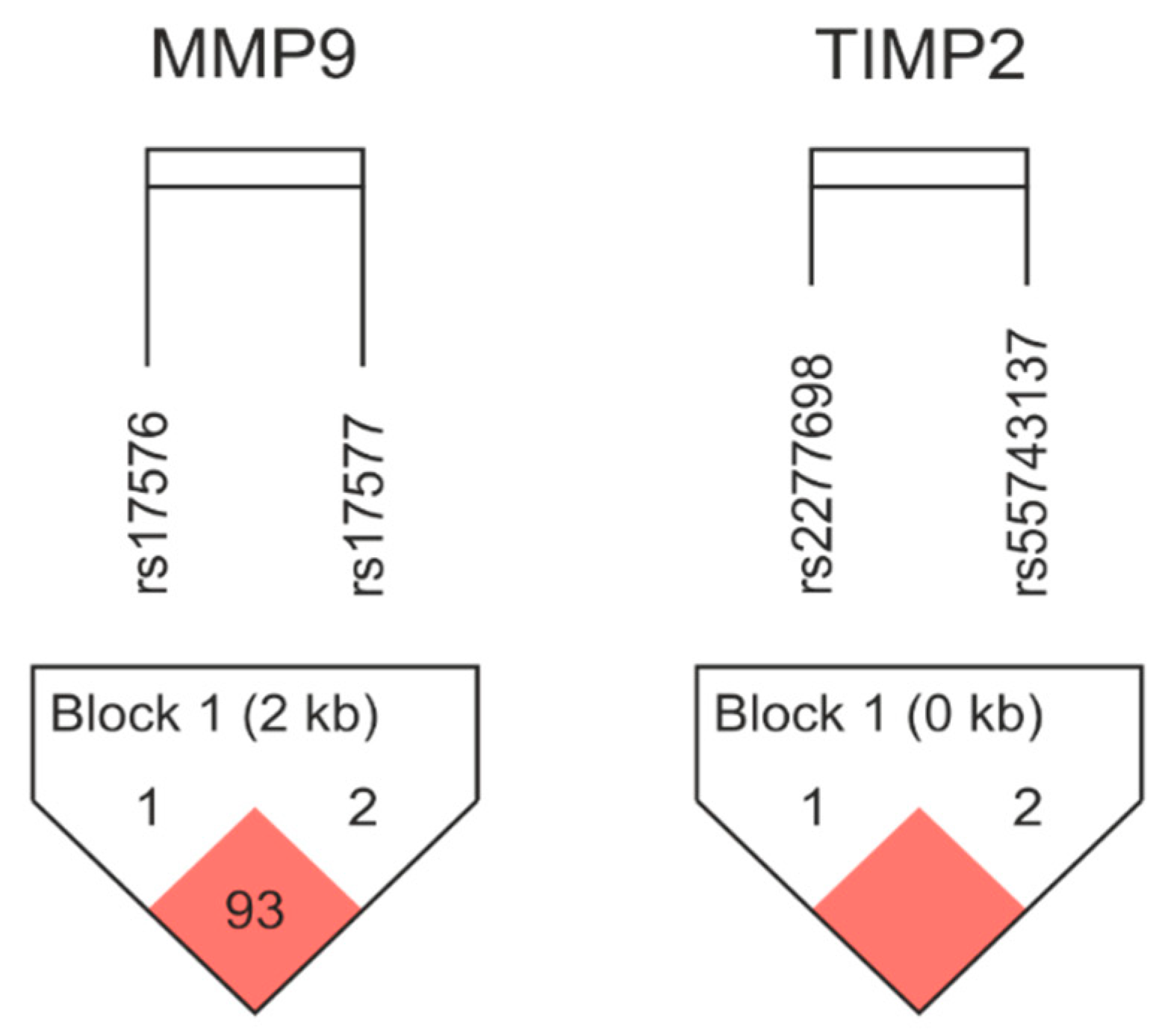
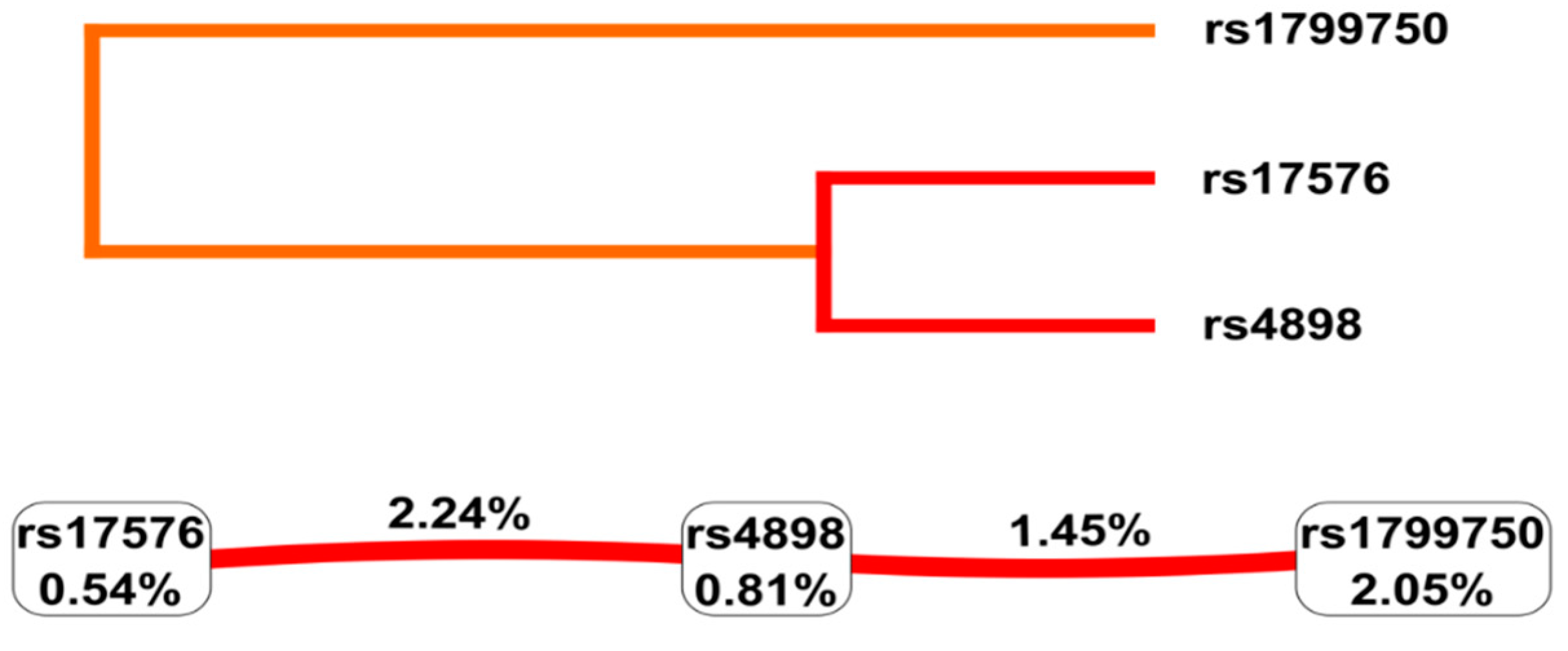
2.2.2. Haplotype Analysis
2.2.3. Gene-Gene Interaction Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical Features
4.3. ROP Diagnosis
4.4. ROP Treatment
4.5. Studied Genetic Variants
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fierson, W.M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013, 131, 189–195. [Google Scholar] [CrossRef]
- Modrzejewska, M.; Bosy-Gąsior, W. Most Up-to-Date Analysis of Epidemiological Data on the Screening Guidelines and Incidence of Retinopathy of Prematurity in Europe-A Literature Review. J. Clin. Med. 2023, 12, 3650. [Google Scholar] [CrossRef]
- Chan-Ling, T.; Gole, G.A.; Quinn, G.E.; Adamson, S.J.; Darlow, B.A. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog. Retin. Eye Res. 2018, 62, 77–119. [Google Scholar] [CrossRef]
- Quinn, G.; Fielder, A. Retinopathy of prematurity. In Taylor and Hoyt’s Paediatric Ophthalmology and Strabismus; Taylor, D.H.C., Ed.; Elsevier Saunders: New York, NY, USA, 2017; pp. 443–455. [Google Scholar]
- Główny Urząd Statystyczny/Rocznik Demograficzny 2022. Demographic Yearbook 2022. Warsaw: Central Statistical Office 2022. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-demograficzny-2022,3,16.html (accessed on 6 October 2023).
- Chmielarz-Czarnocińska, A.; Pawlak, M.; Szpecht, D.; Choręziak, A.; Szymankiewicz-Bręborowicz, M.; Gotz-Więckowska, A. Management of retinopathy of prematurity (ROP) in a Polish cohort of infants. Sci. Rep. 2021, 11, 4522. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Strauss, E.; Januszkiewicz-Lewandowska, D.; Sobaniec, A.; Gotz-Więckowska, A. SELENOP rs3877899 Variant Affects the Risk of Developing Advanced Stages of Retinopathy of Prematurity (ROP). Int. J. Mol. Sci. 2023, 24, 7570. [Google Scholar] [CrossRef]
- Strauss, E.; Gotz-Więckowska, A.; Sobaniec, A.; Chmielarz-Czarnocińska, A.; Szpecht, D.; Januszkiewicz-Lewandowska, D. Hypoxia-Inducible Pathway Polymorphisms and Their Role in the Complications of Prematurity. Genes 2023, 14, 975. [Google Scholar] [CrossRef]
- Kosik, K.; Szpecht, D.; Karbowski, Ł.; Al-Saad, S.R.; Chmielarz-Czarnocińska, A.; Minta, M.; Sowińska, A.; Strauss, E. Hemangioma-related gene polymorphisms in the pathogenesis of intraventricular hemorrhage in preterm infants. Childs Nerv. Syst. 2023, 39, 1589–1594. [Google Scholar] [CrossRef]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix metalloproteinases: A tail of a frog that became a prince. Nature reviews. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef]
- Raza, S.L.; Cornelius, L.A. Matrix metalloproteinases: Pro- and anti-angiogenic activities. J. Investig. Dermatol. Symp. Proc. 2000, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tyagi, S.C. Metalloproteinases as mediators of inflammation and the eyes: Molecular genetic underpinnings governing ocular pathophysiology. Int. J. Ophthalmol. 2017, 10, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Van Den Eynde, K.; Mousa, A.; De Hertogh, G.; Opdenakker, G. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS ONE 2013, 8, e85857. [Google Scholar] [CrossRef]
- Alge-Priglinger, C.S.; Kreutzer, T.; Obholzer, K.; Wolf, A.; Mempel, M.; Kernt, M.; Kampik, A.; Priglinger, S.G. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2009, 5, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, J.G.; Lee, Y.H.; Bae, J.G.; Park, J.H. The association between amniotic fluid-derived inflammatory mediators and the risk of retinopathy of prematurity. Medicine 2022, 101, e29368. [Google Scholar] [CrossRef]
- Magnani, J.E.; Omar, M.; Mercy, P.; Sathrasala, S.; McCaffery, H.; Vartanian, R.J.; Besirli, C.G. Quantitative analysis of tear angiogenic factors in retinopathy of prematurity: A pilot biomarker study. J. AAPOS 2023, 27, 14.E11–14.E16. [Google Scholar] [CrossRef]
- Romero, R.; Velez Edwards, D.R.; Kusanovic, J.P.; Hassan, S.S.; Mazaki-Tovi, S.; Vaisbuch, E.; Kim, C.J.; Chaiworapongsa, T.; Pearce, B.D.; Friel, L.A.; et al. Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2010, 202, 431.E1–431.E34. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.A.; Machiela, M.J. LDpop: An interactive online tool to calculate and visualize geographic LD patterns. BMC Bioinform. 2020, 21, 14. [Google Scholar] [CrossRef]
- World Health Organisation. Preterm Birth: Fact Sheet. 2016. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 1 February 2022).
- Zhu, Y.; Spitz, M.R.; Lei, L.; Mills, G.B.; Wu, X. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001, 61, 7825–7829. [Google Scholar] [PubMed]
- Pereza, N.; Pleša, I.; Peterlin, A.; Jan, Ž.; Tul, N.; Kapović, M.; Ostojić, S.; Peterlin, B. Functional polymorphisms of matrix metalloproteinases 1 and 9 genes in women with spontaneous preterm birth. Dis. Markers 2014, 2014, 171036. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Huo, X.; Lu, D.; Qian, J.; Zhou, J.; Chen, Y.; Xu, L.; Ma, H.; Zhu, J.; Wei, Q.; et al. Functional polymorphisms of matrix metalloproteinase-9 are associated with risk of occurrence and metastasis of lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5433–5439. [Google Scholar] [CrossRef]
- Pandey, M.; Awasthi, S.; Baranwal, S. IL-6: An endogenous activator of MMP-9 in preterm birth. J. Reprod. Immunol. 2020, 141, 103147. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Parry, S.; Urbanek, M.; Sammel, M.; Macones, G.; Kuivaniemi, H.; Romero, R.; Jerome, S. Single Nucleotide Polymorphism in the Matrix Metalloproteinase-1 (MMP-1) Promoter Influences Amnion Cell MMP-1 Expression and Risk for Preterm Premature Rupture of the Fetal Membranes. J. Biol. Chem. 2002, 277, 6296–6302. [Google Scholar] [CrossRef] [PubMed]
- Lathouras, K.; Saso, S.; Tzafetas, M.; Kalinderi, K.; Fidani, S.; Zournatzi, V.; Kyrgiou, M.; Fotopoulou, C.; Ghaem-Maghami, S.; Tzafetas, I. Genetic polymorphisms of matrix metalloproteinases 1-3 and their inhibitor are not associated with premature labor. Future Sci. OA 2018, 4, Fso332. [Google Scholar] [CrossRef]
- Patnaik, S.; Rai, M.; Jalali, S.; Agarwal, K.; Badakere, A.; Puppala, L.; Vishwakarma, S.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; et al. An interplay of microglia and matrix metalloproteinase MMP9 under hypoxic stress regulates the opticin expression in retina. Sci. Rep. 2021, 11, 7444. [Google Scholar] [CrossRef]
- Barnett, J.M.; McCollum, G.W.; Fowler, J.A.; Duan, J.J.; Kay, J.D.; Liu, R.Q.; Bingaman, D.P.; Penn, J.S. Pharmacologic and genetic manipulation of MMP-2 and -9 affects retinal neovascularization in rodent models of OIR. Investig. Ophthalmol. Vis. Sci. 2007, 48, 907–915. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.; Plasencia, F.; Solé-Violán, J.; Blanquer, J.; Labarta, L.; Díaz, C.; Borreguero-León, J.M.; Jiménez, A.; Páramo, J.A.; et al. The 372 T/C genetic polymorphism of TIMP-1 is associated with serum levels of TIMP-1 and survival in patients with severe sepsis. Crit. Care 2013, 17, R94. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Early Treatment for Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, G.H.; Nah, S.; Lee, K.H.; Yang, H.; Kim, Y.M.; Chun, W.; Hong, S.; Kim, S. Association of TIMP-4 gene polymorphism with the risk of osteoarthritis in the Korean population. Rheumatol. Int. 2008, 28, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 May 2023).
- Gonzalez, J.R.; Moreno, V. SNPassoc: SNPs-Based Whole Genome Association Studies. R Package Version 2.0-2. Available online: https://CRAN.R-project.org/package=SNPassoc (accessed on 22 May 2023).
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.W.; Ritchie, M.D.; Moore, J.H. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics 2003, 19, 376–382. [Google Scholar] [CrossRef]
- Botto, L.D.; Khoury, M.J. Commentary: Facing the challenge of gene-environment interaction: The two-by-four table and beyond. Am. J. Epidemiol. 2001, 153, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | I No-ROP (n = 50) | II ROP (n = 50) | II vs. I p-Value (Person) | IIa ROP with Regression (n = 28) | IIb ROP with Treatment (n = 22) | IIb vs. IIa p-Value (Person) |
|---|---|---|---|---|---|---|
| Sex, n (%) | 0.548 a | 0.918 b | ||||
| Female | 25 (50.0) | 22 (44.0) | 12 (42.9) | 10 (45.5) | ||
| Male | 25 (50.0) | 28 (56.0) | 16 (57.1) | 12 (54.5) | ||
| Gestational age (weeks), median (range) | 30 (26–33) | 26 (22–31) | <0.0001 d | 28 (23–31) | 25 (22–30) | 0.005 d |
| Birth weight (grams) | 1350 | 855 | <0.0001 c | 948 | 755 | 0.014 c |
| median (range) | (790–2010) | (432–1500) | (610–1500) | (432–1485) | ||
| Apgar score, median (range) | <0.0001 d 0.0003 d | 0.547 d 0.299 d | ||||
| 1st minute | 6 (1–10) | 4 (1–9) | 5 (1–9) | 4 (1–6) | ||
| 5th minute | 8 (5–10) | 7 (1–10) | 7 (1–9) | 7 (4–10) | ||
| Mode of delivery, n (%) | 18 (32.0) 32 (64.0) | 0.159 a | 0.828 b | |||
| Vaginal | 23 (46.0) | 12 (42.9) | 11 (50.0) | |||
| Caesarean section | 27 (54.0) | 16 (57.1) | 11 (50.0) | |||
| Birth asphyxia, n (%) | 3 (6.0) | 7 (14.0) | 0.317 b | 2 (7.1) | 5 (22.7) | 0.245 b |
| Mechanical ventilation (days) median (range) | 9 (1–53) | 55.5 (3–146) | <0.0001 d | 45 (3–101) | 80 (3–146) | 0.0001 c |
| Intrauterine infection, n (%) | 25 (50.0) | 33 (66.0) | 0.105 a | 17 (60.7) | 16 (72.7) | 0.556 b |
| Late-onset infection, n (%) | 6 (12.0) | 13 (26.0) | 0.126 b | 6 (21.4) | 7 (31.8) | 0.612 b |
| IVH, n (%) | 9 (18.0) | 12 (24.0) | 0.0003 a | 4 (14.3) | 8 (36.4) | 0.017 b |
| BPD, n (%) | 7 (14.0) | 31 (62.0) | <0.0001 a | 14 (50.0) | 17 (77.3) | 0.093 b |
| NEC, n (%) | 15 (30.0) | 33 (66.0) | 0.461 a | 14 (50.0) | 19 (86.4) | 0.139 b |
| Gene and Variant | I No-ROP n (%) | II ROP n (%) | Comparison of Groups II vs. I | |
|---|---|---|---|---|
| OR (95%CI) | p-Value (Person) | |||
| MMP-1 rs1799750 | 1.56 (0.89–2.74) | 0.117 | ||
| 2G | 61 (0.61) | 50 (0.50) | ||
| 1G | 39 (0.39) | 50 (0.50) | ||
| HWE p-value (Person) | 0.814 | 0.157 | ||
| MMP-9 rs17576 | 1.30 (0.73–2.33) | 0.374 | ||
| A | 68 (0.68) | 62 (0.62) | ||
| G | 32 (0.32) | 38 (0.38) | ||
| HWE p-value (Person) | 0.567 | 0.640 | ||
| MMP-9 rs17577 | 0.68 (0.313–1.48) | 0.328 | ||
| G | 82 (0.82) | 87 (0.87) | ||
| A | 18 (0.18) | 13 (0.13) | ||
| HWE p-value (Person) | 0.716 | 0.291 | ||
| TIMP-1 rs4898; All infants | 0.76 (0.43–1.32) | 0.323 | ||
| T | 47 (0.47) | 54 (0.54) | ||
| C | 53 (0.53) | 46 (0.46) | ||
| HWE p-value (Person) | 0.025 | 0.002 | ||
| TIMP-1 rs4898; Female | 0.49 (0.21–1.12) | 0.087 | ||
| T | 23 (0.46) | 28 (0.64) | ||
| C | 27 (0.54) | 16 (0.36) | ||
| HWE p-value (Person) | 0.065 | 0.079 | ||
| TIMP-1 rs4898; Male | 1.07 (0.36–3.14) | 0.909 | ||
| T | 12 (0.48) | 13 (0.46) | ||
| C | 13 (0.52) | 15 (0.54) | ||
| TIMP-2 rs2277698 | 1.87 (0.75–4.68) | 0.175 | ||
| C | 92 (0.92) | 86 (0.86) | ||
| T | 8 (0.08) | 14 (0.14) | ||
| HWE p-value (Person) | 0.539 | 0.231 | ||
| TIMP-2 rs55743137 | 1.37 (0.626–3.00) | 0.428 | ||
| T | 87 (0.87) | 83 (0.83) | ||
| G | 13 (0.13) | 17 (0.17) | ||
| HWE p-value (Person) | 0.846 | 0.578 | ||
| Gene, SNP | Genotypes and Tested Models | I no-ROP n (%) | II ROP n (%) | II vs. I Co-Dominant Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | ||||||||
| OR (95%CI) | p | AIC | AOR (95%CI) | p | AIC | ||||
| MMP-1 rs1799750 | 2G/2G | 19 (38.0) | 15 (30.0) | 1.00 | 0.241 | 141.8 | 1.00 | 0.065 | 83.5 |
| 2G/1G | 23 (46.0) | 20 (40.0) | 1.10 (0.45–2.72) | 1.55 (0.38–6.30) | |||||
| 1G/1G | 8 (16.0) | 15 (30.0) | 2.38 (0.80–7.09) | 6.38 (0.84–48.4) | |||||
| Dominant | 31 (62.0) | 35 (70.0) | 1.43 (0.62–3.29) | 0.398 | 141.9 | 2.25 (0.61–8.34) | 0.219 | 81.8 | |
| Recessive | 8 (16.0) | 15 (30.0) | 2.25 (0.85–5.93) | 0.096101 | 139.8 | 4.14 (0.93–18.33) | 0.061 | 84.3 | |
| Overdominant | 27 (54.0) | 30 (60.0) | 1.28 (0.58–2.82) | 0.544 | 142.3 | 1.33 (0.41–4.27) | 0.631 | 85.7 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 2.22 (0.75–6.58) | 0.144 | 140.4 | 5.01 (1.03–28.4) | 0.048 | 82.0 | |
| MMP-9 rs17576 | AA | 24 (48.0) | 20 (40.0) | 1.00 | 0.689 | 143.9 | 1.00 | 0.454 | 86.4 |
| AG | 20 (40.0) | 22 (44.0) | 1.32 (0.57–3.08) | 1.92 (0.57–6.40) | |||||
| GG | 6 (12.0) | 8 (16.0) | 1.60 (0.48–5.38) | 2.50 (0.38–16.45) | |||||
| Dominant | 26 (52.0) | 30 (60.0) | 1.38 (0.63–3.06) | 0.420 | 142.0 | 2.02 (0.65–6.34) | 0.220 | 84.4 | |
| Recessive | 6 (12.0) | 8 (16.0) | 1.40 (0.45–4.37) | 0.564 | 142.3 | 1.83 (0.31–10.77) | 0.506 | 85.5 | |
| Overdominant | 30 (60.0) | 28 (56.0) | 1.18 (0.53–2.61) | 0.685 | 142.5 | 1.60 (0.51–4.99) | 0.415 | 85.3 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 1.28 (0.73–2.25) | 0.391 | 141.9 | 1.68 (0.72–3.92) | 0.222 | 84.4 | |
| MMP-9 rs17577 | GG | 34 (68.0) | 37 (74.0) | 1.00 | 0.495 | 141.7 | 1.00 | 0.690 | 87.2 |
| GA | 14 (28.0) | 13 (26.0) | 0.85 (0.35–2.07) | 0.85 (0.22–3.21) | |||||
| AA | 2 (4.0) | 0 (0.0) | ― | ― | |||||
| Dominant | 16 (32.0) | 13 (26.0) | 0.75 (0.31–1.78) | 0.508 | 142.2 | 0.77 (0.21–2.83) | 0.692 | 85.8 | |
| Recessive | 2 (4.0) | 0 (0.0) | ― | 0.495 | 139.8 | ― | 0.409 | 85.2 | |
| Overdominant | 36 (72.0) | 37 (74.0) | 0.90 (0.37–2.19) | 0.822 | 142.6 | 0.87 (0.23–3.31) | 0.835 | 85.9 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 0.67 (0.30–1.48) | 0.495 | 141.6 | 0.72 (0.22–2.42) | 0.594 | 85.6 | |
| TIMP-1 rs4898 | Female newborns | ||||||||
| TT | 3 (12.0) | 7 (31.8) | 1.00 | 0.098 | 66.3 | 1.00 | <0.050 | 44.8 | |
| TC | 17 (68.0) | 14 (63.6) | 0.35 (0.08–1.62) | 0.10 (0.01–1.13) | |||||
| CC | 5 (20.0) | 1 (4.5) | 0.09 (0.01–1.08) | 0.02 (0.01–0.81) | |||||
| Dominant | 22 (88.0) | 15 (68.2) | 0.29 (0.06–1.31) | 0.154 | 66.0 | 0.09 (0.01–1.04) | 0.048 | 43.1 | |
| Recessive | 5 (20.0) | 1 (4.5) | 0.19 (0.02–1.78) | 0.194 | 65.9 | 0.36 (0.01–11.3) | 0.554 | 43.4 | |
| Overdominant | 8 (32.0) | 8 (36.3) | 1.21 (0.36–4.07) | 0.768 | 66.1 | 1.50 (0.49–4.59) | 0.515 | 43.3 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 0.09 (0.01–1.04) | 0.047 | 61.7 | 0.02 (0.01–1.07) | 0.050 | 44.6 | |
| Male newborns | |||||||||
| T | 12 (48.0) | 13 (46.4) | 1.00 | 0.909 | 77.3 | 1.00 | 0.189 | 34.9 | |
| C | 13 (52.0) | 15 (53.6) | 1.07 (0.36–3.14) | 0.25 (0.03–1.99) | |||||
| TIMP-2 rs2277698 | CC | 42 (84.0) | 38 (76.0) | 1.00 | 0.397 | 141.4 | 1.00 | 0.249 | 85.2 |
| CT | 8 (16.0) | 10 (20.0) | 1.38 (0.49–3.86) | 1.15 (0.26–5.08) | |||||
| TT | 0 (0.0) | 2 (4.0) | ― | ― | |||||
| Dominant | 8 (16.0) | 12 (24.0) | 1.66 (0.61–4.49) | 0.316 | 141.6 | 1.63 (0.41–6.54) | 0.490 | 85.5 | |
| Recessive | 0 (0.0) | 2 (4.0) | ― | 0.495 | 139.8 | ― | 0.098 | 83.2 | |
| Overdominant | 42 (84.0) | 40 (80.0) | 1.31 (0.47–3.66) | 0.602 | 142.4 | 1.01 (0.23–4.42) | 0.993 | 85.9 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 1.80 (0.73–4.41) | 0.397 | 140.9 | 1.89 (0.59–6.08) | 0.272 | 84.7 | |
| TIMP-2 rs55743137 | TT | 38 (76.0) | 35 (70.0) | 1.00 | 0.730 | 144.0 | 1.00 | 0.452 | 86.3 |
| TG | 11 (22.0) | 13 (26.0) | 1.28 (0.51–3.24) | 1.12 (0.30–4.14) | |||||
| GG | 1 (2.0) | 2 (4.0) | 2.17 (0.19–25.01) | 6.32 (0.31–5130.78) | |||||
| Dominant | 12 (24.0) | 15 (30.0) | 1.36 (0.56–3.30) | 0.499 | 142.2 | 1.43 (0.42–4.88) | 0.571 | 85.6 | |
| Recessive | 1 (2.0) | 2 (4.0) | 2.04 (0.18–23.27) | 0.554 | 142.3 | 6.15 (0.30–125.33) | 0.211 | 84.4 | |
| Overdominant | 39 (78.0) | 37 (74.0) | 1.25 (0.50–3.13) | 0.639 | 142.4 | 1.00 (0.27–3.65) | 0.998 | 85.9 | |
| Log-additive | 50 (50.0) | 50 (50.0) | 1.35 (0.63–2.89) | 0.440 | 142.0 | 1.60 (0.58–4.40) | 0.358 | 85.1 | |
| Gene and SNPs | Haplotypes | Frequency Overall | Frequency ROP, No-ROP | p-Value (Person) |
|---|---|---|---|---|
| MMP-9 rs17576/rs17577 | AG | 0.644 | 0.619, 0.669 | 0.462 |
| GG | 0.201 | 0.251, 0.151 | 0.078 | |
| GA | 0.149 | 0.129, 0.169 | 0.428 | |
| TIMP-2 rs2277698/rs55743137 | CT | 0.850 | 0.830, 0.870 | 0.428 |
| TG | 0.110 | 0.140, 0.080 | 0.175 | |
| CG | 0.040 | 0.030, 0.050 | 0.471 |
| Loci Model | Gene and Variant | Training Balance Accuracy (%) | Testing Balance Accuracy (%) | CVC | Person p-Value |
|---|---|---|---|---|---|
| One locus | MMP-1 rs1799750 | 57.4 | 44.0 | 6/10 | 0.0962 |
| Two loci | MMP-1 rs1799750, TIMP-1 rs4989 | 63.4 | 42.1 | 6/10 | 0.0150 |
| Three loci | MMP-1 rs1799750, MMP-9 rs17576, TIMP-1 rs4989 | 68.9 | 42.3 | 9/10 | 0.0003 |
| Gene | rs Number | Position (GRCh38.p14) | Allele | Variant Type | MAF |
|---|---|---|---|---|---|
| MMP-1 | rs1799750 | chr11:102799765-102799766 | delG | Promoter | delG = 0.4960 |
| MMP-9 | rs17576 | chr20:46011586 | A>G | Coding Gln279Arg | G = 0.3807 |
| MMP-9 | rs17577 | chr20:46014472 | G>A | Coding Arg668Gln | A = 0.1750 |
| TIMP-1 | rs4898 | chrX:47585586 | T>C | Coding Phe124= | C = 0.4650 |
| TIMP-2 | rs2277698 | chr17:78870935 | C>T | Coding Ser101= | T = 0.1252 |
| TIMP-2 | rs55743137 | chr17:78871103 | G>T | Intronic | G = 0.1998 |
| Gene and Variant | Sequence of Primers | Temperature of Primer Attachment | Restriction Enzyme | PCR Products |
|---|---|---|---|---|
| MMP-1 rs179975 | 5′-TGA CTT TTA AAA CAT AGT CTA TGT TCA 3′ 5′ -TCT TGG ATT GAT TTG AGA TAA GTC ATAGC-3′ | 50 °C | AluI | 1G 241, 28 pz 2G 269 pz |
| MMP-9 rs17576 | 5′-GAGAGATGGGATGAACTG-3′ 5′-GTGGTGGAAATGTGGTGT-3′ | 60 °C | MspI (HpaII) | A 252, 187 pz G 187, 129, 123 pz |
| MMP-9 rs17577 | 5′-ACA CGC ACG ACG TCT TCC AGT ATC-3′ 5′-GGG GCA TTT GTT TCC ATT TCC A-3′ | 63 °C | TaqI | G 115, 23 pz A 138 pz |
| TIMP-1 rs4898 | 5’-GCA CAT CAC TAC CTG CAG TCT-3’ 5’-GAA ACA AGC CCA CGA TTT AG-3’ | 54 °C | BauI (BssI) | T 175 pz C 153,22 pz |
| TIMP-2 rs2277698 | 5′-CCA GGA AAT TGG CAG GTA GT-3′ 5′-GAA TTC ACC AAC TGT GTG GC-3′ | 60 °C | BsrI | C 369 pz T 231, 138 pz |
| TIMP-2 rs55743137 | 5′-CCT TTG AAC ATC TGG AAA GAC AA-3′ 5′-TAA CCC ATG TAT TTG CAC TTC CT-3′ | 58 °C | AluI | T 160 pz G 108, 52 pz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choręziak-Michalak, A.; Szpecht, D.; Chmielarz-Czarnocińska, A.; Seremak-Mrozikiewicz, A.; Drews, K.; Kurzawińska, G.; Strauss, E.; Gotz-Więckowska, A. Comprehensive Analysis of the Role of Gene Variants in Matrix Metalloproteinases and Their Tissue Inhibitors in Retinopathy of Prematurity: A Study in the Polish Population. Int. J. Mol. Sci. 2023, 24, 15309. https://doi.org/10.3390/ijms242015309
Choręziak-Michalak A, Szpecht D, Chmielarz-Czarnocińska A, Seremak-Mrozikiewicz A, Drews K, Kurzawińska G, Strauss E, Gotz-Więckowska A. Comprehensive Analysis of the Role of Gene Variants in Matrix Metalloproteinases and Their Tissue Inhibitors in Retinopathy of Prematurity: A Study in the Polish Population. International Journal of Molecular Sciences. 2023; 24(20):15309. https://doi.org/10.3390/ijms242015309
Chicago/Turabian StyleChoręziak-Michalak, Aneta, Dawid Szpecht, Anna Chmielarz-Czarnocińska, Agnieszka Seremak-Mrozikiewicz, Krzysztof Drews, Grażyna Kurzawińska, Ewa Strauss, and Anna Gotz-Więckowska. 2023. "Comprehensive Analysis of the Role of Gene Variants in Matrix Metalloproteinases and Their Tissue Inhibitors in Retinopathy of Prematurity: A Study in the Polish Population" International Journal of Molecular Sciences 24, no. 20: 15309. https://doi.org/10.3390/ijms242015309
APA StyleChoręziak-Michalak, A., Szpecht, D., Chmielarz-Czarnocińska, A., Seremak-Mrozikiewicz, A., Drews, K., Kurzawińska, G., Strauss, E., & Gotz-Więckowska, A. (2023). Comprehensive Analysis of the Role of Gene Variants in Matrix Metalloproteinases and Their Tissue Inhibitors in Retinopathy of Prematurity: A Study in the Polish Population. International Journal of Molecular Sciences, 24(20), 15309. https://doi.org/10.3390/ijms242015309






