Biochemical Responses to the Long-Term Impact of Copper Sulfate (CuSO4) in Tobacco Plants
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Selection of Effective Concentrations and Growth Conditions
4.2. Anatomical and Morphological Characteristics of Plants
4.3. Quantification of Copper
4.4. Biochemical Characteristics
4.4.1. Quantification of Hydrogen Peroxide and Malondialdehyde as Stress Markers
4.4.2. SOD, GPOX and Catalase Activity
4.4.3. Visualization of GPOX Isoforms
4.5. Determination of Total Lignin Content
4.6. Phenolic Compounds
4.7. Metabolic Fingerprinting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Root | Stem | Leaf | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № | Rt, min | m/z, [M-H]- | Formula | Name | Control | Cu 100 µM | Cu 300 µM | Control | Cu 100 µM | Cu 300 µM | Control | Cu 100 µM | Cu 300 µM |
| 1 | 2.09 | 289.07 | C15H14O6 | Catechin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 10.05 | 301.03 | C15H10O7 | Quercetin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 5.92 | 609.15 | C27H30O16 | Rutin | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 8.3 | 227.07 | C14H12O3 | Resveratrol | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 5 | 0.64 | 169.01 | C7H6O5 | Gallic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | 5.6 | 137.02 | C7H6O3 | Salicylic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | 9.23 | 147.04 | C9H8O2 | Cinnamic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 3.96 | 163.04 | C9H8O3 | p-Coumaric acid | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 9 | 4.61 | 193.05 | C10H10O4 | Ferulic acid | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| 10 | 2.34 | 167.03 | C8H8O4 | Vanillic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11 | 2.74 | 197.05 | C9H10O5 | Syringic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 12 | 4.91 | 121.01 | C7H6O2 | Benzoic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13 | 0.62 | 125.02 | C6H6O3 | Phloroglucinol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Compounds | Root | Stem | Leaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № | Rt, min | m/z, [M-H]- | Control | Cu 100 µM | Cu 300 µM | Control | Cu 100 µM | Cu 300 µM | Control | Cu 100 µM | Cu 300 µM |
| 1 | 0.61 | 341.11 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 3 | 0.87 | 249.13 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 3 | 1.08 | 146.96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 4 | 1.3 | 203.09 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 5 | 2.07 | 191.04 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | 2.52 | 121.03 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 2.82 | 472.15 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 8 | 2.91 | 401.15 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| 9 | 3.16 | 194.91 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 3.48 | 470.24 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 11 | 3.81 | 468.22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 12 | 3.89 | 468.22 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 13 | 4.22 | 176.02 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| 14 | 7.38 | 187.11 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 15 | 7.51 | 187.09 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 16 | 8.78 | 282.12 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 17 | 9.33 | 312.13 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 18 | 9.78 | 301.04 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
References
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Ghulam Murtaza, N.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; 867p. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Mansour, S.A. Practical Food Safety: Contemporary Issues and Future Directions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; 640p. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Zhang, Y.; Zhang, A.; Wang, Z.; Yang, Y. Copper stress-induced phytotoxicity associated with photosynthetic characteristics and lignin metabolism in wheat seedlings. Ecotoxicol. Environ. Saf. 2023, 254, 114739. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, D.S.; Tugbaeva, A.S.; Ermoshin, A.A.; Kiseleva, I.S. Response reactions of Zinnia elegans seedlings to the impact of different copper ions concentrations. AIP Conf. Proc. 2021, 2388, 030034. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, A.; Ördög, A.; Szepesi, A.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef]
- Lequeux, H.; Hermans, C.; Lutts, S.; Verbruggen, N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010, 48, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.S.; Pető, A.; Lehotai, N.; Feigl, G.; Erdei, L. Long-term copper (Cu2+) exposure impacts on auxin, nitric oxide (NO) metabolism and morphology of Arabidopsis thaliana L. Plant Growth Regul. 2012, 68, 151–159. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of heavy metal stress on phenolic compounds accumulation in winter wheat plants. Molecules 2022, 28, 241. [Google Scholar] [CrossRef]
- Tugbaeva, A.; Ermoshin, A.; Wuriyanghan, H.; Maleva, M.; Borisova, G.; Kiseleva, I. Copper stress enhances the lignification of axial organs in Zinnia elegans. Horticulturae 2022, 8, 558. [Google Scholar] [CrossRef]
- Wu, J.; Hu, J.; Wang, L.; Zhao, L.; Ma, F. Responses of Phragmites australis to copper stress: A combined analysis of plant morphology, physiology and proteomics. Plant Biol. 2021, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Maleva, M.; Borisova, G.; Filimonova, E.; Lukina, N.; Chukina, N.; Ermoshin, A.; Tugbaeva, A.; Voropaeva, O. Adaptive redox reactions promote naturalization of rare orchid Epipactis atrorubens on serpentine dumps post asbestos mining. Horticulturae 2022, 8, 603. [Google Scholar] [CrossRef]
- Kumar, A.; Borisova, G.; Maleva, M.; Tripti; Shiryaev, G.; Tugbaeva, A.; Sobenin, A.; Kiseleva, I. Biofertilizer based on biochar and metal-tolerant plant growth promoting rhizobacteria alleviates copper impact on morphophysiological traits in Brassica napus L. Microorganisms 2022, 10, 2164. [Google Scholar] [CrossRef]
- Elleuch, A.; Chaâbene, Z.; Grubb, D.C.; Drira, N.; Mejdoub, H.; Khemakhem, B. Morphological and biochemical behavior of fenugreek (Trigonella foenum-graecum) under copper stress. Ecotoxicol. Environ. Saf. 2013, 98, 46–53. [Google Scholar] [CrossRef]
- Michalak, M. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Meychik, N.; Nikolaeva, Y.; Kushunina, M.; Yermakov, I. Contribution of apoplast to short-term copper uptake by wheat and mung bean roots. Funct. Plant Biol. 2016, 43, 403–412. [Google Scholar] [CrossRef]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; El Ferjani, E. Cell wall accumulation of cu ions and modulation of lignifying enzymes in primary leaves of bean seedlings exposed to excess copper. Biol. Trace Elem. Res. 2011, 139, 97–107. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Ivanov, V.B.; Bystrova, E.I.; Seregin, I.V. Comparative impacts of heavy metals on root growth as related to their specificity and selectivity. Rus. J. Plant Physiol. 2003, 50, 398–406. [Google Scholar] [CrossRef]
- Alkan, M.; Doğan, M. Adsorption of copper (II) onto perlite. J. Colloid Interface Sci. 2001, 243, 280–291. [Google Scholar] [CrossRef]
- El-Bayaa, A.A.; Badawy, N.A.; Abd Alkhalik, E. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Cosi, E.; Meneguzzo, S.; Sgherri, C.; Navari-Izzo, F. Uptake and translocation of copper in Brassicaceae. J. Plant Nutr. 2003, 26, 1065–1083. [Google Scholar] [CrossRef]
- Nikolic, M.; Cesco, S.; Monte, R.; Tomasi, N.; Gottardi, S.; Zamboni, A.; Pinton, R.; Varanini, Z. Nitrate transport in cucumber leaves is an inducible process involving an increase in plasma membrane H+-ATPase activity and abundance. BMC Plant Biol. 2012, 12, 6. Available online: https://www.mdpi.com/2673-7140/3/3/40 (accessed on 20 August 2023). [CrossRef]
- Tugbaeva, A.S.; Ermoshin, A.A.; Wuriyanghan, H.; Kiseleva, I.S. Lignification in zinnia (Zinnia elegans Jacq.) stem sections of different age: Biochemical and molecular genetic traits. Horticulturae 2023, 9, 410. [Google Scholar] [CrossRef]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, L.-M.; Liu, Z.-H. Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci. 2005, 168, 855–861. [Google Scholar] [CrossRef]
- Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trend Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Sarath, G.; Baird, L.M.; Vogel, K.P.; Mitchell, R.B. Internode structure and cell wall composition in maturing tillers of switchgrass (Panicum virgatum L.). Bioresour. Technol. 2007, 98, 2985–2992. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Tolerance of Silene vulgaris to copper: Population-related comparison of selected physiological parameters. Environ. Toxicol. 2010, 25, 581–592. [Google Scholar] [CrossRef] [PubMed]
- González-Mendoza, D.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Duran-Hernandez, D.; Gutierrez-Miceli, F. Changes in the phenylalanine ammonia lyase activity, total phenolic compounds, and flavonoids in Prosopis glandulosa treated with cadmium and copper. An. Acad. Bras. Cienc. 2018, 90, 1465–1472. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Chee Kong, Y.; Mohd Zain, N.A. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant sambung nyawa (Gynura procumbens (Lour.) Merr). Molecules 2017, 22, 1623. [Google Scholar] [CrossRef]
- Moustakas, N.K.; Ntzanis, H. Estimating flue-cured tobacco leaf area from linear measurements, under mediterranean conditions. Agric. Med. 1998, 128, 226–231. [Google Scholar]
- Berlin, G.P.; Miksche, J.P. Botanical Microtechnique and Cytochemistry; Iowa State University Press: Ames, IA, USA, 1976; 321p. [Google Scholar]
- Soukup, A. Selected simple methods of plant cell wall histochemistry and staining for light microscopy. Methods Mol. Biol. 2014, 1080, 25–40. [Google Scholar] [CrossRef]
- Roccotiello, E.; Marescotti, P.; Di Piazza, S.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Biodiversity in Metal-Contaminated Sites—Problem and Perspective. In A Case Study, Biodiversity in Ecosystems—Linking Structure and Function; Blanco, J., Ed.; IntechOpen: London, UK, 2015; 1865p. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonics is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 287–297. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, L. Superoxide dismutase: Improved assays and an assay applicable toacrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantitiesof protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Araújo, P.; Sampaio Mayer, J.L.; Paes Leme, A.F.; Mazzafera, P. Enzymatic activity and proteomic profile of class III peroxidases during sugarcane stem development. Plant Physiol Biochem. 2012, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, C.; Chen, M.; Yue, F.; Ralph, J. A facile spectroscopic method for measuring lignin content in lignocellulosic biomass. Green Chem. 2021, 23, 5106–5112. [Google Scholar] [CrossRef]
- Franke, R.; McMichael, C.M.; Meyer, K.; Shirley, A.M.; Cusumano, J.C.; Chapple, C. Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J. 2000, 22, 223–234. [Google Scholar] [CrossRef]
- Esteves Costa, C.A.; Coleman, W.; Dube, M.; Rodrigues, A.E.; Rodrigues Pinto, P.C. Assessment of key features of lignin from lignocellulosic crops: Stalks and roots of corn, cotton, sugarcane, and tobacco. Ind. Crops Prod. 2016, 92, 136–148. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid.-Based Complement. Altern.Med. 2019, 2019, 5410923. [Google Scholar] [CrossRef]
- Stasevich, O.V.; Likhtarovich, Y.S.; Shemet, S.N. Analysis of ferulic acid in plants containing phenylpropanoids. Proc. BSTU 2014, 4, 181–184. [Google Scholar]
- Hassan, A.E.; Heneidak, S.; Gowayed, S.M.H. Comparative studies of some Triticum species by grain protein and amino acids analyses. J. Agron. 2007, 6, 286–293. [Google Scholar] [CrossRef]

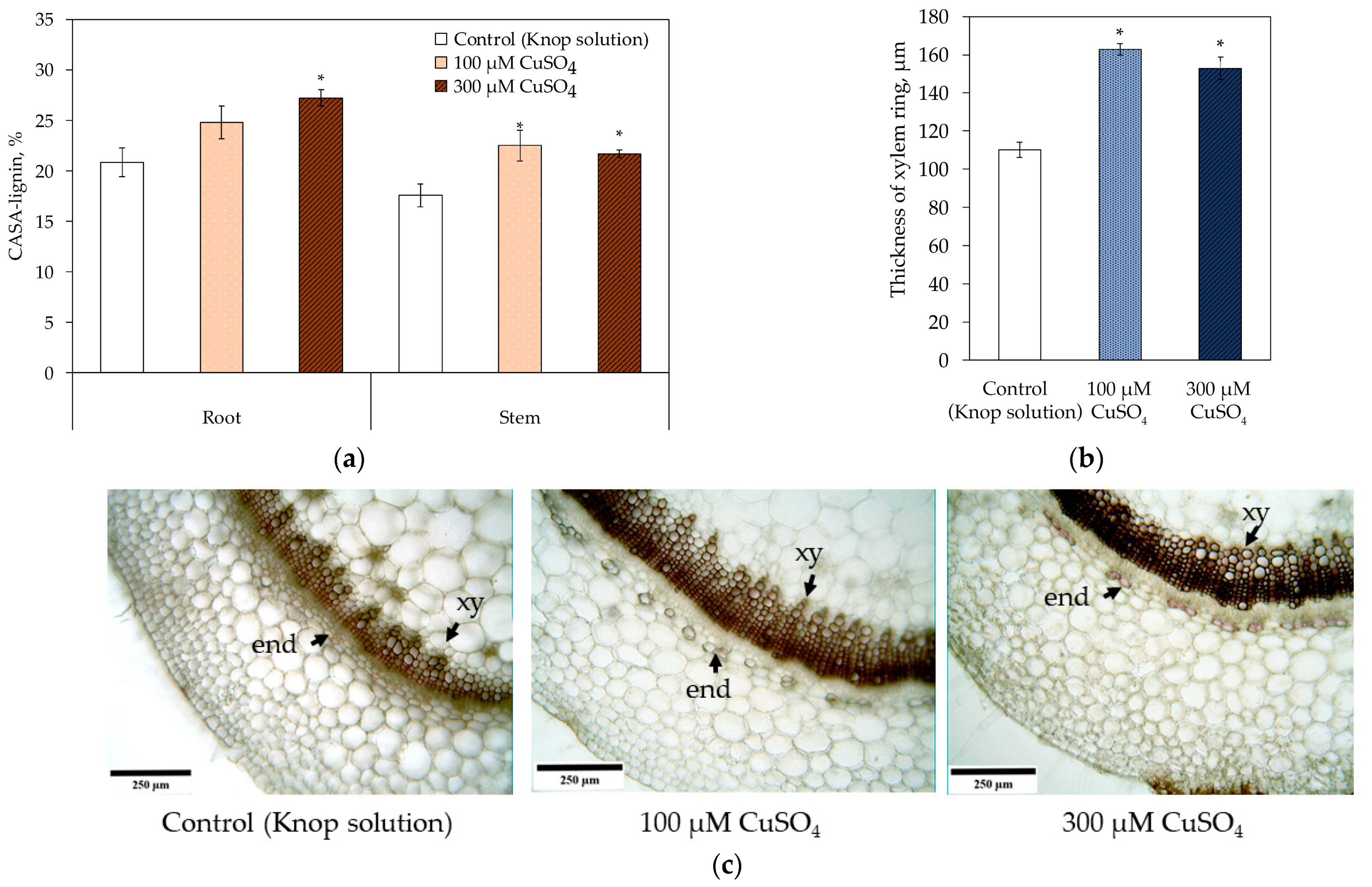
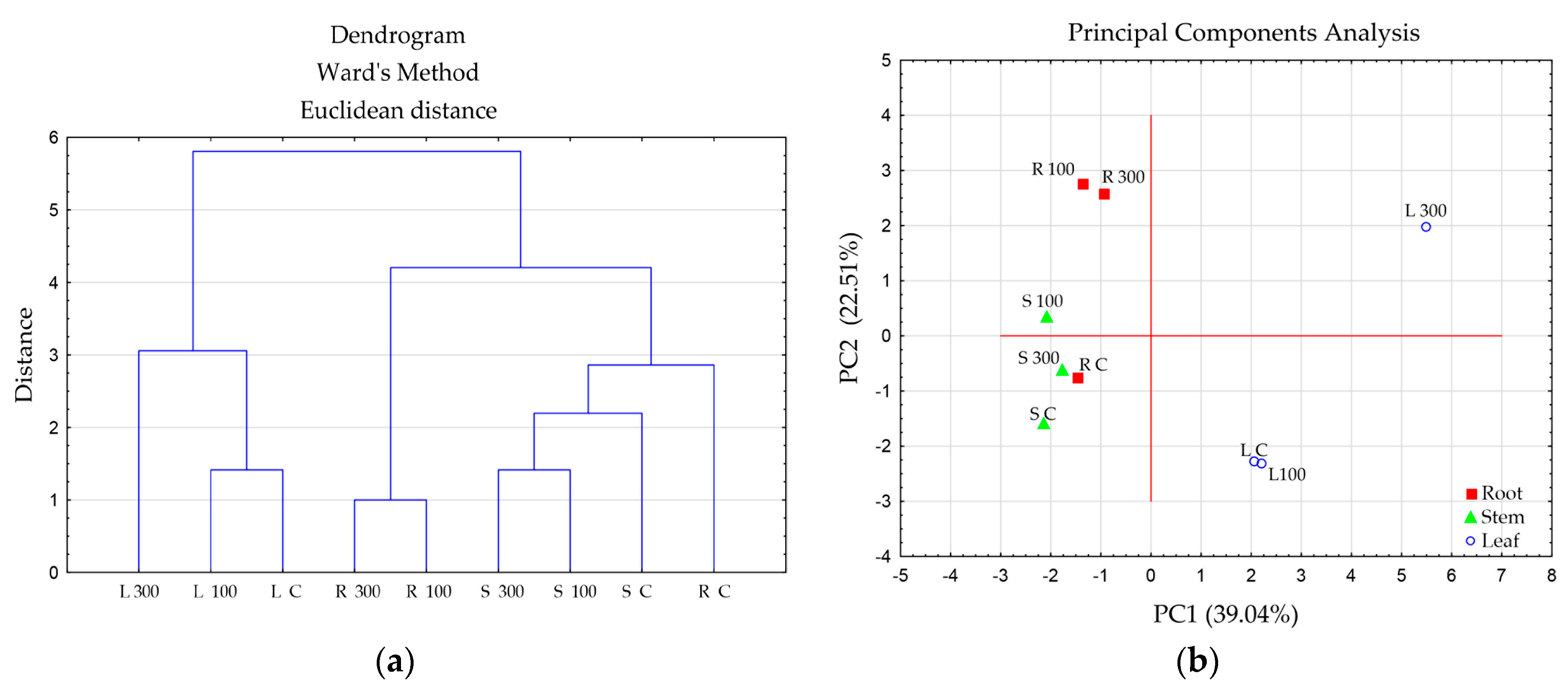
| Treatment | Root Length, cm | Root Diameter, μm | Stem Height, cm | Stem Diameter, μm | Area of the 5th Leaf, cm2 |
|---|---|---|---|---|---|
| Control (Knop solution) | 10.5 ± 0.5 1 | 1146 ± 58 | 11.1 ± 0.4 | 2598 ± 13 | 61.0 ± 2.2 |
| 100 μM CuSO4 | 9.6 ± 0.8 | 1593 ± 45 * | 12.9 ± 0.5 * | 2609 ± 29 * | 69.0 ± 2.4 * |
| 300 μM CuSO4 | 7.3 ± 0.8 * | 1764 ± 64 * | 11.6 ± 0.6 | 2787 ± 24 * | 57.0 ± 2.3 |
| Treatment | Copper Amount, µg g−1 DW | BCF | TF | |||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | Stem/Root | Leaf/Root | |
| Control (Knop solution) | 31.36 ± 1.09 1 | 14.84 ± 0.56 | 9.52 ± 0.44 | 1.11 | 0.53 | 0.34 | 0.47 | 0.30 |
| 100 μM CuSO4 | 723.24 ± 39.78 * | 31.08 ± 1.31 * | 27.44 ± 1.26 * | 2.28 | 0.10 | 0.09 | 0.04 | 0.04 |
| 300 μM CuSO4 | 810.88 ± 32.43 * | 44.80 ± 2.46 * | 33.21 ± 1.86 * | 1.01 | 0.06 | 0.04 | 0.06 | 0.04 |
| Treatment | H2O2, µmol g−1 DW | MDA, µmol g−1 DW | ||||
|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | |
| Control (Knop solution) | 7.70 ± 0.19 1 | 8.51 ± 0.29 | 37.23 ± 0.76 | 42.40 ± 1.59 | 22.28 ± 1.01 | 28.74 ± 2.31 |
| 100 μM CuSO4 | 5.43 ± 0.16 * | 5.45 ± 0.28 * | 12.05 ± 0.16 * | 35.46 ± 1.61 * | 23.83 ± 0.61 | 25.79 ± 1.50 |
| 300 μM CuSO4 | 12.28 ± 0.29 * | 7.55 ± 0.14 | 25.24 ± 0.55 * | 49.90 ± 0.55 * | 33.63 ± 1.74 * | 59.99 ± 2.68 * |
| Treatment | Root | Stem | ||||||
|---|---|---|---|---|---|---|---|---|
| Stele Cross-Sectional Diameter, mm | Stele Area, % | Cortex Thickness, µm | Cell Wall Thickness of Metaxylem Vessels, µm | Stele Cross-Sectional Diameter, mm | Stele Area, % | Cortex Thickness, µm | Cell Wall Thickness of Metaxylem Vessels, µm | |
| Control (Knop solution) | 650 ± 42 1 | 32.2 | 172 ± 8 | 2.74 ± 0.03 | 1738 ± 19 | 44.7 | 403 ± 18 | 2.91 ± 0.14 |
| 100 μM CuSO4 | 1326 ± 47 * | 38.5 | 250 ± 13 * | 2.94 ± 0.10 * | 1938 ± 20 * | 48.0 | 395 ± 11 | 2.97 ± 0.06 |
| 300 μM CuSO4 | 1077 ± 41 * | 37.3 | 244 ± 10 * | 2.92 ± 0.09 * | 1903 ± 41 * | 46.6 | 415 ± 19 | 3.21 ± 0.08 * |
| Organ | Treatment | Free Phenolics | Bound Phenolics | Proportions (%) of Phenolic Acids in Hydrolysate | ||
|---|---|---|---|---|---|---|
| Ferulic Acid | Cinnamic Acid | p-Coumaric Acid | ||||
| Root | Control (Knop solution) | 62.35 ± 2.66 1 | 7.13 ± 0.18 | 5.6 | 2.5 | 4.5 |
| 100 μM CuSO4 | 49.30 ± 1.07 * | 4.42 ± 0.05 * | 9.8 | 8.2 | 10.8 | |
| 300 μM CuSO4 | 56.39 ± 2.11 * | 4.96 ± 0.05 * | 7.0 | 5.2 | 9.7 | |
| Stem | Control (Knop solution) | 65.65 ± 1.77 | 3.44 ± 0.08 | 5.7 | 6.7 | 9.7 |
| 100 μM CuSO4 | 36.60 ± 1.04 * | 3.31 ± 0.03 | 7.6 | 3.5 | 7.4 | |
| 300 μM CuSO4 | 44.23 ± 2.02 * | 4.78 ± 0.05 * | 3.2 | 1.8 | 2.8 | |
| Leaf | Control (Knop solution) | 37.10 ± 2.13 | 8.39 ± 0.12 | 39.5 | 21.8 | 25.3 |
| 100 μM CuSO4 | 36.01 ± 2.73 | 7.57 ± 0.23 | 31.4 | 12.6 | 28.3 | |
| 300 μM CuSO4 | 30.17 ± 0.91 * | 4.74 ± 0.13 * | 7.0 | 6.1 | 6.9 | |
| Name | Root | Stem | Leaf | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (Knop s.) | 100 μM CuSO4 | 300 μM CuSO4 | Control (Knop s.) | 100 μM CuSO4 | 300 μM CuSO4 | Control (Knop s.) | 100 μM CuSO4 | 300 μM CuSO4 | ||
| Root | Control (Knop s.) | 1 | 0.67 | 0.59 | 0.67 | 0.63 | 0.67 | 0.43 | 0.33 | 0.34 |
| 100 μM CuSO4 | 1 | 0.95 | 0.53 | 0.78 | 0.70 | 0.44 | 0.38 | 0.48 | ||
| 300 μM CuSO4 | 1 | 0.56 | 0.82 | 0.64 | 0.47 | 0.40 | 0.50 | |||
| Stem | Control (Knop s.) | 1 | 0.80 | 0.80 | 0.40 | 0.31 | 0.22 | |||
| 100 μM CuSO4 | 1 | 0.92 | 0.53 | 0.35 | 0.45 | |||||
| 300 μM CuSO4 | 1 | 0.53 | 0.47 | 0.45 | ||||||
| Leaf | Control (Knop s.) | 1 | 0.83 | 0.59 | ||||||
| 100 μM CuSO4 | 1 | 0.53 | ||||||||
| 300 μM CuSO4 | 1 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugbaeva, A.S.; Ermoshin, A.A.; Kiseleva, I.S. Biochemical Responses to the Long-Term Impact of Copper Sulfate (CuSO4) in Tobacco Plants. Int. J. Mol. Sci. 2023, 24, 15129. https://doi.org/10.3390/ijms242015129
Tugbaeva AS, Ermoshin AA, Kiseleva IS. Biochemical Responses to the Long-Term Impact of Copper Sulfate (CuSO4) in Tobacco Plants. International Journal of Molecular Sciences. 2023; 24(20):15129. https://doi.org/10.3390/ijms242015129
Chicago/Turabian StyleTugbaeva, Anastasia S., Alexander A. Ermoshin, and Irina S. Kiseleva. 2023. "Biochemical Responses to the Long-Term Impact of Copper Sulfate (CuSO4) in Tobacco Plants" International Journal of Molecular Sciences 24, no. 20: 15129. https://doi.org/10.3390/ijms242015129
APA StyleTugbaeva, A. S., Ermoshin, A. A., & Kiseleva, I. S. (2023). Biochemical Responses to the Long-Term Impact of Copper Sulfate (CuSO4) in Tobacco Plants. International Journal of Molecular Sciences, 24(20), 15129. https://doi.org/10.3390/ijms242015129






