Clinical Phenotypes of Progressive Supranuclear Palsy—The Differences in Interleukin Patterns
Abstract
:1. Introduction
2. Results
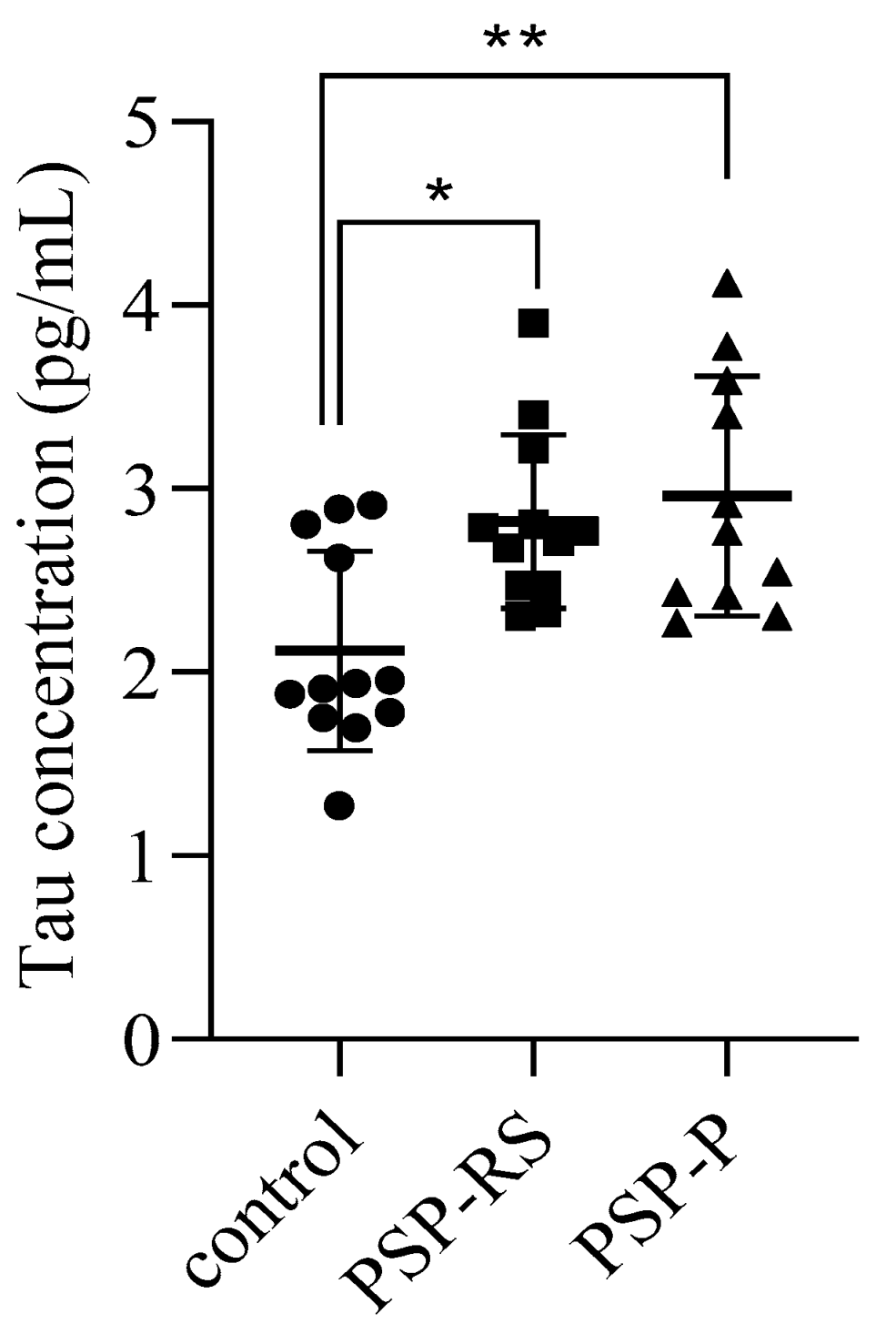
2.1. Cerebrospinal Levels of Total Tau
2.2. Serum Interleukins
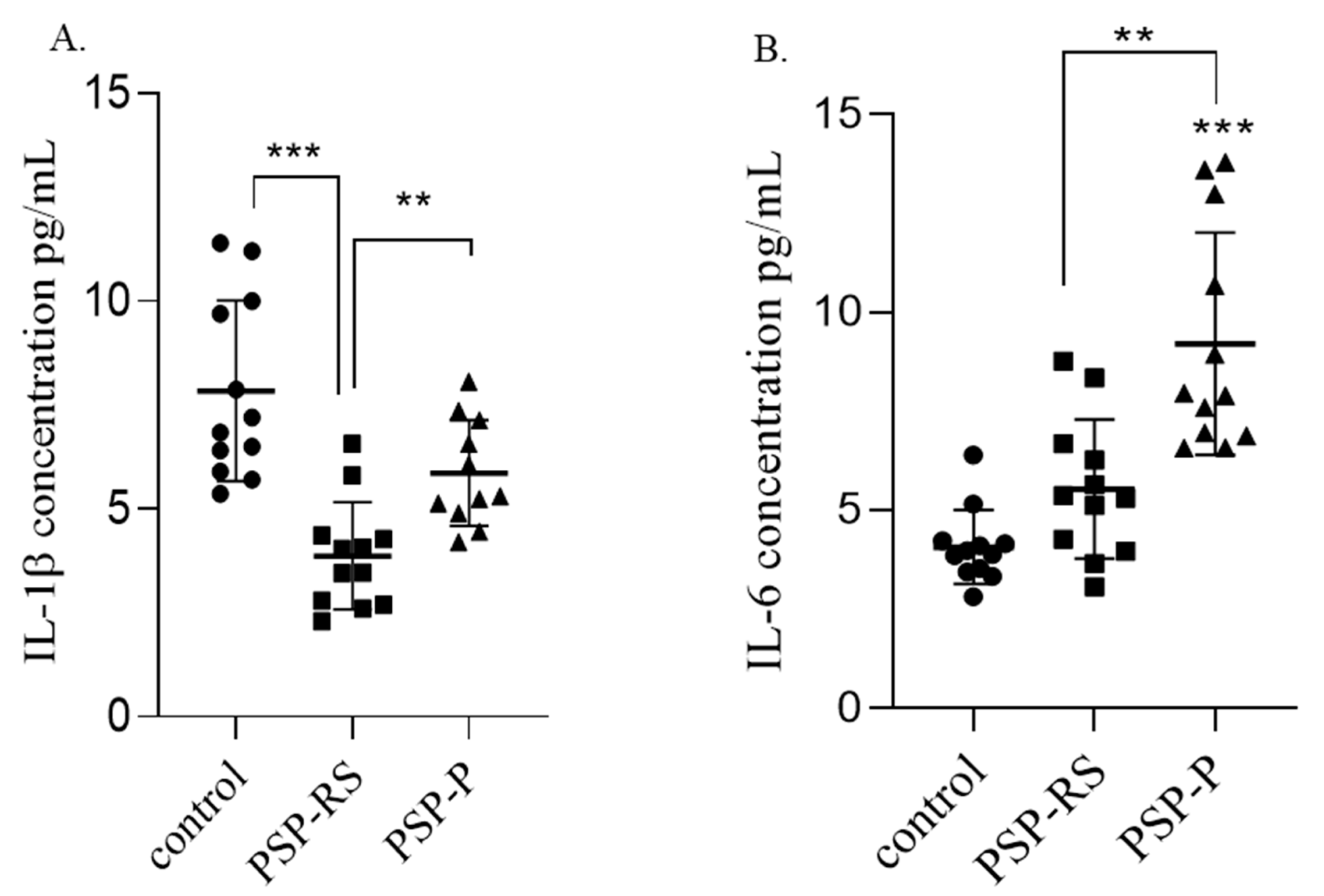
2.2.1. IL-1β
2.2.2. IL-6
2.3. Interleukins in the Cerebrospinal Fluid
2.3.1. IL-1β
2.3.2. IL-6
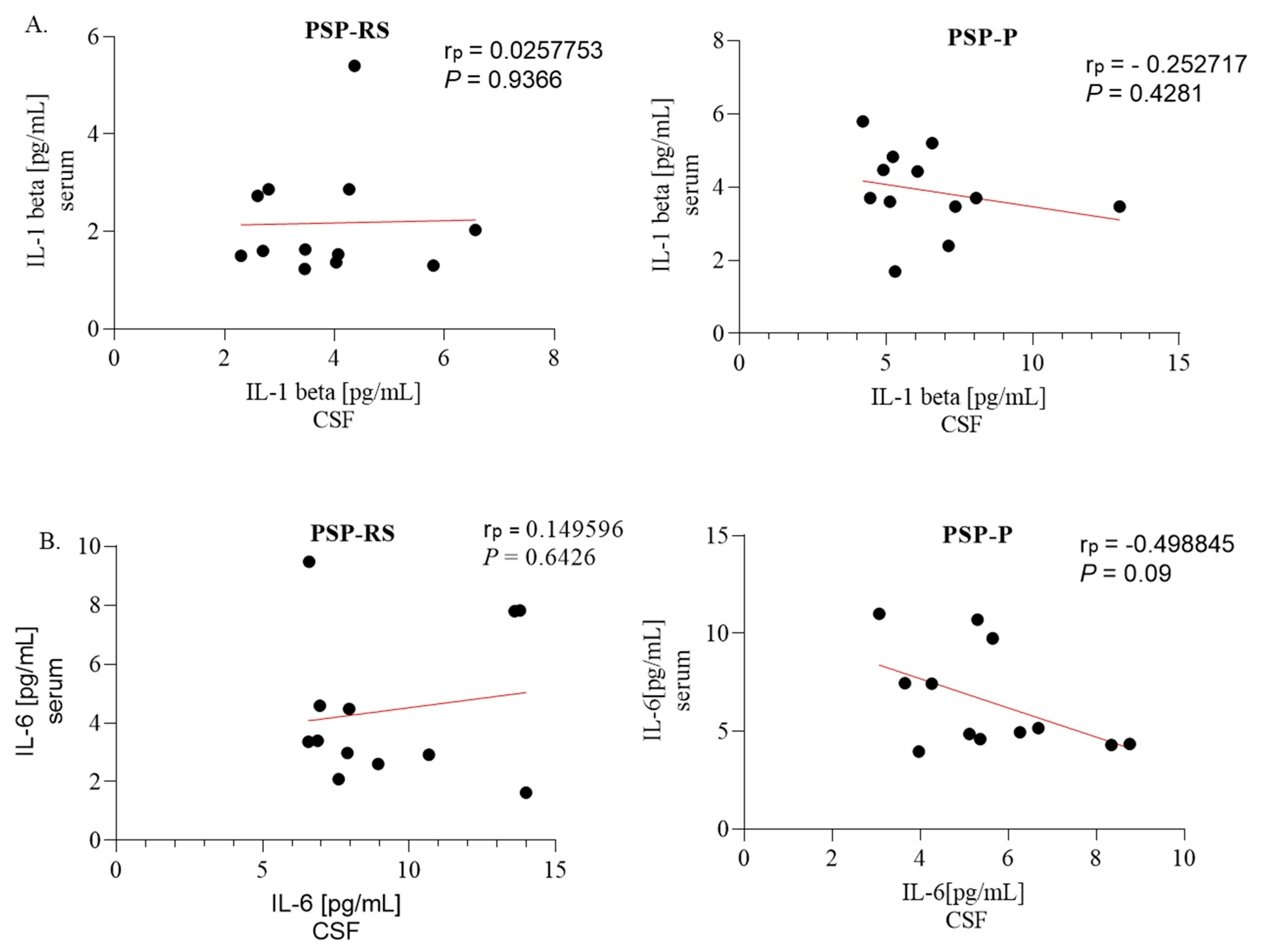
2.4. Correlations between Serum and Cerebrospinal Fluid Expression of Interleukins
2.5. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios—Results of Blood Analysis among Progressive Supranuclear Palsy Patients
2.6. Correlation of Interleukin Levels with Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio
3. Discussion
4. Material and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4R-tau | tauopathy involving four microtubule-binding repeats |
| AD | Alzheimer’s Disease |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| FTD | frontotemporal dementia |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| MSA | multiple system atrophy |
| NLR | neutrophil-to-lymphocyte ratio |
| NMDA | N-methyl-D-aspartate |
| PD | Parkinson’s Disease |
| PLR | platelet-to-lymphocyte ratio |
| PSP | progressive supranuclear palsy |
| PSP-P | progressive supranuclear palsy-parkinsonism predominant |
| PSP-RS | progressive supranuclear palsy with Richardson’s syndrome |
| SD | standard deviation |
| TNF-α | tumor necrosis factor-alpha |
References
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Progressive Supranuclear Palsy-Parkinsonism Predominant (PSP-P)-A Clinical Challenge at the Boundaries of PSP and Parkinson’s Disease (PD). Front. Neurol. 2020, 11, 180. [Google Scholar] [CrossRef]
- Yamada, T.; McGeer, P.L.; McGeer, E.G. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci. Lett. 1992, 135, 99–102. [Google Scholar] [CrossRef]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Microglial Activation and Inflammation as a Factor in the Pathogenesis of Progressive Supranuclear Palsy (PSP). Front. Neurosci. 2020, 14, 893. [Google Scholar] [CrossRef]
- Nübling, G.; Schuberth, M.; Feldmer, K.; Giese, A.; Holdt, L.M.; Teupser, D.; Lorenzl, S. Cathepsin S increases tau oligomer formation through limited cleavage, but only IL-6, not cathepsin S serum levels correlate with disease severity in the neurodegenerative tauopathy progressive supranuclear palsy. Exp. Brain Res. 2017, 235, 2407–2412. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Jankovic, J.; Goetz, C.; Brandel, J.P.; Lai, E.C.; Wenning, G.; D’Olhaberriague, L.; Verny, M.; Chaudhuri, K.R.; et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 1996, 46, 922–930. [Google Scholar] [CrossRef]
- Rydbirk, R.; Elfving, B.; Folke, J.; Pakkenberg, B.; Winge, K.; Brudek, T.; Aznar, S. Increased prefrontal cortex interleukin-2 protein levels and shift in the peripheral T cell population in progressive supranuclear palsy patients. Sci. Rep. 2019, 9, 7781. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy. 2001, 102, 5–14. [Google Scholar]
- Hosseini, S.; Shafiabadi, N.; Khanzadeh, M.; Ghaedi, A.; Ghorbanzadeh, R.; Azarhomayoun, A.; Bazrgar, A.; Pezeshki, J.; Bazrafshan, H.; Khanzadeh, S. Neutrophil to lymphocyte ratio in Parkinson’s disease: A systematic review and meta-analysis. BMC Neurol. 2023, 23, 333. [Google Scholar] [CrossRef]
- Xu, C.; Cai, L.; Yi, T.; Yi, X.; Hu, Y. Neutrophil-to-lymphocyte ratio is associated with stroke progression and functional outcome in patients with ischemic stroke. Brain Behav. 2023, 24, e3261. [Google Scholar] [CrossRef]
- Gao, H.; Yi, Y. Association of Monocyte to Lymphocyte, Neutrophil to Lymphocyte, and Platelet to Lymphocyte Ratios with Non-Healing Lower Extremity Ulcers in Patients with Type 2 Diabetes. Int. J. Low Extrem. Wounds 2023, 13, 15347346231197884. [Google Scholar] [CrossRef]
- Garcea, G.; Ladwa, N.; Neal, C.P.; Metcalfe, M.S.; Dennison, A.R.; Berry, D.P. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J. Surg. 2011, 35, 868–872. [Google Scholar] [CrossRef]
- Inci, I.; Kusbeci, O.Y.; Eskut, N. The neutrophil-to-lymphocyte ratio as a marker of peripheral inflammation in progressive supranuclear palsy: A retrospective study. Neurol. Sci. 2020, 41, 1233–1237. [Google Scholar] [CrossRef]
- Alster, P.; Madetko, N.; Friedman, A. Neutrophil-to-lymphocyte ratio (NLR) at boundaries of Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol. Neurochir. Polska 2021, 55, 97–101. [Google Scholar] [CrossRef]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Polska 2022, 56, 148–155. [Google Scholar] [CrossRef]
- Starhof, C.; Winge, K.; Heegaard NH, H.; Skogstrand, K.; Friis, S.; Hejl, A. Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J. Neuroinflamm. 2018, 15, 305. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Surova, Y.; Widner, H.; Zetterberg, H.; Hansson, O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Zarezadeh Mehrabadi, A.; Aghamohamadi, N.; Khoshmirsafa, M.; Aghamajidi, A.; Pilehforoshha, M.; Massoumi, R.; Falak, R. The roles of interleukin-1 receptor accessory protein in certain inflammatory conditions. Immunology 2022, 166, 38–46. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Hennessy, E.; Murray, C.L.; Nazmi, A.; Delaney, H.J.; Healy, D.; Fagan, S.G.; Rooney, M.; Stewart, E.; Lewis, A.; et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer’s disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimer’s Dement. 2021, 17, 1735–1755. [Google Scholar] [CrossRef]
- Wang, R.P.; Huang, J.; Chan, K.W.Y.; Leung, W.K.; Goto, T.; Ho, Y.S.; Chang, R.C. IL-1β and TNF-α play an important role in modulating the risk of periodontitis and Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863 Pt A, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Quillen, D.; Hughes, T.M.; Craft, S.; Howard, T.; Register, T.; Suerken, C.; Hawkins, G.A.; Milligan, C. Levels of Soluble Interleukin 6 Receptor and Asp358Ala Are Associated with Cognitive Performance and Alzheimer Disease Biomarkers. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200095. [Google Scholar] [CrossRef] [PubMed]
- Brosseron, F.; Maass, A.; Kleineidam, L.; Ravichandran, K.A.; Kolbe, C.C.; Wolfsgruber, S.; Santarelli, F.; Häsler, L.M.; McManus, R.; Ising, C.; et al. Serum IL-6, sAXL, and YKL-40 as systemic correlates of reduced brain structure and function in Alzheimer’s disease: Results from the DELCODE study. Alzheimer’s Res. Ther. 2023, 15, 13. [Google Scholar] [CrossRef]
- Mrak, R.E.; Griffin, W.S. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol. Aging 2001, 22, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Griffin, W.S. Interleukin-1 and the immunogenetics of Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 2000, 59, 471–476. [Google Scholar] [CrossRef]
- Griffin, W.S.; Mrak, R.E. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer’s disease. J. Leukoc. Biol. 2002, 72, 233–238. [Google Scholar] [CrossRef]
- Sjögren, M.; Folkesson, S.; Blennow, K.; Tarkowski, E. Increased intrathecal inflammatory activity in frontotemporal dementia: Pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1107–1111. [Google Scholar] [CrossRef]
- Todd, L.; Palazzo, I.; Suarez, L.; Liu, X.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 2019, 16, 118. [Google Scholar] [CrossRef]
- Willis, E.F.; MacDonald, K.P.A.; Nguyen, Q.H.; Garrido, A.L.; Gillespie, E.R.; Harley, S.B.R.; Bartlett, P.F.; Schroder, W.A.; Yates, A.G.; Anthony, D.C.; et al. Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell 2020, 180, 833–846.e16. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Jia, X.; Wang, Q.; Li, Y.; Hu, M.; Tian, L.; Yang, J.; Xing, W.; Zhang, W.; et al. Neuroprotection by IFN-γ via astrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget 2017, 8, 40065–40078. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Dong, Y. Acute and subacute IL-1β administrations differentially modulate neuroimmune and neurotrophic systems: Possible implications for neuroprotection and neurodegeneration. J. Neuroinflamm. 2013, 10, 826. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Quattrone, A.; Vescio, B.; Nigro, S.; Arabia, G.; Nisticò, R.; Novellino, F.; Salsone, M.; Arcuri, P.; et al. Magnetic Resonance Parkinsonism Index for evaluating disease progression rate in progressive supranuclear palsy: A longitudinal 2-year study. Park. Relat. Disord. 2020, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Quattrone, A.; Morelli, M.; Nigro, S.; Quattrone, A.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nisticò, R.; Salsone, M.; Novellino, F.; et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Park. Relat. Disord. 2018, 54, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener. Dis. 2008, 5, 339–346. [Google Scholar] [CrossRef]
- Lenka, A.; Pasha, S.A.; Mangalore, S.; George, L.; Jhunjhunwala, K.R.; Bagepally, B.S.; Naduthota, R.M.; Saini, J.; Yadav, R.; Pal, P.K. Role of corpus callosum volumetry in differentiating the subtypes of progressive supranuclear palsy and early Parkinson’s disease. Mov. Disord. Clin. Pract. 2017, 4, 552–558. [Google Scholar] [CrossRef]



| Control Group | PSP-RS | PSP-P | |
|---|---|---|---|
| IL-1β in CSF (pg/mL) | |||
| mean value | 7.838889 | 3.868889 | 6.448833 |
| median | 7.016667 | 3.75 | 5.683334 |
| SD | 2.17092 | 1.292941 | 2.384032 |
| IL-1β in the serum (pg/mL) | |||
| mean value | 1.538889 | 2.172222 | 3.897222 |
| median | 1.5 | 1.616667 | 3.7 |
| SD | 0.816476 | 0.63 | 1.144637 |
| IL-6 in CSF (pg/mL) | |||
| mean value | 4.071962 | 5.537339 | 9.286164 |
| median | 3.926681 | 5.331976 | 7.924135 |
| SD | 0.929435 | 1.762108 | 2.949349 |
| IL-6 in the serum (pg/mL) | |||
| mean value | 3.910656 | 4.427359 | 7.182641 |
| median | 3.397149 | 3.68391 | 5.169043 |
| SD | 1.204799 | 1.510545 | 2.573167 |
| Diagnosis | Neutrophils (×103) | Platelets (×103) | Lymphocytes (×103) | NLR | PLR |
|---|---|---|---|---|---|
| PSP-RS | 4.43 | 210.17 | 1.96 | 2.41 | 112.29 |
| PSP-P | 4.65 | 232 | 2.20 | 2.26 | 113.23 |
| PSP-P | PSP-RS | Healthy Control | |
|---|---|---|---|
| Number of participants | 12 | 12 | 12 |
| Age (years) (mean) | 55–80 (66.67) | 64–75 (68.7) | 35–69 (50) |
| Sex (male:female) | 7:5 | 7:5 | 5:7 |
| Disease duration (years) (mean) | 3–6 (4.5) | 3–6 (3.5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madetko-Alster, N.; Otto-Ślusarczyk, D.; Wiercińska-Drapało, A.; Koziorowski, D.; Szlufik, S.; Samborska-Ćwik, J.; Struga, M.; Friedman, A.; Alster, P. Clinical Phenotypes of Progressive Supranuclear Palsy—The Differences in Interleukin Patterns. Int. J. Mol. Sci. 2023, 24, 15135. https://doi.org/10.3390/ijms242015135
Madetko-Alster N, Otto-Ślusarczyk D, Wiercińska-Drapało A, Koziorowski D, Szlufik S, Samborska-Ćwik J, Struga M, Friedman A, Alster P. Clinical Phenotypes of Progressive Supranuclear Palsy—The Differences in Interleukin Patterns. International Journal of Molecular Sciences. 2023; 24(20):15135. https://doi.org/10.3390/ijms242015135
Chicago/Turabian StyleMadetko-Alster, Natalia, Dagmara Otto-Ślusarczyk, Alicja Wiercińska-Drapało, Dariusz Koziorowski, Stanisław Szlufik, Joanna Samborska-Ćwik, Marta Struga, Andrzej Friedman, and Piotr Alster. 2023. "Clinical Phenotypes of Progressive Supranuclear Palsy—The Differences in Interleukin Patterns" International Journal of Molecular Sciences 24, no. 20: 15135. https://doi.org/10.3390/ijms242015135
APA StyleMadetko-Alster, N., Otto-Ślusarczyk, D., Wiercińska-Drapało, A., Koziorowski, D., Szlufik, S., Samborska-Ćwik, J., Struga, M., Friedman, A., & Alster, P. (2023). Clinical Phenotypes of Progressive Supranuclear Palsy—The Differences in Interleukin Patterns. International Journal of Molecular Sciences, 24(20), 15135. https://doi.org/10.3390/ijms242015135







