A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases
Abstract
:1. Introduction
2. Carotenoids: Properties, Structures, and Functions
3. Aging
4. The Effect of Carotenoids on Aging
5. The Effect of Carotenoids on Aging-Related Diseases
5.1. Cardiovascular Diseases and Carotenoids
5.2. Skin Health and Carotenoids
5.3. Liver Diseases and Carotenoids
5.4. Diabetes Mellitus and Carotenoids
5.5. Age-Related Macular Degeneration and Carotenoids
5.6. Diabetic Retinopathy and Carotenoids
5.7. Dry Eye Diseases and Carotenoids
5.8. Neurodegenerative Diseases and Carotenoids
5.8.1. Alzheimer’s Disease
5.8.2. Parkinson’s Disease
6. Methods
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bruins, M.J.; Van Dael, P.; Eggersdorfer, M. The Role of Nutrients in Reducing the Risk for Noncommunicable Diseases during Aging. Nutrients 2019, 11, 85. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ji, S. Cellular senescence: Molecular mechanisms and pathogenicity. J. Cell. Physiol. 2018, 233, 9121–9135. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Alugoju, P.; Krishna Swamy, V.K.D.; Anthikapalli, N.V.A.; Tencomnao, T. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 16, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Ye, B.; Cui, Y.; Geng, R.; Liu, S.; Zhang, Y.; Guo, W.; Fu, S. Astaxanthin Alleviates Nonalcoholic Fatty Liver Disease by Regulating the Intestinal Flora and Targeting the AMPK/Nrf2 Signal Axis. J. Agric. Food Chem. 2022, 70, 10620–10634. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Analy. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Feng, L.; Nie, K.; Jiang, H.; Fan, W. Effects of lutein supplementation in age-related macular degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar] [CrossRef]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients. 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharm. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharm. 2018, 9, 521. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessi, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary Antioxidants, Circulating Antioxidant Concentrations, Total Antioxidant Capacity, and Risk of All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2018, 9, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharm. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Therond, P. Dommages créés aux biomolécules (lipides, protéines, ADN) par le stress oxydant [Oxidative stress and damages to biomolecules (lipids, proteins, DNA)]. Ann. Pharm. Fr. 2006, 64, 383–389. [Google Scholar] [CrossRef]
- Tian, L.; Wen, Y.; Li, S.; Zhang, P.; Wang, Y.; Wang, J.; Cao, K.; Du, L.; Wang, N.; Jie, Y. Benefits and Safety of Astaxanthin in the Treatment of Mild-to-Moderate Dry Eye Disease. Front. Nutr. 2022, 8, 796951. [Google Scholar] [CrossRef]
- Bhattarai, G.; So, H.S.; Kieu, T.T.T.; Kook, S.H.; Lee, J.C.; Jeon, Y.M. Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients 2021, 13, 3575. [Google Scholar] [CrossRef]
- Leh, H.E.; Sopian, M.M.; Abu Bakar, M.H.; Lee, L.K. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the Type II diabetes mellitus patients: A case-control study. Ann. Med. 2021, 53, 1059–1065. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Kan, J.; Wang, M.; Liu, Y.; Liu, H.; Chen, L.; Zhang, X.; Huang, C.; Liu, B.Y.; Gu, Z.; Du, J. A novel botanical formula improves eye fatigue and dry eye: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2020, 112, 334–342. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People-A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 7, 817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, D. Protective effects of astaxanthin on diabetic cardiomyopathy in rats. CyTA–J. Food 2018, 16, 909–915. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.M.; Mastaloudis, A.F.; Hester, S.N.; Gray, R.; Kern, D.; Namkoong, J.; Draelos, Z.D. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J. Cosmet. Derm. 2017, 16, 491–499. [Google Scholar] [CrossRef]
- Martín-Pozuelo, G.; González-Barrio, R.; Barberá, G.G.; Albalat, A.; García-Alonso, J.; Mullen, W.; Mischak, H.; Periago, M.J. Tomato Juice Consumption Modifies the Urinary Peptide Profile in Sprague-Dawley Rats with Induced Hepatic Steatosis. Int. J. Mol. Sci. 2016, 17, 1789. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cui, W.; Wang, L.; Xiong, Y.; Liu, L.; Sun, X.; Hao, L. Lutein prevents high fat diet-induced atherosclerosis in ApoE-deficient mice by inhibiting NADPH oxidase and increasing PPAR expression. Lipids 2015, 50, 261–273. [Google Scholar] [CrossRef]
- Sila, A.; Ghlissi, Z.; Kamoun, Z.; Makni, M.; Nasri, M.; Bougatef, A.; Sahnoun, Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur. J. Nutr. 2015, 54, 301–307. [Google Scholar] [CrossRef]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. Protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharm. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Leite, J.O.; DeOgburn, R.; Smyth, J.A.; Clark, R.M.; Fernandez, M.L. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Huang, C.Y.; Kiefer, R.; Lee, S.D.; Maurya, N.; Velmurugan, B.K. Cardiovascular Disease and Possible Ways in Which Lycopene Acts as an Efficient Cardio-Protectant against Different Cardiovascular Risk Factors. Molecules 2022, 27, 3235. [Google Scholar] [CrossRef] [PubMed]
- Wolak, T.; Paran, E. Can carotenoids attenuate vascular aging? Vasc. Pharm. 2013, 59, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, L.; Kark, J.D.; Gomez-Gracia, E.; Martin, B.C.; Steck, S.E.; Kardinaal, A.F.; Ringstad, J.; Thamm, M.; Masaev, V.; Riemersma, R.; et al. Lycopene and myocardial infarction risk in the EURAMIC Study. Am. J. Epidemiol. 1997, 146, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharm. Res. 2019, 149, 104477. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Schwartz, S.; Frank, E.; Gierhart, D.; Simpson, P.; Frumento, R. Zeaxanthin-based dietary supplement and topical serum improve hydration and reduce wrinkle count in female subjects. J. Cosmet. Derm. 2016, 15, e13–e20. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Bavarsad, N.; Mapar, M.A.; Safaezadeh, M.; Latifi, S.M. A double-blind, placebo-controlled randomized trial of skin-lightening cream containing lycopene and wheat bran extract on melasma. J. Cosmet. Derm. 2021, 20, 1795–1800. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Qi, Y.; Chen, X.; Deng, J.; Zhang, Y.; Li, R.; Fan, J. The effect of tomato and lycopene on clinical characteristics and molecular markers of UV-induced skin deterioration: A systematic review and meta-analysis of intervention trials. Crit. Rev. Food Sci. Nutr. 2023, 6, 1–20. [Google Scholar] [CrossRef]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Derm. 2020, 19, 22–27. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochem. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Chen, J.C.; Wang, T.J.; Zheng, H.Y.; Chen, W.C.; Chang, P.Y.; Lin, Y.W. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem. Pharm. 2016, 105, 91–100. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, Q.; Orfila, C.; Zhao, J.; Zhang, L. Systematic Review and Meta-Analysis on the Effects of Astaxanthin on Human Skin Ageing. Nutrients 2021, 13, 2917. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179–1184. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, A.; Gramdorf, S.; Müller, R.H.; Kurz, T. Beta-carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008, 73, N1–N6. [Google Scholar] [CrossRef]
- Murillo, A.G.; DiMarco, D.M.; Fernandez, M.L. The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease. Biology 2016, 5, 42. [Google Scholar] [CrossRef]
- Christensen, K.; Lawler, T.; Mares, J. Dietary Carotenoids and Non-Alcoholic Fatty Liver Disease among US Adults, NHANES 2003⁻2014. Nutrients 2019, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; Martín-Pozuelo, G.; González-Barrio, R.; Navarro-González, I.; Pallarés, F.J.; Santaella, M.; García-Alonso, J.; Sevilla, Á.; Periago-Castón, M.J. Ameliorative Effect of Spinach on Non-Alcoholic Fatty Liver Disease Induced in Rats by a High-Fat Diet. Int. J. Mol. Sci. 2019, 20, 1662. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Althagafy, H.S.; Abd-Alhameed, E.K.; Al-Thubiani, W.S.; Hassanein, E.H.M. Promising hepatoprotective effects of lycopene in different liver diseases. Life Sci. 2022, 310, 121131. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel Action of Carotenoids on Non-Alcoholic Fatty Liver Disease: Macrophage Polarization and Liver Homeostasis. Nutrients 2016, 8, 391. [Google Scholar] [CrossRef]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Machate, D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef] [PubMed]
- Atlas, I.D. Diabetes around the World in 2021. Available online: https://diabetesatlas.org/ (accessed on 30 August 2023).
- Roglic, G. WHO Global Report on Diabetes: A Summary. 2023. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 30 August 2023).
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef] [PubMed]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp. Biol. Med. 2018, 243, 613–620. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut Microbiota Dysbiosis Drives and Implies Novel Therapeutic Strategies for Diabetes Mellitus and Related Metabolic Diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Glukhareva, T.V.; Danilova, I.G.; Kovaleva, E.G. Natural antioxidants in diabetes treatment and management: Prospects of astaxanthin. Crit. Rev. Food Sci. Nutr. 2022, 62, 5005–5028. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, W.Q.; Cao, Y.; He, L.P.; Guan, K.; Ling, W.H.; Chen, Y.M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Suganuma, H.; Shimizu, S.; Hayashi, H.; Sawada, K.; Tokuda, I.; Ihara, K.; Nakaji, S. Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome. Nutrients 2020, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients 2020, 12, 2310. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Satoh, T.; Gupta, R.C. Chapter 38—Astaxanthin: Health Benefits and Toxicity. In Nutraceuticals; Ramesh, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 531–539. [Google Scholar]
- Chen, Q.; Tao, J.; Li, G.; Zheng, D.; Tan, Y.; Li, R.; Tian, L.; Li, Z.; Cheng, H.; Xie, X. Astaxanthin ameliorates experimental diabetes-induced renal oxidative stress and fibronectin by upregulating connexin43 in glomerular mesangial cells and diabetic mice. Eur. J. Pharm. 2018, 840, 33–43. [Google Scholar] [CrossRef]
- Pan, F.; Cui, W.; Gao, L.; Shi, X.; Yang, H.; Hu, Y.; Li, M. Serum lutein is a promising biomarker for type 2 diabetes mellitus and diabetic kidney disease in the elderly. J. Clin. Lab. Anal. 2022, 36, e24350. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef]
- Quansah, D.Y.; Ha, K.; Jun, S.; Kim, S.A.; Shin, S.; Wie, G.A.; Joung, H. Associations of Dietary Antioxidants and Risk of Type 2 Diabetes: Data from the 2007–2012 Korea National Health and Nutrition Examination Survey. Molecules 2017, 22, 1664. [Google Scholar] [CrossRef]
- Zhu, N.W.; Yin, X.L.; Lin, R.; Fan, X.L.; Chen, S.J.; Zhu, Y.M.; Zhao, X.Z. Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia. Neural Regen. Res. 2020, 15, 332–341. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Shi, H.; Yin, J.; Xu, T.; Zhu, R.; et al. Lycopene ameliorates islet function and down-regulates the TLR4/MyD88/NF-κB pathway in diabetic mice and Min6 cells. Food Funct. 2023, 14, 5090–5104. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Dziedziak, J.; Kasarełło, K.; Cudnoch-Jędrzejewska, A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants 2021, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y. Recent Advances and the Mechanism of Astaxanthin in Ophthalmological Diseases. J. Ophthalmol. 2022, 2022, 8071406. [Google Scholar] [CrossRef]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochem. 2009, 48, 4798–4807. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic retinopathy for the non-ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2017, 2017, 9702820. [Google Scholar] [CrossRef]
- Benlarbi-Ben Khedher, M.; Hajri, K.; Dellaa, A.; Baccouche, B.; Hammoum, I.; Boudhrioua-Mihoubi, N.; Dhifi, W.; Ben Chaouacha-Chekir, R. Astaxanthin inhibits aldose reductase activity in Psammomys obesus, a model of type 2 diabetes and diabetic retinopathy. Food Sci. Nutr. 2019, 7, 3979–3985. [Google Scholar] [CrossRef] [PubMed]
- Lawler, T.; Korger, J.; Liu, Y.; Liu, Z.; Pak, J.W.; Barrett, N.; Blodi, B.; Domalpally, A.; Johnson, E.; Wallace, R.; et al. Carotenoids in Age-Related Eye Disease Study Investigators. Serum and Macular Carotenoids in Relation to Retinal Vessel Caliber Fifteen Years Later, in the Second Carotenoids in Age-Related Eye Disease Study. Invest. Ophthalmol. Vis. Sci. 2021, 62, 20. [Google Scholar] [CrossRef]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. A Systematic Review of Carotenoids in the Management of Diabetic Retinopathy. Nutrients 2021, 13, 2441. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.Ö.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent Advances in Studies on the Therapeutic Potential of Dietary Carotenoids in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Katare, D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes Metab. Syndr. 2016, 10, 144–149. [Google Scholar] [CrossRef]
- Glade, M.J. Oxidative stress and cognitive longevity. Nutrition 2010, 26, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, S.; Jeziorek, M. The role of nutrition in Alzheimer’s disease. Rocz. Panstw. Zakl. Hig. 2021, 72, 29–39. [Google Scholar] [PubMed]
- Boccardi, V.; Arosio, B.; Cari, L.; Bastiani, P.; Scamosci, M.; Casati, M.; Ferri, E.; Bertagnoli, L.; Ciccone, S.; Rossi, P.D.; et al. Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 2020, 59, 119–126. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimers Dement. 2017, 3, 432–439. [Google Scholar] [CrossRef]
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.F.; Barberger-Gateau, P. Plasma Carotenoids Are Inversely Associated With Dementia Risk in an Elderly French Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 683–688. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Association between leukocyte telomere length and serum carotenoid in US adults. Eur. J. Nutr. 2017, 56, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Ellis, A.C. Dietary Antioxidants and Parkinson’s Disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.Y.; Tan, E.K.; Yuan, J.M.; Koh, W.P.; Tan, L.C.S. Dietary Antioxidants and Risk of Parkinson’s Disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, J.; Shim, E.; Chung, E.J.; Jang, S.H.; Koh, S.B. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s Disease. Nutr. Res. Pr. 2017, 11, 114–120. [Google Scholar] [CrossRef]
- Yang, F.; Wolk, A.; Håkansson, N.; Pedersen, N.L.; Wirdefeldt, K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 2017, 32, 1631–1636. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2016, 19, 237–246. [Google Scholar] [CrossRef]
- Park, H.A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. Dietary antioxidants associated with slower progression of parkinsonian signs in older adults. Nutr. Neurosci. 2022, 25, 550–557. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
| Carotenoids | Sources | References |
|---|---|---|
astaxanthin | Microalgae (Haematococcus pluvialis), pineapples (Phaffia rhodozyma), some fungi and bacteria, salmon, krill, peaches, sturgeons, carrots, shells | [12,13] |
zeaxanthin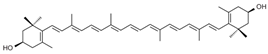 | Corn, lavender, spinach, lettuce, broccoli, egg yolk, green leafy vegetables | [14] |
β-carotene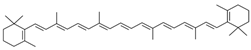 | Carrot, orange, sweet potatoes, broccoli, mango | [2,7] |
lutein | Egg yolk, lettuce, parsley, spinach, basil | [2,15,16,17] |
lycopene | Tomato, rosehip, watermelon, grapefruit, guava, papaya | [18,19,20] |
| Year | Species | Findings on Carotenoid Intake | References |
|---|---|---|---|
| 2022 | Mice | Astaxanthin significantly reduced HFD-induced fat deposition, obesity, and fatty liver. | [13] |
| 2021 | Human | Oral administration of astaxanthin improved symptoms and signs in middle-aged and elderly patients with mild to moderate DED. | [26] |
| 2021 | Mice | Astaxanthin improved periodontal destruction and oxidative systemic complications triggered by hyperglycemia in type I diabetes. | [27] |
| 2021 | Human | Lycopene intake and peripheral antioxidant level in T2DM patients had a positive correlation while HbA1c and FPG levels were reduced by higher lycopene intake. | [28] |
| 2020 | Human | Dietary astaxanthin intake delayed LDL oxidation. | [29] |
| 2020 | Human | The combination of lutein ester, zeaxanthin blackcurrant, chrysanthemum, and goji berry extracts improved eye fatigue, dry eye, and macular function. | [30] |
| 2018 | Human | Orally taken astaxanthin reduced skin moisture loss and skin roughness. | [31] |
| 2018 | Rat | Astaxanthin administered intraperitoneally significantly lowered blood sugar in rats. | [32] |
| 2017 | Human | Dietary lycopene intake reduced the risk of CVD by 17%. | [33] |
| 2017 | Human | The product rich in lycopene reduced the intensity of erythema formation. | [34] |
| 2016 | Rat | Lycopene improved liver fibrosis and reduced abnormal intra- and extrahepatic angiogenesis in rats with cirrhosis. Rats fed lycopene-rich tomato juice for 5 weeks did not develop NAFLD. | [35] |
| 2016 | Human | Oral supplementation (isomers of 10 mg of lutein and 2 mg of zeaxanthin) improved skin conditions and brightened the skin. | [36] |
| 2015 | Mice | Dietary lutein intake prevented HFD-induced atherosclerosis in ApoE-deficient mice. | [37] |
| 2015 | Rat | Astaxanthin originating from shrimp reduced oxidative damage and prevented pathological changes in diabetic rats. Daily administration of astaxanthin in diabetic rats decreased blood sugar. | [38] |
| 2013 | Mice | Astaxanthin application (100 mg/kg) inhibited retinal dysfunction in a mouse model of light-induced retinal damage. | [39] |
| 2011 | Guinea pig | Lutein prevented cholesterol deposition in the aortic tissue of atherosclerotic guinea pigs. | [40] |
| 2011 | Human | Astaxanthin supplementation for 3 weeks suppressed lipid peroxidation in obese adults in Korea. | [41] |
| Diseases | Lycopene | Lutein | β-Carotene | Astaxanthin | Zeaxanthin |
|---|---|---|---|---|---|
| Cardiovascular diseases | Reducing LDL [1] | Prevention of lipid peroxidation [2] | No data | Increase NO bioavailability [3,4] | No data |
| Neurodegenerative diseases | Healing of motor function [5] Regulation of the length of the telomer [6] | Improving cognitive function [7] | Healing of motor function [5] Regulation of the length of the telomere [6] | Protection against neural apoptosis and preventing neuroinflammation [8] | No data |
| Diabetes mellitus | Decreasing HbA1c [9] | No data | Decreasing adipose tissue [10] Decreasing triglyceride and insulin resistance [11] | Protecting β cells [12] Anti-lipid peroxidation activity [13] Decreasing blood sugar [14] | No data |
| Skin diseases | Photoprotection activity [15] | Improving hydration and elasticity [16,17] | Protection against photodamage and skin cancer [16,18] | Suppressing oxidative damage [19] DNA repair [20] | Anti-wrinkle [15] |
| Liver diseases | ROS extinguishion and antioxidant effect [22] | No data | Hepatoprotective effect [23] | Lipid peroxidation inhibition [24] | No data |
| Eye diseases | No data | Increasing visual acuity [25] | No data | Decreasing ROS level in retina [26] Improving the symptoms of DED [27] | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. https://doi.org/10.3390/ijms242015199
Bakac ER, Percin E, Gunes-Bayir A, Dadak A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. International Journal of Molecular Sciences. 2023; 24(20):15199. https://doi.org/10.3390/ijms242015199
Chicago/Turabian StyleBakac, Elif Rabia, Ece Percin, Ayse Gunes-Bayir, and Agnes Dadak. 2023. "A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases" International Journal of Molecular Sciences 24, no. 20: 15199. https://doi.org/10.3390/ijms242015199
APA StyleBakac, E. R., Percin, E., Gunes-Bayir, A., & Dadak, A. (2023). A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. International Journal of Molecular Sciences, 24(20), 15199. https://doi.org/10.3390/ijms242015199







