Acute Phase Protein Orosomucoid (Alpha-1-Acid Glycoprotein) Predicts Delayed Cerebral Ischemia and 3-Month Unfavorable Outcome after Aneurysmal Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Results
2.1. Cohort Characteristics
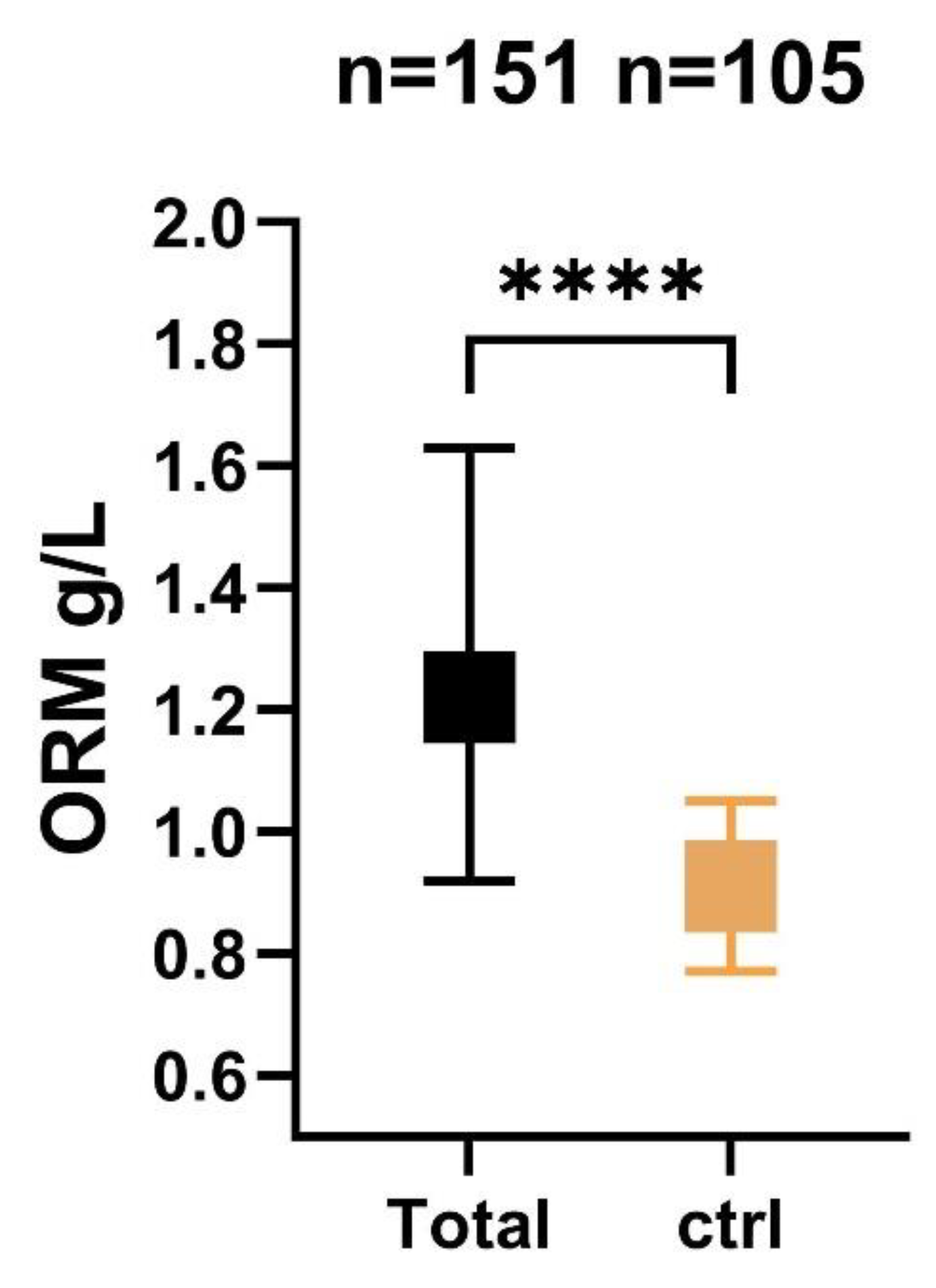
2.2. Association of Serum ORM Level with Admission Severity Scores
2.3. Serum ORM Levels in Relation to DCI and 3-Month Outcome
2.4. Variables Associated with Higher Serum ORM Level
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical and Outcome Data Definitions
4.3. Sample Collection and Processing Protocol
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; van Gelder, J.M. The probability of sudden death from rupture of intracranial aneurysms: A meta-analysis. Neurosurgery 2002, 51, 1105–1107. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Desai, M.; Hinduja, A.; Rubinos, C.A.; Mansueto, G.; Singh, P.; Domeniconi, G.G.; Ikram, A.; et al. Pathophysiology of Early Brain Injury and Its Association with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage: A Review of Current Literature. J. Clin. Med. 2023, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, D.C.; Jayaraman, K.; Yuan, J.Y.; Diwan, D.; Vellimana, A.K.; Osbun, J.W.; Chatterjee, A.R.; Athiraman, U.; Dhar, R.; Zipfel, G.J. Early Brain Injury After Subarachnoid Hemorrhage: Incidence and Mechanisms. Stroke 2023, 54, 1426–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, Q.; Zhao, Y.H.; Chen, X. Toll-like receptor-4 pathway as a possible molecular mechanism for brain injuries after subarachnoid hemorrhage. Int. J. Neurosci. 2020, 130, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Williams, J.P.; Weiser, M.R.; Pechet, T.T.; Kobzik, L.; Moore, F.D., Jr.; Hechtman, H.B. alpha 1-Acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. Am. J. Physiol. 1997, 273, G1031–G1035. [Google Scholar]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. Alpha(1)-acid glycoprotein: An acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Luo, Z.; Lei, H.; Sun, Y.; Liu, X.; Su, D.F. Orosomucoid, an acute response protein with multiple modulating activities. J. Physiol. Biochem. 2015, 71, 329–340. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Vrethem, M.; Ohman, S.; von Schenck, H.; Forsberg, P.; Olsson, J.E. Comparison of concentration of orosomucoid in serum and cerebrospinal fluid in different neurological diseases. Acta Neurol. Scand. 1987, 75, 328–331. [Google Scholar] [CrossRef]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care 2015, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Sörensson, J.; Matejka, G.L.; Ohlson, M.; Haraldsson, B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am. J. Physiol. 1999, 276, H530–H534. [Google Scholar] [CrossRef]
- Kuebler, J.F.; Toth, B.; Yokoyama, Y.; Bland, K.I.; Rue, L.W., 3rd; Chaudry, I.H. Alpha1-acid-glycoprotein protects against trauma-hemorrhagic shock. J. Surg. Res. 2004, 119, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, G.; Zeng, M.; Fu, B.M. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc. Res. 2010, 80, 148–157. [Google Scholar] [CrossRef]
- Komori, H.; Watanabe, H.; Shuto, T.; Kodama, A.; Maeda, H.; Watanabe, K.; Kai, H.; Otagiri, M.; Maruyama, T. α(1)-Acid glycoprotein up-regulates CD163 via TLR4/CD14 protein pathway: Possible protection against hemolysis-induced oxidative stress. J. Biol. Chem. 2012, 287, 30688–30700. [Google Scholar] [CrossRef]
- Hanafy, K.A. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J. Neuroinflammation 2013, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhou, W.; Yan, Z.; Qu, M.; Bu, X. Toll-like Receptor 4 (TLR4) is associated with cerebral vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 2015, 55, 878–884. [Google Scholar] [CrossRef]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021, 10, e021845. [Google Scholar] [CrossRef]
- Gunnarsson, P.; Levander, L.; Påhlsson, P.; Grenegård, M. alpha(1)-acid glycoprotein (AGP)-induced platelet shape change involves the Rho/Rho kinase signalling pathway. Thromb. Haemost. 2009, 102, 694–703. [Google Scholar]
- Costello, M.; Fiedel, B.A.; Gewurz, H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature 1979, 281, 677–678. [Google Scholar] [CrossRef]
- Jo, M.; Kim, J.H.; Song, G.J.; Seo, M.; Hwang, E.M.; Suk, K. Astrocytic Orosomucoid-2 Modulates Microglial Activation and Neuroinflammation. J. Neurosci. 2017, 37, 2878–2894. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [PubMed]
- Daemen, M.A.; Heemskerk, V.H.; van’t Veer, C.; Denecker, G.; Wolfs, T.G.; Vandenabeele, P.; Buurman, W.A. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000, 102, 1420–1426. [Google Scholar] [CrossRef]
- Ligresti, G.; Aplin, A.C.; Dunn, B.E.; Morishita, A.; Nicosia, R.F. The acute phase reactant orosomucoid-1 is a bimodal regulator of angiogenesis with time- and context-dependent inhibitory and stimulatory properties. PLoS ONE 2012, 7, e41387. [Google Scholar] [CrossRef] [PubMed]
- Öngöz Dede, F.; Ballı, U.; Durmuşlar, M.C.; Bozkurt Doğan, Ş.; Avcı, B.; Ayas, B.; Tunçel, Ö.K. Analysis of Orosomucoid and C-Reactive Protein Levels in Gingival Tissue And Serum of Rats with Experimental Periodontitis: Comparison at different time points in disease progression. J. Exp. Clin. Med. 2017, 34, 269–274. [Google Scholar]
- Sann, L.; Bienvenu, F.; Bienvenu, J.; Bourgeois, J.; Bethenod, M. Evolution of serum prealbumin, C-reactive protein, and orosomucoid in neonates with bacterial infection. J. Pediatr. 1984, 105, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Kustán, P.; Kőszegi, T.; Miseta, A.; Péter, I.; Ajtay, Z.; Kiss, I.; Németh, B. Urinary Orosomucoid A Potential Marker Of Inflammation In Psoriasis. Int. J. Med. Sci. 2018, 15, 1113–1117. [Google Scholar] [CrossRef]
- Csecsei, P.; Olah, C.; Varnai, R.; Simon, D.; Erdo-Bonyar, S.; Berki, T.; Czabajszki, M.; Zavori, L.; Schwarcz, A.; Molnar, T. Different Kinetics of Serum ADAMTS13, GDF-15, and Neutrophil Gelatinase-Associated Lipocalin in the Early Phase of Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 11005. [Google Scholar] [CrossRef]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]

| Favorable (N = 71) | Unfavorable (N = 80) | p-Value | |
|---|---|---|---|
| Age, years | 52 ± 12 | 58 ± 12 | 0.002 |
| Female | 51 (72) | 65 (81) | 0.529 |
| Hypertension | 31 (44) | 41 (51) | 0.393 |
| NIDDM | 3 (4) | 14 (17) | 0.015 |
| Smoking | 14 (20) | 14 (18) | 0.754 |
| mFisher score | 3 (2–3) | 3 (3–4) | 0.001 |
| WFNS | 1 (1–2) | 4 (2–5) | <0.001 |
| SEBES | 1 (1–2) | 3 (1–4) | <0.001 |
| Aneurysm location | |||
| ICA | 11 (15) | 7 (9) | 0.670 |
| MCA | 18 (25) | 22 (28) | 0.325 |
| ACoA | 25 (36) | 26 (33) | 0.410 |
| PCom | 6 (9) | 6 (7) | 0.980 |
| ACA | 3 (4) | 8 (10) | 0.088 |
| VB | 8 (11) | 11 (13) | 0.650 |
| CRP (mg/L) | 8.3 (3–17) | 27 (9–73) | <0.001 |
| NLR | 4.2 (3–7) | 6.4 (4–11) | 0.002 |
| WBC (G/L) | 11.4 (8.7–13.4) | 12.2 (10.4–15.7) | 0.028 |
| Neutrophile (G/L) | 8 (6.5–10.8) | 9.9 (7.5–12.9) | 0.015 |
| Lymphocyte (G/L) | 1.7 (1.1–2.3) | 1.4 (1.1–2) | 0.093 |
| Glucose (mmol/L) | 7.2 (6.2–9.4) | 8.1 (7.3–9.5) | 0.145 |
| Creatinine | 60 (51–68) | 61 (49–73) | 0.654 |
| Hydrocephalus | 23 (32.4) | 59 (73.8) | <0.001 |
| Intraparenchymal hematoma | 2 (3) | 19 (24) | <0.001 |
| Extraventricular drainage | 12 (17) | 57 (71) | <0.001 |
| Decompressive craniotomy | 0 (0) | 19 (24) | <0.001 |
| Mechanical ventilation | 7 (10) | 60 (75) | <0.001 |
| infection | 6 (10) | 26 (32) | 0.004 |
| Macrovascular vasospasm | 12 (17) | 30 (37) | 0.007 |
| Delayed cerebral ischemia | 4 (7) | 30 (37) | <0.001 |
| Variable | Multivariate | ||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| DCI | |||
| Model a | 1.227 | 1.068–1.410 | 0.004 |
| Model b | 1.467 | 1.091–1.971 | 0.011 |
| Model c | 1.574 | 1.095–2.260 | 0.014 |
| Poor clinical outcome at 3 months | |||
| Model a | 1.518 | 1.257–1.833 | <0.001 |
| Model b | 1.432 | 1.145–1.791 | 0.002 |
| Model c | 1.308 | 1.016–1.684 | 0.037 |
| Cut-off Value | AUC | p-Value | 95% CI | Sensitivity (%) | Specificity (%) | Power | |
|---|---|---|---|---|---|---|---|
| DCI | 1.270 | 0.713 | 0.009 | 0.562–0.864 | 68.8 | 60 | AC |
| Poor functional outcome at 3 months | 1.200 | 0.793 | <0.001 | 0.694–0.891 | 72.5 | 68.3 | AC |
| ORM Concentrations (Categorized According to the Optimal Cut-off (≤1.20 g/L vs. >1.20 g/L) | |||
|---|---|---|---|
| Variable | OR (95% CI) | p | C |
| Age | 0.985 (0.949–1.022) | 0.423 | 0.440 |
| Gender | |||
| Female | Reference | ||
| Male | 0.800 (0.311–2.056) | 0.643 | 0.476 |
| Smoking | |||
| No | Reference | ||
| Yes | 0.595 (0.154–2.292) | 0.450 | 0.472 |
| WFNS | 1.944 (1.378–2.741) | <0.001 | 0.738 |
| mFisher-score | 1.854 (1.095–3.139) | 0.022 | 0.676 |
| CRP | 1.023 (1.005–1.042) | 0.014 | 0.690 |
| Neutrophile count | 1.191 (1.040–1.363) | 0.011 | 0.690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavori, L.; Varnai, R.; Molnar, T.; Szirmay, B.; Farkas, N.; Schwarcz, A.; Csecsei, P. Acute Phase Protein Orosomucoid (Alpha-1-Acid Glycoprotein) Predicts Delayed Cerebral Ischemia and 3-Month Unfavorable Outcome after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 15267. https://doi.org/10.3390/ijms242015267
Zavori L, Varnai R, Molnar T, Szirmay B, Farkas N, Schwarcz A, Csecsei P. Acute Phase Protein Orosomucoid (Alpha-1-Acid Glycoprotein) Predicts Delayed Cerebral Ischemia and 3-Month Unfavorable Outcome after Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences. 2023; 24(20):15267. https://doi.org/10.3390/ijms242015267
Chicago/Turabian StyleZavori, Laszlo, Reka Varnai, Tihamer Molnar, Balazs Szirmay, Nelli Farkas, Attila Schwarcz, and Peter Csecsei. 2023. "Acute Phase Protein Orosomucoid (Alpha-1-Acid Glycoprotein) Predicts Delayed Cerebral Ischemia and 3-Month Unfavorable Outcome after Aneurysmal Subarachnoid Hemorrhage" International Journal of Molecular Sciences 24, no. 20: 15267. https://doi.org/10.3390/ijms242015267
APA StyleZavori, L., Varnai, R., Molnar, T., Szirmay, B., Farkas, N., Schwarcz, A., & Csecsei, P. (2023). Acute Phase Protein Orosomucoid (Alpha-1-Acid Glycoprotein) Predicts Delayed Cerebral Ischemia and 3-Month Unfavorable Outcome after Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences, 24(20), 15267. https://doi.org/10.3390/ijms242015267








