Preparation of NIn-Methyl-6-[18F]fluoro- and 5-Hydroxy-7-[18F]fluorotryptophans as Candidate PET-Tracers for Pathway-Specific Visualization of Tryptophan Metabolism
Abstract
1. Introduction
2. Results and Discussion
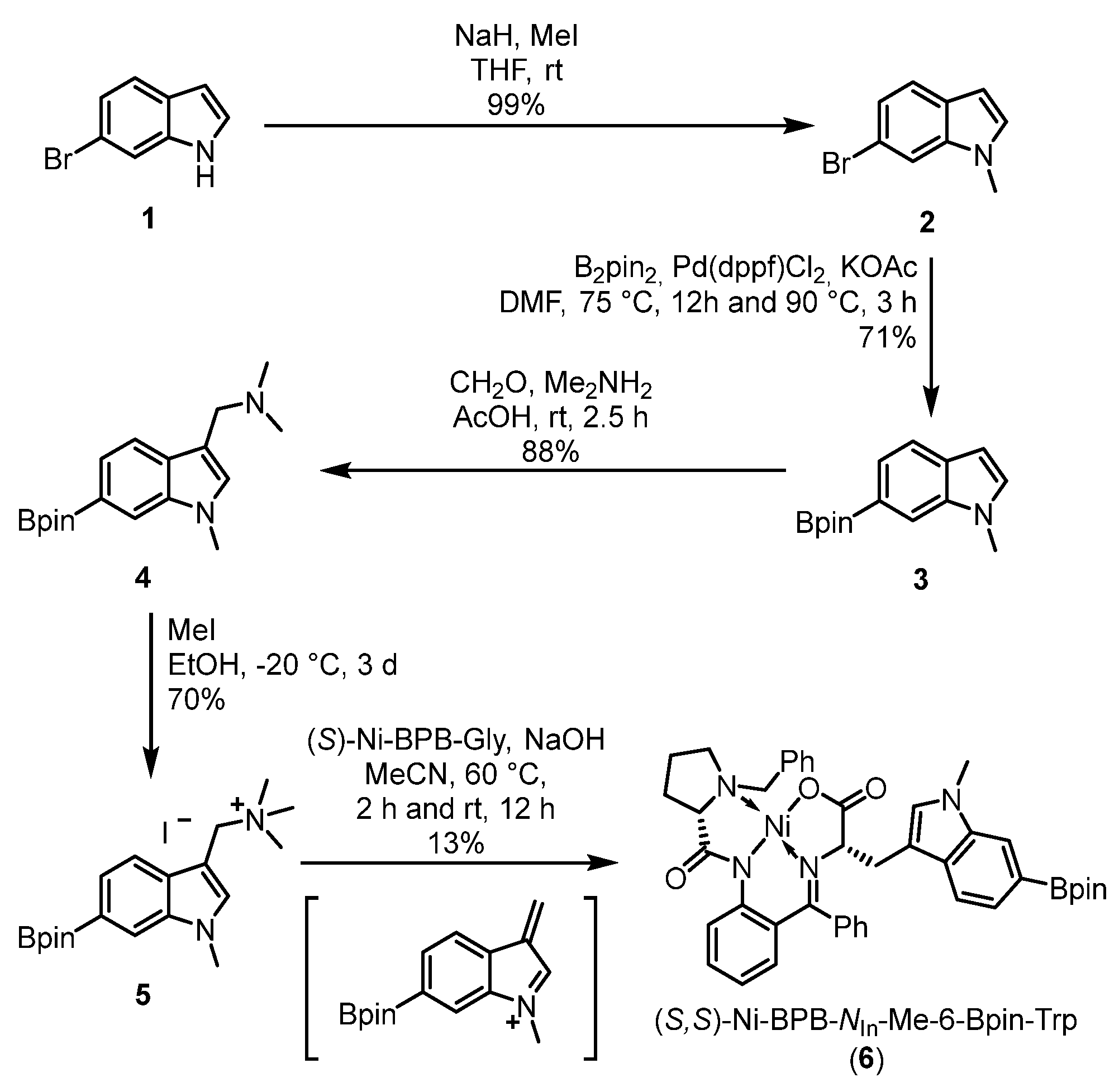
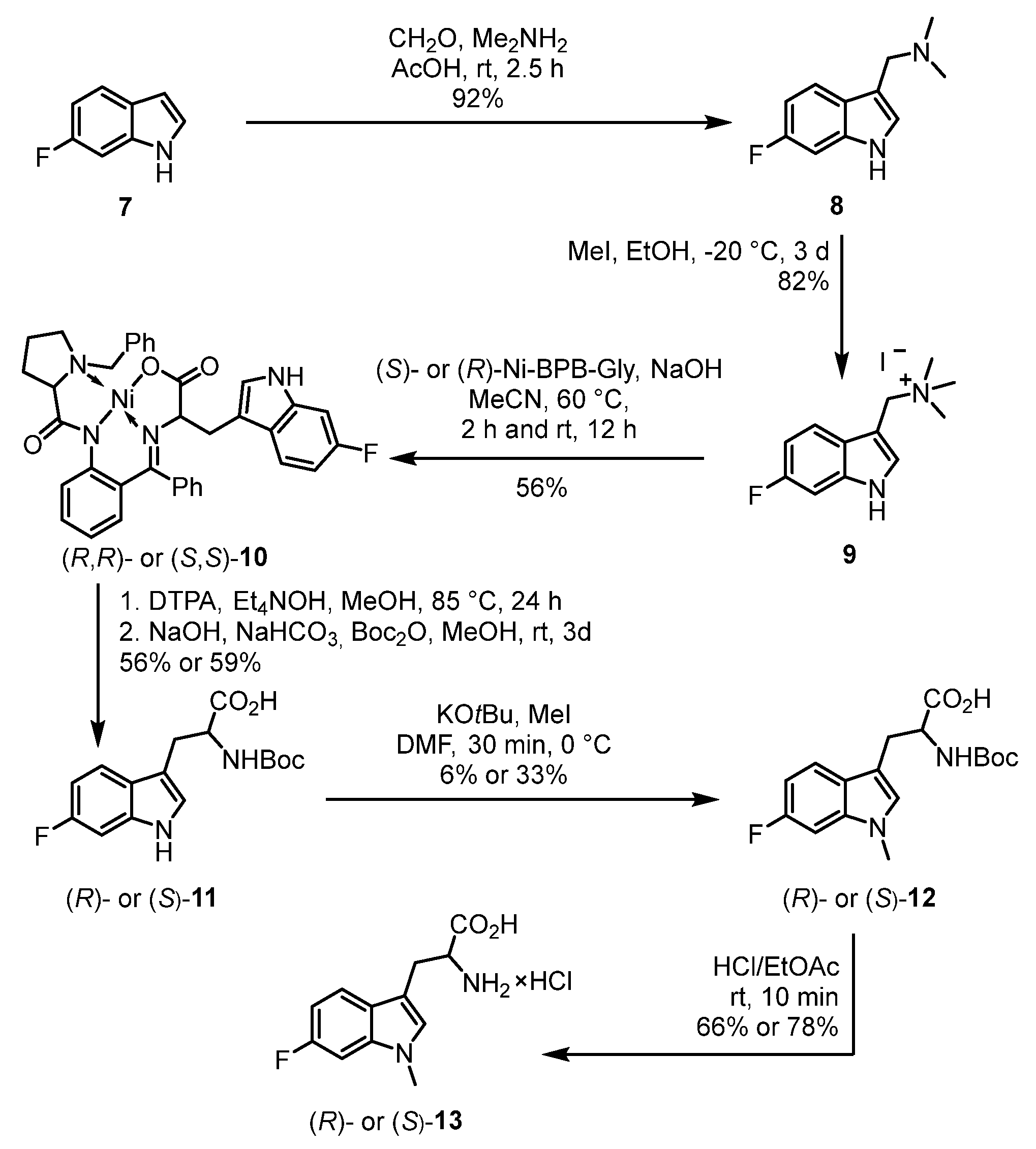
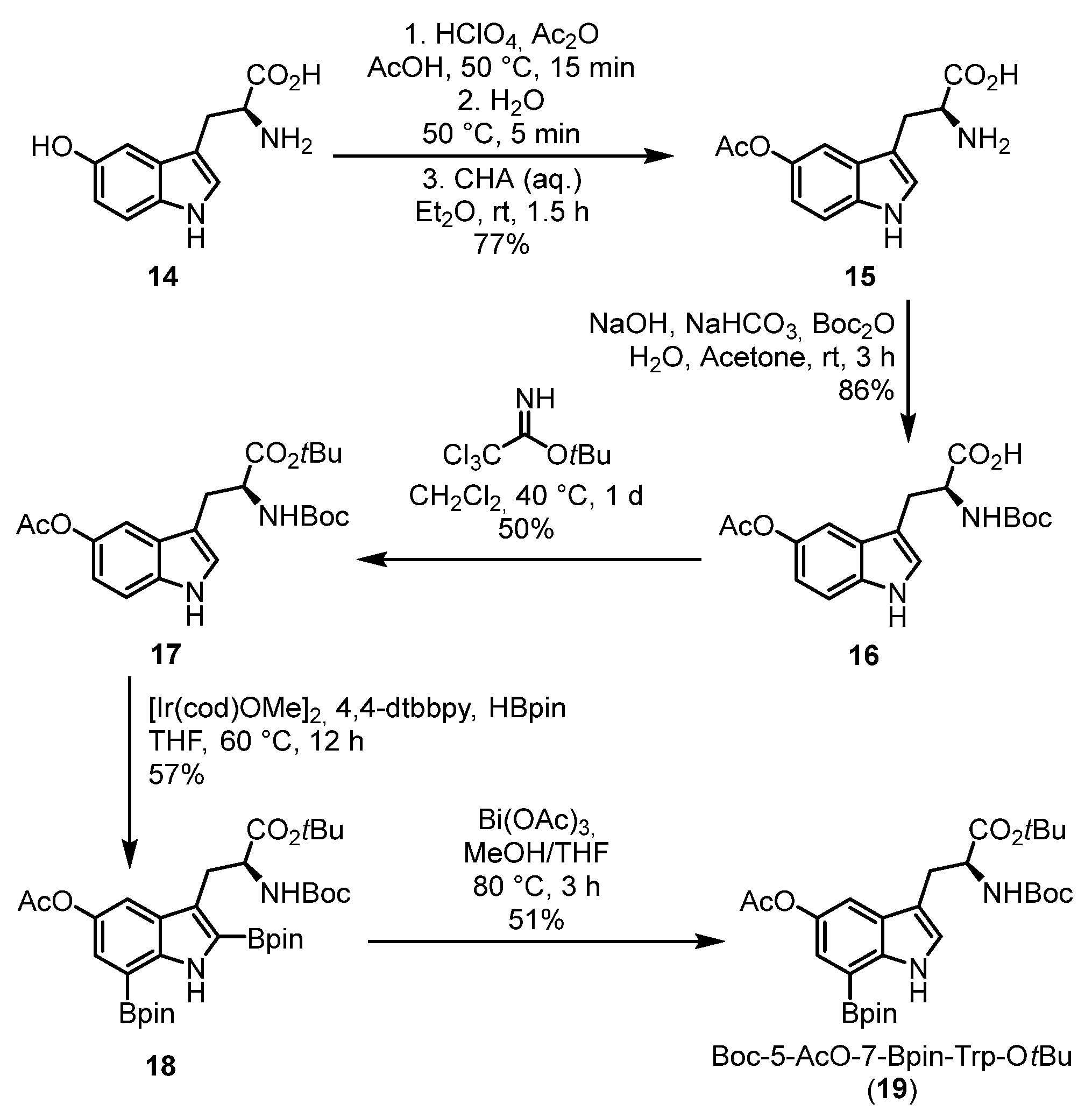
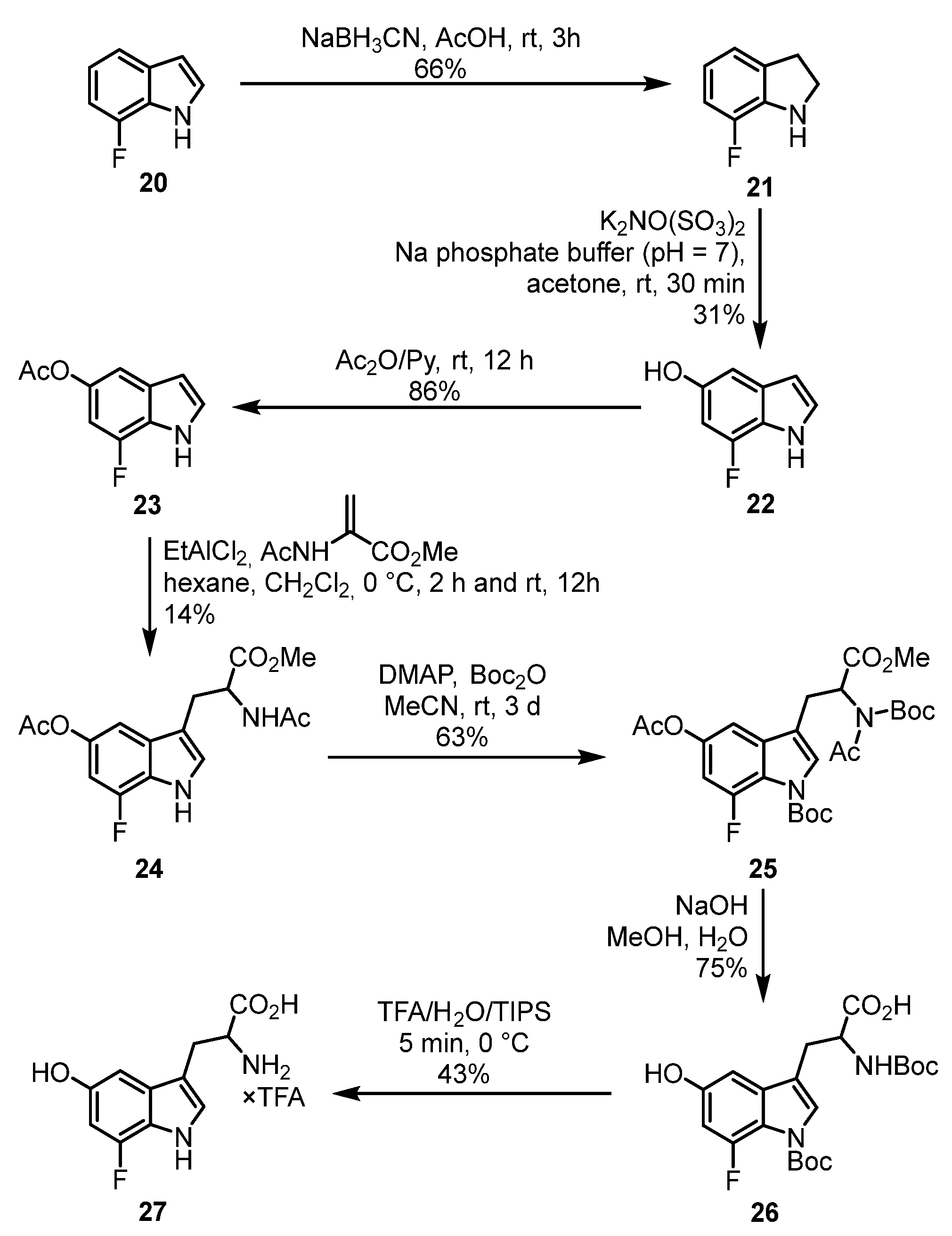
2.1. Chemistry
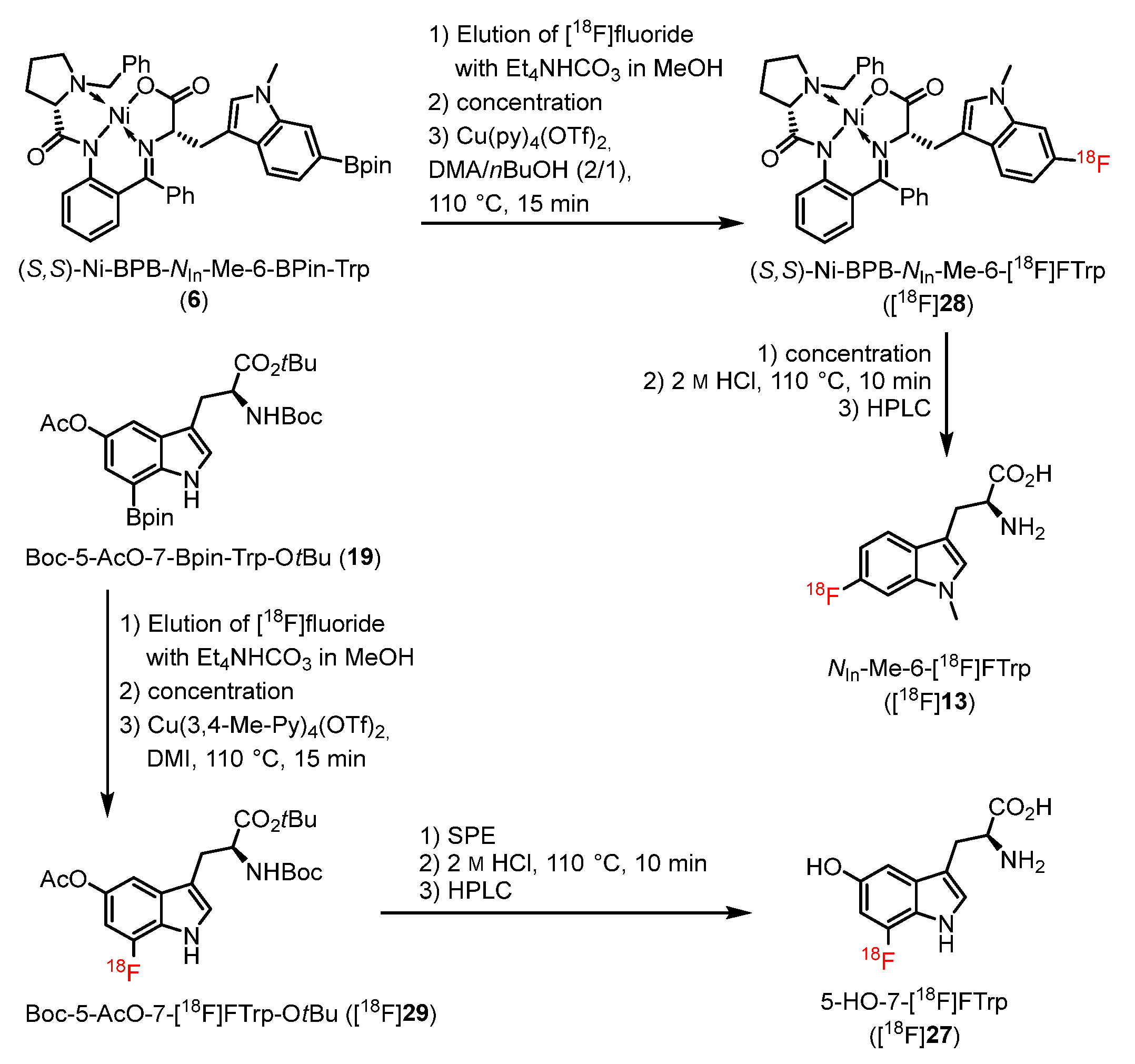
2.2. Radiosyntheses
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. 6-Bromo-1-methyl-1H-indole (2) [57]
3.1.3. 1-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole (3)
3.1.4. N,N-Dimethyl-1-[1-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl]methanamine (4)
3.1.5. N,N,N-Trimethyl-1-[1-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl]methanaminium Iodide (5)
3.1.6. (S,S)-Ni-BPB-NIn-methyl-6-Bpin-tryptophan (6)
3.1.7. 1-(6-Fluoro-1H-indol-3-yl)-N,N-dimethylmethanamine (8)
3.1.8. 1-(6-Fluoro-1H-indol-3-yl)-N,N,N-trimethylmethanaminium Iodide (9)
3.1.9. (R,R)- or (S,S)-Ni(II)-BPB-6-fluoro-tryptophan [(R,R)- or (S,S)-10] [40]
3.1.10. (R)- or (S)-2-[(tert-Butoxycarbonyl)amino]-3-(6-fluoro-1H-indol-3-yl)propanoic Acid [(R)- or (S)-11]
3.1.11. (R)- or (S)-2-[(tert-Butoxycarbonyl)amino]-3-(6-fluoro-1-methyl-1H-indol-3-yl)propanoic Acid [(R)- or (S)-12]
3.1.12. (R)- or (S)-2-Amino-3-(6-fluoro-1-methyl-1H-indol-3-yl)propanoic Acid Hydrochloride [(R)- or (S)-13 × HCl]
3.1.13. (S)-3-(5-Acetoxy-1H-indol-3-yl)-2-aminopropanoic Acid (15) [44]
3.1.14. (S)-3-(5-Acetoxy-1H-indol-3-yl)-2-[(tert-butoxycarbonyl)amino]propanoic Acid (16) [45]
3.1.15. tert-Butyl (S)-3-(5-acetoxy-1H-indol-3-yl)-2-[(tert-butoxycarbonyl)amino]propanoate (17)
3.1.16. tert-Butyl (S)-3-[5-acetoxy-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl]-2-[(tert-butoxycarbonyl)amino]propanoate (18)
3.1.17. tert-Butyl (S)-3-[5-acetoxy-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl]-2-[(tert-butoxycarbonyl)amino]propanoate (19)
3.1.18. 7-Fluoroindoline (21) [59]
3.1.19. 5-Hydroxy-7-fluoroindole (22)
3.1.20. 5-Acetoxy-7-fluoroindole (23) [61]
3.1.21. Methyl 2-Acetamido-3-(5-acetoxy-7-fluoro-1H-indol-3-yl)propanoate (24) [50]
3.1.22. tert-Butyl 5-Acetoxy-3-{2-[N-(tert-butoxycarbonyl)acetamido]-3-methoxy-3-oxopropyl}-7-fluoro-1H-indole-1-carboxylate (25)
3.1.23. 3-[1-(tert-Butoxycarbonyl)-7-fluoro-5-hydroxy-1H-indol-3-yl]-2-[(tert-butoxycarbonyl)amino]propanoic Acid (26)
3.1.24. 2-Amino-3-(7-fluoro-5-hydroxy-1H-indol-3-yl)propanoic Acid Trifluoroacetate (27 × TFA)
3.2. Radiochemistry
3.2.1. General
3.2.2. Semi-Preparative HPLC
3.2.3. Analytical HPLC
3.2.4. (S)-2-Amino-3-(6-[18F]fluoro-1-methyl-1H-indol-3-yl)propanoic Acid (NIn-Me-6-[18F]FTrp, [18F]13)
3.2.5. 2-Amino-3-(7-[18F]fluoro-5-hydroxy-1H-indol-3-yl)propanoic Acid (5-HO-7-[18F]FTrp, [18F]27)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.S.; Terentis, A.C.; King, N.J.C.; Thomas, S.R. Role of Indoleamine 2,3-Dioxygenase in Health and Disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, J.-T.; Tan, L. The Kynurenine Pathway in Neurodegenerative Diseases: Mechanistic and Therapeutic Considerations. J. Neurol. Sci. 2012, 323, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zamanakou, M.; Germenis, A.E.; Karanikas, V. Tumor Immune Escape Mediated by Indoleamine 2,3-Dioxygenase. Immunol. Lett. 2007, 111, 69–75. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Überall, F.; Fuchs, D. The Potential of Targeting Indoleamine 2,3-Dioxygenase for Cancer Treatment. Expert Opin. Ther. Targets 2015, 19, 605–615. [Google Scholar] [CrossRef]
- Olivier, B. Serotonin: A Never-Ending Story. Eur. J. Pharmacol. 2015, 753, 2–18. [Google Scholar] [CrossRef]
- Švob Štrac, D.; Pivac, N.; Mück-Šeler, D. The Serotonergic System and Cognitive Function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Politis, M.; Niccolini, F. Serotonin in Parkinson’s Disease. Behav. Brain Res. 2015, 277, 136–145. [Google Scholar] [CrossRef]
- Takano, H. Cognitive Function and Monoamine Neurotransmission in Schizophrenia: Evidence From Positron Emission Tomography Studies. Front. Psychiatry 2018, 9, 228. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, G. Associations Among Monoamine Neurotransmitter Pathways, Personality Traits, and Major Depressive Disorder. Front. Psychiatry 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Vancassel, S.; Capuron, L.; Castanon, N. Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Muzik, O.; Chugani, D.C.; Chakraborty, P.; Mangner, T.; Chugani, H.T. Analysis of [C-11]Alpha-Methyl-Tryptophan Kinetics for the Estimation of Serotonin Synthesis Rate In Vivo. J. Cereb. Blood Flow Metab. 1997, 17, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.R.; Michelhaugh, S.K.; Klinger, N.V.; Kupsky, W.J.; Polin, L.A.; Muzik, O.; Juhász, C.; Mittal, S. Tryptophan PET Imaging of the Kynurenine Pathway in Patient-Derived Xenograft Models of Glioblastoma. Mol. Imaging 2016, 15, 153601211664488. [Google Scholar] [CrossRef]
- Diksic, M.; Nagahiro, S.; Sourkes, T.L.; Yamamoto, Y.L. A New Method to Measure Brain Serotonin Synthesis in Vivo. I. Theory and Basic Data for a Biological Model. J. Cereb. Blood Flow Metab. 1990, 10, 1–12. [Google Scholar] [CrossRef]
- Chugani, D.C.; Muzik, O. α[C-11]Methyl-Tryptophan PET Maps Brain Serotonin Synthesis and Kynurenine Pathway Metabolism. J. Cereb. Blood Flow Metab. 2000, 20, 2–9. [Google Scholar] [CrossRef]
- Atkins, H.L.; Christman, D.R.; Fowler, J.S.; Hauser, W.; Hoyte, R.M.; Klopper, J.F.; Lin, S.S.; Wolf, A.P. Organic Radiopharmaceuticals Labeled with Isotopes of Short Half-Life. V. 18F-Labeled 5- and 6-Fluorotryptophan. J. Nucl. Med. 1972, 13, 713–719. [Google Scholar]
- Henrottin, J.; Zervosen, A.; Lemaire, C.; Sapunaric, F.; Laurent, S.; Van den Eynde, B.; Goldman, S.; Plenevaux, A.; Luxen, A. N1-Fluoroalkyltryptophan Analogues: Synthesis and in Vitro Study as Potential Substrates for Indoleamine 2,3-Dioxygenase. ACS Med. Chem. Lett. 2015, 6, 260–265. [Google Scholar] [CrossRef]
- Xin, Y.; Cai, H. Improved Radiosynthesis and Biological Evaluations of L- and D-1-[18F]Fluoroethyl-Tryptophan for PET Imaging of IDO-Mediated Kynurenine Pathway of Tryptophan Metabolism. Mol. Imaging Biol. 2017, 19, 589–598. [Google Scholar] [CrossRef]
- Zischler, J.; Kolks, N.; Modemann, D.; Neumaier, B.; Zlatopolskiy, B.D. Alcohol-Enhanced Cu-Mediated Radiofluorination. Chem.-A Eur. J. 2017, 23, 3251–3256. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Schäfer, D.; Urusova, E.A.; Guliyev, M.; Bannykh, O.; Endepols, H.; Neumaier, B. Discovery of 7-[18F]Fluorotryptophan as a Novel Positron Emission Tomography (PET) Probe for the Visualization of Tryptophan Metabolism in Vivo. J. Med. Chem. 2018, 61, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Habermeier, A.; Graf, J.; Sandhöfer, B.F.; Boissel, J.-P.; Roesch, F.; Closs, E.I. System L Amino Acid Transporter LAT1 Accumulates O-(2-Fluoroethyl)-l-Tyrosine (FET). Amino Acids 2015, 47, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Beamish, C.; McGirr, R.; Demarco, J.; Cockburn, N.; Krokowski, D.; Lee, T.-Y.; Kovacs, M.; Hatzoglou, M.; Dhanvantari, S. Characterization of 5-(2-18F-Fluoroethoxy)-L-Tryptophan for PET Imaging of the Pancreas. F1000Research 2016, 5, 1851. [Google Scholar] [CrossRef]
- John, F.; Muzik, O.; Mittal, S.; Juhász, C. Fluorine-18-Labeled PET Radiotracers for Imaging Tryptophan Uptake and Metabolism: A Systematic Review. Mol. Imaging Biol. 2020, 22, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Booth, E.S.; Basran, J.; Lee, M.; Handa, S.; Raven, E.L. Substrate Oxidation by Indoleamine 2,3-Dioxygenase. J. Biol. Chem. 2015, 290, 30924–30930. [Google Scholar] [CrossRef]
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-Containing Oxygenases. Chem. Rev. 1996, 96, 2841–2888. [Google Scholar] [CrossRef]
- Bosin, T.R.; Buckpitt, A.R.; Maickel, R.P. Substrate Specificity of Aromatic-L-Amino Acid Decarboxylase. Life Sci. 1974, 14, 899–908. [Google Scholar] [CrossRef]
- Chauhan, N.; Thackray, S.J.; Rafice, S.A.; Eaton, G.; Lee, M.; Efimov, I.; Basran, J.; Jenkins, P.R.; Mowat, C.G.; Chapman, S.K.; et al. Reassessment of the Reaction Mechanism in the Heme Dioxygenases. J. Am. Chem. Soc. 2009, 131, 4186–4187. [Google Scholar] [CrossRef]
- Roberts, K.M.; Fitzpatrick, P.F. Mechanisms of Tryptophan and Tyrosine Hydroxylase. IUBMB Life 2013, 65, 350–357. [Google Scholar] [CrossRef]
- Rosenthal, J.; Schuster, D.I. The Anomalous Reactivity of Fluorobenzene in Electrophilic Aromatic Substitution and Related Phenomena. J. Chem. Educ. 2003, 80, 679. [Google Scholar] [CrossRef]
- Leeds, J.M.; Brown, P.J.; McGeehan, G.M.; Brown, F.K.; Wiseman, J.S. Isotope Effects and Alternative Substrate Reactivities for Tryptophan 2,3-Dioxygenase. J. Biol. Chem. 1993, 268, 17781–17786. [Google Scholar] [CrossRef] [PubMed]
- Chanut, E.; Trouvin, J.H.; Bondoux, D.; Gardier, A.; Launay, J.M.; Jacquot, C. Metabolism of 6-Fluoro-DL-Tryptophan and Its Specific Effects on the Rat Brain Serotoninergic Pathway. Biochem. Pharmacol. 1993, 45, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Cumming, P.; Häusser, M.; Martin, W.R.W.; Grierson, J.; Adam, M.J.; Ruth, T.J.; McGeer, E.G. Kinetics of in Vitro Decarboxylation and the in Vivo Metabolism of 2-18F- and 6-18F-FluoroDOPA in the Hooded Rat. Biochem. Pharmacol. 1988, 37, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Neels, O.C.; Koopmans, K.P.; Jager, P.L.; Vercauteren, L.; van Waarde, A.; Doorduin, J.; Timmer-Bosscha, H.; Brouwers, A.H.; de Vries, E.G.E.; Dierckx, R.A.J.O.; et al. Manipulation of [11C]-5-Hydroxytryptophan and 6-[18F]Fluoro-3,4-Dihydroxy-L-Phenylalanine Accumulation in Neuroendocrine Tumor Cells. Cancer Res. 2008, 68, 7183–7190. [Google Scholar] [CrossRef]
- Nahmias, C.; Wahl, L.; Chirakal, R.; Firnau, G.; Garnett, E.S. A Probe for Intracerebral Aromatic Amino-acid Decarboxylase Activity: Distribution and Kinetics of [18F]6-fluoro-L-m-tyrosine in the Human Brain. Mov. Disord. 1995, 10, 298–304. [Google Scholar] [CrossRef]
- DeJesus, O.T.; Endres, C.J.; Shelton, S.E.; Nickles, R.J.; Holden, J.E. Evaluation of Fluorinated m-Tyrosine Analogs as PET Imaging Agents of Dopamine Nerve Terminals: Comparison with 6-FluoroDOPA. J. Nucl. Med. 1997, 38, 630–636. [Google Scholar]
- Stadlwieser, J.F.; Dambaur, M.E. Convenient Synthesis of 1H-Indol-1-Yl Boronatesvia Palladium(0)-Catalyzed Borylation of Bromo-1H-Indoles with ‘Pinacolborane’. Helv. Chim. Acta 2006, 89, 936–946. [Google Scholar] [CrossRef]
- Ishiyama, T.; Ishida, K.; Miyaura, N. Synthesis of Pinacol Arylboronates via Cross-Coupling Reaction of Bis(Pinacolato)Diboron with Chloroarenes Catalyzed by Palladium(0)–Tricyclohexylphosphine Complexes. Tetrahedron 2001, 57, 9813–9816. [Google Scholar] [CrossRef]
- Somei, M.; Kizu, K.; Kunimoto, M.; Yamada, F. The Chemistry of Indoles. XXIV. Syntheses of 3-Indoleacetic Acid and 3-Indoleacetonitrile Having a Halogeno Group and a Carbon Functional Group at the 4-Position. Chem. Pharm. Bull. 1985, 33, 3696–3708. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Bulychev, A.G.; Vitt, S.V.; Struchkov, Y.T.; Batsanov, A.S.; Timofeeva, T.V.; Tsyryapkin, V.A.; Ryzhov, M.G.; Lysova, L.A. General Method of Diastereo- and Enantioselective Synthesis of β-Hydroxy-α-Amino Acids by Condensation of Aldehydes and Ketones with Glycine. J. Am. Chem. Soc. 1985, 107, 4252–4259. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Bakhmutov, V.I.; Chernoglazova, N.I.; Kochetkov, K.A.; Vitt, S.V.; Garbalinskaya, N.S.; Belikov, V.M. General Method for the Asymmetric Synthesis of α-Amino Acids via Alkylation of the Chiral Nickel(II) Schiff Base Complexes of Glycine and Alanine. J. Chem. Soc. Perkin Trans. 1 1988, 2, 305–312. [Google Scholar] [CrossRef]
- Ellis, T.K.; Hochla, V.M.; Soloshonok, V.A. Efficient Synthesis of 2-Aminoindane-2-Carboxylic Acid via Dialkylation of Nucleophilic Glycine Equivalent. J. Org. Chem. 2003, 68, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Modemann, D.; Zlatopolskiy, B.D.; Urusova, E.; Zischler, J.; Craig, A.; Ermert, J.; Guliyev, M.; Endepols, H.; Neumaier, B. 2-[18F]Fluorophenylalanine: Synthesis by Nucleophilic 18F-Fluorination and Preliminary Biological Evaluation. Synthesis 2019, 51, 664–676. [Google Scholar] [CrossRef]
- Viret, J. 5-Acetyloxy-L-Tryptophane and Its Salts, Process for Their Preparation and Their Therapeutical Uses. EU Patent 0058111 B1, 23 October 1985. [Google Scholar]
- Lutz, C.; Simon, W.; Werner-Simon, S.; Müller, C.; Hechler, T.; Kulke, M. Method for Synthesizing Amanitins. U.S. Patent 10961277 B2, 30 March 2021. [Google Scholar]
- Orlovskaya, V.V.; Modemann, D.J.; Kuznetsova, O.F.; Fedorova, O.S.; Urusova, E.A.; Kolks, N.; Neumaier, B.; Krasikova, R.N.; Zlatopolskiy, B.D. Alcohol-Supported Cu-Mediated 18F-Fluorination of Iodonium Salts under “Minimalist” Conditions. Molecules 2019, 24, 3197. [Google Scholar] [CrossRef] [PubMed]
- Loach, R.P.; Fenton, O.S.; Amaike, K.; Siegel, D.S.; Ozkal, E.; Movassaghi, M. C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines. J. Org. Chem. 2014, 79, 11254–11263. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Tyagarajan, S.; Perera, D.; Krska, S.W.; Maligres, P.E.; Smith, M.R.; Maleczka, R.E. Bismuth Acetate as a Catalyst for the Sequential Protodeboronation of Di- and Triborylated Indoles. Org. Lett. 2016, 18, 1554–1557. [Google Scholar] [CrossRef]
- Zimmer, H.; Lankin, D.C.; Horgan, S.W. Oxidations with Potassium Nitrosodisulfonate (Fremy’s Radical). Teuber Reaction. Chem. Rev. 1971, 71, 229–246. [Google Scholar] [CrossRef]
- Angelini, E.; Balsamini, C.; Bartoccini, F.; Lucarini, S.; Piersanti, G. Switchable Reactivity of Acylated α, β-Dehydroamino Ester in the Friedel−Crafts Alkylation of Indoles by Changing the Lewis Acid. J. Org. Chem. 2008, 73, 5654–5657. [Google Scholar] [CrossRef]
- Lohray, B.B.; Bhushan, V.; Rao, B.P.; Madhavan, G.R.; Murali, N.; Rao, K.N.; Reddy, A.K.; Rajesh, B.M.; Reddy, P.G.; Chakrabarti, R.; et al. Novel Euglycemic and Hypolipidemic Agents. 1. J. Med. Chem. 1998, 41, 1619–1630. [Google Scholar] [CrossRef]
- Bruncko, M.; Dai, Y.; Ding, H.; Doherty, G.A.; Elmore, S.W.; Hasvold, L.; Hexamer, L.; Kunzer, A.; Mantei, R.A.; McClellan, W.J.; et al. Apoptosis-Inducing Agents for the Treatment of Cancer and Immune and Autoimmune Diseases. U.S. Patent 2010/0160322 A1, 24 June 2010. [Google Scholar]
- Zischler, J.; Krapf, P.; Richarz, R.; Zlatopolskiy, B.D.; Neumaier, B. Automated Synthesis of 4-[18F]Fluoroanisole, [18F]DAA1106 and 4-[18F]FPhe Using Cu-Mediated Radiofluorination under “Minimalist” Conditions. Appl. Radiat. Isot. 2016, 115, 133–137. [Google Scholar] [CrossRef]
- Richarz, R.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Neumaier, B.; Zlatopolskiy, B.D. Neither Azeotropic Drying, nor Base nor Other Additives: A Minimalist Approach to 18F-Labeling. Org. Biomol. Chem. 2014, 12, 8094–8099. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Kolks, N.; Urusova, E.A.; Zischler, J.; Brugger, M.; Endepols, H.; Neumaier, B.; Zlatopolskiy, B.D. Preparation of Labeled Aromatic Amino Acids via Late-Stage 18F-Fluorination of Chiral Nickel and Copper Complexes. Chem. Commun. 2020, 56, 9505–9508. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Kolks, N.; Smets, D.; Haseloer, A.; Gröner, B.; Urusova, E.A.; Endepols, H.; Neumaier, F.; Ruschewitz, U.; Klein, A.; et al. Next Generation Copper Mediators for the Efficient Production of 18F-Labeled Aromatics. Chem. Eur. J. 2022, 29, e202202965. [Google Scholar] [CrossRef]
- Rawson, D.J.; Dack, K.N.; Dickinson, R.P.; James, K. The Design and Synthesis of a Novel Series of Indole Derived Selective ETA Antagonists. Bioorg. Med. Chem. Lett. 2002, 12, 125–128. [Google Scholar] [CrossRef]
- Nudelman, A.; Bechor, Y.; Falb, E.; Fischer, B.; Wexler, B.A.; Nudelman, A. Acetyl Chloride-Methanol as a Convenient Reagent for: A) Quantitative Formation of Amine Hydrochlorides B) Carboxylate Ester Formation C) Mild Removal of N-t-Boc-Protective Group. Synth. Commun. 1998, 28, 471–474. [Google Scholar] [CrossRef]
- Sato, K.; Sugimoto, H.; Rikimaru, K.; Imoto, H.; Kamaura, M.; Negoro, N.; Tsujihata, Y.; Miyashita, H.; Odani, T.; Murata, T. Discovery of a Novel Series of Indoline Carbamate and Indolinylpyrimidine Derivatives as Potent GPR119 Agonists. Bioorg. Med. Chem. 2014, 22, 1649–1666. [Google Scholar] [CrossRef] [PubMed]
- Moser, W.; Howie, R.A. Nitrosodisulphonates. Part I. Fremy’s Salt (Potassium Nitrosodisulphonate). J. Chem. Soc. A Inorg. Phys. Theor. 1968, 1968, 3039–3043. [Google Scholar] [CrossRef]
- Nagano, T.; Okabe, T.; Kojima, H.; Saito, N.; Nakano, H.; Abe, M.; Tanaka, A.; Honma, T.; Yokoyama, S.; Tsuganezawa, K.; et al. Anticancer Agent. EU Patent 2565192 A1, 6 March 2013. [Google Scholar]
- Humpert, S.; Hoffmann, C.; Neumaier, F.; Zlatopolskiy, B.D.; Neumaier, B. Validation of Analytical HPLC with Post-Column Injection as a Method for Rapid and Precise Quantification of Radiochemical Yields. J. Chromatogr. B 2023, 1228, 123847. [Google Scholar] [CrossRef]
- Schlosser, M.; Ginanneschi, A.; Leroux, F. In search of simplicity and flexibility: A rational access to twelve fluoroindolecarboxylic acids. Eur. J. Org. Chem. 2006, 2006, 2956–2969. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolks, N.; Neumaier, F.; Neumaier, B.; Zlatopolskiy, B.D. Preparation of NIn-Methyl-6-[18F]fluoro- and 5-Hydroxy-7-[18F]fluorotryptophans as Candidate PET-Tracers for Pathway-Specific Visualization of Tryptophan Metabolism. Int. J. Mol. Sci. 2023, 24, 15251. https://doi.org/10.3390/ijms242015251
Kolks N, Neumaier F, Neumaier B, Zlatopolskiy BD. Preparation of NIn-Methyl-6-[18F]fluoro- and 5-Hydroxy-7-[18F]fluorotryptophans as Candidate PET-Tracers for Pathway-Specific Visualization of Tryptophan Metabolism. International Journal of Molecular Sciences. 2023; 24(20):15251. https://doi.org/10.3390/ijms242015251
Chicago/Turabian StyleKolks, Niklas, Felix Neumaier, Bernd Neumaier, and Boris D. Zlatopolskiy. 2023. "Preparation of NIn-Methyl-6-[18F]fluoro- and 5-Hydroxy-7-[18F]fluorotryptophans as Candidate PET-Tracers for Pathway-Specific Visualization of Tryptophan Metabolism" International Journal of Molecular Sciences 24, no. 20: 15251. https://doi.org/10.3390/ijms242015251
APA StyleKolks, N., Neumaier, F., Neumaier, B., & Zlatopolskiy, B. D. (2023). Preparation of NIn-Methyl-6-[18F]fluoro- and 5-Hydroxy-7-[18F]fluorotryptophans as Candidate PET-Tracers for Pathway-Specific Visualization of Tryptophan Metabolism. International Journal of Molecular Sciences, 24(20), 15251. https://doi.org/10.3390/ijms242015251




