Advances in Polyoxometalates as Electron Mediators for Photocatalytic Dye Degradation
Abstract
:1. Introduction
2. Photocatalytic Degradation of Different Dyes with POM-Based Materials
2.1. Methylene Blue (MB)
| Catalyst | Synthesis Method | Irradiation | Catalyst Dosage (mg/L) | MB Dosage (mg/L) | pH | Time (min) | Removal Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1st | nth | ||||||||
| PMo12V3 | Hydrothermal | UV | 500 | 10 | - | 65 | 99.3 | ~/5th | [53] |
| PMo11V/g-C3N4 | Dipping | Vis | 400 | 10 | - | 120 | 94.7 | - | [56] |
| P2W18Sn3/Nd-TiO2 | One-Pot Synthesis | UV | - | 10 | 3 | 5 | 91.0 | - | [57] |
| Mn-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 95.6 | ~/5th | [62] |
| Ag-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 94.4 | ~/5th | [66] |
| Zn-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 96.1 | ~/5th | [67] |
| [Ag5(pz)6][BW12] | Hydrothermal | UV | 500 | 15 | - | 140 | 93.2 | ~/5th | [68] |
| [Hpip]2.5[BW12] | Heating reflux | Vis | 800 | 20 | - | 24 | 97.1 | 90.0/5th | [74] |
| [Cu3(pz)4]2[As3Mo8V4]2 | Hydrothermal | UV | 400 | 10 | - | 120 | 97.8 | 94.3/5th | [75] |
| Cu2(L1)2(Mo8O26)0.5 | Hydrothermal | UV | 105 | 3.2 | 6.8 | 7 | 97.9 | 96.0/4th | [76] |
| Cu2(L2)3(Mo4O13)2 | Hydrothermal | UV | 500 | 3.2 | - | 90 | 99.1 | ~/5th | [78] |
| Ce2(BINDI)(Mo6O19) | Heating reflux | Vis | 300 | 3.2 | - | 27 | 96.0 | ~/3rd | [80] |
| Fe3O4/Ag/Cu2(PCA)4(P2Mo5) | Sonochemical | Vis | 166.7 | 15 | - | 26 | 98.7 | 97.5/6th | [81] |
| P@Cu-AndCOF P@Ni-AndCOF | Solvothermal | UV-Vis | 100 | 50 | - | 660 | 89.0 79.3 | - | [83] |
| (Hbpp)2CoCd(P4Mo6)2 | Hydrothermal | Vis | 2000 | 32 | 6.8 | 180 | 96.0 | ~/5th | [85] |
| Fe4Fe6(P4Mo6)2 Fe4Fe2(P4Mo6)2 | Solvothermal | Vis | 1000 | 19 | - | 180 | 98.0 99.0 | ~/10th | [88] |
2.2. Rhodamine B (RhB)
| Catalyst | Synthesis Method | Irradiation | Catalyst Dosage (mg/L) | RhB Dosage (mg/L) | pH | Time (min) | Removal Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1st | nth | ||||||||
| CoW12/TiO2 | Sol-gel/hydrothermal | Vis | 5000 | 15 | 5 | 30 | 100 | ~/4th | [95] |
| Co-PMo12/N-TiO2 | One-pot synthesis | Vis | 400 | 10 | 7 | 40 | 98.0 | ~/4th | [98] |
| Ag3PW12/TiO2 | Hydrothermal | Vis | 1000 | 10 | - | 120 | 95.0 | ~/4th | [101] |
| ZnO/Ag4SiW ZnO/Cs3PW12 | Precipitation | Simulated sunlight | 300 | 50 | - | 60 | 92.3 72.7 | 84.8/3rd 65.3/3rd | [106] |
| Co2(bpy)3][SiW12] | Hydrothermal | UV | 400 | 50 | - | 120 | 92.0 | ~/3rd | [109] |
| [Cu8(CPT)8](SiW12)(CH3CN)4 | Solvothermal | Vis | 375 | 10 | - | 70 | 96.6 | ~/4th | [110] |
| Ag-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 90.6 | ~/5th | [66] |
| Zn-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 91.1 | ~/5th | [67] |
| Mn-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 94.1 | ~/5th | [62] |
| [Cu3(en)]4(pdc)[BW12] [CuI5(pz)6][BW12] | Hydrothermal | UV | 500 | 10 | - | 150 | 91.3 92.6 | ~/5th | [114] |
| [Cu3(pz)4]2[As3Mo8V4]2 | Hydrothermal | UV | 400 | 10 | - | 120 | 97.4 | 92.7/5th | [75] |
| Cu(bipy)4(P2Mo5) | Hydrothermal | Vis | 300 | 30 | - | 180 | 89.6 | ~/4th | [115] |
| Cu2(L2)3(Mo4O13)2 | Hydrothermal | UV | 500 | 4.79 | - | 90 | 98.7 | ~/4th | [78] |
| P@Ni-AndCOF P@Cu-AndCOF | Solvothermal | UV-Vis | 100 | 50 | - | 660 | 71.3 83.0 | - | [83] |
2.3. Methyl Orange (MO)
| Catalyst | Synthesis Method | Irradiation | Catalyst Dosage (mg/L) | MO Dosage (mg/L) | pH | Time (min) | Removal Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1st | nth | ||||||||
| Pt/PMo12/TiO2 | Electrospinning/calcination and photoreduction | Vis | 1000 | 20 | 1 | 180 | 88.1 | ~/5th | [119] |
| Bi/PMo12/TiO2 | Electrospinning/calcination and hydrothermal | Vis | 1000 | 40 | 1 | 180 | 92.5 | ~/8th | [123] |
| Ag/PMo10V2/TiO2 | Heating reflux and photoreduction | Vis | 1000 | 20 | - | 30 | 100 | - | [124] |
| P2W18Sn3/Nd-TiO2 | One-Pot Synthesis | UV | - | 10 | 3 | 5 | 98.4 | 95.0/5th | [57] |
| Fe3O4@SiO2@[TiO2/PW12]10 | Layer-by-Layer method | UV | 2000 | 10 | 2 | 100 | 83.9 | - | [126] |
| Co2Co4(SiW10)2/Fe2O3 | Precipitation | UV | 1000 | 10 | 1 | 90 | 76.2 | 69.4/3rd | [128] |
| [Ag5(pz)6][BW12] | Hydrothermal | UV | 500 | 15 | - | 140 | 90.9 | ~/5th | [68] |
| Ag-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 92.4 | ~/5th | [66] |
| Zn-BTC@Ag5[BW12] | Grinding | UV | 500 | 15 | - | 140 | 95.2 | ~/5th | [67] |
| [Cu3(pz)4]2[As3Mo8V4]2 | Hydrothermal | UV | 400 | 10 | - | 120 | 96.8 | 91.4/5th | [75] |
| (DETA)3.5Fe(P4Mo6)2 | Hydrothermal | UV | 60 | 20 | - | 20 | 99.8 | - | [131] |
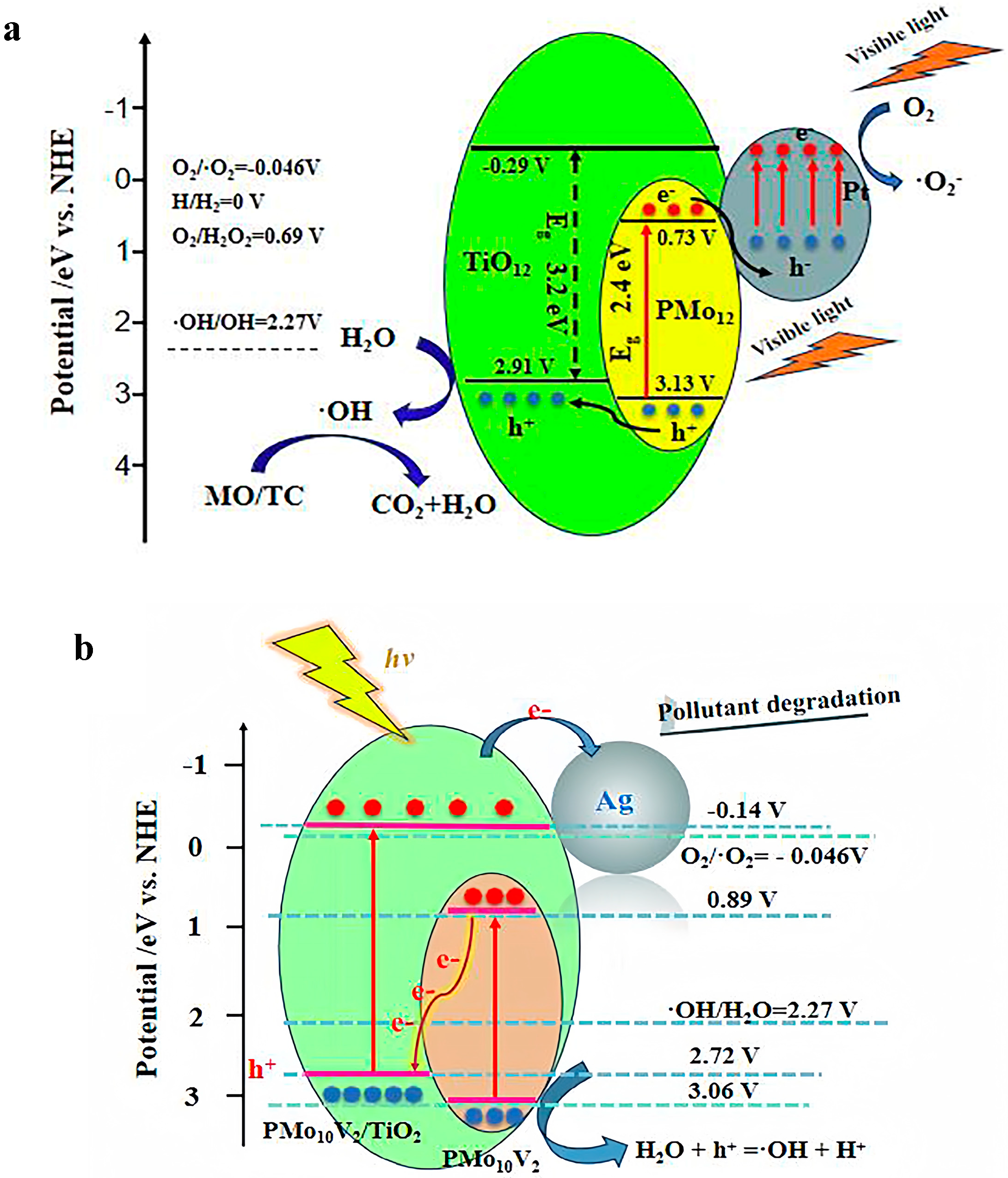
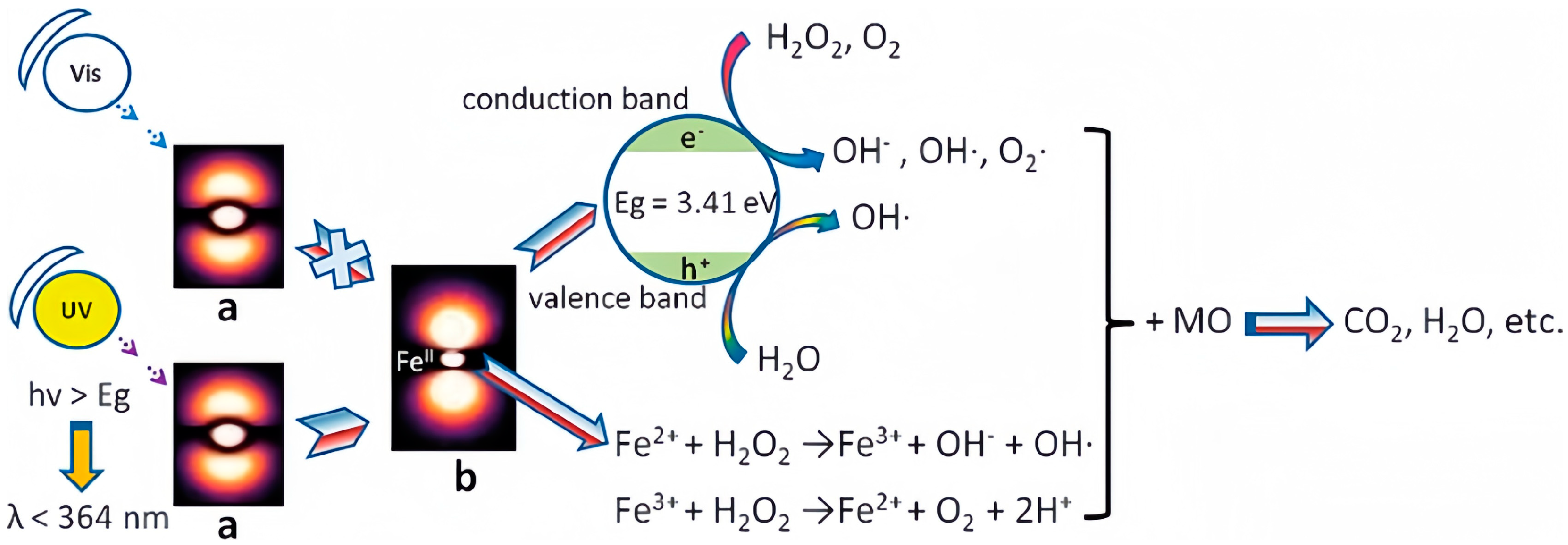
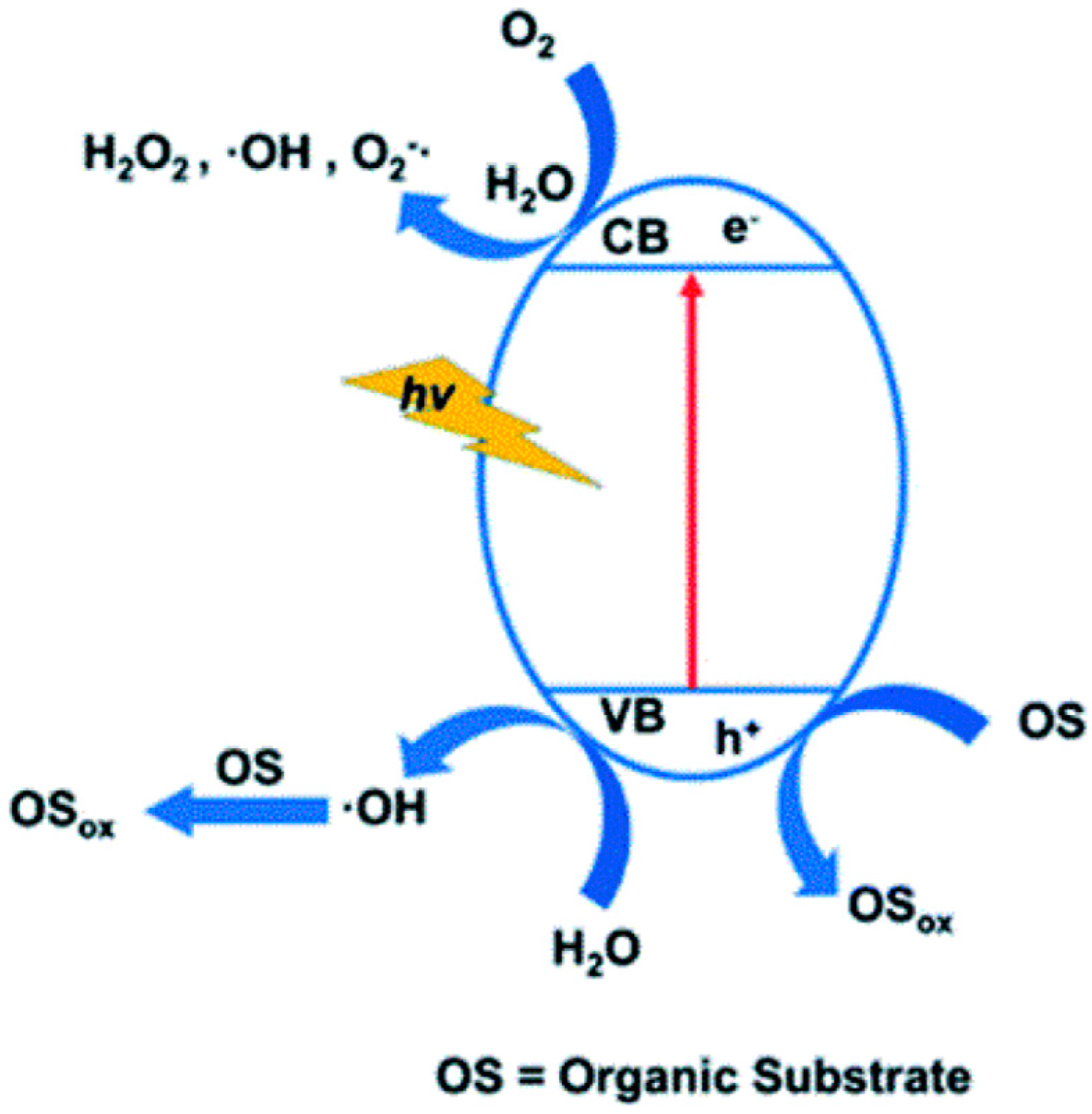
3. Photodegradation Mechanism of Dyes and Enhancement Strategies for POM-Based Materials
3.1. Photodegradation Mechanism of Dyes
3.2. Enhancement Strategies for POM-Based Materials
- (1)
- (2)
- (3)
- (4)
- Introduction of Conjugated Structures: The incorporation of conjugated structures, such as benzene rings or carbazole moieties, into the POMs framework extends its light-absorption range, enabling broader visible light utilization [145];
- (5)
- (1)
- Dipping: Simple but may result in uneven impregnation, especially for large materials.
- (2)
- Grinding: Easy but challenging to control particle size, leading to non-uniformity of materials.
- (3)
- Heating Reflux: Versatile for various reactions, offering precise temperature control but requiring reflux equipment and involving intricate procedures.
- (4)
- One-Pot Synthesis: Suitable for complex multi-component materials, saving time and resources by avoiding intermediate steps, but necessitates precise reaction control and may produce byproducts.
- (5)
- Hydrothermal and Solvothermal Methods: Ideal for synthesizing crystals, nanoparticles, and complex structures, with control over material size and shape. However, they typically involve high-temperature and high-pressure conditions.
4. Application Potential of POM-Based Materials
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Hulme, A.N.; McNab, H.; Quye, A. The natural constituents of historical textile dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Li, W.; Li, Y.; Wang, G.; Li, A.; Li, H. Efficient removal of acid dyes using permanent magnetic resin and its preliminary investigation for advanced treatment of dyeing effluents. J. Clean. Prod. 2020, 251, 119694. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, S.; Zhang, L.; Zhou, P.; Zhong, Y.; Xu, H.; Mao, Z. Oversized macroporous flower-like Cu9S8 used as an efficient peroxidase mimetic enzyme for the degradation of organic dyes. J. Phys. Chem. Solids 2022, 163, 110534. [Google Scholar] [CrossRef]
- Chang, J.S.; Lin, C.Y. Decolorization kinetics of a recombinant Escherichia coli strain harboring azo-dye-decolorizing determinants from Rhodococcus sp. Biotechnol. Lett. 2001, 23, 631–636. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Didier de Vasconcelos, G.M.; Mulinari, J.; de Arruda Guelli Ulson de Souza, S.M.; Ulson de Souza, A.A.; de Oliveira, D.; de Andrade, C.J. Biodegradation of azo dye-containing wastewater by activated sludge: A critical review. World J. Microbiol. Biotechnol. 2021, 37, 101. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R.; Yan, W.; Wu, M.; Zhou, Y.; Gao, C. Antibacterial polyvinyl alcohol nanofiltration membrane incorporated with Cu(OH)2 nanowires for dye/salt wastewater treatment. Sci. Total Environ. 2022, 817, 152897. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Advances in application of cotton-based adsorbents for heavy metals trapping, surface modifications and future perspectives. Ecotoxicol. Environ. Saf. 2020, 201, 110825. [Google Scholar] [CrossRef]
- Ghosh, T.; Ullah, K.; Nikam, V.; Park, C.Y.; Meng, Z.D.; Oh, W.C. The characteristic study and sonocatalytic performance of CdSe–graphene as catalyst in the degradation of azo dyes in aqueous solution under dark conditions. Ultrason. Sonochem. 2013, 20, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Hekmati, M.; Ziyadi, H.; Ghasemi, E.; Esmaeili, D. Hibiscus sabdariffa extract modified magnetic polymer nanocomposite for azo dyes removal from aqueous samples. Mater. Chem. Phys. 2021, 267, 124608. [Google Scholar] [CrossRef]
- Chakravarthi, B.; Mathkala, V.; Palempalli, U.M.D. Degradation and detoxification of congo red azo dye by immobilized laccase of streptomyces sviceus. J. Pure Appl. Microbiol. 2021, 15, 864–876. [Google Scholar] [CrossRef]
- Li, Y.; Cao, P.; Wang, S.; Xu, X. Research on the treatment mechanism of anthraquinone dye wastewater by algal-bacterial symbiotic system. Bioresour. Technol. 2022, 347, 126691. [Google Scholar] [CrossRef]
- Murphree, S.S. Heterocyclic dyes: Preparation, properties, and applications. Prog. Heterocycl. Chem. 2011, 22, 21–58. [Google Scholar]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.Y.; Bhaduri, B.; Kurkuri, M. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism—A review. Sustain. Mater. Technol. 2022, 31, e00378. [Google Scholar] [CrossRef]
- Gad, H.M.H.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Lafi, R.; Hafiane, A. Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J. Taiwan Inst. Chem. Eng. 2016, 58, 424–433. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Verma, N.; Yadav, S.; Marí, B.; Mittal, A.; Jindal, J. Synthesis and charcterization of coupled ZnO/SnO2 photocatalysts and their activity towards degradation of cibacron red dye. Trans. Indian Ceram. Soc. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Maruthanayagam, A.; Mani, P.; Kaliappan, K.; Chinnappan, S. In vitro and in silico studies on the removal of methyl orange from aqueous solution using Oedogonium subplagiostomum AP1. Water Air Soil Pollut. 2020, 231, 232. [Google Scholar] [CrossRef]
- Aljuaid, A.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Abdulaziz, O.; Alsharif, A.; Alsaiari, J.A.; Saih, M.; Alotaibi, R.T.; Khan, I. g-C3N4 based photocatalyst for the efficient photodegradation of toxic methyl orange dye: Recent modifications and future perspectives. Molecules 2023, 28, 3199. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.C.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Wan, X.Y.; Zhan, Y.Q.; Long, Z.H.; Zeng, G.Y.; He, Y. Core@ double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem. Eng. J. 2017, 330, 491–504. [Google Scholar] [CrossRef]
- Hu, X.S.; Liang, R.; Sun, G.X. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Huang, H.; Cao, W.; Li, T. Removal of rhodamine B and Cr(VI) from aqueous solutions by a polyoxometalate adsorbent. Chem. Eng. Res. Des. 2015, 100, 192–202. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- D’Cruz, B.; Amin, M.O.; Al-Hetlani, E. Polyoxometalate-based materials for the removal of contaminants from wastewater: A review. Ind. Eng. Chem. Res. 2021, 60, 10960–10977. [Google Scholar] [CrossRef]
- Müller, A.; Roy, S. Oxomolybdates: From structures to functions in a new era of nanochemistry. In The Chemistry of Nanomaterials: Synthesis, Properties and Applications, 2nd ed.; Rao, C.N.R., Müller, A., Cheetham, A.K., Eds.; Wiley: Hoboken, NJ, USA, 2004; pp. 452–475. ISBN 9783527306862. [Google Scholar]
- D’Cruz, B.; Samuel, J.; Sreedhar, M.K.; George, L. Green synthesis of novel polyoxoanions of tungsten containing phosphorus as a heteroatom: Characterization, non-isothermal decomposition kinetics and photocatalytic activity. New J. Chem. 2014, 38, 5436–5444. [Google Scholar] [CrossRef]
- Hutin, M.; Rosnes, M.H.; Long, D.L.; Cronin, L. Polyoxometalates: Synthesis and structure–from building blocks to emergent materials. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 241–269. ISBN 9780080965291. [Google Scholar]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Carraro, M.; Gross, S. Hybrid materials based on the embedding of organically modified transition metal oxoclusters or polyoxometalates into polymers for functional applications: A review. Materials 2014, 7, 3956–3989. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Y.; Huang, B.; Xiao, Z.; Wu, P. Application of polyoxometalates in photocatalytic degradation of organic pollutants. Nanoscale Adv. 2021, 3, 4646–4658. [Google Scholar] [CrossRef]
- Suzuki, K.; Mizuno, N.; Yamaguchi, K. Polyoxometalate photocatalysis for liquid-phase selective organic functional group transformations. ACS Catal. 2018, 8, 10809–10825. [Google Scholar] [CrossRef]
- Stuckart, M.; Monakhov, K.Y. Polyoxometalate encapsulation into metal–organic frameworks: The way towards functional nanomaterials for water splitting. J. Mater. Chem. A 2018, 6, 17849–17853. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ng, K.H.; Cheng, C.K.; Nur, H.; Nurhadi, M.; Arumugam, M. Photocatalytic remediation of organic waste over Keggin-based polyoxometalate materials: A review. Chemosphere 2021, 263, 128244. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Dong, X.; Wang, H.; Dong, F. In situ FT-IR investigation on the reaction mechanism of visible light photocatalytic NO oxidation with defective g-C3N4. Sci. Bull. 2018, 63, 117–125. [Google Scholar] [CrossRef]
- Wang, X.L.; Rong, X.; Lin, H.Y.; Liu, D.N.; Wang, X.; Liu, G.C.; Song, G. Metal ions induced various polymolybdate-based metal–organic complexes with a pyridyl-amide-carboxylate ligand: Synthesis, structures and selective separation of cationic dyes. Polyhedron 2017, 126, 92–99. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Lv, J.H.; Yu, K.; Wang, L.; Zhang, H.; Wang, S.; Zhou, B.B. One-step synthesis of two Wells–Dawson arsenotungstate hybrids via M–O–M bridges for efficient adsorption and selective separation of organic pollutants. CrystEngComm 2017, 19, 5653–5661. [Google Scholar] [CrossRef]
- Yi, F.Y.; Zhu, W.; Dang, S.; Li, J.P.; Wu, D.; Li, Y.H.; Sun, Z.M. Polyoxometalates-based heterometallic organic–inorganic hybrid materials for rapid adsorption and selective separation of methylene blue from aqueous solutions. Chem. Commun. 2015, 51, 3336–3339. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Dang, M.; Wang, Y.; Zhang, B.; Ren, H.; Lv, L.; Xia, A. Dual defects and build-in electric field mediated direct Z-scheme W18O49/g-C3N4−x heterojunction for photocatalytic NO removal and organic pollutant degradation. J. Colloid Interface Sci. 2021, 582, 212–226. [Google Scholar] [CrossRef]
- Fujimoto, S.; Cameron, J.M.; Wei, R.J.; Kastner, K.; Robinson, D.; Sans, V.; Newton, G.N.; Oshio, H. A simple approach to the visible-light photoactivation of molecular metal oxides. Inorg. Chem. 2017, 56, 12169–12177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, F.; Liu, S.M.; Liu, S.X.; Lai, X.Y.; Li, X.H.; Lu, Y.; Li, Y.G.; Hu, C.W.; Shi, Z.; et al. Aminated graphene oxide impregnated with photocatalytic polyoxometalate for efficient adsorption of dye pollutants and its facile and complete photoregeneration. Small 2017, 13, 1603174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Liu, Y.; Ma, Y.H.; Li, T.R.; Li, Y.H.; Xie, T.F.; Dong, S.S. Photo-Fenton degradation of emerging pollutants over Fe-POM nanoparticle/porous and ultrathin g-C3N4 nanosheet with rich nitrogen defect: Degradation mechanism, pathways, and products toxicity assessment. Appl. Catal. B 2020, 278, 119349. [Google Scholar] [CrossRef]
- Li, D.D.; Ma, P.T.; Niu, J.Y.; Wang, J.P. Recent advances in transition-metal-containing Keggin-type polyoxometalate-based coordination polymers. Coord. Chem. Rev. 2019, 392, 49–80. [Google Scholar] [CrossRef]
- Srivani, A.; Venkateswara Rao, K.T.; Prasad, P.S.; Lingaiah, N. Role of vanadium in Keggin heteropoly molybdate supported on titania catalysts for oxidation reactions. J. Chem. Sci. 2014, 126, 467–472. [Google Scholar] [CrossRef]
- Li, F.R.; Ji, T.; Chen, W.L. A tri-vanadium-capped Keggin phosphomolybdate: Synthesis, characterization, photocatalytic and bifunctional electrocatalytic properties. Tungsten 2021, 4, 99–108. [Google Scholar] [CrossRef]
- Zhang, Y.M.; An, C.W.; Zhang, D.F.; Liu, T.; Yan, J.S.; Zhang, J. Photocatalytic activity of vanadium-substituted polyoxometalate doped magnetic carbon nitride towards antibiotics. Russ. J. Inorg. Chem. 2021, 66, 679–683. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; An, C.; Liu, H.; Wu, Q. Preparation of vanadium-substituted polyoxometalate doped carbon nitride hybrid materials POM/g-C3N4 and their photocatalytic oxidation performance. Mater. Lett. 2020, 262, 126954. [Google Scholar] [CrossRef]
- Pazhooh, P.; Khoshnavazi, R.; Bahrami, L.; Naseri, E. Synthesis and photocatalytic activity assessing of the TiO2 nanocomposites modified by some lanthanide ions and tin-derivative sandwich-type polyoxometalates. J. Iran. Chem. Soc. 2018, 15, 1775–1783. [Google Scholar] [CrossRef]
- Roy, S.; Vemuri, V.; Maiti, S.; Manoj, K.S.; Subbarao, U.; Peter, S.C. Two Keggin-based isostructural POMOF hybrids: Synthesis, crystal structure, and catalytic properties. Inorg. Chem. 2018, 57, 12078–12092. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.; Blanco, C.; Raymundo-Piñero, E.; Khomenko, V.; Béguin, F.; Santamaría, R. Effects of thermal treatment of activated carbon on the electrochemical behaviour in supercapacitors. Electrochim. Acta 2007, 52, 4969–4973. [Google Scholar] [CrossRef]
- Wang, D.G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Ramadan, M.; Ahmed, N.; ElNaga, A.O.A.; Alalawy, H.H.; Zaki, T.; Shaban, S.A.; Hassan, H.B.; Allam, N.K. Metal–Organic frameworks encapsulated with vanadium-substituted heteropoly acid for highly stable asymmetric supercapacitors. J. Energy Storage 2020, 28, 101292. [Google Scholar] [CrossRef]
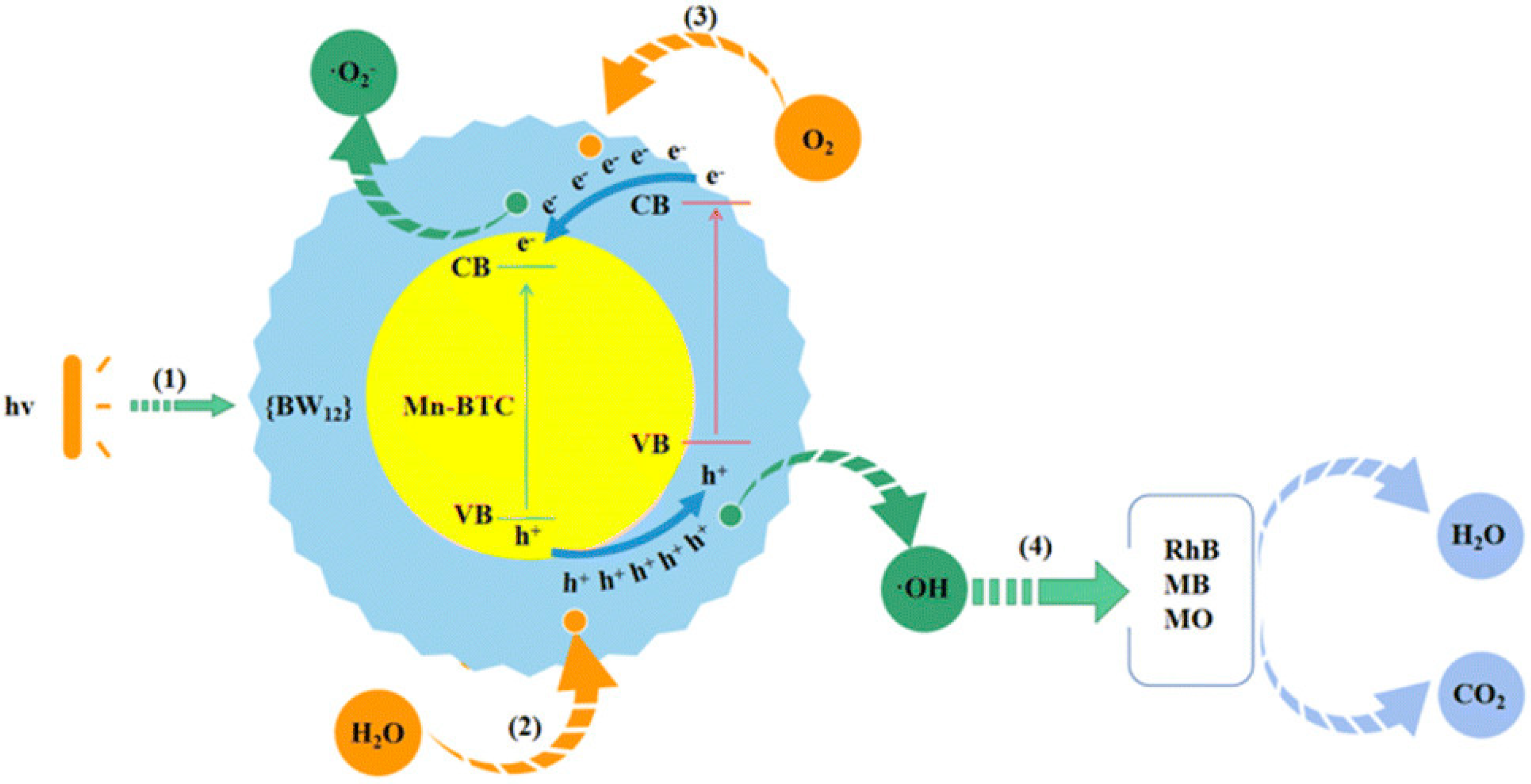
- Shi, C.; Kang, N.; Wang, C.; Yu, K.; Lv, J.; Wang, C.; Zhou, B. An inorganic-organic hybrid nanomaterial with a core-shell structure constructed by using Mn-BTC and Ag5[BW12O40] for supercapacitors and photocatalytic dye degradation. Nanoscale Adv. 2022, 4, 4358–4365. [Google Scholar] [CrossRef]
- Yue, L.; Cao, Y.; Han, Y.; Li, Z.; Luo, X.; Liu, Y. Preparation of core-shell structured Fe3O4@Sn-MOF composite and photocatalytic performance. J. Alloys Compd. 2021, 870, 159339. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.; Duan, L.; Liu, R.; Wu, H.; Hou, T.; Jiang, X.; Gao, H. Influence of interface combination of RGO-photosensitized SnO2 @RGO core-shell structures on their photocatalytic performance. Appl. Surf. Sci. 2017, 391, 627–634. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Kwon, S.; Zhang, F.; Stephen, B.; Kim, K.K.; Jung, R.; Kwon, S.; Chung, K.; Yang, W. Photocatalytic improvement of Mn-adsorbed g-C3N4. Appl. Catal. B Environ. 2017, 206, 271–281. [Google Scholar] [CrossRef]
- Yu, L.; Ning, K.; Chunmei, W.; Kai, Y.; Jinghua, L.; Chunxiao, W.; Baibin, Z. A hybrid borotungstate-coated metal-organic framework with supercapacitance, photocatalytic dye degradation and H2O2 sensing properties. Dalton Trans. 2022, 51, 7613–7621. [Google Scholar] [CrossRef]
- Wang, L.; Kang, N.; Gong, L.; Wang, C.; Yu, K.; Wang, C.; Zhou, B. A novel core-shell structured hybrid composed of zinc homobenzotrizoate and silver borotungstate with supercapacitor and photocatalytic dye degradation performance. J. Energy Storage 2022, 46, 103873. [Google Scholar] [CrossRef]
- Song, Z.J.; Wang, L.Y.; Kang, N.; Yu, K.; Lv, J.; Zhou, B. 3D host-guest material of {Ag (pz)} modified {BW12O40} with supercapacitor, photocatalytic dye degradation and H2O2 sensing performances. J. Solid State Chem. 2023, 323, 124038. [Google Scholar] [CrossRef]
- Cameron, J.M.; Wales, D.J.; Newton, G.N. Shining a light on the photo-sensitisation of organic–inorganic hybrid polyoxometalates. Dalton. Trans. 2018, 47, 5120–5136. [Google Scholar] [CrossRef] [PubMed]
- Azcarate, I.; Ahmed, I.; Farha, R.; Goldmann, M.; Wang, X.; Xu, H.; Hasenknopf, B.; Lacôte, E.; Ruhlmann, L. Synthesis and characterization of conjugated Dawson-type polyoxometalate–porphyrin copolymers. Dalton Trans. 2013, 42, 12688–12698. [Google Scholar] [CrossRef]
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Lassalle-Kaiser, B.; Mialane, P.; Fontecave, M.; Mellot-Draznieks, C.; Dolbecq, A. A fully noble metal-free photosystem based on cobalt-polyoxometalates immobilized in a porphyrinic metal-organic framework for water oxidation. J. Am. Chem. Soc. 2018, 140, 3613–3618. [Google Scholar] [CrossRef]
- Iqbal, A.; Asif, H.M.; Zhou, Y.S.; Zhang, L.J.; Wang, T.; Shehzad, F.K.; Ren, X. From simplicity to complexity in grafting Dawson-type polyoxometalates on porphyrin, leading to the formation of new organic-inorganic hybrids for the investigation of third-order optical nonlinearities. Inorg. Chem. 2019, 58, 8763–8774. [Google Scholar] [CrossRef]
- Zhu, S.L.; Xu, X.; Ou, S.; Zhao, M.; He, W.L.; Wu, C.D. Assembly of a metalloporphyrin-polyoxometalate hybrid material for highly efficient activation of molecular oxygen. Inorg. Chem. 2016, 55, 7295–7300. [Google Scholar] [PubMed]
- Jamshidi, A.; Mohammadi Zonoz, F.; Wei, Y. A new Keggin-based organic-inorganic nanohybrid in the role of a dual-purpose catalyst. J. Chem. Sci. 2020, 132, 37. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.; Song, Y.; Su, Z.; Chen, J. The 3D POMOFs based two AsIII-capped Keggin arsenomolybdates with four VIV substituted: Synthesis, structures and properties. J. Solid State Chem. 2020, 291, 121639. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, Q.Q.; Cui, Z.W.; Shi, S.; Long, J.; Wang, X.; Fei, B. A novel octamolybdate-based organic–inorganic hybrid as photo-Fenton-like catalyst for degradation of methylene blue. Appl. Organomet. Chem. 2023, 37, e6966. [Google Scholar] [CrossRef]
- Fei, B.L.; Deng, N.P.; Wang, J.H.; Liu, Q.B.; Long, J.Y.; Li, Y.G.; Mei, X. A heteropoly blue as environmental friendly material: An excellent heterogeneous Fenton-like catalyst and flocculent. J. Hazard. Mater. 2017, 340, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; An, J.D.; Wang, T.T.; Li, Y.; Ding, B. Hydrothermal assembly, structural diversity, and photocatalytic characterization of two polyoxometalates-based hybrid CuII and CuI coordination polymers with 2,6-(1,2,4-triazole -4-yl)pyridine. Inorg. Nano-Met. Chem. 2020, 51, 976–984. [Google Scholar] [CrossRef]
- Hao, Z.C.; Wang, S.C.; Yang, Y.J.; Cui, G.H. Syntheses, structural diversities and photocatalytic properties of three nickel (II) coordination polymers based semi-bis(benzimidazole) and aromatic dicarboxylic acid ligands. Polyhedron 2020, 181, 114466. [Google Scholar] [CrossRef]
- Liu, J.J.; Fu, J.J.; Liu, T.; Cheng, F. Photochromic polyoxometalate/naphthalenediimide hybrid structure with visble -light-driven dye degradation. J. Solid State Chem. 2022, 312, 123236. [Google Scholar] [CrossRef]
- Zhan, S.; Li, C.; Tian, H.; Ma, C.; Liu, H.; Luo, J.; Li, M. Synthesis, characterization and dye removal behavior of core–shell–shell Fe3O4/Ag/polyoxometalates ternary nanocomposites. Nanomaterials 2019, 9, 1255. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, H.L.; Zhang, W.X.; Cheng, W.Z.; Liu, X.; Li, X.M.; Wu, J.H. Synthesis and characterization of polymer-coated AgZnO nanoparticles with enhanced photocatalytic activity. RSC Adv. 2014, 4, 44011–44017. [Google Scholar] [CrossRef]
- Rani, S.; Tariq, M.; Bhatti, M.H.; Abdelmohsen, S.A.M.; Alanazi, M.M.; Khan, M.A.; Asif, H.M.; Nadeem, M.; Khan, R. A simplistic approach for the synthesis of covalent organic frameworks (COFs) comprising of tetrafunctionalized porphyrin and polyoxometalates to uncover catalytic applications. Opt. Mater. 2023, 138, 113672. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhang, X.B.; Li, Y.L.; Huang, S.L.; Yang, G.Y. Polyoxometalate functionalized architectures. Coord. Chem. Rev. 2020, 414, 213260. [Google Scholar] [CrossRef]
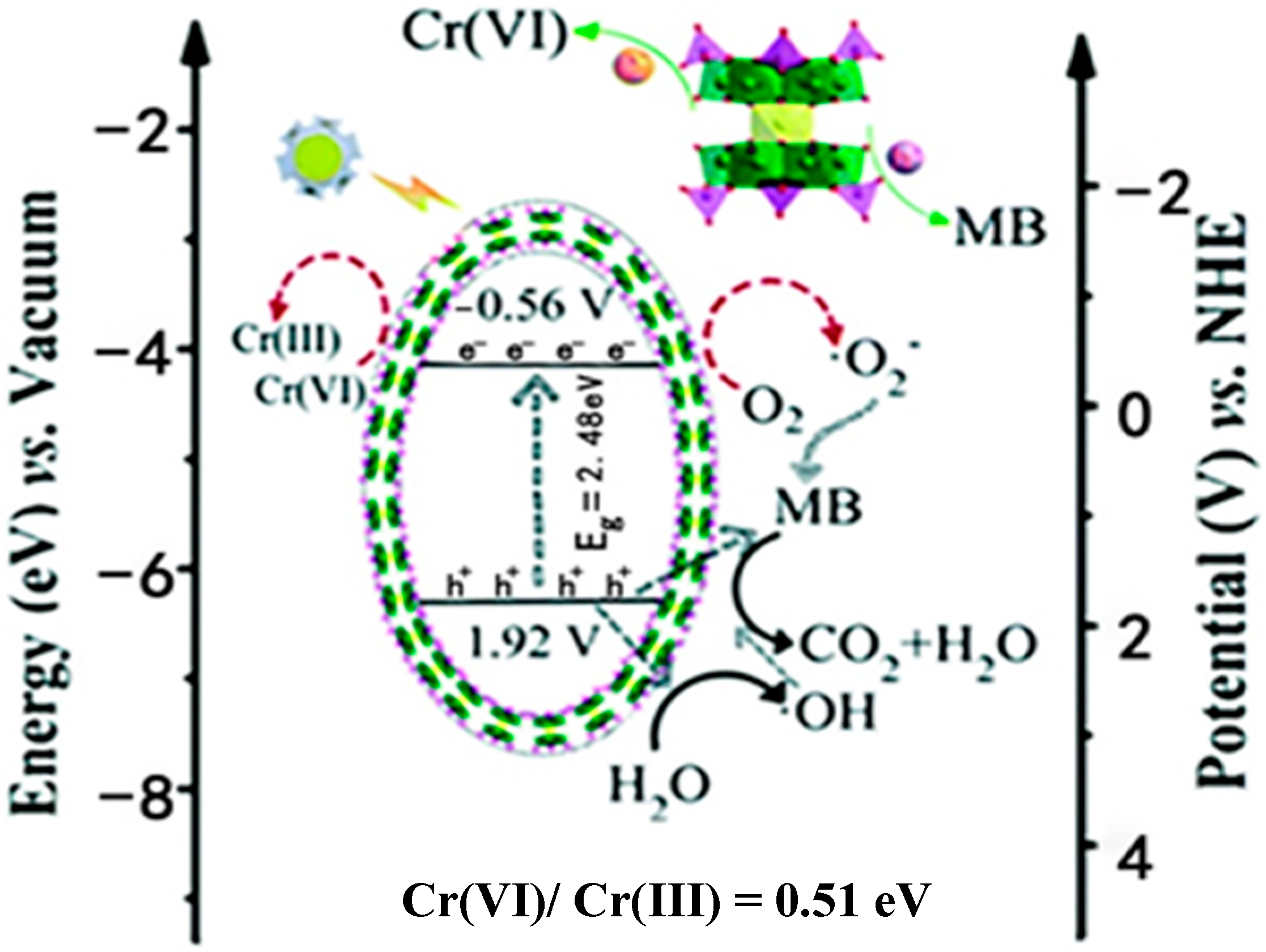
- Tian, X.; Zhang, Y.; Ma, Y.; Zhao, Q.; Han, Z. Hourglass-type polyoxometalate-based crystalline materials as efficient cooperating photocatalysts for the reduction of Cr(VI) and oxidation of dyes. Catal. Sci. Technol. 2020, 10, 2593–2601. [Google Scholar] [CrossRef]
- Chang, W.J.; Jiang, Y.C.; Wang, S.L.; Lii, K.H. Hydrothermal synthesis of a three-dimensional organic-inorganic hybrid network formed by poly(oxomolybdophosphate) anions and nickel coordination cations. Inorg. Chem. 2006, 45, 6586–6588. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.L.; Au, C.T.; Yin, S.F. Cu2O/BiVO4 heterostructures: Synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Guo, H.L.; Xing, X.X.; Mao, S.X.; Feng, T.; Fan, Y.H.; Qin, Z.J.; Pang, J.Y.; Bai, Y.; Dang, D.B. Two three-dimensional Fe(II) frameworks based on P(4)Mo(6) tetrameric clusters exhibiting efficient visible-light photocatalytic properties for the degradation of Cr(VI) and methylene blue. Dalton Trans. 2022, 51, 18090–18098. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Koodali, R.T.; Zhao, D. Photocatalytic degradation of aqueous organic pollutants using titania supported periodic mesoporous silica. Energy Environ. Sci. 2010, 3, 608–614. [Google Scholar] [CrossRef]
- Xing, Z.P.; Zhang, J.Q.; Cui, J.Y.; Yin, J.W.; Zhao, T.Y.; Kuang, J.Y.; Xiu, Z.Y.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Q.; Pang, W. Photocatalytic degradation of textile dye X-3B using polyoxometalate–TiO2 hybrid materials. J. Hazard. Mater. 2007, 141, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, Y.; Ma, F.; Li, H.; Chen, L.; Guo, Y. Design of ordered mesoporous H3PW12O40-titania materials and their photocatalytic activity to dye methyl orange degradation. Catal. Commun. 2010, 11, 839–843. [Google Scholar] [CrossRef]
- Khoshnavazi, R.; Fereydouni, S.; Bahrami, L. Enhanced photocatalytic activity of nanocomposites of TiO2 doped with Zr, Y or Ce polyoxometalates for degradation of methyl orange dye. Water Sci. Technol. 2016, 73, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Rafiee, E.; Eavani, S. Photocatalytic removal of toxic dyes, liquorice and tetracycline wastewaters by a mesoporous photocatalyst under irradiation of different lamps and sunlight. J. Environ. Manag. 2022, 313, 115023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Chen, L. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor. Catal. Today 2011, 175, 283–292. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Rafiee, E.; Eavani, S. Introducing of a novel polyoxometalate-based organic-inorganic hybrid: Insights into electochemical property-photoactivity relationship. J. Mater. Sci. Mater. Electron. 2021, 32, 1121–1138. [Google Scholar] [CrossRef]
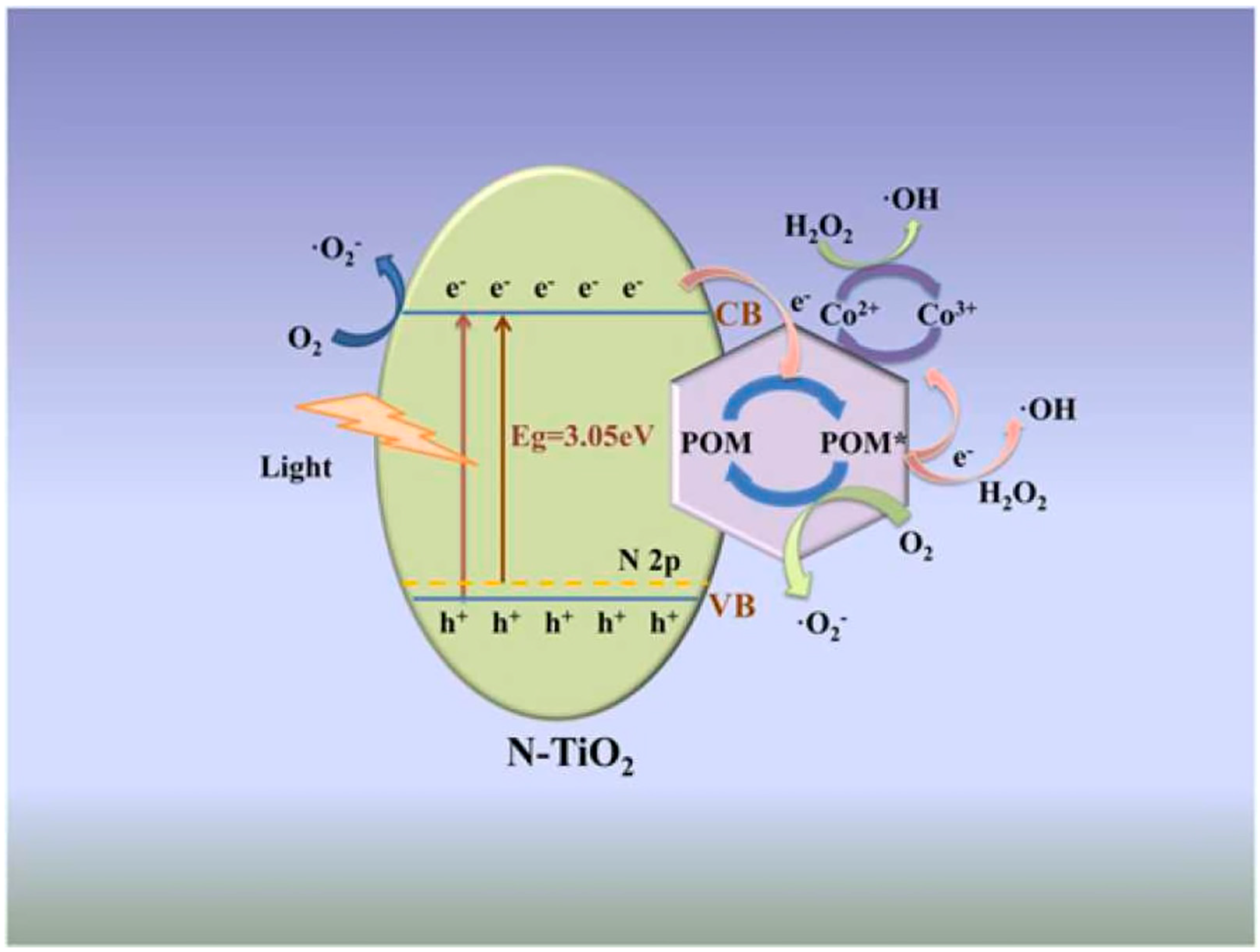
- Li, S.; Zhang, J.; Cao, Y.; Yang, Y.; Xie, T.; Lin, Y. Visible light assisted heterogeneous photo-Fenton-like degradation of rhodamine B based on the Co-POM/N-TiO2 composites: Catalyst properties, photogenerated carrier transfer and degradation mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129248. [Google Scholar] [CrossRef]
- Fei, B.L.; Zhong, J.K.; Deng, N.P.; Wang, J.H.; Liu, Q.B.; Li, Y.G.; Mei, X. A novel 3D heteropoly blue type photo-Fenton-like catalyst and its ability to remove dye pollution. Chemosphere 2018, 197, 241–250. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Guo, Y.; Ma, F.; Li, H.; Chen, L.; Guo, Y. Design of mesostructured H3PW12O40–titania materials with controllable structural orderings and pore geometries and their simulated sunlight photocatalytic activity towards diethyl phthalate degradation. Appl. Catal. B Environ. 2010, 99, 364–375. [Google Scholar] [CrossRef]
- Cai, S.; Shi, S.; Li, H.; Bai, Y.; Dang, D. Construction of self-sufficient Z-scheme Ag3PW12O40/TiO2 photocatalysts for the improved visible-light-driven photo-degradation of rhodamine B. Res. Chem. Intermed. 2018, 44, 7769–7788. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two different roles of metallic Ag on Ag/AgX/BiOX (X = Cl, Br) visible light photocatalysts: Surface plasmon resonance and Z-Scheme bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Yan, T.; Tian, J.; Guan, W.; Qiao, Z.; Li, W.; You, J.; Huang, B. Ultra-low loading of Ag3PO4 on hierarchical In2S3 microspheres to improve the photocatalytic performance: The cocatalytic effect of Ag and Ag3PO4. Appl. Catal. B Environ. 2017, 202, 84–94. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
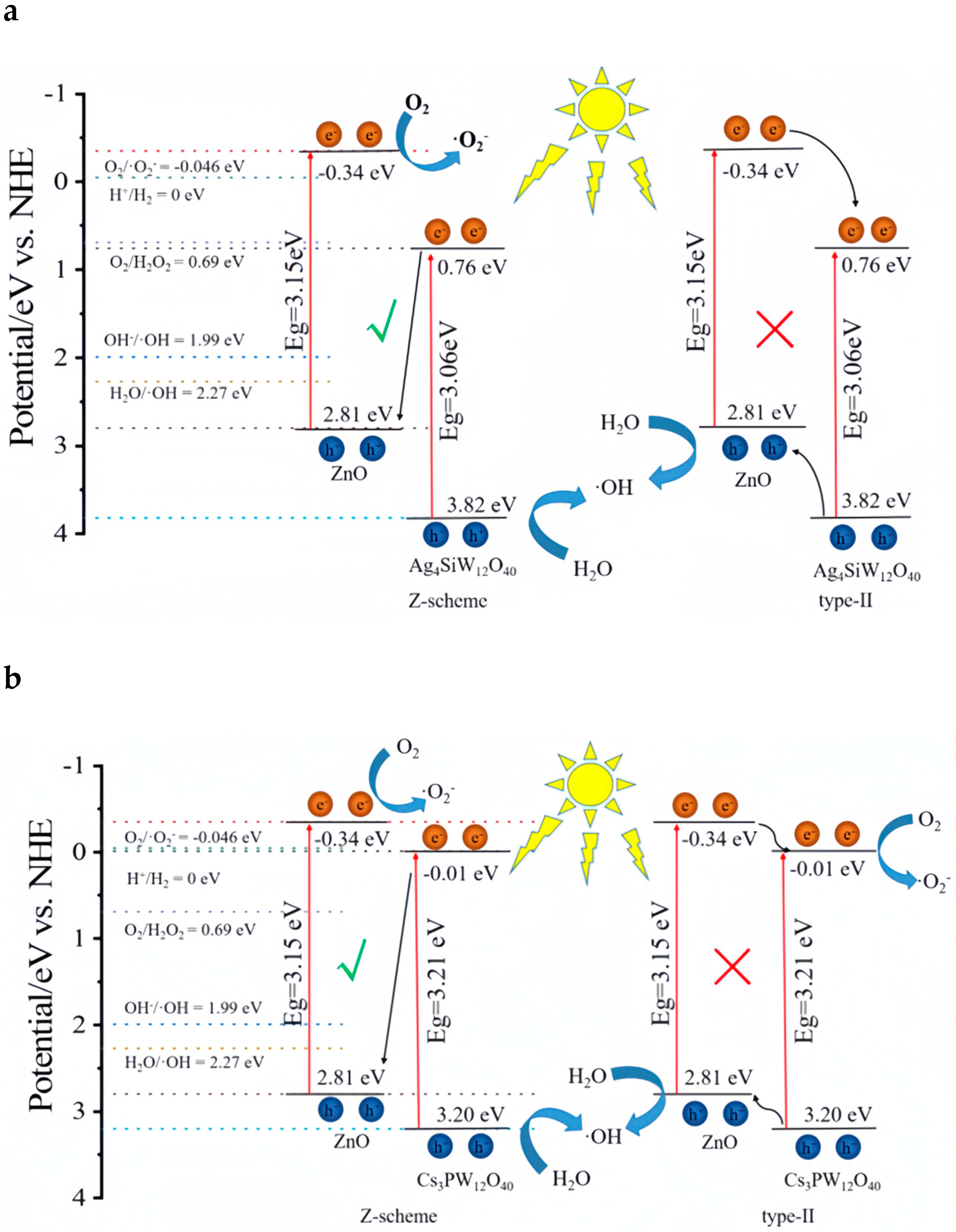
- Guo, R.; Bai, L.; Dong, G.; Chai, D.; Lang, K.; Mou, Z.; Zhao, M. Construction of ZnO/Keggin polyoxometalate Nano-heterojunction catalyst for efficient removal of rhodamine B in aqueous solution. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1599–1615. [Google Scholar] [CrossRef]
- Shi, H.F.; Yan, G.; Zhang, Y.; Tan, H.Q.; Zhou, W.Z.; Ma, Y.Y.; Li, Y.G.; Chen, W.; Wang, E. Ag/AgxH3–xPMo12O40 nanowires with enhanced visible-light-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2016, 9, 422–430. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Wang, X.; Wang, P.; Yu, J. Enhanced visible-light photocatalytic activity of Bi2WO6 nanoparticles by Ag2O cocatalyst. Appl. Catal. B Environ. 2012, 111–112, 326–333. [Google Scholar] [CrossRef]
- Zhang, Z.; Gómez-García, C.J.; Wu, Q.; Xin, J.; Pang, H.; Ma, H.; Chai, D.; Li, S.; Zhao, C. Synthesis of a polyoxometalate-encapsulated metal–organic framework via in situ ligand transformation showing highly catalytic activity in both hydrogen evolution and dye degradation. Inorg. Chem. 2022, 61, 11830–11836. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Zheng, Y.P.; Shi, D.Y.; Fang, S.M. An acid-base resistant polyoxometalate-based metal–organic framework constructed from {Cu4Cl}7+ and {Cu2 (CO2)4} clusters for photocatalytic degradation of organic dye. J. Solid State Chem. 2020, 287, 121384. [Google Scholar] [CrossRef]
- Rahmati, R.; Nayebi, B.; Ayati, B. Investigating the effect of hydrogen peroxide as an electron acceptor in increasing the capability of slurry photocatalytic process in dye removal. Water Sci. Technol. 2021, 83, 2414–2423. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked formation of H2O2 during the hydroxyl radical-scavenging process when using alcohols as scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, L.; Du, N.; Wang, C.; Yu, K.; Wang, C.; Zhou, B. {BW12O40} hybrids modified by in situ synthesized rigid ligand with supercapacitance and photocatalytic properties. Inorg. Chem. 2021, 60, 16357–16369. [Google Scholar] [PubMed]
- Liang, Z.; Cheng, H.; Zhang, X.; Mao, Q. Two polyoxometalates based on {P2Mo5} catalysts: Synthesis, characterization, and photocatalytic degradation of RhB. J. Mol. Liq. 2023, 377, 121483. [Google Scholar] [CrossRef]
- Guo, Y.H.; Hu, C.W.; Jiang, S.C.; Guo, C.X.; Yang, Y.; Wang, E. Heterogeneous photodegradation of aqueous hydroxy butanedioic acid by microporous polyoxometalates. Appl. Catal. B Environ. 2002, 36, 9–17. [Google Scholar] [CrossRef]
- Shi, H.F.; Yu, Y.C.; Zhang, Y.; Feng, X.J.; Zhao, X.Y.; Tan, H.Q.; Khan, S.U.; Li, Y.G.; Wang, E. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Zhao, Y.; Tan, H.; Zhao, Z.; Shi, H.; Wang, E.; Li, Y.G. AgxH3−xPMo12O40/Ag nanorods/g-C3N4 1D/2D Z-scheme heterojunction for highly efficient visible-light photocatalysis. Dalton Trans. 2019, 48, 6484–6491. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, T.; Zhang, Y.; Tan, H.; Shen, W.; Wang, W.; Li, Y.G.; Wang, E. Pt/POMs/TiO2 composite nanofibers with an enhanced visible-light photocatalytic performance for environmental remediation. Dalton Trans. 2019, 48, 13353–13359. [Google Scholar] [CrossRef]
- Yu, L.H.; Shao, Y.; Li, D.Z. Direct combination of hydrogen evolution from water and methane conversion in a photocatalytic system over Pt/TiO2. Appl. Catal. B Environ. 2017, 204, 216–223. [Google Scholar] [CrossRef]
- Gong, J.Y.; Imbault, A.; Farnood, R. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination. Appl. Catal. B Environ. 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Luo, B.F.; Xu, D.B.; Li, D.; Wu, G.L.; Wu, M.M.; Shi, W.D.; Chen, M. Fabrication of a Ag/Bi3TaO7 plasmonic photocatalyst with enhanced photocatalytic activity for degradation of tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 17061–17069. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhu, H.W.; Jin, T.; Chen, L.; Zhang, J.Y.; Qiao, K.Y.; Chen, Z. Construction of Bi/Polyoxometalate doped TiO2 composite with efficient visible-light photocatalytic performance: Mechanism insight, degradation pathway and toxicity evaluation. Appl. Surf. Sci. 2023, 615, 156310. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Zhao, Y.; Sun, H.; Tan, H.; Qiu, T.; Zhao, Z.; Zhao, X.; Cheng, S.; Li, Y. Polyoxometalates-doped TiO2/Ag hybrid heterojunction: Removal of multiple pollutants and mechanism investigation. Environ. Sci. Nano 2021, 8, 3855–3864. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Niu, P.; Wang, D.; Wang, A.; Liang, Y.; Wang, X. Fabrication of bifunctional TiO2/POM microspheres using a Layer-by-Layer method and photocatalytic activity for methyl orange degradation. J. Nanomater. 2018, 2018, 4212187. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, W.; Sun, C.; Han, L.; Qin, C.; Chen, M.; Wang, X.; Wang, E.; Su, Z. Oxidative polyoxometalates modified graphitic carbon nitride for visible-light CO2 reduction. ACS Appl. Mater. Interfaces 2017, 9, 11689–11695. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, L.; Guan, Y.L.; Fu, L.J.; Wang, H.; Chi, J.Y.; Jiang, Z.Q.; Wang, X.D.; Yu, H.H. Synthesis, characterisation and photocatalytic properties of visible light responsive Co2Co4(SiW10O37)2/Fe2O3 composites. Chem. Zvesti 2022, 76, 6997–7006. [Google Scholar] [CrossRef]
- Li, S.J.; Liu, S.X.; Li, C.C.; Ma, F.J.; Liang, D.D.; Zhang, W.; Tan, R.K.; Zhang, Y.Y.; Xu, L. Reactivity of polyoxoniobates in acidic solution: Controllable assembly and disassembly based on niobium-substituted germanotungstates. Chem. Eur. J. 2010, 16, 13435–13442. [Google Scholar] [CrossRef]
- Keita, B.; Liu, T.; Nadjo, L. Synthesis of remarkably stabilized metal nanostructures using polyoxometalates. J. Mater. Chem. 2009, 19, 19–33. [Google Scholar] [CrossRef]
- Chen, W.H.; Hu, Z.B.; Zhou, J.C.; Xiong, J.H.; Luo, J.S.; Zhang, H.Q.; Mi, J.X. Two new Sandwich-type phosphomolybdates: Thermal decomposition and photocatalytic degradation behavior of a UV-excited solid-phase Fenton catalyst. Eur. J. Inorg. Chem. 2019, 2019, 3015–3022. [Google Scholar] [CrossRef]
- Zou, J.P.; Chen, Y.; Zhu, M.; Wang, D.K.; Luo, X.B.; Luo, S.L. Semiconductor-based nanocomposites for photodegradation of organic pollutants. In Nanomaterials for the Removal of Pollutants and Resource Reutilization, 2nd ed.; Luo, X.B., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–28. ISBN 9780128148372. [Google Scholar]
- Feng, J.; Xiong, S.; Wang, Y. Atomic layer deposition of hybrid metal oxides on carbon nanotube membranes for photodegradation of dyes. Compos. Commun. 2019, 12, 39–46. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (•OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Ong, B.C.; Lim, H.K.; Tay, C.Y.; Lim, T.T.; Dong, Z.L. Polyoxometalates for bifunctional applications: Catalytic dye degradation and anticancer activity. Chemosphere 2022, 286, 131869. [Google Scholar] [CrossRef] [PubMed]
- Valipour, A.; Rezvani, M.A.; Alina Asli, M.; Oveisi, M.; Mahmoodi, N.M. Bi-amino surface functionalized polyoxometalate nanocomposite as an environmentally friendly catalyst: Synthesis and dye degradation. Water Sci. Technol. 2017, 75, 2381–2389. [Google Scholar]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Alharthi, F.A.; AlFawaz, A.; Ahmad, N. Photocatalytic degradation of anionic dye using well-crystalline SnWO4 polyoxometalate. Phys. Scr. 2022, 97, 085813. [Google Scholar] [CrossRef]
- Lei, P.; Chen, C.; Yang, J.; Wanhong, M.; Zhao, J.; Zang, L. Degradation of dye pollutants by immobilized polyoxometalate with H2O2 under visible-light irradiation. Environ. Sci. Technol. 2005, 39, 8466–8474. [Google Scholar] [CrossRef]
- Yu, Z.T.; Liao, Z.L.; Jiang, Y.S. Li, G.H.; Chen, J.S. Water-insoluble Ag–U–Organic assemblies with photocatalytic activity. Chem. Eur. J. 2005, 11, 2642–2650. [Google Scholar] [CrossRef]
- Yu, X.S.; Cui, H.J.; Wang, Q.Z.; Li, J.S.; Su, F.; Zhang, L.C.; Sang, X.J.; Zhu, Z.M. Construction and visible-light photocatalytic performance of carboxyethyltin/transition metal–functionalized wheel-like tungstophosphates. Appl. Organomet. Chem. 2020, 34, e5720. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, D.; Wang, Q.; Yan, J.; Wu, Q. Photocatalytic degradation of textile dye X-3B using TiW11Ti/SiO2 hybrid materials. Catal. Commun. 2019, 126, 10–14. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, L.; Wang, H.J.; Sun, J.W. Asymmetrical modification of Keggin polyoxometalates by sextuple Ag–N coordination polymeric chains: Synthesis, structure and selective separation of cationic dyes. J. Solid State Chem. 2021, 296, 121986. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, Y.; Zeng, F.; Si, C.; Zhang, D.; Xu, W.; Shi, J. Advances in Polyoxometalates as Electron Mediators for Photocatalytic Dye Degradation. Int. J. Mol. Sci. 2023, 24, 15244. https://doi.org/10.3390/ijms242015244
Li R, Wang Y, Zeng F, Si C, Zhang D, Xu W, Shi J. Advances in Polyoxometalates as Electron Mediators for Photocatalytic Dye Degradation. International Journal of Molecular Sciences. 2023; 24(20):15244. https://doi.org/10.3390/ijms242015244
Chicago/Turabian StyleLi, Ruyue, Yaqi Wang, Fei Zeng, Cuiqing Si, Dan Zhang, Wenbiao Xu, and Junyou Shi. 2023. "Advances in Polyoxometalates as Electron Mediators for Photocatalytic Dye Degradation" International Journal of Molecular Sciences 24, no. 20: 15244. https://doi.org/10.3390/ijms242015244
APA StyleLi, R., Wang, Y., Zeng, F., Si, C., Zhang, D., Xu, W., & Shi, J. (2023). Advances in Polyoxometalates as Electron Mediators for Photocatalytic Dye Degradation. International Journal of Molecular Sciences, 24(20), 15244. https://doi.org/10.3390/ijms242015244





