OXPHOS Organization and Activity in Mitochondria of Plants with Different Life Strategies
Abstract
:1. Introduction
2. Results
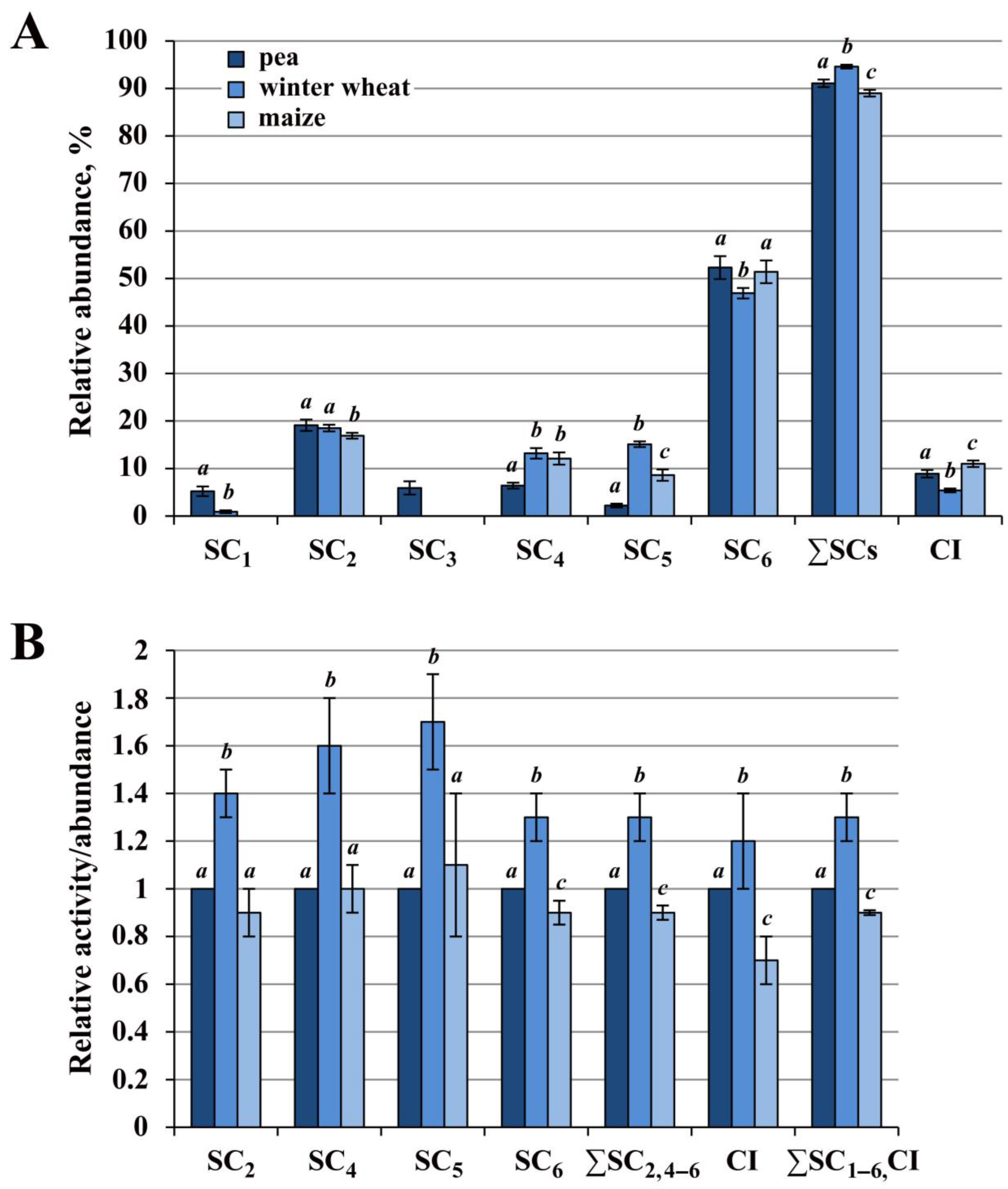
2.1. Relative Abundance and Activity of Superassembled and Free Complex I in Mitochondria of Pea, Winter Wheat and Maize
2.1.1. Winter Wheat Complex I Had the Highest Activity
2.2. Pea complex II Was More Digitonin-Resistant Compared to Winter Wheat and Maize Enzyme
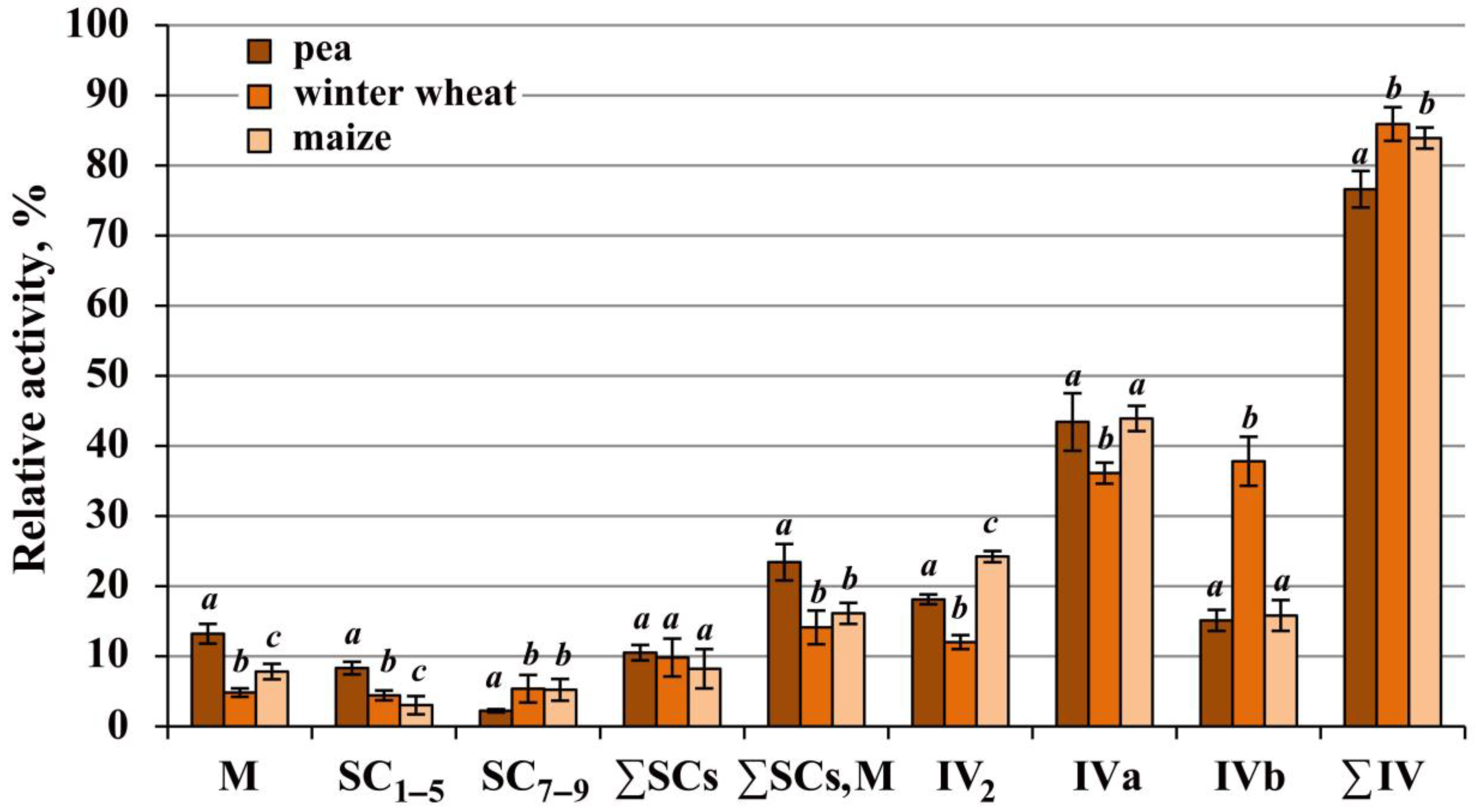
2.3. Most of the Complex IV Population in All Species Was Digitonin-Sensitive and Solubilized As Free Monomers and Dimers
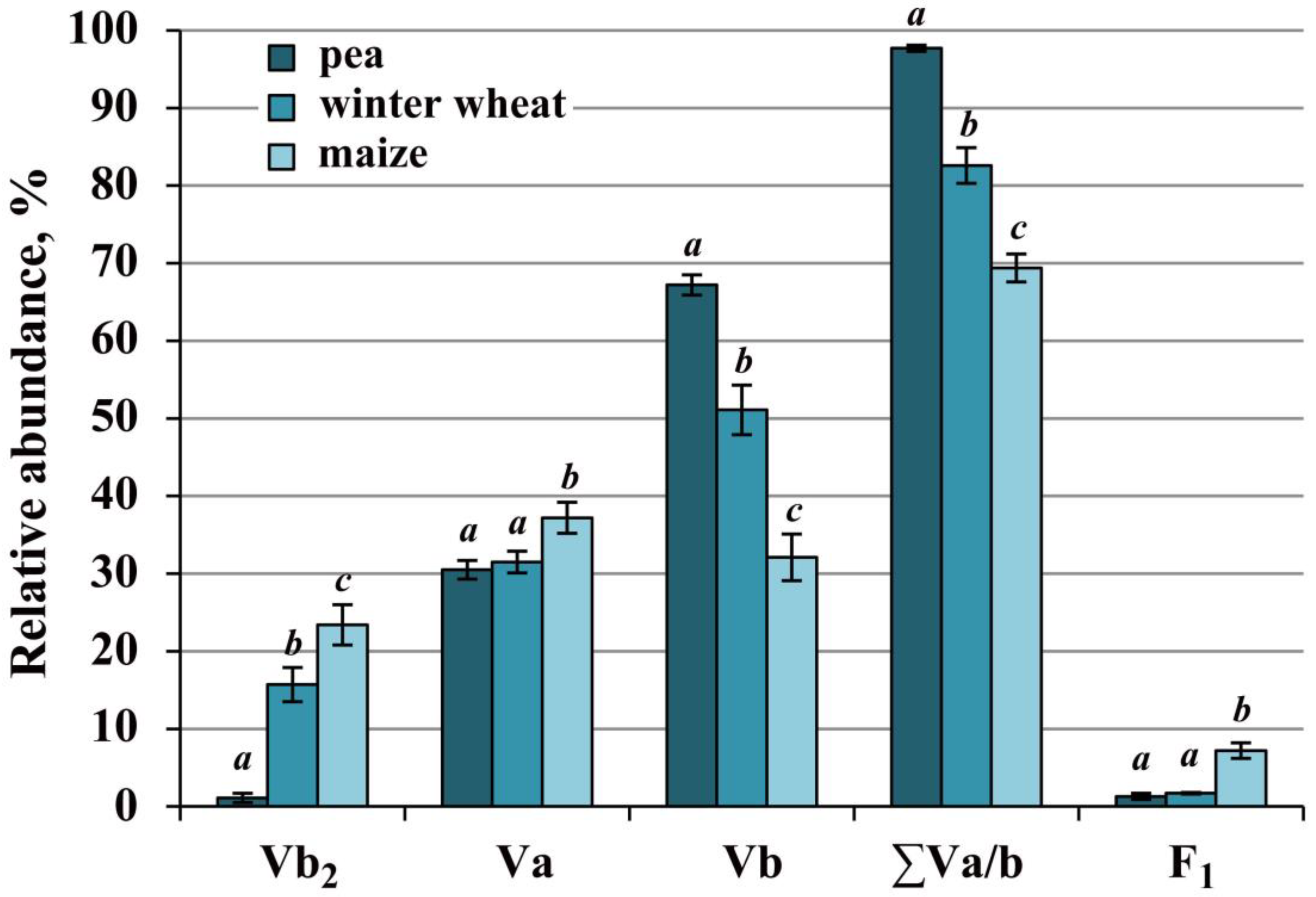
2.4. Complex V of Cereals Was Highly Stable, Whereas the Pea Enzyme Was More Active
3. Discussion
3.1. The OXPHOS Architecture in Pea, Winter Wheat, and Maize Shoot Mitochondria has Conserved Features
3.2. Distinctive Features of the OXPHOS in Frost-Tolerant Winter Wheat
3.3. OXPHOS Features in the C4 Plant Maize
3.4. High-Energy Pea OXPHOS
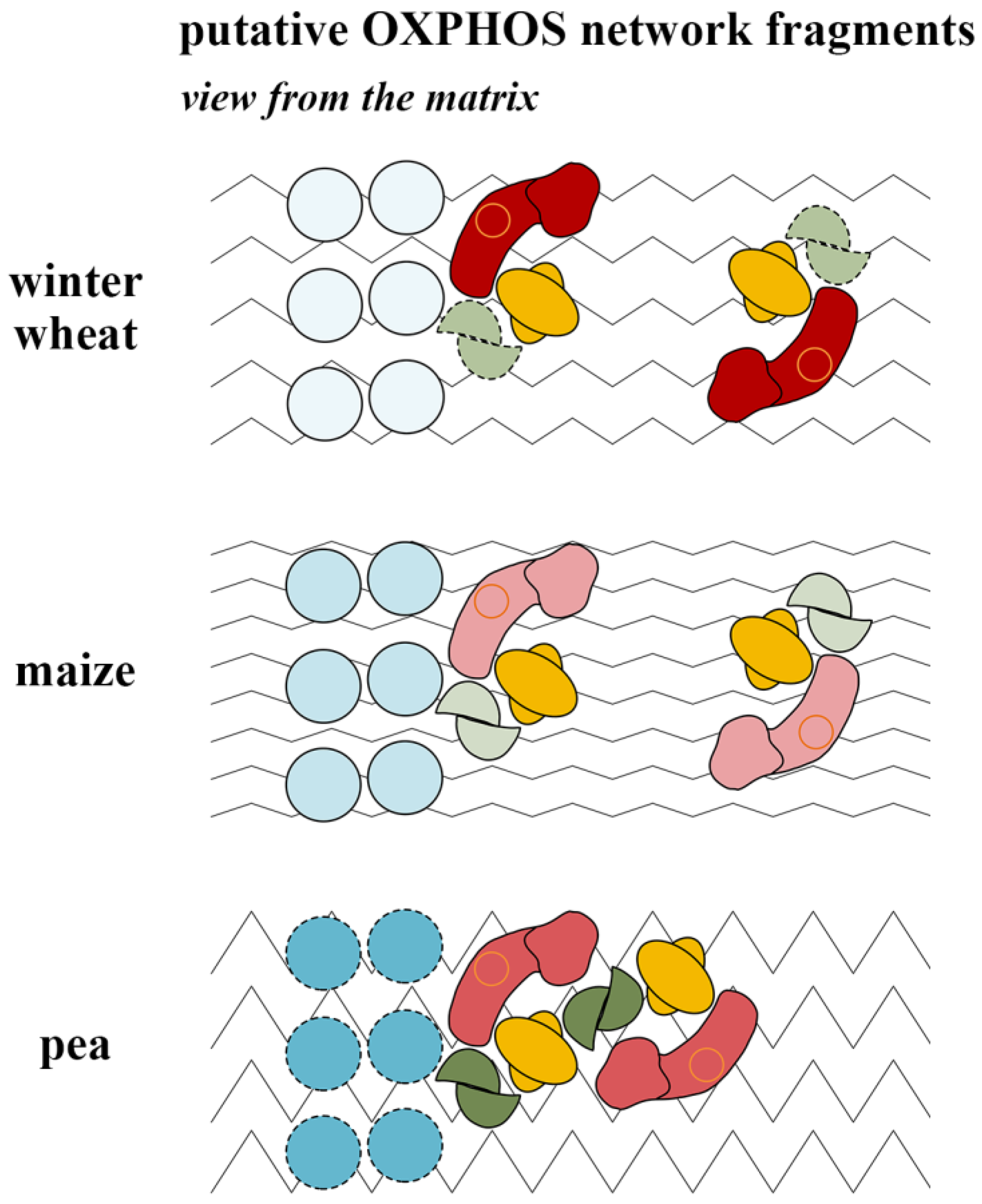
3.5. Putative Oxphosomic Organization in the Mitochondria of the Studied Species
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Mitochondria
4.3. Digitonin Solubilization of Mitochondria
4.4. One-Dimensional BN-PAGE and in-Gel Activity Staining of OXPHOS Complexes
4.5. Determination of Molecular Weights of OXPHOS Supercomplexes and Complexes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Rakhmankulova, Z.F. Respiratory supercomplexes of plant mitochondria: Structure and possible functions. Russ. J. Plant Physiol. 2014, 61, 721–732. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radic. Biol. Med. 2021, 167, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M. Protein crowding in the inner mitochondrial membrane. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148305. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Eubel, H.; Keegstra, W.; Boekema, E.J.; Braun, H.P. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc. Natl. Acad. Sci. USA 2005, 102, 3225–3229. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Sunderhaus, S.; Braun, H.P.; Boekema, E.J. Characterization of dimeric ATP synthase and cristae membrane ultrastructure from Saccharomyces and Polytomella mitochondria. FEBS Lett. 2006, 580, 3427–3432. [Google Scholar] [CrossRef]
- Bultema, J.B.; Braun, H.P.; Boekema, E.J.; Kouril, R. Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 60–67. [Google Scholar] [CrossRef]
- Gu, J.; Wu, M.; Guo, R.; Yan, K.; Lei, J.; Gao, N.; Yang, M. The architecture of the mammalian respirasome. Nature 2016, 537, 639–643. [Google Scholar] [CrossRef]
- Letts, J.A.; Fiedorczuk, K.; Sazanov, L.A. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef]
- Davies, K.M.; Blum, T.B.; Kühlbrandt, W. Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc. Natl. Acad. Sci. USA 2018, 115, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.; Nitharwal, R.G.; Fedotovskaya, O.; Schäfer, J.; Guo, H.; Kuang, Q.; Benlekbir, S.; Sjöstrand, D.; Ädelroth, P.; Rubinstein, J.L.; et al. Structure of a functional obligate complex III2IV2 respiratory supercomplex from Mycobacterium smegmatis. Nat. Struct. Mol. Biol. 2018, 25, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.M.; Lukoyanova, N.; Zhang, Y.; Cabrera-Orefice, A.; Arnold, S.; Meunier, B.; Pinotsis, N.; Maréchal, A. Structure of yeast cytochrome c oxidase in a supercomplex with cytochrome bc1. Nat. Struct. Mol. Biol. 2019, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Guo, F.; Letts, J.A. Atomic structures of respiratory complex III2, complex IV, and supercomplex III2-IV from vascular plants. eLife 2021, 10, e62047. [Google Scholar] [CrossRef]
- Klusch, N.; Dreimann, M.; Senkler, J.; Rugen, N.; Kühlbrandt, W.; Braun, H.P. Cryo-EM structure of the respiratory I + III2 supercomplex from Arabidopsis thaliana at 2 Å resolution. Nat. Plants 2023, 9, 142–156. [Google Scholar] [CrossRef]
- Maldonado, M.; Fan, Z.; Abe, K.M.; Letts, J.A. Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata. Nat. Plants 2023, 9, 157–168. [Google Scholar] [CrossRef]
- Acín-Pérez, R.; Fernández-Silva, P.; Peleato, M.L.; Pérez-Martos, A.; Enriquez, J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell 2008, 32, 529–539. [Google Scholar] [CrossRef]
- Cogliati, S.; Cabrera-Alarcón, J.L.; Enriquez, J.A. Regulation and functional role of the electron transport chain supercomplexes. Biochem. Soc. Trans. 2021, 49, 2655–2668. [Google Scholar] [CrossRef]
- Ukolova, I.V.; Kondakova, M.A.; Kondratov, I.G.; Sidorov, A.V.; Borovskii, G.B.; Voinikov, V.K. New insights into the organisation of the oxidative phosphorylation system in the example of pea shoot mitochondria. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148264. [Google Scholar] [CrossRef]
- Chen, C.; Ko, Y.; Delannoy, M.; Ludtke, S.J.; Chiu, W.; Pedersen, P.L. Mitochondrial ATP synthasome: Three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J. Biol. Chem. 2004, 279, 31761–31768. [Google Scholar] [CrossRef]
- Nůsková, H.; Mráček, T.; Mikulová, T.; Vrbacký, M.; Kovářová, N.; Kovalčíková, J.; Pecina, P.; Houštěk, J. Mitochondrial ATP synthasome: Expression and structural interaction of its components. Biochem. Biophys. Res. Commun. 2015, 464, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, J.A. Supramolecular organization of respiratory complexes. Annu. Rev. Physiol. 2016, 78, 533–561. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; Pérez-Pérez, R.; Lobo-Jarne, T.; Harbour, M.E.; Ding, S.; Peñas, A.; Diaz, F.; Moraes, C.T.; Fearnley, I.M.; Zeviani, M.; et al. Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. EMBO J. 2020, 39, e102817. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Carrozzo, R.; Santorelli, F.M.; Schägger, H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta 2006, 1757, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Nübel, E.; Wittig, I.; Kerscher, S.; Brandt, U.; Schägger, H. Two-dimensional native electrophoretic analysis of respiratory supercomplexes from Yarrowia lipolytica. Proteomics 2009, 9, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Strecker, V.; Wumaier, Z.; Wittig, I.; Schägger, H. Large pore gels to separate mega protein complexes larger than 10 MDa by blue native electrophoresis: Isolation of putative respiratory strings or patches. Proteomics 2010, 10, 3379–3387. [Google Scholar] [CrossRef]
- Miranda-Astudillo, H.; Colina-Tenorio, L.; Jiménez-Suárez, A.; Vázquez-Acevedo, M.; Salin, B.; Giraud, M.F.; Remacle, C.; Cardol, P.; González-Halphen, D. Oxidative phosphorylation supercomplexes and respirasome reconstitution of the colorless alga Polytomella sp. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 434–444. [Google Scholar] [CrossRef]
- Yaguzhinsky, L.S.; Yurkov, V.I.; Krasinskaya, I.P. On the localized coupling of respiration and phosphorylation in mitochondria. Biochim. Biophys. Acta 2006, 1757, 408–414. [Google Scholar] [CrossRef]
- Møller, I.M.; Rasmusson, A.G.; Van Aken, O. Plant mitochondria—Past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Krause, F.; Reifschneider, N.H.; Vocke, D.; Seelert, H.; Rexroth, S.; Dencher, N.A. “Respirasome”-like supercomplexes in green leaf mitochondria of spinach. J. Biol. Chem. 2004, 279, 48369–48375. [Google Scholar] [CrossRef]
- Braun, H.P. The Oxidative phosphorylation system of the mitochondria in plants. Mitochondrion 2020, 53, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Eubel, H.; Jänsch, L.; Braun, H.P. New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol. 2003, 133, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, J.; Braun, H.P.; Schmitz, U.; Colditz, F. The mitochondrial proteome of the model legume Medicago truncatula. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1658–1668. [Google Scholar] [CrossRef]
- Senkler, J.; Senkler, M.; Eubel, H.; Hildebrandt, T.; Lengwenus, C.; Schertl, P.; Schwarzländer, M.; Wagner, S.; Wittig, I.; Braun, H.P. The mitochondrial complexome of Arabidopsis thaliana. Plant J. 2017, 89, 1079–1092. [Google Scholar] [CrossRef]
- Farhat, N.; Hichri, S.; Hildebrandt, T.M.; Debez, A.; Braun, H.P. Composition and stability of the oxidative phosphorylation system in the halophile plant Cakile maritima. Front. Plant Sci. 2019, 10, 1010. [Google Scholar] [CrossRef]
- Eubel, H.; Heinemeyer, J.; Braun, H.P. Identification and characterization of respirasomes in potato mitochondria. Plant Physiol. 2004, 134, 1450–1459. [Google Scholar] [CrossRef]
- Pomortsev, A.V.; Dorofeev, N.V.; Katysheva, N.B.; Peshkova, A.A. Changes in dehydrin composition in winter cereal crowns during winter survival. Biol. Plant. 2017, 61, 394–398. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Grabelnych, O.I.; Sumina, O.N.; Funderat, S.P.; Pobezhimova, T.P.; Voinikov, V.K.; Kolesnichenko, A.V. The distribution of electron transport between the main cytochrome and alternative pathways in plant mitochondria during short-term cold stress and cold hardening. J. Therm. Biol. 2004, 29, 165–175. [Google Scholar] [CrossRef]
- Smith, J.J.; McFeters, G.A. Mechanisms of INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J. Microbiol. Methods 1997, 29, 161–175. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Litvinova, E.G.; Zakharchenko, M.V.; Khunderyakova, N.V.; Fadeev, R.S.; Teplova, V.V.; Fedotcheva, T.A.; Beloborodova, N.V.; Kondrashova, M.N. Substrate-specific reduction of tetrazolium salts by isolated mitochondria, tissues, and leukocytes. Biochem. Biokhimiia 2017, 82, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Klusch, N.; Senkler, J.; Yildiz, Ö.; Kühlbrandt, W.; Braun, H.P. A ferredoxin bridge connects the two arms of plant mitochondrial complex I. Plant Cell. 2021, 33, 2072–2091. [Google Scholar] [CrossRef] [PubMed]
- Sazanov, L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015, 16, 375–388. [Google Scholar] [CrossRef]
- Gazizova, N.; Rakhmatullina, D.; Minibayeva, F. Effect of respiratory inhibitors on mitochondrial complexes and ADP/ATP translocators in the Triticum aestivum roots. Plant Physiol. Biochem. 2020, 151, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Schikowsky, C.; Senkler, J.; Braun, H.P. SDH6 and SDH7 Contribute to anchoring succinate dehydrogenase to the inner mitochondrial membrane in Arabidopsis thaliana. Plant Physiol. 2017, 173, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Taylor, N.L.; Narsai, R.; Eubel, H.; Whelan, J.; Millar, A.H. Functional and composition differences between mitochondrial complex II in Arabidopsis and rice are correlated with the complex genetic history of the enzyme. Plant Mol. Biol. 2010, 72, 331–342. [Google Scholar] [CrossRef]
- Gilkerson, R.W.; Selker, J.M.; Capaldi, R.A. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 2003, 546, 355–358. [Google Scholar] [CrossRef]
- Schägger, H.; Pfeiffer, K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 2001, 276, 37861–37867. [Google Scholar] [CrossRef]
- Ramírez-Aguilar, S.J.; Keuthe, M.; Rocha, M.; Fedyaev, V.V.; Kramp, K.; Gupta, K.J.; Rasmusson, A.G.; Schulze, W.X.; van Dongen, J.T. The composition of plant mitochondrial supercomplexes changes with oxygen availability. J. Biol. Chem. 2011, 286, 43045–43053. [Google Scholar] [CrossRef]
- Lenaz, G.; Tioli, G.; Falasca, A.I.; Genova, M.L. Complex I function in mitochondrial supercomplexes. Biochim. Biophys. Acta. Bioenerg. 2016, 1857, 991–1000. [Google Scholar] [CrossRef]
- Schägger, H.; de Coo, R.; Bauer, M.F.; Hofmann, S.; Godinot, C.; Brandt, U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J. Biol. Chem. 2004, 279, 36349–36353. [Google Scholar] [CrossRef] [PubMed]
- Sunderhaus, S.; Klodmann, J.; Lenz, C.; Braun, H.P. Supramolecular structure of the OXPHOS system in highly thermogenic tissue of Arum maculatum. Plant Physiol. Biochem. 2010, 48, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Membrane fluidity and compositional changes in response to high temperature stress in wheat. In Physiological, Molecular, and Genetic Perspectives of Wheat Improvement; Wani, S.H., Mohan, A., Singh, G.P., Eds.; Springer: Cham, Switzerland, 2021; pp. 115–123. [Google Scholar] [CrossRef]
- Makarenko, S.P.; Konstantinov, Y.M.; Khotimchenko, S.V.; Konenkina, T.A.; Arziev, A.S. Fatty acid composition of mitochondrial membrane lipids in cultivated (Zea mays) and wild (Elymus sibiricus) grasses. Rus. J. Plant Physiol. 2003, 50, 487–491. [Google Scholar] [CrossRef]
- Grabel’nykh, O.I.; Kirichenko, K.A.; Pobezhimova, T.P.; Borovik, O.A.; Pavlovskaya, N.S.; Lyubushkina, I.V.; Koroleva, N.A.; Voinikov, V.K. Influence of cold shock on the fatty acid composition and functional state of mitochondria in hardened and nondhardened seedlings of winter wheat. Biol. Membr. 2014, 31, 204–217. [Google Scholar] [CrossRef]
- Stupnikova, I.; Benamar, A.; Tolleter, D.; Grelet, J.; Borovskii, G.; Dorne, A.J.; Macherel, D. Pea seed mitochondria are endowed with a remarkable tolerance to extreme physiological temperatures. Plant Physiol. 2006, 140, 326–335. [Google Scholar] [CrossRef]
- Kolesnichenko, A.V.; Pobezhimova, T.P.; Grabelnykh, O.I.; Voinikov, V.K. Stress CSP310 protein uncouples oxidative phosphorylation otherwise than other plant uncoupling proteins do. Rus. J. Plant Physiol. 2003, 50, 224–231. [Google Scholar] [CrossRef]
- Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985, 54, 1015–1069. [Google Scholar] [CrossRef]
- Nesterov, S.; Chesnokov, Y.; Kamyshinsky, R.; Panteleeva, A.; Lyamzaev, K.; Vasilov, R.; Yaguzhinsky, L. Ordered clusters of the complete oxidative phosphorylation system in cardiac mitochondria. Int. J. Mol. Sci. 2021, 22, 1462. [Google Scholar] [CrossRef]
- Fuchs, P.; Rugen, N.; Carrie, C.; Elsässer, M.; Finkemeier, I.; Giese, J.; Hildebrandt, T.M.; Kühn, K.; Maurino, V.G.; Ruberti, C.; et al. Single organelle function and organization as estimated from Arabidopsis mitochondrial proteomics. Plant J. 2020, 101, 420–441. [Google Scholar] [CrossRef]
- Benamar, A.; Tallon, C.; Macherel, D. Membrane integrity and oxidative properties of mitochondria isolated from imbibing pea seeds after priming or accelerated ageing. Seed Sci. Res. 2003, 13, 35–45. [Google Scholar] [CrossRef]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Schertl, H.; Braun, H.P. Activity measurements of mitochondrial enzymes in native gels. In Plant Mitochondria: Methods and Protocols; Whelan, J., Murcha, M.W., Eds.; Springer Science+Business Media LLC: New York, NY, USA, 2015; pp. 131–138. [Google Scholar] [CrossRef]
- Wittig, I.; Beckhaus, T.; Wumaier, Z.; Karas, M.; Schägger, H. Mass estimation of native proteins by blue native electrophoresis: Principles and practical hints. Mol. Cell. Proteom. 2010, 9, 2149–2161. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukolova, I.V.; Borovskii, G.B. OXPHOS Organization and Activity in Mitochondria of Plants with Different Life Strategies. Int. J. Mol. Sci. 2023, 24, 15229. https://doi.org/10.3390/ijms242015229
Ukolova IV, Borovskii GB. OXPHOS Organization and Activity in Mitochondria of Plants with Different Life Strategies. International Journal of Molecular Sciences. 2023; 24(20):15229. https://doi.org/10.3390/ijms242015229
Chicago/Turabian StyleUkolova, Irina V., and Gennadii B. Borovskii. 2023. "OXPHOS Organization and Activity in Mitochondria of Plants with Different Life Strategies" International Journal of Molecular Sciences 24, no. 20: 15229. https://doi.org/10.3390/ijms242015229
APA StyleUkolova, I. V., & Borovskii, G. B. (2023). OXPHOS Organization and Activity in Mitochondria of Plants with Different Life Strategies. International Journal of Molecular Sciences, 24(20), 15229. https://doi.org/10.3390/ijms242015229





