Abstract
TCR-like chimeric antigen receptor (CAR-T) cell therapy has emerged as a game-changing strategy in cancer immunotherapy, offering a broad spectrum of potential antigen targets, particularly in solid tumors containing intracellular antigens. In this study, we investigated the cytotoxicity and functional attributes of in vitro-generated T-lymphocytes, engineered with a TCR-like CAR receptor precisely targeting the cancer testis antigen MAGE-A4. Through viral transduction, T-cells were genetically modified to express the TCR-like CAR receptor and co-cultured with MAGE-A4-expressing tumor cells. Flow cytometry analysis revealed a significant surge in cells expressing activation markers CD69, CD107a, and FasL upon encountering tumor cells, indicating robust T-cell activation and cytotoxicity. Moreover, immune transcriptome profiling unveiled heightened expression of pivotal T-effector genes involved in immune response and cell proliferation regulation. Additionally, multiplex assays also revealed increased cytokine production and cytotoxicity driven by granzymes and soluble Fas ligand (sFasL), suggesting enhanced anti-tumor immune responses. Preliminary in vivo investigations revealed a significant deceleration in tumor growth, highlighting the therapeutic potential of these TCR-like CAR-T cells. Further investigations are warranted to validate these revelations fully and harness the complete potential of TCR-like CAR-T cells in overcoming cancer’s resilient defenses.
1. Introduction
In recent years, significant strides have been made in the development of personalized treatment methods tailored specifically to address the needs of individuals afflicted with malignant neoplasms [1]. The rapid advancements in this scientific field have led to the emergence and meticulous evaluation of a diverse range of genetically modified lymphocytes. Notably, within this repertoire of lymphocytes, T-cells have been equipped with chimeric antigen receptors (CAR-T cells), intricately engineered to selectively recognize surface molecules present on tumor cells. Additionally, T-cells have been expertly engineered to incorporate artificially introduced T-cell receptors (TCR-engineered T-cells), granting them the precise specificity desired for effective therapeutic interventions [2,3,4].
CAR-T lymphocytes recognize surface antigen epitopes, which comprise 14–26% of the total cell proteome [5], limiting the number of potential effective and specific targets for anticancer CAR-T therapy. However, the intracellular domain of the chimeric receptor can be modified to transmit extra costimulatory signals that activate T-cells, ensuring resistance to the immunosuppressive tumor microenvironment and depletion [6,7,8].
TCR-engineered T-cells, similar to natural T-lymphocytes, recognize the peptide-MHC complex (pMHC), which can be derived from antigens from any cellular compartment, including neoantigens, antigens of oncogenic viruses, and germline cancer antigens (cancer-testis antigens) [9]. At the same time, proper activation of TCR-engineered T-cells requires a full-fledged co-stimulatory signal, which can be challenging to implement in an immunosuppressive tumor microenvironment. However, the efficiency of anticancer therapy can be improved by following immunization with pMHC-carrying agents such as MHC monomers or antigen-presenting cells [10].
A potential approach to combining the advantages and avoiding the disadvantages of CAR-T-cell and TCR-engineered cell therapy can be through the application of lymphocytes with a TCR-like CAR-receptor [11,12]. In this scenario, the antigen-recognition domain is composed of a single-chain variable fragment (scFv) derived from a TCR-like antibody that specifically targets the desired epitope-MHC complex. Meanwhile, the intracellular signaling domain of these lymphocytes is similar to that of CAR-T cells. Consequently, lymphocytes with a TCR-like CAR receptor can be directed to antigens at any cellular location [13]. Modifications to the CAR receptor’s intracellular signaling domain, as well as vaccination with pMHC-carrying agents, may promote its function.
This paper describes the generation, as well as the phenotypic, and functional properties of lymphocytes with a TCR-like CAR receptor, the antigen-recognizing region of which is specific to the p230–239 epitope (GVYDGREHTV) of the tumor antigen MAGE-A4 (melanoma-associated antigen family A4), and the intracellular domain contains the activation motif GITR (glucocorticoid-induced TNFR-related protein, TNFRSF18, CD357).
MAGE-A4 levels were found to be significantly higher in malignant neoplasms of the head and neck, esophagus, stomach, ovaries, endometrial, and other organs [14,15,16]. MAGE-A4 is a cancer-embryonic antigen that is primarily expressed in immune-privileged tissues such as the testicles and placenta. This makes it a promising candidate for targeted antitumor therapy with low toxicity. Previous efforts to target MAGE-A4 using protein and peptide vaccines, as well as TCR-engineered lymphocytes, have demonstrated limited clinical effectiveness [17]. Therefore, the quest for alternative approaches to anti-MAGE-A4 therapy remains relevant.
We assumed that the inclusion of the GITR activation motif within the intracellular domain of the CAR construct in the proposed approach holds the potential to improve its therapeutic efficacy. This enhancement can be attributed to the robust T-cell costimulatory activity exerted by GITR [18,19]. Moreover, the intracellular signaling mediated by GITR, resulting in NF-kB activation, remains unimpeded by the inhibitory effects of PD-1 signaling [20].
In our work, we employed in vitro transduction with viral vectors to generate TCR-like CAR-T cells. We then conducted an extensive investigation to evaluate the phenotype, activation status, and cytotoxic potential of these TCR-like CAR-T cells upon exposure to MAGEA4-positive tumor cell lines. Additionally, we assessed the therapeutic potential of these cells using an in vivo melanoma model. The findings in this study may provide substantial insights for the advancement of anticancer treatment through the utilization of genetically modified lymphocytes.
2. Results
2.1. Anti-MAGE-A4 TCR-like CAR-T Cells Manifest Cytotoxicity Markers
We conducted an in-depth analysis of flow cytometry data from T-lymphocyte cells using HSNE dimensionality reduction. An analysis was conducted on the transduced T-cells after co-cultivating them with the SK-Mel-37 cell line expressing MAGE-A4, with the aim of accurately classifying cells based on their marker expression. The clustering analysis unveiled distinct subpopulations of T-cells. Notably, the predominant subpopulation consisted of CD8+ CD69+ CD107a+ FasL+ CD40L− 4-1BB− lymphocytes, comprising approximately 68.47 ± 3.041% of the T-cells. Additionally, CD4+ FasL+ CD69+ CD107a+ CD40L− 4-1BB− cells constituted 26.12 ± 3.780%, while CD8+ CD69+ CD107a+ FasL+ CD40L− 4-1BB− lymphocytes accounted for 5.411 ± 1.167% (mean ± standard error of the mean, n = 6) (Figure 1 and Figure 2).
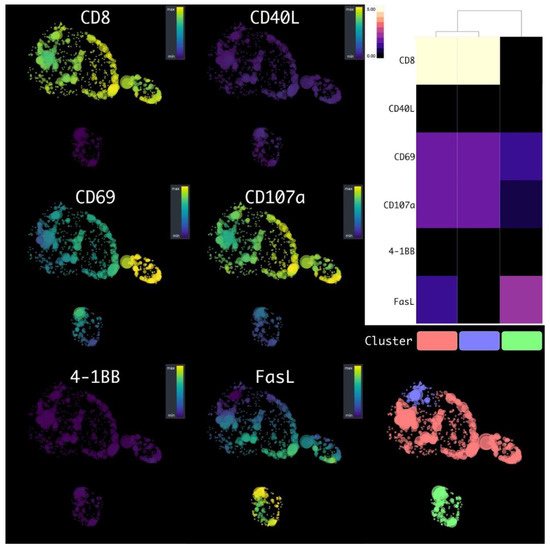
Figure 1.
HSNE plots of the transduced T-cells, after the co-cultivation with tumor cell lines, with a flow cytometry data marker overlay. Clusters are color-labeled in accordance with the heatmap.
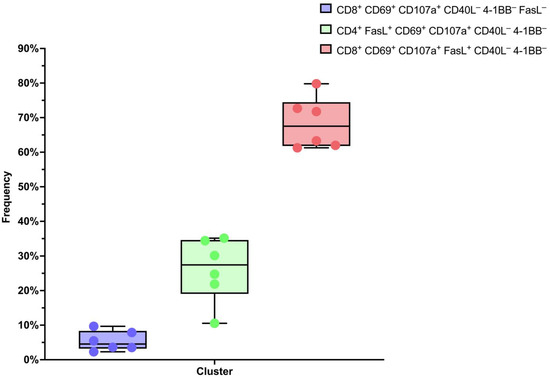
Figure 2.
Box plots of the percentage composition of each sample.
2.2. Transcriptional Profiling Reveals Immunological Features of Generated TCR-like CAR-T Cells
We then conducted differential gene expression analysis of the transduced T-cells’ immune transcriptome before and after the co-cultivation with the SK-Mel-37 cell line expressing MAGE-A4. We considered q-values < 0.005 and log2 (Fold change) > 0.847 or log2 (Fold change) < −0.847 significant (Figure 3).
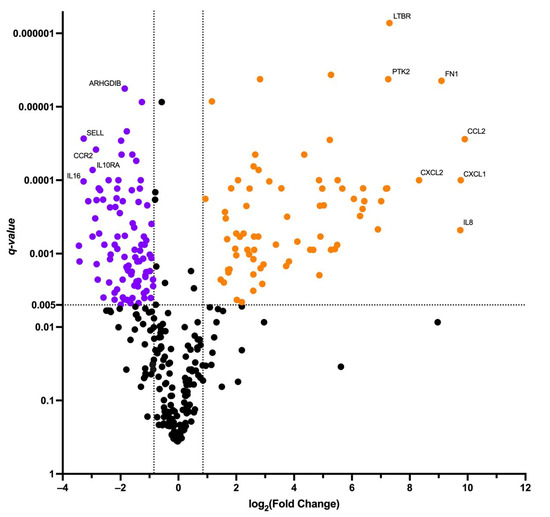
Figure 3.
Volcano plot of differentially expressed genes. Orange dots depict up-regulated genes, purple dots depict down-regulated genes.
The analysis revealed a set of genes with altered expression levels. Among the up-regulated genes were: AHR, APP, ATG12, ATG7, BCL6, BST1, C1QBP, C1R, C1S, C3, CCL2, CCL20, CCL3, CCL4, CD22, CD276, CD59, CD9, CEBPB, CFI, CIITA, CSF1, CSF2, CTNNB1, CXCL1, CXCL10, CXCL11, CXCL2, EDNRB, EGR1, EGR2, FCGR2A, FN1, GZMB, HLA-DMA, HLA-DMB, HLA-DPA1, HLA-DPB1, HLA-DRA, HLA-DRB1, HLA-DRB3, ICAM1, IFNG, IL13, IL13RA1, IL1A, IL1B, IL1RAP, IL2, IL22, IL3, IL6, IL6ST, IL8, IRAK2, ITGA6, LIF, LTBR, NCAM1, NT5E, PLAU, PLAUR, PRKCD, PSMB5, PSMB7, PTK2, SMAD5, SOCS3, SPP1, TBX21, TGFBI, THY1, TLR2, TNFRSF9.
Conversely, the down-regulated genes comprised: ADA, ARHGDIB, B2M, BAX, BCL2, CASP1, CASP8, CCL5, CCND3, CCR2, CCR5, CD244, CD247, CD27, CD28, CD3D, CD3E, CD45RB, CD5, CD53, CD6, CD7, CD79B, CD8A, CD8B, CD96, CD99, CISH, CSF2RB, CX3CR1, CXCR4, DPP4, FOXP3, GBP5, GZMA, HLA-A, HLA-B, ICAM2, ICAM3, ICOS, IFITM1, IKBKE, IKZF1, IL10RA, IL12RB1, IL16, IL2RG, IL4R, IRAK4, ITGAL, ITGAM, ITGB2, JAK1, JAK2, JAK3, KLRC4, LAIR1, LCK, LCP2, LEF1, MAP4K1, MR1, MUC1, MYD88, NCF4, NFATC3, PDGFRB, POU2F2, PRDM1, PRF1, PSMB10, PTGER4, PYCARD, RARRES3, S1PR1, SELL, SELPLG, SIGIRR, SLAMF1, SLAMF6, STAT2, STAT4, STAT5B, TAP1, TAP2, TGFB1, TGFBR1, TLR1, TMEM173, TNFSF12, TNFSF8, UBE2L3, ZAP70.
After that, we conducted gene set enrichment analysis of the up-regulated genes in gene ontology biological process terms (Figure 4, Table 1).
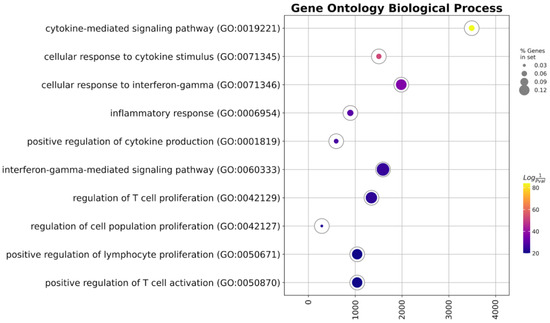
Figure 4.
GSEA of the up-regulated genes.

Table 1.
Gene ontology enrichment analysis of the up-regulated genes.
2.3. TCR-like CAR-T Cells Exhibit Antigen-Dependent Activation and Cytotoxicity In Vitro
We proceeded to investigate the antigen-dependent activation and cytotoxic potential of transduced T-lymphocytes. The transduced T-cells, acting as effectors, were co-cultured with human tumor cell lines SK-Mel-37, NW-Mel-38, and HCT-116 for a duration of 6–8 h, maintaining an effector-to-target ratio of 5:1. Our analysis revealed a significant increase in the cytotoxic response against cell lines expressing the target antigen in comparison to the MAGEA4 negative line. Interestingly, there were no notable differences observed in the cytotoxicity levels between the NW-MEL-38 and SK-MEL-37 cell lines (Figure 5).
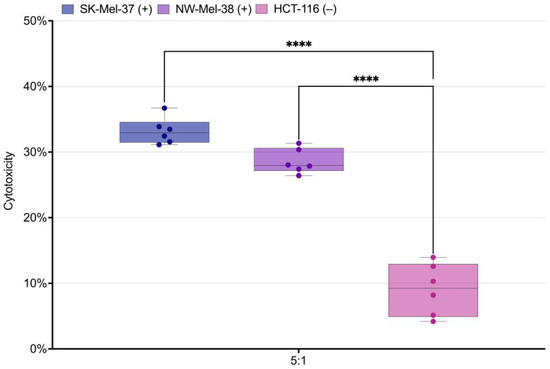
Figure 5.
Box plot of the measured cytotoxicity for the SK-Mel-37, NW-Mel-38, and HCT-116 cell lines. **** stands for q-value < 0.0001, (+) stands for the presence of the target antigen expression, (−) stands for the absence of the target antigen expression.
2.4. TCR-like CAR-T Cells Elicit the Secretion of Key Cytokines in Response to Tumor Cells
We conducted a comprehensive analysis of cytokine secretion in the conditioned media from two groups: transduced cells and non-transduced cells, both cultured with SK-Mel-37 tumor cells. The results of our analysis demonstrated significant differences in the secretion levels of pivotal cytokines: granzyme B, IFN-gamma, TNF-alpha, sFasL, and IL-2 (Figure 6, Table 2). Notably, the transduced cells exhibited substantially higher secretion levels of these cytokines compared to the non-transduced cells in response to the SK-Mel-37 tumor cells.
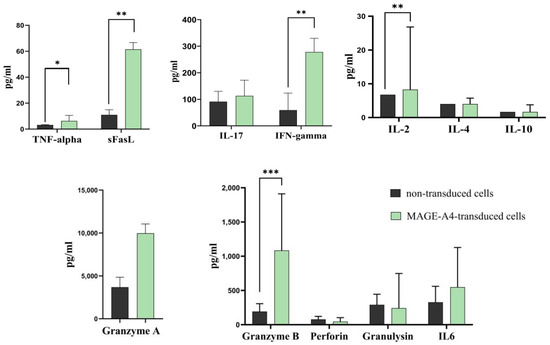
Figure 6.
Bar plot of the cytokines secreted by transduced T-cells following co-cultivation with tumor cell lines expressing the target antigen. Each bar’s height represents the median levels of cytokines, and the error bars indicate the interquartile range. Significance levels are indicated by asterisks, with *** denoting a p-value < 0.001, ** representing a p-value < 0.01, and * indicating a p-value < 0.05.

Table 2.
Quantitative Analysis of Cytokine Production Levels in MAGEA4-Transduced T-Cell after Co-Culture with the SK-MEL-37 Line.
2.5. Anti-MAGEA4 TCR-like CAR Slows Tumor Growth in Melanoma Model In Vivo
To evaluate the efficacy of MAGEA4-directed TCR-like CAR-T cells in a solid tumor model in vivo, we introduced human melanoma cells SK-Mel-37 expressing MAGEA4 into NRG mice. Once the average tumor volume reached 100 mm3, we randomly assigned the mice into three groups (untreated, control, experimental), each consisting of eight mice. In the control group, we administered non-transduced T-cells, while in the experimental group, we treated the mice with anti-MAGE-A4 TCR-like CAR-T cells. Both treated groups received a single dose of 8 million cells per mouse, while the untreated group did not receive any treatment. Starting from day 22, mice treated with Anti-MAGE-A4 TCR-like CAR-T cells exhibited a significant reduction in tumor growth compared to the untreated group. Furthermore, a single dose of anti-MAGE-A4 TCR-like CAR-T cells significantly slowed tumor growth compared to mice in the control group, starting from day 32 (Figure 7).
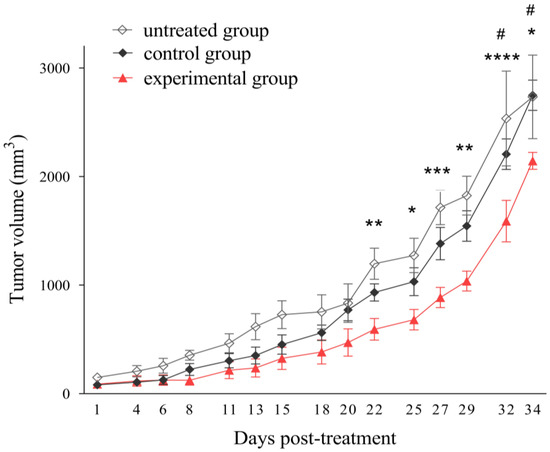
Figure 7.
Tumor growth impairment with MAGE-A4-Specific TCR-like CAR-T-cell treatment in a melanoma model. SK-Mel-37 tumor cells (5 million/mouse) were administered to NRG mice (n = 24). Once the tumors reached sizes of 80–100 mm3, the control group received non-transduced T-cells, the experimental group was treated with anti-MAGE-A4 TCR-like CAR-T cells (8 million/mouse intravenously), and the untreated group also served as a control. The asterisks (*) indicate statistically significant differences between the experimental and untreated groups, with corresponding p-values: **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Grids (#) represent significant disparities between the experimental and control groups: # p < 0.05, determined by two-way ANOVA.
3. Discussion
In this study, we embarked on a comprehensive exploration of the intricate mechanisms involved when a TCR-like CAR (chimeric antigen receptor) binds to a pMHC (peptide–major histocompatibility complex) on the surface of tumor cells. This binding event triggers a cascade of intracellular signaling events within the TCR-like CAR-T cells, ultimately activating them to seek out and eliminate tumor cells. Importantly, this TCR-like CAR system mimics the functionality of natural T-cell receptors (TCRs) but offers distinct advantages over traditional transgenic TCRs [21]. This novel receptor design, akin to affinity-enhanced TCRs, holds the potential to revolutionize T-cell-based immunotherapies, offering improved antigen specificity and recognition capabilities [22,23].
Our investigation was centered on analyzing flow cytometry data, employing HSNE (hierarchical stochastic neighbor embedding) dimensionality reduction, to delve into the dynamic interplay of immune responses. Specifically, we focused on T-cell interactions with the SK-Mel-37 cell line expressing MAGE-A4, an antigen of interest in melanoma immunotherapy. The outcomes of our analysis unveiled a diverse landscape of T-cell subpopulations, each defined by unique marker expression profiles.
One particularly intriguing T-cell subpopulation stood out: CD8+ CD69+ CD107a+ FasL+ CD40L− 4-1BB− lymphocytes. This subset exhibited characteristics strongly associated with cytotoxicity and the targeted eradication of tumor cells. The presence of CD107a granular membrane proteins reinforced the notion that these T-cells play a pivotal role in eliminating tumor cells [24]. Equally noteworthy was the expression of Fas ligand (FasL), hinting at a potential mechanism by which these T-cells induce apoptosis in target tumor cells [25]. Adding another layer of complexity to our findings, we identified CD4+ FasL+ CD69+ CD107a+ CD40L− 4-1BB− cells, suggesting that CD4+ T-cells might also participate in cytotoxic functions. Traditionally, CD8+ T-cells have held the spotlight as the primary effectors of cytotoxic responses. However, emerging research points to the capacity of CD4+ T-cells to exhibit cytotoxic capabilities under specific conditions [26], opening new avenues for our understanding of anti-tumor immunity.
Moreover, the early activation marker CD69 signals that these T-cell subpopulations swiftly responded upon encountering MAGE-A4-expressing tumor cells [27].
Beyond the phenotypic characterization of T-cell subsets, our research extended to a comparative analysis of the immune transcriptome. We scrutinized gene expression in transduced T-cells both before and after co-cultivation with MAGE-A4-expressing SK-Mel-37 cells. This comprehensive analysis, coupled with gene set enrichment analysis (GSEA) of up-regulated genes, offered compelling evidence of immune function activation. We observed the up-regulation of pivotal immune-related genes known for their cytotoxic effects, including GZMB (granzyme B) and IFNG (interferon-gamma). This suggests a robust immune response capable of enhancing the anti-tumor potential of transduced T-cells [28].
One notable observation was the significant upregulation of the FCGR2A gene, which encodes CD32 receptors. These receptors are crucial for binding to the Fc portion of IgG, highlighting their essential role in bridging innate and adaptive immunity. Interestingly, our research hinted at the potential of CD4+ T-cells to influence the expression of CD32 receptors, both on the cell surface and intracellularly [29].
Furthermore, our investigation unveiled an increased transcriptional activity of genes encoding chemokines. These genes play established roles in directing T-cell trafficking, facilitating infiltration into tumor microenvironments, governing migratory patterns, and orchestrating intricate cellular interactions [30]. Some of these chemokines could potentially emerge as significant products of activated CD4+ lymphocytes, akin to traditional cytokines. Notably, the interaction between CD4+ T-cells and antigen-presenting dendritic cells (DCs) was found to induce the production of CCL3 and CCL4 chemokines, which bind to CCR5 on naive CD8+ T-cells, promoting their migration to sites of immune activation [31].
One of the central players highlighted in our research was IL-2 (Interleukin-2), known for its potent mitogenic and growth-inducing properties. Extensive prior studies have primarily explored IL-2 signaling in antigen receptor-activated CD4+ and CD8+ T-cells. IL-2 plays a pivotal role in precisely regulating cytokine receptors, transcription factors, chromatin regulators, and effector cytokines, significantly influencing T-cell fate. Among these factors, T-bet, encoded by Tbx21, emerges as a critical regulator [32]. It plays a multifaceted role in the differentiation and effector functions of cell-mediated immunity. Specifically, T-bet’s influence extends to T helper type 1 (Th1) cell differentiation, particularly through its regulation of interferon-gamma (IFNG) expression. This regulatory role extends to cytotoxic CD8+ T-cells and natural killer (NK) cells, impacting the expression of IFN-gamma, perforin, and granzyme B [33,34,35]. Furthermore, collaborative mechanisms, such as the interplay between T-bet and Bcl-6 in regulating Th1 gene expression patterns, contribute to maintaining IFNG expression within a balanced range [36].
Our transcriptome analysis may indicate the coexistence of effector differentiation and memory development within a subset of cells, consistent with previous research. Importantly, Bcl6 has been closely linked to the generation and proliferation of memory CD8+ T-cells [37,38]. This promising characteristic could potentially enhance both rapid proliferation and sustained immune responses in vivo. However, further investigations are warranted, particularly concerning the metabolic aspects of this regulation, offering the prospect of deeper insights into the realms of immunology and immunotherapy.
Notably, our research showcased a down-regulation of crucial regulatory T-cell (Treg) function genes, including FOXP3, TGFB1, and TGFBR1 [39,40]. This observation implies a potential reduction in the immunosuppressive microenvironment within the T-cell response. Complementing these findings, our comprehensive analysis of various cytokine proteins indicated no significant secretion levels of the anti-inflammatory cytokines IL-10 and IL-4 (Figure 6) [41,42]. These observations could be attributed to the co-stimulatory GITR signaling previously mentioned [18]. The upregulation of GITR on effector T-cells upon TCR activation has been shown to inhibit the suppressive activity of Tregs and enhance the survival of effector T-cells [43]. This modulation of the immune landscape potentially creates a more favorable environment for CAR-T-cell activity, further augmenting their anti-tumor effects.
Intriguingly, our investigation into gene expression at the protein level has unveiled increased production of granzyme B, IFN-gamma, TNF-alpha, and soluble Fas ligand (sFasL) pin-transduced TCR-like CAR-T cells upon encountering SK-Mel-37 tumor cells. These findings are consistent with the established mechanism of CAR-T-cell therapy [28], suggesting that the cytotoxic activity of TCR-like CAR-T cells involves a combination of degranulation and ligand-based lytic pathways, which may work synergistically or additively. Particularly, it is interesting to note that FasL can enhance lytic action even in instances of poor or hampered degranulation [44,45].
Furthermore, the investigation of the immune transcriptome revealed a down-regulation in the expression of the perforin gene (PRF1). This was supported by cytokine analysis (Figure 6), which indicated no notable production of perforin. These findings point to a possible mechanism in which granzyme B-mediated activity is independent of perforin. However, the precise molecular mechanisms underlying granzyme delivery remain unclear, as conflicting reports have emerged, leading to disparate findings in the scientific literature [46].
It is essential to acknowledge the potential translational and clinical implications of our findings. Although our in vitro approach provided valuable insights, it is vital to consider its limitations, particularly in assessing potential off-target effects and toxicity. Previous studies have indicated that single-chain variable fragments (scFvs), such as those used in CARs, can exhibit cross-reactivity with different peptides, recognizing unique conformations of MHC proteins bound to specific peptides or directly binding to peptides, akin to T-cell receptors (TCRs) [47,48]. Furthermore, the release of cytokines by activated T-cells during treatment can lead to immune-related adverse events such as cytokine release syndrome (CRS) and neurotoxicity [49].
To bridge the gap between preclinical research and clinical translation, we conducted in vivo studies using immunodeficient mice bearing melanoma tumors. Our preliminary and preclinical findings demonstrated a significant reduction in tumor growth in the experimental group compared to both the untreated and control groups. Although complete tumor regression was not attained, the observed reduction in tumor growth is particularly promising. This is noteworthy, especially considering that only a single dose of anti-MAGE-A4 TCR-like CAR-T cells was administered in this study. Given the well-documented dose–response relationship in CART-cell therapy, the optimization of treatment strategies through precise dosage adjustments, the exploration of synergistic combination therapies, and the implementation of multiple dosages exhibit significant potential for enhancing treatment efficacy [50,51].
In summary, our study provides a comprehensive understanding of TCR-like CAR T-cell responses, offering promising insights into improving immunotherapy strategies for cancer treatment.
4. Materials and Methods
4.1. Study Population and Interventions
Venous blood sampling was conducted on a cohort of six healthy adult donors. The average age of the healthy blood donors was determined to be 27.33 ± 6.34 years (mean ± standard error of the mean).
4.2. PBMC Isolation
We performed venous blood collection, drawing up to 5 mL of blood, and preserved the samples in EDTA-containing tubes. We isolated peripheral blood mononuclear cells (PBMCs) from whole blood samples using a conventional Ficoll–Urografin (PanEco, Moscow, Russia) density gradient method. Briefly, we diluted the peripheral blood with an equal volume of RPMI-1640 medium (Biolot, St. Petersburg, Russia). The diluted blood was then layered on top of a Ficoll–Urografin solution (ρ = 1.077 g/L) and subjected to centrifugation at 400× g and room temperature for 40 min. Subsequently, the mononuclear cells were harvested from an opalescent layer located at the phase boundary, spanning the entire cross-section of the tube.
4.3. Retroviral Particles
We were provided with a gamma-retroviral vector by Prof. H. Shiku from Mie University Graduate School of Medicine, Japan. This vector contains an insert encoding a specialized T-cell receptor (CAR) designed exclusively to target the p230–239 epitope (GVYDGREHTV) of the tumor antigen MAGE-A4 (Scheme 1).
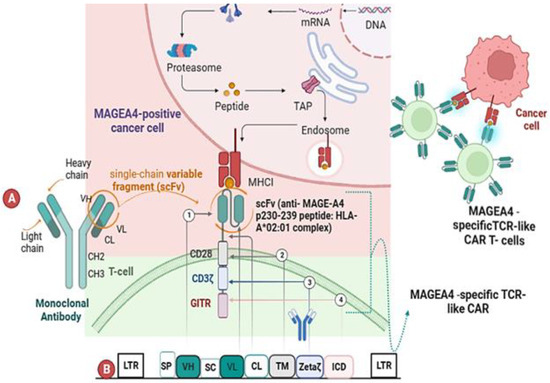
Scheme 1.
The scheme of the pMAGE-A4 transfer MMLV plasmid. (A) Illustrates the structural composition of a TCR-like CAR, which consists of the following structural elements: ① an extracellular domain responsible for antigen recognition, consisting of a single-chain variable fragment (scFv) that specifically interacts with the p230–239 anti-MAGE-A4 peptide bound to HLA-A*02:01; ② a transmembrane domain derived from the CD28 receptor; ③ the CD3ζ molecule acting as the primary signal transducer; ④ the intracellular domain of GITR, which mimics co-stimulation and enhances the TCR recognition by the antigen-presenting cell. (B) Presents a schematic representation of the vector encoding MAGEA4-specific TCR-like CAR receptor cells. The vector comprises various key elements, as depicted in the schematic, including the following: LTR (long terminal repeat), SP (signal peptide), VH (heavy chain variable domain), VL (light chain variable domain), along with a hinge binding section of the constant light chain (CL). Additionally, it includes SC (single chain), TM (transmembrane domain), ICD (intracellular domain), GITR (glucocorticoid-induced TNFR family-related protein), and CH (heavy chain constant domain).
4.4. Induction of T-Cell Proliferation
We stimulated the proliferation of peripheral blood mononuclear cells (PBMCs) by immobilizing retronectin at a concentration of 25 mg/mL (Takara Bio, Kusatsu, Japan) and CD3 antibodies at a dose of 5 mg/mL (Biolegend, San Diego, CA, USA) onto the wells of 12-well plates (TPP, Trasadingen, Switzerland). The PBMCs were cultured at a concentration of 0.5 × 106 cells/mL in 2 mL of GT-T551 medium (Takara Bio, Japan) supplemented with 300 U/mL IL-2 (Roncoleukin, Biotech, Moscow, Russia) and 0.6% human blood serum of group AB. The plates were then incubated in a humidified atmosphere with 5% CO2 at a temperature of 37 °C. During the 2nd and 3rd days of cultivation, we actively replenished half of the culture medium volume with a fresh portion of IL-2.
4.5. Retroviral Transduction of the Anti-CD3 Primed PBMCs
To perform retroviral transduction, we thawed 1 mL of a retrovirus solution in a water bath at 37 °C. Then, we diluted it four times in PBS (Biolot, St. Petersburg, Russia) with 2% human serum albumin (Microgen, Tomsk, Russia), and a 5% glucose-citrate buffer. We added the diluted retroviral solution to retronectin-coated wells of 24-well plates and centrifuged the plate with the retrovirus solution for 2 h at 32 °C at 2000× g (Jouan MR 23, Nantes, France). After the liquid content was removed, the wells were washed twice with PBS containing 2% albumin. Subsequently, 1.5–2 × 105 PBMCs primed with anti-CD3 antibodies were added to each well, resuspending them in 1.5 mL of medium supplemented with IL-2 (300 U/mL), and the cell suspension plates were centrifuged at 1000× g for 10 min at 32 °C. The cell plates were placed in a humidified atmosphere with 5% CO2 at 37 °C. The next day, a second round of transduction was performed by transferring the cells into the wells of new 24-well plates with retroviral particles. The cells were then centrifuged at 1000× g for 10 min at 32 °C, which allowed for an increase in the efficiency of transduction of 10–15%. After that, the cells were incubated in a humid atmosphere at 37 °C containing CO2. Following 6–8 h, the cells were transferred to new 6-well plates (TPP, Trasadingen, Switzerland) supplemented with an equivalent volume of GT-T551 culture medium and 300 U/mL IL-2. On days 9–10 after initiating the protocol, an aliquot of cells was collected to assess transduction efficiency and determine the cell phenotype. On the 11th day from the start of the protocol, the transduced cells were co-cultured with tumor cell lines to evaluate their cytotoxic activity in vitro. As a control, we used cells primed with anti-CD3 and stimulated with IL-2, to which no retrovirus was added, and no transduction was performed.
4.6. Evaluation of the Efficiency of Transduction
We employed biotin-SP (long spacer) AffiniPure F(ab′)2 Fragment Goat Anti-Mouse IgG, F(ab′)2 fragment specific (Jackson Immunoresearch, West Grove, PA, USA) to assess the transduction efficiency of PBMCs. The cells were stained with these antibodies at a 1:1000 dilution and incubated for 20 min in the dark at room temperature. After two washes, we added streptavidin-PE at a dilution of 1:600, along with monoclonal antibodies targeting surface markers (anti-CD3-AF700 (Biolegend, USA)) and Zombie Aqua vital dye (Biolegend, USA) to evaluate the culture’s viability. Staining was conducted for 20 min at room temperature in the dark. The stained cells were then washed with PBS containing 0.1% NaN3 and subjected to analysis using an Attune NxT flow cytometer (Thermo Fisher, Waltham, MA, USA). We gated cells from debris, singlets from the cells, alive cells from the singlets, CD3-positive cells from the alive cells, and anti-Fab-positive cells from the CD3-positive cells. We observed 50 ± 12.28% retroviral transduction of said T-cells (mean ± standard deviation), with a minimum of 37.7% and a maximum of 62.2% (Scheme 2).
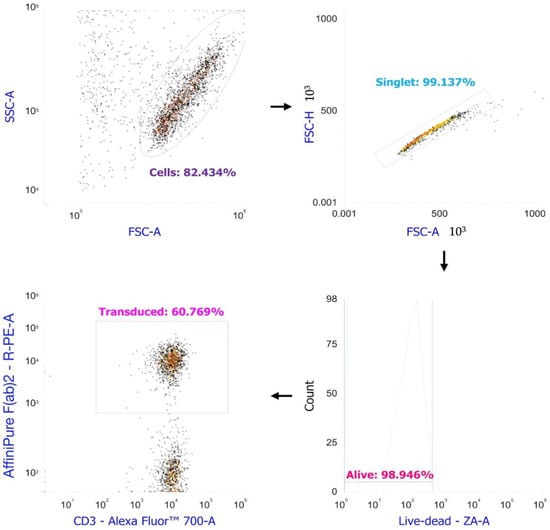
Scheme 2.
Retroviral transduction estimation of the anti-CD3 primed PBMCs by flow cytometry.
4.7. Cell Lines
We utilized human melanoma cell lines NW-Mel-38 and SK-Mel-37 as cell lines that express the MAGEA4 target antigen. For our experiments, we used the HCT-116 cell line as a negative control, which does not express the MAGE-A4 target antigen. The tumor cell lines were cultivated in culture flasks with a planting concentration of 50–75 thousand cells/mL until they reached the log-phase of growth. RPMI-1640 medium supplemented with 10% FCS (Hyclone, Logan, UT, USA), 2 mM L-glutamine (Biolot, Russia), 5 × 10−5 mM mercaptoethanol (Sigma, Cibolo, TX, USA), 25 mM HEPES (Sigma, USA), 80 µg/mL gentamicin (Krka, Novo Mesto, Slovenia), and 100 µg/mL ampicillin (Sintez, Kurgan, Russia) were used for cell culture. After detachment from the plastic surface using trypsin-versen solution (Biolot, St. Petersburg, Russia), the cells were seeded into the wells (4–5 × 104 cells/well) of a 96-well flat-bottomed culture plate (TPP, Switzerland) for 16–17 h, ensuring proper adhesion of the tumor cells to the plastic. The cell lines NW-Mel-38 and SK-Mel-37 were obtained from the collection of cell cultures at the Graduate School of Medicine, Mie University, under the supervision of Professor H. Shiku.
4.8. Phenotyping for Markers of Activation and Cytotoxicity by Flow Cytometry
We aimed to explore the antigen-dependent activation level and cytotoxic potential of transduced T-lymphocytes by co-culturing the studied T-cells with tumor target cells. The adherent tumor cells (target) were initially cultured for 16–17 h, after which they were replaced with fresh culture medium, and T-cells (effectors) were introduced to achieve an effector: target ratio of 5:1. We co-cultured the transduced T-cells with human tumor lines SK-MEL-37, NW-Mel-38, and HCT-116 for 4–5 h. We conducted an analysis of activation and cytotoxicity marker expression using flow cytometry, employing a range of monoclonal antibodies, including anti-CD3-AF700 (cat #300324), anti-CD8-PeCy7 (cat #344712), anti-CD4-Bv570 (cat #300534), CD137(4-1BB)-BV711TM (cat #309832), anti-CD154 (CD40L)-PerCP/Cy5 (cat #310834), anti-CD69-AF647 (cat #310918), anti-CD178 (FasL)-BV421TM (cat #306412), and anti-CD107a-APC/Cyanine7 (cat #328630). All antibodies were sourced from Biolegend (USA) and used in strict accordance with the manufacturer’s instructions. For MAGEA4-specific cell labeling, we utilized anti-Fab as previously described. Following the staining process, the cells were washed with PBS containing 0.1% NaN3 and subsequently analyzed using an Attune NxT flow cytometer (Thermo Fisher, USA). We then gated cells from debris, singlets from the cells, alive cells from the singlets, and CD3-positive cells from the alive cells, and exported them as.fsc files in the conventional gating software for the Attune NxT Flow cytometer. We then transformed the .fcs files to .csv files using a custom Python 3 code via Jupyter Notebooks. We performed arcsinh-transformation with the automatically selected cofactors of the flow cytometry data contained in the .csv files to automatically split cells into negative and positive for all the markers with the R script (https://github.com/janinemelsen/Single-cell-analysis-flow-cytometry/blob/master/scripts/CSV_to_transformed_normalized_FCS_git.R, accessed on 16 July 2023) published by Melsen et al. [52]. We then performed simultaneous batch correction and data normalization by fdaNorm and exported the corrected .csv files as .fcs with the R script (https://github.com/janinemelsen/Single-cell-analysis-flow-cytometry/blob/master/scripts/clustering_dimensionalityreduction_pseudotime_git.R, accessed on 16 July 2023) originally published by Melsen et al. [52].
4.9. HSNE Dimensionality Reduction and Clustering
We normalized the .fcs files into the Cytosplore app [53] and subjected them to an HSNE dimensionality reduction. We used CD4, CD8, CD40L, CD69, CD107a, CD137 (4-1BB), and FasL (CD178) for dimensionality reduction. We then exported the frequencies of the cells per cluster and visualized them in GraphPad Prism 9.4 as box plots.
4.10. Magnetic Separation of Transduced T-Cells after Co-Culturing with Tumor Cells
To investigate the gene expression profile, we isolated MAGEA4-positive cells from the overall cell population following retroviral transduction. We accomplished this by adding biotin-SP (long spacer) AffiniPure F(ab′)2 Fragment Goat Anti-Mouse IgG, F(ab′)2 fragment-specific antibodies to the cells on day 11 of the experiment (10 µL of antibodies per 10 × 106 cells), followed by a 20 min incubation in a cold Versene solution supplemented with 0.5% bovine serum albumin. After two washes, we added magnetic beads conjugated with MojoSort™ Streptavidin Nanobeads (Biolegend, USA, cat #480016) to the cells at a rate of 10 µL of beads per 10 × 106 cells. We then washed and sorted the cells using a MojoSort™ Magnet (Biolegend, USA, cat #480019). The sorted cells were allowed to rest for 16–17 h in a culture medium supplemented with IL-2 (300 U/mL). Next, we co-cultured these cells with adherent MAGEA4-positive tumor cells (SK-Mel-37) at an effector-to-target ratio of 5:1, maintaining the co-culture for 2 h to activate key genes that regulate the immune response. Following the co-cultivation with tumor cells, we separated the transduced T-cells from the tumor cells using intensive pipetting in PBS. To eliminate any tumor cell contamination, we conducted positive magnetic sorting for the MojoSort™ Human CD45 Nanobeads (Biolegend, USA, cat #480029) marker. The viability of the magnetically sorted cells was then assessed using a Countess 3 Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA, USA) and trypan blue staining, revealing a cell viability of over 92%.
4.11. Total RNA Extraction
We isolated total RNA from 300.000 to 600.00 cells with the Total RNA Purification Plus Kit (Norgen Biotek, Thorold, ON, Canada). We measured the concentration and quality of the total RNA in each sample on a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). We froze the total RNA at −80 °C until analysis.
4.12. Gene Expression Profiling by Nanostring
We performed gene expression profiling with the help of the Nanostring nCounter SPRINT Profiler analytical system using 100 ng of total RNA from each sample. We used an nCounter Human Immunology v2 panel to analyze the total RNA samples. The nCounter Human Immunology v2 panel consists of 579 immune and inflammation-associated genes, 15 housekeeping genes, and eight negative and six positive controls. The samples (n = 3–6) were subjected to an overnight hybridization reaction at 65 °C, where 5–14 μL of total RNA was combined with 3 μL of nCounter Reporter probes, 0–7 μL of DEPC-treated water, 11 μL of hybridization buffer, and with 5 μL of nCounter capture probes (total reaction volume = 33 μL). After the hybridization of the probes to targets of interest in the samples, the number of target molecules was determined on the nCounter digital analyzer. We performed normalization and QC in nSolver 4 using added synthetic positive controls and the 15 housekeeping genes included in the panel. We then performed background thresholding on the normalized data to remove non-expressing genes. The background level was determined as: mean of all NEG controls + 2SD of all NEG controls + mean of the POS_E controls.
4.13. Differential Gene Expression Testing
We performed differential gene expression using multiple t-tests (with Q < 0.005) in GraphPad Prism 9.4. The Volcano plot was created in GraphPad Prism 9.4. The GSEA was done using GSEApy [54].
4.14. LDH Cytotoxicity Assay
After allowing the adherent tumor cells (target) to incubate for 16–17 h, we replaced the culture medium with X-VIVO 15 serum-free medium (Lonza) and then added T-cells (effectors) to achieve an effector: the target ratio of 5:1 Cytotoxicity was assessed by determining the lactate dehydrogenase (LDH) activity in the conditioned medium of transduced T-cells and human tumor lines SK-MEL-37, NW-Mel-38, and HCT-116 for a duration of for 6–8 h, using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (G1780, Promega Corporation, Madison, WI, USA). LDH activity was measured through a 30 min coupled enzymatic assay, which converts the tetrazolium salt INT into red formazan. The absorbance of visible light was determined using a standard 96-well plate reader (Varioskan, Thermo Fisher Scientific), and the intensity of the color formed was found to be directly proportional to the number of lysed cells. For statistical analysis of LDH cytotoxicity, we employed one-way ANOVA with Dunnett correction for multiple testing (with q-value < 0.0001) using GraphPad Prism 9.4.
4.15. Quantification of Cytokine Production
We co-cultured transduced cells with SK-Mel-37 tumor cells at an effector: target ratio of 5:1 for 48 h. Co-culture supernatants were collected and immediately frozen until the time of analysis. We employed the LEGENDplex™ Human CD8/NK Panel (13-plex) with a Filter Plate kit (Cat. Number: 740267, Lot Number: B330268, Biolegend, USA) to assess the cytokine content in the conditioned media. The analysis was performed following the manufacturer’s instructions. For each sample, 25 µL of conditioned medium was used. Subsequently, we compared the resulting cytokine profiles for transduced cells cultured with SK-Mel-37 tumor cells to those of non-transduced cells cultured with SK-Mel-37 tumor cells, aiming to identify any differences in cytokine production between the two experimental conditions. Statistical analysis was conducted using GraphPad Prism 10.0.0 software (GraphPad Software, San Diego, CA, USA). The Mann–Whitney test was used to compare between the two groups. Cytokines were categorized based on their respective concentration levels. The data are presented as median and the interquartile range.
4.16. In Vivo Efficacy
NRG immunodeficient mice of the NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ strain were utilized. The study was conducted at the Center for Genetic Resources of Laboratory Animals within the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences (RFMEFI62119X0023). Both male and female mice, aged 8 weeks, were included in the study, all with SPF (specific pathogen-free) status. These mice were housed in single-sex family groups, comprising 2–5 individuals per group, within individually ventilated cages (IVC) using the Opti Mice system provided by Animal Care Systems. These cages maintained controlled environmental conditions, including a temperature range of 21–24 °C, relative humidity levels between 30–50%, and a lighting regimen of 12:12 light: dark cycle. The mice were fed a diet from Ssniff (Soest, Germany) and had access to reverse osmosis water enriched with a mineral mixture ad libitum.
For the melanoma model, 5 million SK-MEL-37 tumor cells were subcutaneously implanted near the right scapula of the experimental animals suspended in 100 µL RPMI medium. The experiments strictly adhered to humane and ethical standards outlined in the European Community directive (86/609/EEC). Mice were closely monitored every 2–3 days for changes in skin condition, motor activity, and behavior. If mice displayed signs of toxicity (e.g., curvature, hunching, reduced activity), a body weight loss exceeding 20%, or a significant increase in tumor volume, they were euthanized in accordance with ethical guidelines for animal care. Planned euthanasia was carried out using CO2 overdose, followed by cervical dislocation. Tumor volumes were precisely determined using caliper measurements and the formula V = a × b2 × 0.52 (where ‘a’ represents length and ‘b’ represents width). When the average tumor volume reached 100 mm3, the mice were randomly distributed into three groups. The control group received 8 million non-transduced T-cells intravenously, the experimental group received anti-MAGEA4 TCR-like CAR-T cells in the same manner, and the third group remained untreated. Statistical analysis was conducted using GraphPad Prism 10.0.0 software (GraphPad Software, USA). The two-way ANOVA test was used to compare between the groups. Tumor volumes are presented as mean ± standard error of the mean.
5. Conclusions
In the course of this investigation, we conducted comprehensive in vitro assessments to evaluate the cytotoxic activity of MAGE-A4-specific CAR-T lymphocytes against the SK-Mle-37 tumor cell line expressing the target MAGE-A4 antigen. Our diverse in vitro functional assays not only corroborated tumor-specific cytotoxicity but also elucidated the presence of early activation markers, contributing to augmented antitumor activity. The observed enhancement in cytotoxic activity of the genetically modified T-lymphocytes can be attributed to the inclusion of GITR in the CAR receptor construct. Our initial in vivo experiments demonstrated a significant deceleration in tumor growth in a murine melanoma model. These findings underscore the potential of targeted immunotherapies for solid tumors and provide a valuable foundation for further research and clinical development. As we move forward, optimizing dosing regimens and conducting rigorous safety assessments will be crucial in advancing this promising therapeutic approach, while additional investigations into potential off-target effects and long-term efficacy will be pivotal for a more comprehensive understanding of its potential.
Author Contributions
Conceptualization, Y.A. and H.S.; methodology, J.S., M.F., J.P., A.A., K.V., J.L., E.Z. and O.S.; validation, data curation, formal analysis, R.P.-Z., O.P.-Z., S.A. and M.V.; writing—original draft preparation, A.A.; writing—review and editing, A.A. and J.S.; visualization, A.A.; project administration, funding acquisition, A.S. and S.S.; supervision, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of the Russian Science Foundation, project number 21-65-00004 (https://rscf.ru/project/21-65-00004/, accessed on 20 April 2021).
Institutional Review Board Statement
Blood sampling from healthy adult donors was approved by the local ethics committee of the Research Institute of Fundamental and Clinical Immunology at meeting No. 129 on 17 February 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current research are accessible from the corresponding author upon an email request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rath, J.A.; Arber, C. Engineering Strategies to Enhance TCR-Based Adoptive T Cell Therapy. Cells 2020, 9, 1485. [Google Scholar] [CrossRef]
- Huang, R.; Li, X.; He, Y.; Zhu, W.; Gao, L.; Liu, Y.; Gao, L.; Wen, Q.; Zhong, J.F.; Zhang, C.; et al. Recent Advances in CAR-T Cell Engineering. J. Hematol. Oncol. 2020, 13, 86. [Google Scholar] [CrossRef]
- Chodon, T.; Comin-Anduix, B.; Chmielowski, B.; Koya, R.C.; Wu, Z.; Auerbach, M.; Ng, C.; Avramis, E.; Seja, E.; Villanueva, A.; et al. Adoptive Transfer of MART-1 T-Cell Receptor Transgenic Lymphocytes and Dendritic Cell Vaccination in Patients with Metastatic Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 2457–2465. [Google Scholar] [CrossRef]
- Abramson, J.S. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus. Med. Rev. 2020, 34, 29–33. [Google Scholar] [CrossRef]
- Fagerberg, L.; Jonasson, K.; von Heijne, G.; Uhlén, M.; Berglund, L. Prediction of the Human Membrane Proteome. Proteomics 2010, 10, 1141–1149. [Google Scholar] [CrossRef]
- Yu, L.; Huang, L.; Lin, D.; Lai, X.; Wu, L.; Liao, X.; Liu, J.; Zeng, Y.; Liang, L.; Zhang, G.; et al. GD2-Specific Chimeric Antigen Receptor-Modified T Cells for the Treatment of Refractory and/or Recurrent Neuroblastoma in Pediatric Patients. J. Cancer Res. Clin. Oncol. 2022, 148, 2643–2652. [Google Scholar] [CrossRef]
- Liu, H.; Lei, W.; Zhang, C.; Yang, C.; Wei, J.; Guo, Q.; Guo, X.; Chen, Z.; Lu, Y.; Young, K.H.; et al. CD19-Specific CAR T Cells That Express a PD-1/CD28 Chimeric Switch-Receptor Are Effective in Patients with PD-L1–Positive B-Cell Lymphoma. Clin. Cancer Res. 2021, 27, 473–484. [Google Scholar] [CrossRef]
- Singh, N.; Frey, N.V.; Engels, B.; Barrett, D.M.; Shestova, O.; Ravikumar, P.; Cummins, K.D.; Lee, Y.G.; Pajarillo, R.; Chun, I.; et al. Antigen-Independent Activation Enhances the Efficacy of 4-1BB-Costimulated CD22 CAR T Cells. Nat. Med. 2021, 27, 842–850. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-Engineered T Cell Therapy in Solid Tumors: State of the Art and Perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef]
- Ishihara, M.; Nishida, Y.; Kitano, S.; Kawai, A.; Muraoka, D.; Momose, F.; Harada, N.; Miyahara, Y.; Seo, N.; Hattori, H.; et al. A Phase 1 Trial of NY-ESO-1-Specific TCR-Engineered T-Cell Therapy Combined with a Lymph Node-Targeting Nanoparticulate Peptide Vaccine for the Treatment of Advanced Soft Tissue Sarcoma. Int. J. Cancer 2023, 152, 2554–2566. [Google Scholar] [CrossRef]
- Raskin, S.; Van Pelt, S.; Toner, K.; Balakrishnan, P.B.; Dave, H.; Bollard, C.M.; Yvon, E. Novel TCR-like CAR-T Cells Targeting an HLA*0201-Restricted SSX2 Epitope Display Strong Activity against Acute Myeloid Leukemia. Mol. Ther. Methods Clin. Dev. 2021, 23, 296–306. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Xiong, W.; Yin, B.; Huang, Y.; Chu, J.; Xing, C.; Qian, C.; Du, Y.; Duan, T.; et al. Development of a TCR-like Antibody and Chimeric Antigen Receptor against NY-ESO-1/HLA-A2 for Cancer Immunotherapy. J. Immunother. Cancer 2022, 10, e004035. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishikawa, H. Engineering Strategies for Broad Application of TCR-T- and CAR-T-Cell Therapies. Int. Immunol. 2021, 33, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Zajac, P.; Schultz-Thater, E.; Tornillo, L.; Sadowski, C.; Trella, E.; Mengus, C.; Iezzi, G.; Spagnoli, G.C. MAGE-A Antigens and Cancer Immunotherapy. Front. Med. 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Z.; Liu, Y.; Wang, X.; Liu, X.; Gao, Y.; Jin, Y. The Expression of Cancer-Testis Antigen in Ovarian Cancer and the Development of Immunotherapy. Am. J. Cancer Res. 2022, 12, 681–694. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Zhang, L. Expression of Cancer-Testis Antigens in Esophageal Cancer and Their Progress in Immunotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 281–291. [Google Scholar] [CrossRef]
- Alsalloum, A.; Shevchenko, J.A.; Sennikov, S. The Melanoma-Associated Antigen Family A (MAGE-A): A Promising Target for Cancer Immunotherapy? Cancers 2023, 15, 1779. [Google Scholar]
- Golubovskaya, V.M.; Berahovich, R.; Xu, Q.; Zhou, H.; Xu, S.; Guan, J.; Harto, H.; Li, L.; Wu, L. GITR Domain inside CAR Co-Stimulates Activity of CAR-T Cells against Cancer. Front. Biosci. (Landmark Ed.) 2018, 23, 2245–2254. [Google Scholar] [CrossRef]
- He, Y.; Vlaming, M.; van Meerten, T.; Bremer, E. The Implementation of TNFRSF Co-Stimulatory Domains in CAR-T Cells for Optimal Functional Activity. Cancers 2022, 14, 299. [Google Scholar] [CrossRef]
- Hauer, J.; Püschner, S.; Ramakrishnan, P.; Simon, U.; Bongers, M.; Federle, C.; Engelmann, H. TNF Receptor (TNFR)-Associated Factor (TRAF) 3 Serves as an Inhibitor of TRAF2/5-Mediated Activation of the Noncanonical NF-KappaB Pathway by TRAF-Binding TNFRs. Proc. Natl. Acad. Sci. USA 2005, 102, 2874–2879. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wei, W.; Li, Y. TCR Engineered T Cells for Solid Tumor Immunotherapy. Exp. Hematol. Oncol. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q. Novel Chimeric Antigen Receptor T Cells Based on T-Cell Receptor-like Antibodies. Blood Sci. 2019, 1, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and Viable Identification of Antigen-Specific CD8+ T Cells by a Flow Cytometric Assay for Degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The Role of CD95 and CD95 Ligand in Cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef]
- Hombach, A.A.; Abken, H. Most Do, but Some Do Not: CD4+CD25− T Cells, but Not CD4+CD25+ Treg Cells, Are Cytolytic When Redirected by a Chimeric Antigen Receptor (CAR). Cancers 2017, 9, 112. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Holgado, M.P.; Sananez, I.; Raiden, S.; Geffner, J.R.; Arruvito, L. CD32 Ligation Promotes the Activation of CD4(+) T Cells. Front. Immunol. 2018, 9, 2814. [Google Scholar] [CrossRef]
- Hedrick, J.A.; Zlotnik, A. Chemokines and Lymphocyte Biology. Curr. Opin. Immunol. 1996, 8, 343–347. [Google Scholar] [CrossRef]
- Castellino, F.; Huang, A.Y.; Altan-Bonnet, G.; Stoll, S.; Scheinecker, C.; Germain, R.N. Chemokines Enhance Immunity by Guiding Naive CD8+ T Cells to Sites of CD4+ T Cell–Dendritic Cell Interaction. Nature 2006, 440, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A Novel Transcription Factor, T-Bet, Directs Th1 Lineage Commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.M.; Juedes, A.; Szabo, S.J.; von Herrath, M.; Glimcher, L.H. Antigen-Driven Effector CD8 T Cell Function Regulated by T-Bet. Proc. Natl. Acad. Sci. USA 2003, 100, 15818–15823. [Google Scholar] [CrossRef]
- Townsend, M.J.; Weinmann, A.S.; Matsuda, J.L.; Salomon, R.; Farnham, P.J.; Biron, C.A.; Gapin, L.; Glimcher, L.H. T-Bet Regulates the Terminal Maturation and Homeostasis of NK and Valpha14i NKT Cells. Immunity 2004, 20, 477–494. [Google Scholar] [CrossRef]
- Oestreich, K.J.; Huang, A.C.; Weinmann, A.S. The Lineage-Defining Factors T-Bet and Bcl-6 Collaborate to Regulate Th1 Gene Expression Patterns. J. Exp. Med. 2011, 208, 1001–1013. [Google Scholar] [CrossRef]
- Ichii, H.; Sakamoto, A.; Kuroda, Y.; Tokuhisa, T. Bcl6 Acts as an Amplifier for the Generation and Proliferative Capacity of Central Memory CD8+ T Cells. J. Immunol. 2004, 173, 883–891. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Tang, S.; Zhou, L.; Huang, C.; Cao, Y.; Huang, H.; Wu, X.; Meng, D.; Ye, L.; et al. Cutting Edge: Transcription Factor BCL6 Is Required for the Generation, but Not Maintenance, of Memory CD8+ T Cells in Acute Viral Infection. J. Immunol. 2019, 203, 323–327. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Levings, M.K.; Bacchetta, R.; Schulz, U.; Roncarolo, M.G. The Role of IL-10 and TGF-Beta in the Differentiation and Effector Function of T Regulatory Cells. Int. Arch. Allergy Immunol. 2002, 129, 263–276. [Google Scholar] [CrossRef]
- Sad, S.; Mosmann, T.R. Interleukin (IL) 4, in the Absence of Antigen Stimulation, Induces an Anergy-like State in Differentiated CD8+ TC1 Cells: Loss of IL-2 Synthesis and Autonomous Proliferation but Retention of Cytotoxicity and Synthesis of Other Cytokines. J. Exp. Med. 1995, 182, 1505–1515. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and Viral IL-10 Strongly Reduce Antigen-Specific Human T Cell Proliferation by Diminishing the Antigen-Presenting Capacity of Monocytes via Downregulation of Class II Major Histocompatibility Complex Expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Kloepper, J.; Ren, J.; Tay, R.E.; Kazer, S.W.; Kiner, E.; Krishnan, S.; Posada, J.M.; Ghosh, M.; Mamessier, E.; et al. Targeting Treg Cells with GITR Activation Alleviates Resistance to Immunotherapy in Murine Glioblastomas. Nat. Commun. 2021, 12, 2582. [Google Scholar] [CrossRef] [PubMed]
- Meiraz, A.; Garber, O.G.; Harari, S.; Hassin, D.; Berke, G. Switch from Perforin-Expressing to Perforin-Deficient CD8(+) T Cells Accounts for Two Distinct Types of Effector Cytotoxic T Lymphocytes In Vivo. Immunology 2009, 128, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hassin, D.; Garber, O.G.; Meiraz, A.; Schiffenbauer, Y.S.; Berke, G. Cytotoxic T Lymphocyte Perforin and Fas Ligand Working in Concert Even When Fas Ligand Lytic Action Is Still Not Detectable. Immunology 2011, 133, 190–196. [Google Scholar] [CrossRef]
- Cullen, S.P.; Martin, S.J. Mechanisms of Granule-Dependent Killing. Cell Death Differ. 2008, 15, 251–262. [Google Scholar] [CrossRef]
- Mareeva, T.; Martinez-Hackert, E.; Sykulev, Y. How a T Cell Receptor-like Antibody Recognizes Major Histocompatibility Complex-Bound Peptide. J. Biol. Chem. 2008, 283, 29053–29059. [Google Scholar] [CrossRef]
- Maruta, M.; Ochi, T.; Tanimoto, K.; Asai, H.; Saitou, T.; Fujiwara, H.; Imamura, T.; Takenaka, K.; Yasukawa, M. Direct Comparison of Target-Reactivity and Cross-Reactivity Induced by CAR- and BiTE-Redirected T Cells for the Development of Antibody-Based T-Cell Therapy. Sci. Rep. 2019, 9, 13293. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Abila, B.; Mostafa Kamel, Y. CAR-T: What Is Next? Cancers 2023, 15, 663. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, C.; Dai, H.; Wu, Z.; Han, X.; Guo, Y.; Chen, D.; Wei, J.; Ti, D.; Liu, Z.; et al. Low-Dose Decitabine Priming Endows CAR T Cells with Enhanced and Persistent Antitumour Potential via Epigenetic Reprogramming. Nat. Commun. 2021, 12, 409. [Google Scholar] [CrossRef]
- Rotte, A.; Frigault, M.J.; Ansari, A.; Gliner, B.; Heery, C.; Shah, B. Dose-Response Correlation for CAR-T Cells: A Systematic Review of Clinical Studies. J. Immunother. Cancer 2022, 10, e005678. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; van Ostaijen-Ten Dam, M.M.; Lankester, A.C.; Schilham, M.W.; van den Akker, E.B. A Comprehensive Workflow for Applying Single-Cell Clustering and Pseudotime Analysis to Flow Cytometry Data. J. Immunol. 2020, 205, 864–871. [Google Scholar] [CrossRef]
- Höllt, T.; Pezzotti, N.; van Unen, V.; Koning, F.; Eisemann, E.; Lelieveldt, B.; Vilanova, A. Cytosplore: Interactive Immune Cell Phenotyping for Large Single-Cell Datasets. Comput. Graph. Forum 2016, 35, 171–180. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Peltz, G. GSEApy: A Comprehensive Package for Performing Gene Set Enrichment Analysis in Python. Bioinformatics 2023, 39, btac757. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).