Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches
Abstract
:1. Introduction
2. Biology of Tendon Aging
2.1. Tendon Cellular Changes during Aging
2.1.1. Tendon Cellularity and Cell Morphological Properties during Aging
2.1.2. Cell Motility, Metabolism and Self-Renewal Capacity during Aging
2.1.3. Cell Differentiation and ECM-Secreting Potential during Aging
| Age-Related Changes | Description | Reference | |
|---|---|---|---|
| Cell physical property | Cellularity |
| [3,32,39,40,41,42,43] |
| [16] | ||
| [23] | ||
| [18,24,25,26] | ||
| Cell morphology |
| [18,19,23] | |
| [32,40] | ||
| [32,44] | ||
| [45] | ||
| Cytoskeletal organization |
| [18,39] | |
| [17] | ||
| Stiffness |
| [18,19] | |
| Cell behaviors | Cell adhesion and migration |
| [14] |
| [17] | ||
| Metabolic activity |
| [46] | |
| [22] | ||
| [17] | ||
| Cell–cell interactions |
| [31] | |
| Proliferation (self-renewal) capacity |
| [14,34] | |
| [17] | ||
| Differentiation |
| [14,34,35] | |
| [14,26,28] | ||
| [47] | ||
| [48] | ||
| [16,32,41] |
2.2. Biology of Cellular Tendon ECM during Aging
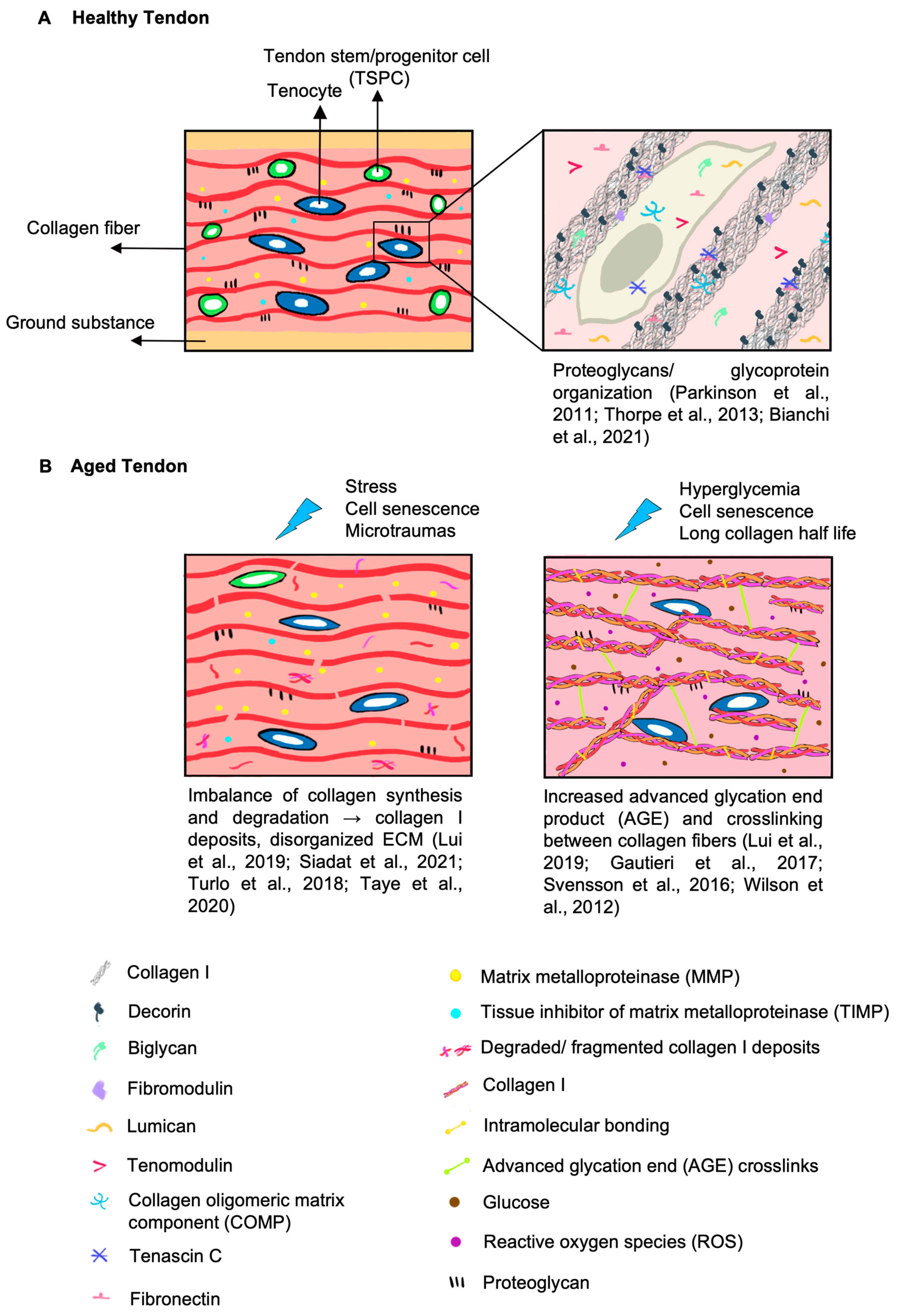
2.2.1. Changes in Collagens during Aging
2.2.2. Changes in Noncollagenous ECM during Aging
2.3. Tendon Biomechanical Properties and Functions during Aging
| Parameter | Definition and Human Physiological Range | Age-Related Molecular and Structural Changes | Age-Related Biomechanical Changes |
|---|---|---|---|
| Tensile strength | The maximum force the tendon can withstand in tension before tear. Achilles [104] 59 ± 18 MPa Patellar [105] 58.7 ± 16.3 MPa | Human
| |
| Stiffness | The extent of resistance to elastic deformation in response to the applied force. Achilles [104] 685 ± 262 N/mm Patellar [113] 4434 ± 562 N/mm | Human
| |
| Tensile modulus | The slope of the stress–strain curve in the elastic deformation region that measures stiffness [116]. Achilles [119] 822 ± 211 MPa Patellar [98] 660 ± 266 MPa | Similar contributing biological factors as mentioned with additional factors: | Human |
| Viscoelasticity | Represented by tendons exhibiting viscous and elastic characteristics when undergoing deformation Decreased dynamic modulus indicates less resistance to strain and a reduced ability to properly transfer force [41]. |
| Human
|
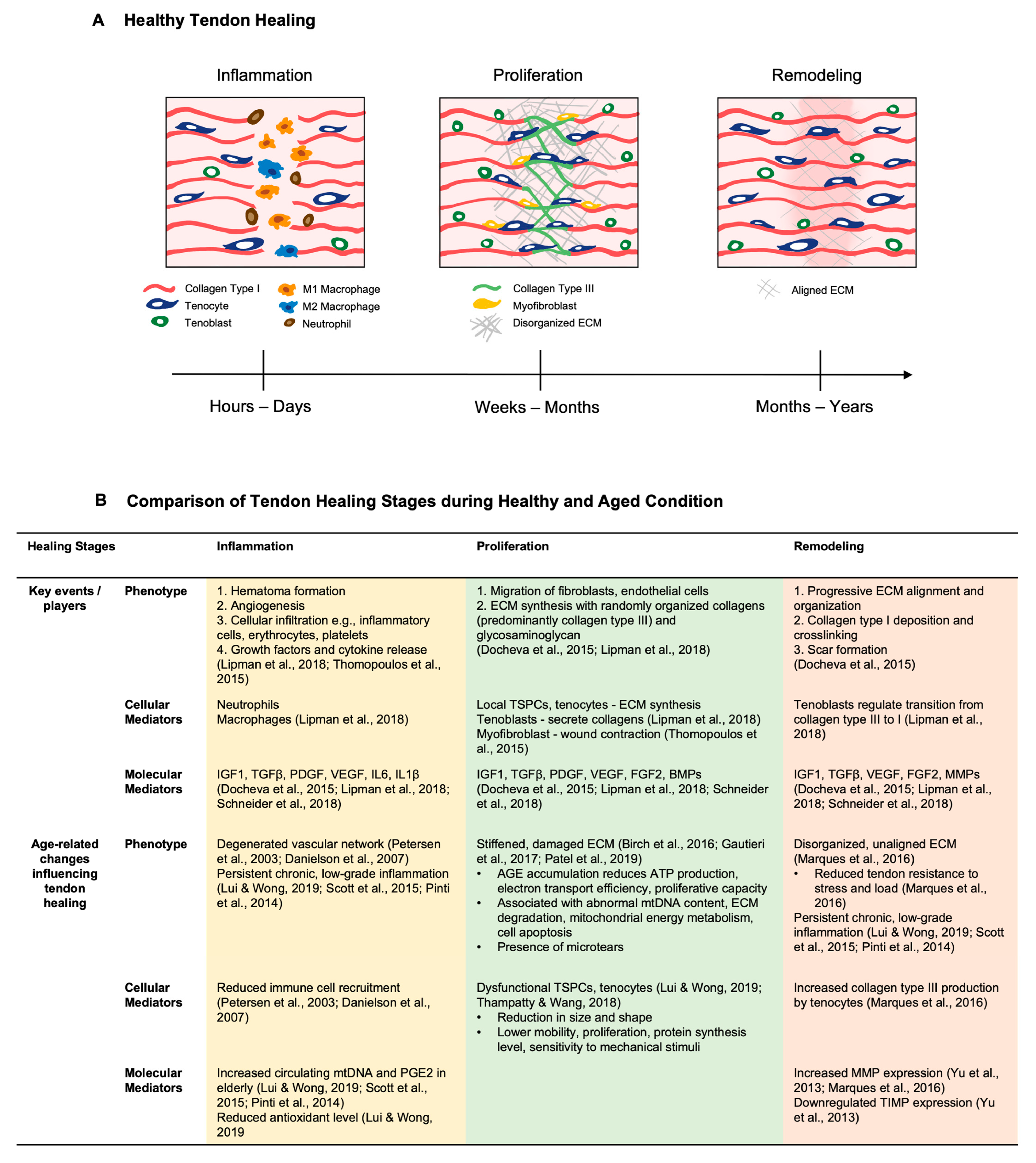
3. Features of Tendon Healing during Aging
3.1. General Tendon Healing Phases
3.2. Impact of Aging on the Tendon Healing Response
4. Treatment Approaches for Tendon-Aging-Associated Injuries and Diseases
4.1. Diet Suggestions
4.2. Physical Activity
4.3. Surgical Considerations for Tendon Injury in Elderly Patients
4.4. Advanced Cell Rejuvenation Strategies
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=Between%202015%20and%202050%2C%20the,%2D%20and%20middle%2Dincome%20countries (accessed on 12 July 2020).
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y.; Wong, C.M. Biology of Tendon Stem Cells and Tendon in Aging. Front. Genet. 2019, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Ashe, M.C. Common tendinopathies in the upper and lower extremities. Curr. Sports Med. Rep. 2006, 5, 233–241. [Google Scholar] [CrossRef]
- Riasat, K.; Bardell, D.; Goljanek-Whysall, K.; Clegg, P.D.; Peffers, M.J. Epigenetic mechanisms in Tendon Ageing. Br. Med. Bull. 2020, 135, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.C.; Li, Y.J.; Chen, M.H.; Lu, P.P.; Rui, Y.F. Tendon stem/progenitor cell ageing: Modulation and rejuvenation. World J. Stem Cells 2019, 11, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, P.; Zheng, M.; Gao, J.; Liu, D.; Wang, A.; Zheng, Q.; Leys, T.; Tai, A.; Zheng, M. Challenges and perspectives of tendon-derived cell therapy for tendinopathy: From bench to bedside. Stem Cell Res. Ther. 2022, 13, 444. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Lafuente-Merchan, M.; Ciriza, J.; Saenz-Del-Burgo, L.; Pedraz, J.L. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J. Control Release 2021, 333, 448–486. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Chuen, F.S.; Chuk, C.Y.; Ping, W.Y.; Nar, W.W.; Kim, H.L.; Ming, C.K. Immunohistochemical characterization of cells in adult human patellar tendons. J. Histochem. Cytochem. 2004, 52, 1151–1157. [Google Scholar] [CrossRef]
- Dyment, N.A.; Galloway, J.L. Regenerative biology of tendon: Mechanisms for renewal and repair. Curr. Mol. Biol. Rep. 2015, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Akinbiyi, T.; Xu, L.; Ramcharan, M.; Leong, D.J.; Ros, S.J.; Colvin, A.C.; Schaffler, M.B.; Majeska, R.J.; Flatow, E.L.; et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell 2010, 9, 911–915. [Google Scholar] [CrossRef]
- Birch, H.L.; Peffers, M.J.; Clegg, P.D. Influence of Ageing on Tendon Homeostasis. Adv. Exp. Med. Biol. 2016, 920, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; McDermott, B.T.; Goodship, A.E.; Clegg, P.D.; Birch, H.L. Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand. J. Med. Sci. Sports 2016, 26, 684–693. [Google Scholar] [CrossRef]
- Arnesen, S.M.; Lawson, M.A. Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech. Ageing Dev. 2006, 127, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kiderlen, S.; Polzer, C.; Radler, J.O.; Docheva, D.; Clausen-Schaumann, H.; Sudhop, S. Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochem. Biophys. Res. Commun. 2019, 509, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Zu, H.; Wang, J.H.; Wang, Q.M. Aging-related viscoelasticity variation of tendon stem cells (TSCs) characterized by quartz thickness shear mode (TSM) resonators. Sens Actuators 2015, 210, 369–380. [Google Scholar] [CrossRef]
- Ilić, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377, 539–544. [Google Scholar] [CrossRef]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Floridi, A.; Ippolito, E.; Postacchini, F. Age-related changes in the metabolism of tendon cells. Connect. Tissue Res. 1981, 9, 95–97. [Google Scholar] [CrossRef]
- Kohler, J.; Popov, C.; Klotz, B.; Alberton, P.; Prall, W.C.; Haasters, F.; Muller-Deubert, S.; Ebert, R.; Klein-Hitpass, L.; Jakob, F.; et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013, 12, 988–999. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.D.; Liu, J.P.; Wang, H.S.; Liu, X.M.; Wang, Q.; Cai, X.H. miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone 2015, 71, 210–216. [Google Scholar] [CrossRef]
- Alberton, P.; Dex, S.; Popov, C.; Shukunami, C.; Schieker, M.; Docheva, D. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 2015, 24, 597–609. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Tao, X.; Wang, G.; Wang, Q.; Liu, X. The role of Pin1 protein in aging of human tendon stem/progenitor cells. Biochem. Biophys. Res. Commun. 2015, 464, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Xiao, L.; Dai, G.; Lu, P.; Wang, Y.; Rui, Y. AQP1 modulates tendon stem/progenitor cells senescence during tendon aging. Cell Death Dis. 2020, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, F. Downregulation of FOXP1 correlates with tendon stem/progenitor cells aging. Biochem. Biophys. Res. Commun. 2018, 504, 96–102. [Google Scholar] [CrossRef]
- Rui, Y.F.; Chen, M.H.; Li, Y.J.; Xiao, L.F.; Geng, P.; Wang, P.; Xu, Z.Y.; Zhang, X.P.; Dai, G.C. CTGF Attenuates Tendon-Derived Stem/Progenitor Cell Aging. Stem Cells Int. 2019, 2019, 6257537. [Google Scholar] [CrossRef]
- Cury, D.P.; Dias, F.J.; Miglino, M.A.; Watanabe, I.S. Structural and Ultrastructural Characteristics of Bone-Tendon Junction of the Calcaneal Tendon of Adult and Elderly Wistar Rats. PLoS ONE 2016, 11, e0153568. [Google Scholar] [CrossRef] [PubMed]
- Popov, C.; Kohler, J.; Docheva, D. Activation of EphA4 and EphB2 Reverse Signaling Restores the Age-Associated Reduction of Self-Renewal, Migration, and Actin Turnover in Human Tendon Stem/Progenitor Cells. Front. Aging Neurosci. 2015, 7, 246. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Naito, K.; Goto, K.; Kojima, Y.; Furuhata, A.; Igarashi, M.; Nagaoka, I.; Kaneko, K. Effect of aging on the tendon structure and tendon-associated gene expression in mouse foot flexor tendon. Biomed. Rep. 2019, 10, 238–244. [Google Scholar] [CrossRef]
- Ruzzini, L.; Abbruzzese, F.; Rainer, A.; Longo, U.G.; Trombetta, M.; Maffulli, N.; Denaro, V. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2856–2866. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Moderate Exercise Mitigates the Detrimental Effects of Aging on Tendon Stem Cells. PLoS ONE 2015, 10, e0130454. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, L.; Dai, G.; Lu, P.; Zhang, Y.; Li, Y.; Ni, M.; Rui, Y. Inhibition of JAK-STAT Signaling Pathway Alleviates Age-Related Phenotypes in Tendon Stem/Progenitor Cells. Front. Cell Dev. Biol. 2021, 9, 650250. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Brochhausen, C.; Pfeifer, C.G.; Alt, V.; Docheva, D. Aged Tendon Stem/Progenitor Cells Are Less Competent to Form 3D Tendon Organoids Due to Cell Autonomous and Matrix Production Deficits. Front. Bioeng. Biotechnol. 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.; Gabrelow, C.; Pierce, J.; Gibb, E.; Elliott, J. Retinoic acid receptor signaling preserves tendon stem cell characteristics and prevents spontaneous differentiation in vitrox. Stem Cell Res. Ther. 2016, 7, 45. [Google Scholar] [CrossRef]
- Li, Y.; Dai, G.; Shi, L.; Lin, Y.; Chen, M.; Li, G.; Rui, Y. The Potential Roles of Tendon Stem/Progenitor Cells in Tendon Aging. Curr. Stem Cell Res. Ther. 2019, 14, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Knecht, R.S.; Tinguely, Y.; Eskibozkurt, G.E.; Wang, C.S.; Mooney, D.J. Aging and matrix viscoelasticity affect multiscale tendon properties and tendon derived cell behavior. Acta Biomater. 2022, 143, 63–71. [Google Scholar] [CrossRef]
- Siadat, S.M.; Zamboulis, D.E.; Thorpe, C.T.; Ruberti, J.W.; Connizzo, B.K. Tendon Extracellular Matrix Assembly, Maintenance and Dysregulation Throughout Life. Adv. Exp. Med. Biol. 2021, 1348, 45–103. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef]
- Nie, D.; Zhang, J.; Zhou, Y.; Sun, J.; Wang, W.; Wang, J.H. Rapamycin Treatment of Tendon Stem/Progenitor Cells Reduces Cellular Senescence by Upregulating Autophagy. Stem Cells Int. 2021, 2021, 6638249. [Google Scholar] [CrossRef] [PubMed]
- Riggin, C.N.; Weiss, S.N.; Rodriguez, A.B.; Raja, H.; Chen, M.; Schultz, S.M.; Sehgal, C.M.; Soslowsky, L.J. Increasing Vascular Response to Injury Improves Tendon Early Healing Outcome in Aged Rats. Ann. Biomed. Eng. 2022, 50, 587–600. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Majima, T.; Nagashima, K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol. Scand. 1994, 152, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, E.; Natali, P.G.; Postacchini, F.; Accinni, L.; De Martino, C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J. Bone Jt. Surg. Am. Vol. 1980, 62, 583–598. [Google Scholar] [CrossRef]
- Kannus, P.; Paavola, M.; Józsa, L. Aging and degeneration of tendons. In Tendon Injuries; Springer: Berlin/Heidelberg, Germany, 2005; pp. 25–31. [Google Scholar]
- Han, W.; Wang, B.; Liu, J.; Chen, L. The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells. Acta Biochim. Et Biophys. Sin. 2017, 49, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Fang, Y.; Cheung, K.; Wei, T.K.J.; Clegg, P.D.; Birch, H.L. Transcriptome analysis of ageing in uninjured human Achilles tendon. Arthritis Res. Ther. 2015, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Parkinson, J.; Samiric, T.; Ilic, M.Z.; Cook, J.; Handley, C.J. Involvement of proteoglycans in tendinopathy. J. Musculoskelet. Neuronal Interact. 2011, 11, 86–93. [Google Scholar]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative Strategies in Tendon Tissue Engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef]
- Turlo, A.J.; Ashraf Kharaz, Y.; Clegg, P.D.; Anderson, J.; Peffers, M.J. Donor age affects proteome composition of tenocyte-derived engineered tendon. BMC Biotechnol. 2018, 18, 2. [Google Scholar] [CrossRef]
- Taye, N.; Karoulias, S.Z.; Hubmacher, D. The “other” 15-40%: The Role of Non-Collagenous Extracellular Matrix Proteins and Minor Collagens in Tendon. J. Orthop. Res. 2020, 38, 23–35. [Google Scholar] [CrossRef]
- Gautieri, A.; Passini, F.S.; Silvan, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef]
- Svensson, R.B.; Heinemeier, K.M.; Couppe, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. (1985) 2016, 121, 1237–1246. [Google Scholar] [CrossRef]
- Wilson, S.L.; Guilbert, M.; Sulé-Suso, J.; Torbet, J.; Jeannesson, P.; Sockalingum, G.D.; Yang, Y. The effect of collagen ageing on its structure and cellular behaviour. In Proceedings of Dynamics and Fluctuations in Biomedical Photonics IX; SPIE: Bellingham, WA, USA; pp. 125–134.
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P.W., Hart, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Screen, H.R.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Gracey, E.; Burssens, A.; Cambré, I.; Schett, G.; Lories, R.; McInnes, I.B.; Asahara, H.; Elewaut, D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef]
- Wang, H.; Dai, G.C.; Li, Y.J.; Chen, M.H.; Lu, P.P.; Zhang, Y.W.; Zhang, M.; Cao, M.M.; Rui, Y.F. Targeting senescent tendon stem/progenitor cells to prevent or treat age-related tendon disorders. Stem Cell Rev. Rep. 2023, 19, 680–693. [Google Scholar] [CrossRef]
- Gehwolf, R.; Wagner, A.; Lehner, C.; Bradshaw, A.D.; Scharler, C.; Niestrawska, J.A.; Holzapfel, G.A.; Bauer, H.C.; Tempfer, H.; Traweger, A. Pleiotropic roles of the matricellular protein Sparc in tendon maturation and ageing. Sci. Rep. 2016, 6, 32635. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y. Tendinopathy in diabetes mellitus patients-Epidemiology, pathogenesis, and management. Scand. J. Med. Sci. Sports 2017, 27, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Seyer-Hansen, K.; Bailey, A.J. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim. Biophys. Acta 1981, 677, 313–317. [Google Scholar] [CrossRef]
- Kostrominova, T.Y.; Brooks, S.V. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age 2013, 35, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.; Screen, H.R.; Clegg, P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.M.; Archer, M.; Rai, J.; Weis, M.; Fernandes, R.J.; Eyre, D.R. Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon. Matrix Biol. Plus 2021, 12, 100070. [Google Scholar] [CrossRef]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Mayne, R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997, 72, 352–361. [Google Scholar]
- Tresoldi, I.; Oliva, F.; Benvenuto, M.; Fantini, M.; Masuelli, L.; Bei, R.; Modesti, A. Tendon’s ultrastructure. Muscles Ligaments Tendons J. 2013, 3, 2–6. [Google Scholar] [CrossRef]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Del Buono, A.; Oliva, F.; Osti, L.; Maffulli, N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-Dependent Alterations of MMPs, TIMPs and Tendon Structure in Human Achilles Tendons after Acute Rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Hannafin, J.A. The mature athlete: Aging tendon and ligament. Sports Health 2014, 6, 41–48. [Google Scholar] [CrossRef]
- Thankam, F.G.; Dilisio, M.F.; Gross, R.M.; Agrawal, D.K. Collagen I: A kingpin for rotator cuff tendon pathology. Am. J. Transl. Res. 2018, 10, 3291–3309. [Google Scholar]
- Yu, T.-Y.; Pang, J.-H.S.; Wu, K.P.-H.; Chen, M.J.; Chen, C.-H.; Tsai, W.-C. Aging is associated with increased activities of matrix metalloproteinase-2 and-9 in tenocytes. BMC Musculoskelet. Disord. 2013, 14, 2. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Dressler, M.R.; Butler, D.L.; Boivin, G.P. Age-related changes in the biomechanics of healing patellar tendon. J. Biomech. 2006, 39, 2205–2212. [Google Scholar] [CrossRef]
- Ribitsch, I.; Gueltekin, S.; Keith, M.F.; Minichmair, K.; Peham, C.; Jenner, F.; Egerbacher, M. Age-related changes of tendon fibril micro-morphology and gene expression. J. Anat. 2020, 236, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Boros, K.; Freemont, T. Physiology of ageing of the musculoskeletal system. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 203–217. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Streeter, I.; Pinchbeck, G.L.; Goodship, A.E.; Clegg, P.D.; Birch, H.L. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 2010, 285, 15674–15681. [Google Scholar] [CrossRef] [PubMed]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef]
- Xu, X.; Ha, P.; Yen, E.; Li, C.; Zheng, Z. Small Leucine-Rich Proteoglycans in Tendon Wound Healing. Adv. Wound Care 2022, 11, 202–214. [Google Scholar] [CrossRef]
- Kalamajski, S.; Aspberg, A.; Oldberg, A. The decorin sequence SYIRIADTNIT binds collagen type I. J. Biol. Chem. 2007, 282, 16062–16067. [Google Scholar] [CrossRef] [PubMed]
- Al Makhzoomi, A.K.; Kirk, T.B.; Dye, D.E.; Allison, G.T. The influence of glycosaminoglycan proteoglycan side chains on tensile force transmission and the nanostructural properties of Achilles tendons. Microsc. Res. Tech. 2022, 85, 233–243. [Google Scholar] [CrossRef]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Glycosaminoglycans of human rotator cuff tendons: Changes with age and in chronic rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.J.; Ehrle, A.; Comerford, E.J.; Canty-Laird, E.G.; Mead, A.; Clegg, P.D.; Maddox, T.W. Intrafascicular chondroid-like bodies in the ageing equine superficial digital flexor tendon comprise glycosaminoglycans and type II collagen. J. Orthop. Res. 2021, 39, 2755–2766. [Google Scholar] [CrossRef]
- Leiphart, R.; Shetye, S.; Weiss, S.; Dyment, N.; Soslowsky, L.J. Induced Knockdown of Decorin, Alone and in Tandem with Biglycan Knockdown, Directly Increases Aged Tendon Viscoelasticity. J. Biomech. Eng. 2020, 142, 111006. [Google Scholar] [CrossRef]
- Beach, Z.M.; Dekhne, M.S.; Rodriguez, A.B.; Weiss, S.N.; Adams, T.H.; Adams, S.M.; Sun, M.; Birk, D.E.; Soslowsky, L.J. Decorin knockdown is beneficial for aged tendons in the presence of biglycan expression. Matrix Biol. Plus 2022, 15, 100114. [Google Scholar] [CrossRef]
- Smith, R.K.; Gerard, M.; Dowling, B.; Dart, A.J.; Birch, H.L.; Goodship, A.E. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: A proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet. J. Suppl. 2002, 34, 241–244. [Google Scholar] [CrossRef]
- Wang, T.; Wagner, A.; Gehwolf, R.; Yan, W.; Passini, F.S.; Thien, C.; Weissenbacher, N.; Lin, Z.; Lehner, C.; Teng, H.; et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 2021, 13, eabe5738. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites 2022, 12, 125. [Google Scholar] [CrossRef]
- Vogel, H.G. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech. Ageing Dev. 1980, 14, 283–292. [Google Scholar] [CrossRef]
- Avery, N.C.; Bailey, A.J. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: Relevance to aging and exercise. Scand. J. Med. Sci. Sports 2005, 15, 231–240. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Hayashi, K.; Yamamoto, N.; Nagashima, K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 7–10. [Google Scholar] [CrossRef]
- Delabastita, T.; Bogaerts, S.; Vanwanseele, B. Age-Related Changes in Achilles Tendon Stiffness and Impact on Functional Activities: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2018, 27, 116–127. [Google Scholar] [CrossRef]
- Johnson, G.A.; Tramaglini, D.M.; Levine, R.E.; Ohno, K.; Choi, N.Y.; Woo, S.L. Tensile and viscoelastic properties of human patellar tendon. J. Orthop. Res. 1994, 12, 796–803. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Kjaer, M. The impact of loading, unloading, ageing and injury on the human tendon. J. Physiol. 2019, 597, 1283–1298. [Google Scholar] [CrossRef]
- LaCroix, A.S.; Duenwald-Kuehl, S.E.; Brickson, S.; Akins, T.L.; Diffee, G.; Aiken, J.; Vanderby, R., Jr.; Lakes, R.S. Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann. Biomed. Eng. 2013, 41, 1120–1128. [Google Scholar] [CrossRef]
- Ackerman, J.E.; Bah, I.; Jonason, J.H.; Buckley, M.R.; Loiselle, A.E. Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J. Orthop. Res. 2017, 35, 2716–2724. [Google Scholar] [CrossRef]
- Stenroth, L.; Peltonen, J.; Cronin, N.J.; Sipilä, S.; Finni, T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J. Appl. Physiol. (1985) 2012, 113, 1537–1544. [Google Scholar] [CrossRef]
- Turan, A.; Teber, M.A.; Yakut, Z.I.; Unlu, H.A.; Hekimoglu, B. Sonoelastographıc assessment of the age-related changes of the Achilles tendon. Med. Ultrason. 2015, 17, 58–61. [Google Scholar] [CrossRef]
- Lewis, G.; Shaw, K.M. Tensile properties of human tendo Achillis: Effect of donor age and strain rate. J. Foot Ankle Surg. 1997, 36, 435–445. [Google Scholar] [CrossRef]
- Hashemi, J.; Chandrashekar, N.; Slauterbeck, J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin. Biomech. 2005, 20, 645–652. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef]
- Viidik, A.; Nielsen, H.M.; Skalicky, M. Influence of physical exercise on aging rats: II. Life-long exercise delays aging of tail tendon collagen. Mech. Ageing Dev. 1996, 88, 139–148. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Skalicky, M.; Viidik, A. Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech. Ageing Dev. 1998, 100, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.F.; Scholze, M.; Babian, C.; Scheidt, H.; Vielmuth, F.; Waschke, J.; Ondruschka, B.; Hammer, N. Water-content related alterations in macro and micro scale tendon biomechanics. Sci. Rep. 2019, 9, 7887. [Google Scholar] [CrossRef]
- Birch, H.; Smith, T.; Tasker, T.; Goodship, A. Age related changes to mechanical and matrix properties in human Achilles tendon. In Proceedings of the Transactions of the 47th Annual Meeting of the Orthpaedic Research Society, San Francisco, CA, USA, 25–28 February 2001; The Society: New York, NY, USA, 2001; p. 713. [Google Scholar]
- Pardes, A.M.; Beach, Z.M.; Raja, H.; Rodriguez, A.B.; Freedman, B.R.; Soslowsky, L.J. Aging leads to inferior Achilles tendon mechanics and altered ankle function in rodents. J. Biomech. 2017, 60, 30–38. [Google Scholar] [CrossRef]
- Hansen, P.; Bojsen-Moller, J.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Mechanical properties of the human patellar tendon, in vivo. Clin. Biomech. 2006, 21, 54–58. [Google Scholar] [CrossRef]
- Stammers, M.; Ivanova, I.M.; Niewczas, I.S.; Segonds-Pichon, A.; Streeter, M.; Spiegel, D.A.; Clark, J. Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon. J. Biol. Chem. 2020, 295, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Torgutalp, S.S.; Babayeva, N.; Tas, S.; Donmez, G.; Korkusuz, F. Effects of hyperlipidemia on patellar tendon stiffness: A shear wave elastography study. Clin. Biomech. 2020, 75, 104998. [Google Scholar] [CrossRef] [PubMed]
- McCrum, C.; Leow, P.; Epro, G.; Konig, M.; Meijer, K.; Karamanidis, K. Alterations in Leg Extensor Muscle-Tendon Unit Biomechanical Properties With Ageing and Mechanical Loading. Front. Physiol. 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.K.; Arruda, E.M.; Brooks, S.V. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J. Appl. Physiol. (1985) 2011, 111, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Zaseck, L.W.; Miller, R.A.; Brooks, S.V. Rapamycin Attenuates Age-associated Changes in Tibialis Anterior Tendon Viscoelastic Properties. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Wren, T.A.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Mechanical properties of the human achilles tendon. Clin. Biomech. 2001, 16, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Stark, R.J.; Goodship, A.E.; Birch, H.L. Mechanical properties of the equine superficial digital flexor tendon relate to specific collagen cross-link levels. Equine Vet. J. Suppl. 2010, 42, 538–543. [Google Scholar] [CrossRef]
- Vafek, E.C.; Plate, J.F.; Friedman, E.; Mannava, S.; Scott, A.T.; Danelson, K.A. The effect of strain and age on the mechanical properties of rat Achilles tendons. Muscles Ligaments Tendons J. 2017, 7, 548–553. [Google Scholar] [CrossRef]
- Dressler, M.R.; Butler, D.L.; Wenstrup, R.; Awad, H.A.; Smith, F.; Boivin, G.P. A potential mechanism for age-related declines in patellar tendon biomechanics. J. Orthop. Res. 2002, 20, 1315–1322. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Udeze, C.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R. Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: A possible mechanism for age-related tendinopathy? Eur. Cell Mater. 2013, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zhu, C.; Suen, H.C.; Huang, S.; Liao, J.; Ker, D.F.E.; Tuan, R.S.; Wang, D. Tenogenic induction of human adipose-derived stem cells by soluble tendon extracellular matrix: Composition and transcriptomic analyses. Stem Cell Res. Ther. 2022, 13, 380. [Google Scholar] [CrossRef]
- Wang, D.; Pun, C.C.M.; Huang, S.; Tang, T.C.M.; Ho, K.K.W.; Rothrauff, B.B.; Yung, P.S.H.; Blocki, A.M.; Ker, E.D.F.; Tuan, R.S. Tendon-derived extracellular matrix induces mesenchymal stem cell tenogenesis via an integrin/transforming growth factor-beta crosstalk-mediated mechanism. FASEB J. 2020, 34, 8172–8186. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Hope, M.; Saxby, T.S. Tendon healing. Foot Ankle Clin. 2007, 12, 553–567. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Nerlich, M.; Pfeifer, C.G.; Docheva, D. Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J. Exp. Orthop. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, L.; Chen, Y.; Lyu, K.; Zhu, B.; Li, Y.; Liu, X.; Liu, X.; Long, L.; Wang, X.; et al. The Functions and Mechanisms of Basic Fibroblast Growth Factor in Tendon Repair. Front. Physiol. 2022, 13, 852795. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Cao, R.; Morita, H.; Soininen, R.; Chan, K.M.; Liu, B.; Cao, Y.; Tryggvason, K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004, 64, 4699–4702. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- Wojciak, B.; Crossan, J.F. The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin. Exp. Immunol. 1993, 93, 108–114. [Google Scholar] [CrossRef]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal 2009, 3, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef]
- Hattori, N.; Carrino, D.A.; Lauer, M.E.; Vasanji, A.; Wylie, J.D.; Nelson, C.M.; Apte, S.S. Pericellular versican regulates the fibroblast-myofibroblast transition: A role for ADAMTS5 protease-mediated proteolysis. J. Biol. Chem. 2011, 286, 34298–34310. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. The tendon injury response is influenced by decorin and biglycan. Ann. Biomed. Eng. 2014, 42, 619–630. [Google Scholar] [CrossRef]
- Berglund, M.; Reno, C.; Hart, D.A.; Wiig, M. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury: Differences in the regulation between tendon and tendon sheath. J. Hand Surg. Am. 2006, 31, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, W.; Lou, J.; Xing, X.; Tu, Y.; Manske, P.R. Flexor tendon healing in the rat: A histologic and gene expression study. J. Hand Surg. Am. 2003, 28, 814–823. [Google Scholar] [CrossRef]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Korcari, A.; Buckley, M.R.; Loiselle, A.E. Characterization of scar tissue biomechanics during adult murine flexor tendon healing. J. Mech. Behav. Biomed. Mater. 2022, 130, 105192. [Google Scholar] [CrossRef]
- Miyashita, H.; Ochi, M.; Ikuta, Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch. Orthop. Trauma. Surg. 1997, 116, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Thampatty, B.P.; Wang, J.H. Mechanobiology of young and aging tendons: In vivo studies with treadmill running. J. Orthop. Res. 2018, 36, 557–565. [Google Scholar] [CrossRef]
- Marques, A.C.; Albertini, R.; Serra, A.J.; da Silva, E.A.; de Oliveira, V.L.; Silva, L.M.; Leal-Junior, E.C.; de Carvalho, P.T. Photobiomodulation therapy on collagen type I and III, vascular endothelial growth factor, and metalloproteinase in experimentally induced tendinopathy in aged rats. Lasers Med. Sci. 2016, 31, 1915–1923. [Google Scholar] [CrossRef]
- Patel, S.H.; Yue, F.; Saw, S.K.; Foguth, R.; Cannon, J.R.; Shannahan, J.H.; Kuang, S.; Sabbaghi, A.; Carroll, C.C. Advanced Glycation End-Products Suppress Mitochondrial Function and Proliferative Capacity of Achilles Tendon-Derived Fibroblasts. Sci. Rep. 2019, 9, 12614. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Pufe, T.; Zantop, T.; Paulsen, F. Blood supply of the flexor hallucis longus tendon with regard to dancer’s tendinitis: Injection and immunohistochemical studies of cadaver tendons. Foot Ankle Int. 2003, 24, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Danielson, P.; Alfredson, H.; Forsgren, S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc. Res. Tech. 2007, 70, 310–324. [Google Scholar] [CrossRef]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on Pathophysiology. J. Orthop. Sports Phys. Ther. 2015, 45, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; Cevenini, E.; Nasi, M.; De Biasi, S.; Salvioli, S.; Monti, D.; Benatti, S.; Gibellini, L.; Cotichini, R.; Stazi, M.A.; et al. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur. J. Immunol. 2014, 44, 1552–1562. [Google Scholar] [CrossRef]
- Lipman, K.; Wang, C.; Ting, K.; Soo, C.; Zheng, Z. Tendinopathy: Injury, repair, and current exploration. Drug Des. Devel Ther. 2018, 12, 591–603. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Shi, L.; Lu, P.P.; Dai, G.C.; Li, Y.J.; Rui, Y.F. Advanced glycation end productions and tendon stem/progenitor cells in pathogenesis of diabetic tendinopathy. World J. Stem Cells 2021, 13, 1338. [Google Scholar] [CrossRef]
- Skovgaard, D.; Svensson, R.B.; Scheijen, J.; Eliasson, P.; Mogensen, P.; Hag, A.M.; Kjær, M.; Schalkwijk, C.G.; Schjerling, P.; Magnusson, S.P.; et al. An advanced glycation endproduct (AGE)-rich diet promotes accumulation of AGEs in Achilles tendon. Physiol. Rep. 2017, 5, e13215. [Google Scholar] [CrossRef]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Malmgaard-Clausen, N.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of Long-Term Physical Activity and Diet on Skin Glycation and Achilles Tendon Structure. Nutrients 2019, 11, 1409. [Google Scholar] [CrossRef]
- Menzel, E.J.; Reihsner, R. Alterations of biochemical and biomechanical properties of rat tail tendons caused by non-enzymatic glycation and their inhibition by dibasic amino acids arginine and lysine. Diabetologia 1991, 34, 12–16. [Google Scholar] [CrossRef]
- Iqbal, M.; Kenney, P.B.; Al-Humadi, N.H.; Klandorf, H. Relationship between mechanical properties and pentosidine in tendon: Effects of age, diet restriction, and aminoguanidine in broiler breeder hens. Poult. Sci. 2000, 79, 1338–1344. [Google Scholar] [CrossRef]
- Beason, D.P.; Hsu, J.E.; Marshall, S.M.; McDaniel, A.L.; Temel, R.E.; Abboud, J.A.; Soslowsky, L.J. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J. Shoulder Elbow Surg. 2013, 22, 681–686. [Google Scholar] [CrossRef]
- Steplewski, A.; Fertala, J.; Tomlinson, R.; Hoxha, K.; Han, L.; Thakar, O.; Klein, J.; Abboud, J.; Fertala, A. The impact of cholesterol deposits on the fibrillar architecture of the Achilles tendon in a rabbit model of hypercholesterolemia. J. Orthop. Surg. Res. 2019, 14, 172. [Google Scholar] [CrossRef]
- Grewal, N.; Thornton, G.M.; Behzad, H.; Sharma, A.; Lu, A.; Zhang, P.; Reid, W.D.; Granville Alex Scott, D.J. Accumulation of oxidized LDL in the tendon tissues of C57BL/6 or apolipoprotein E knock-out mice that consume a high fat diet: Potential impact on tendon health. PLoS ONE 2014, 9, e114214. [Google Scholar] [CrossRef]
- David, M.A.; Jones, K.H.; Inzana, J.A.; Zuscik, M.J.; Awad, H.A.; Mooney, R.A. Tendon repair is compromised in a high fat diet-induced mouse model of obesity and type 2 diabetes. PLoS ONE 2014, 9, e91234. [Google Scholar] [CrossRef] [PubMed]
- Korntner, S.; Kunkel, N.; Lehner, C.; Gehwolf, R.; Wagner, A.; Augat, P.; Stephan, D.; Heu, V.; Bauer, H.C.; Traweger, A.; et al. A high-glucose diet affects Achilles tendon healing in rats. Sci. Rep. 2017, 7, 780. [Google Scholar] [CrossRef]
- Vieira, C.P.; De Oliveira, L.P.; Da Ré Guerra, F.; Dos Santos De Almeida, M.; Marcondes, M.C.; Pimentel, E.R. Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat. Rec. 2015, 298, 538–545. [Google Scholar] [CrossRef]
- Barbosa, A.W.; Benevides, G.P.; Alferes, L.M.; Salomão, E.M.; Gomes-Marcondes, M.C.; Gomes, L. A leucine-rich diet and exercise affect the biomechanical characteristics of the digital flexor tendon in rats after nutritional recovery. Amino Acids 2012, 42, 329–336. [Google Scholar] [CrossRef]
- Shakibaei, M.; de Souza, P.; van Sickle, D.; Stahlmann, R. Biochemical changes in Achilles tendon from juvenile dogs after treatment with ciprofloxacin or feeding a magnesium-deficient diet. Arch. Toxicol. 2001, 75, 369–374. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e912. [Google Scholar] [CrossRef]
- Galloway, M.T.; Lalley, A.L.; Shearn, J.T. The role of mechanical loading in tendon development, maintenance, injury, and repair. J. Bone Joint Surg. Am. 2013, 95, 1620–1628. [Google Scholar] [CrossRef]
- Guzzoni, V.; Selistre-de-Araújo, H.S.; Marqueti, R.C. Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells 2018, 7, 251. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Arampatzis, A. Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med. Open 2015, 1, 7. [Google Scholar] [CrossRef]
- de Cássia Marqueti, R.; Almeida, J.A.; Nakagaki, W.R.; Guzzoni, V.; Boghi, F.; Renner, A.; Silva, P.E.; Durigan, J.L.Q.; Selistre-de-Araújo, H.S. Resistance training minimizes the biomechanical effects of aging in three different rat tendons. J. Biomech. 2017, 53, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D. Adaptation of the tendon to mechanical usage. J. Musculoskelet. Neuronal Interact. 2006, 6, 174–180. [Google Scholar]
- Maganaris, C.N.; Narici, M.V.; Reeves, N.D. In vivo human tendon mechanical properties: Effect of resistance training in old age. J. Musculoskelet. Neuronal Interact. 2004, 4, 204–208. [Google Scholar]
- Marqueti, R.C.; Durigan, J.L.Q.; Oliveira, A.J.S.; Mekaro, M.S.; Guzzoni, V.; Aro, A.A.; Pimentel, E.R.; Selistre-de-Araujo, H.S. Effects of aging and resistance training in rat tendon remodeling. Faseb. J. 2018, 32, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. Elife 2018, 7, 38069. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.S.; Svensson, R.B.; Gylling, A.T.; Couppé, C.; Magnusson, S.P.; Kjaer, M. Load magnitude affects patellar tendon mechanical properties but not collagen or collagen cross-linking after long-term strength training in older adults. BMC Geriatr. 2019, 19, 30. [Google Scholar] [CrossRef]
- Centner, C.; Lauber, B.; Seynnes, O.R.; Jerger, S.; Sohnius, T.; Gollhofer, A.; König, D. Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared with high-load resistance training. J. Appl. Physiol. (1985) 2019, 127, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Micallef, J.; Pandya, J.; Low, A.K. Management of rotator cuff tears in the elderly population. Maturitas 2019, 123, 9–14. [Google Scholar] [CrossRef]
- Mirzoyan, H.; Handelberg, F.; Pouliart, N. Outcome at 3 to 5 years of a treatment algorithm for rotator cuff tears in an elderly population. Acta Orthop. Belg. 2018, 84, 509–515. [Google Scholar]
- Mansat, P.; Cofield, R.H.; Kersten, T.E.; Rowland, C.M. Complications of rotator cuff repair. Orthop. Clin. N. Am. 1997, 28, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Neviaser, R.J.; Neviaser, T.J. Reoperation for failed rotator cuff repair: Analysis of fifty cases. J. Shoulder Elbow Surg. 1992, 1, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Rodriguez, M.; Patnaik, S.; Wang, P. Spontaneous Iliopsoas Tendon Tear: A Rare Cause of Hip Pain in the Elderly. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Pendse, A.; Kankate, R. Reconstruction of chronic achilles tendon ruptures in elderly patients, with vascularized flexor hallucis longus tendon transfer using single incision technique. Acta Orthop. Belg. 2019, 85, 137–143. [Google Scholar]
- Kim, J.B.; Hong, S.; Wang, S.D.; Kim, C.H. Pectoralis major tendon transfer for recurrent anterior shoulder dislocation after primary surgery in an elderly patient: A case report. Medicine 2019, 98, e14264. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, B.W.; Hekman, K.M.C.; van den Bekerom, M.P.J. Letter to the Editor: The debate of rotator cuff surgery in the elderly is going on! J. Shoulder Elbow Surg. 2019, 28, e245–e246. [Google Scholar] [CrossRef]
- Gumina, S.; Postacchini, F. Open-Surgery Technique. In Rotator Cuff Tear: Pathogenesis, Evaluation and Treatment; Gumina, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 283–296. [Google Scholar] [CrossRef]
- Dideriksen, K.; Boesen, A.P.; Reitelseder, S.; Couppe, C.; Svensson, R.; Schjerling, P.; Magnusson, S.P.; Holm, L.; Kjaer, M. Tendon collagen synthesis declines with immobilization in elderly humans: No effect of anti-inflammatory medication. J. Appl. Physiol. (1985) 2017, 122, 273–282. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, M. Treatment of infected Achilles tendinitis and overlying soft tissue defect using an anterolateral thigh free flap in an elderly patient: A case report. Medicine 2018, 97, e11995. [Google Scholar] [CrossRef] [PubMed]
- Fourniols, E.; Lazennec, J.Y.; Rousseau, M.A. Salvage technique for postoperative infection and necrosis of the Achilles tendon. Orthop. Traumatol. Surg. Res. 2012, 98, 915–920. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, H.S.; Seo, S.G.; Kim, S.W.; Gwak, H.C.; Bae, S.Y. Debridement and Functional Rehabilitation for Achilles Tendon Infection Following Tendon Repair. J. Bone Joint. Surg. Am. 2016, 98, 1161–1167. [Google Scholar] [CrossRef]
- Hennecke, K.; Redeker, J.; Kuhbier, J.W.; Strauss, S.; Allmeling, C.; Kasper, C.; Reimers, K.; Vogt, P.M. Bundles of spider silk, braided into sutures, resist basic cyclic tests: Potential use for flexor tendon repair. PLoS ONE 2013, 8, e61100. [Google Scholar] [CrossRef]
- Younesi, M.; Knapik, D.M.; Cumsky, J.; Donmez, B.O.; He, P.; Islam, A.; Learn, G.; McClellan, P.; Bohl, M.; Gillespie, R.J.; et al. Effects of PDGF-BB delivery from heparinized collagen sutures on the healing of lacerated chicken flexor tendon in vivo. Acta Biomater. 2017, 63, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.R.; Loke, A.M.K.; Tay, S.C. The Effect of Suture Materials on the Biomechanical Performance of Different Flexor Tendon Repairs and the Concept of Construct Efficiency. J. Hand Surg. Asian Pac. Vol. 2018, 23, 243–247. [Google Scholar] [CrossRef]
- Kocaoglu, B.; Ulku, T.K.; Gereli, A.; Karahan, M.; Turkmen, M. Evaluation of absorbable and nonabsorbable sutures for repair of achilles tendon rupture with a suture-guiding device. Foot Ankle Int. 2015, 36, 691–695. [Google Scholar] [CrossRef]
- Cox, J.T.; Shorten, P.L.; Gould, G.C.; Markert, R.J.; Barnett, M.D., Jr.; Laughlin, R.T. Knotted versus knotless suture bridge repair of the achilles tendon insertion: A biomechanical study. Am. J. Sports Med. 2014, 42, 2727–2733. [Google Scholar] [CrossRef]
- Rawson, S.; Cartmell, S.; Wong, J. Suture techniques for tendon repair; a comparative review. Muscles Ligaments Tendons J. 2013, 3, 220–228. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Yang, Q.Q.; Zhang, L.; Tang, J.B. Nanoparticle-coated sutures providing sustained growth factor delivery to improve the healing strength of injured tendons. Acta Biomater. 2021, 124, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, K.; Le, W.; Behn, A.W.; Yao, J. The Effect of Growth Differentiation Factor 8 (Myostatin) on Bone Marrow-Derived Stem Cell-Coated Bioactive Sutures in a Rabbit Tendon Repair Model. Hand 2020, 15, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Thompson, D.L.; Hellenbrand, D.J.; Lee, J.S.; Madura, C.J.; Wesley, M.G.; Dillon, N.J.; Sharma, T.; Enright, C.J.; Murphy, W.L. Sustained release of neurotrophin-3 via calcium phosphate-coated sutures promotes axonal regeneration after spinal cord injury. J. Neurosci. Res. 2016, 94, 645–652. [Google Scholar] [CrossRef]
- Link, A.; Haag, H.; Michel, T.; Denzinger, M.; Wendel, H.P.; Schlensak, C.; Krajewski, S. Development of a Novel Polymer-Based mRNA Coating for Surgical Suture to Enhance Wound Healing. Coatings 2019, 9, 374. [Google Scholar] [CrossRef]
- Varma, S.R.; Jaber, M.; Fanas, S.A.; Desai, V.; Al Razouk, A.M.; Nasser, S. Effect of Hyaluronic Acid in Modifying Tensile Strength of Nonabsorbable Suture Materials: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2020, 10, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Viju, S.; Marian Shilpa, L.; Thilagavathi, G. Functionalized Silk for Surgical Suture Applications; Springer: Singapore, 2019; pp. 49–65. [Google Scholar]
- Tejido-Rastrilla, R.; Ferraris, S.; Goldmann, W.H.; Grünewald, A.; Detsch, R.; Baldi, G.; Spriano, S.; Boccaccini, A.R. Studies on Cell Compatibility, Antibacterial Behavior, and Zeta Potential of Ag-Containing Polydopamine-Coated Bioactive Glass-Ceramic. Materials 2019, 12, 500. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Kumari, S.; Gupta, B. Preparation and biological characterization of plasma functionalized poly (ethylene terephthalate) antimicrobial sutures. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1034–1042. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Schnepf, K.; Goldmann, W.H.; Boccaccini, A.R. Development and Characterization of Bioactive Glass Containing Composite Coatings with Ion Releasing Function for Antibiotic-Free Antibacterial Surgical Sutures. Materials 2019, 12, 423. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, H.J.; Kim, J.S.; Lee, S.J.; Han, H.J. EphB2 signaling-mediated Sirt3 expression reduces MSC senescence by maintaining mitochondrial ROS homeostasis. Free Radic. Biol. Med. 2017, 110, 368–380. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Xiao, L.; Dai, G.; Lu, P.; Rui, Y. Noncanonical Wnt5a signaling regulates tendon stem/progenitor cells senescence. Stem Cell Res. Ther. 2021, 12, 544. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Song, W.; Cai, Z.; Ding, Z.; Chen, D.; Xia, F.; He, Y. Inhibition of IKKβ/NF-κB signaling facilitates tendinopathy healing by rejuvenating inflamm-aging induced tendon-derived stem/progenitor cell senescence. Mol. Ther. Nucleic Acids 2022, 27, 562–576. [Google Scholar] [CrossRef]
- Li, K.; Deng, Y.; Deng, G.; Chen, P.; Wang, Y.; Wu, H.; Ji, Z.; Yao, Z.; Zhang, X.; Yu, B.; et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res. Ther. 2020, 11, 131. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, L.; Zhou, Y.; Lu, X.; Shao, X.; Lin, C.; Yu, K.; Zhang, X.; Hong, J.; Chen, Y. Effect of Hypoxia on Self-Renewal Capacity and Differentiation in Human Tendon-Derived Stem Cells. Med. Sci. Monit. 2017, 23, 1334–1339. [Google Scholar] [CrossRef]
- Walia, B.; Huang, A.H. Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J. Orthop. Res. 2019, 37, 1270–1280. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Cornell, H.R.; Zargar Baboldashti, N.; Thompson, M.S.; Carr, A.J.; Hulley, P.A. Regulation of hypoxia-induced cell death in human tenocytes. Adv. Orthop. 2012, 2012, 984950. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Chandra, I.S.; Kovilam, A.N.; Diaz, C.G.; Volberding, B.T.; Dilisio, M.F.; Radwan, M.M.; Gross, R.M.; Agrawal, D.K. Amplification of Mitochondrial Activity in the Healing Response Following Rotator Cuff Tendon Injury. Sci. Rep. 2018, 8, 17027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, B.; Gao, P. Effects of young extracellular matrix on the biological characteristics of aged tendon stem cells. Adv. Clin. Exp. Med. 2018, 27, 1625–1630. [Google Scholar] [CrossRef]
- Yin, H.; Strunz, F.; Yan, Z.; Lu, J.; Brochhausen, C.; Kiderlen, S.; Clausen-Schaumann, H.; Wang, X.; Gomes, M.E.; Alt, V.; et al. Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. Biomaterials 2020, 236, 119802. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, K.Y.C.; Ng, K.W.K.; Rao, Y.; Zhu, C.; Qi, S.; Tuan, R.S.; Ker, D.F.E.; Wang, D.M. Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches. Int. J. Mol. Sci. 2023, 24, 15183. https://doi.org/10.3390/ijms242015183
Kwan KYC, Ng KWK, Rao Y, Zhu C, Qi S, Tuan RS, Ker DFE, Wang DM. Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches. International Journal of Molecular Sciences. 2023; 24(20):15183. https://doi.org/10.3390/ijms242015183
Chicago/Turabian StyleKwan, Ka Yu Carissa, Ka Wai Kerry Ng, Ying Rao, Chenxian Zhu, Shengcai Qi, Rocky S. Tuan, Dai Fei Elmer Ker, and Dan Michelle Wang. 2023. "Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches" International Journal of Molecular Sciences 24, no. 20: 15183. https://doi.org/10.3390/ijms242015183
APA StyleKwan, K. Y. C., Ng, K. W. K., Rao, Y., Zhu, C., Qi, S., Tuan, R. S., Ker, D. F. E., & Wang, D. M. (2023). Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches. International Journal of Molecular Sciences, 24(20), 15183. https://doi.org/10.3390/ijms242015183