Revealing Further Insights into Astringent Seeds of Chinese Fir by Integrated Metabolomic and Lipidomic Analyses
Abstract
:1. Introduction
2. Results
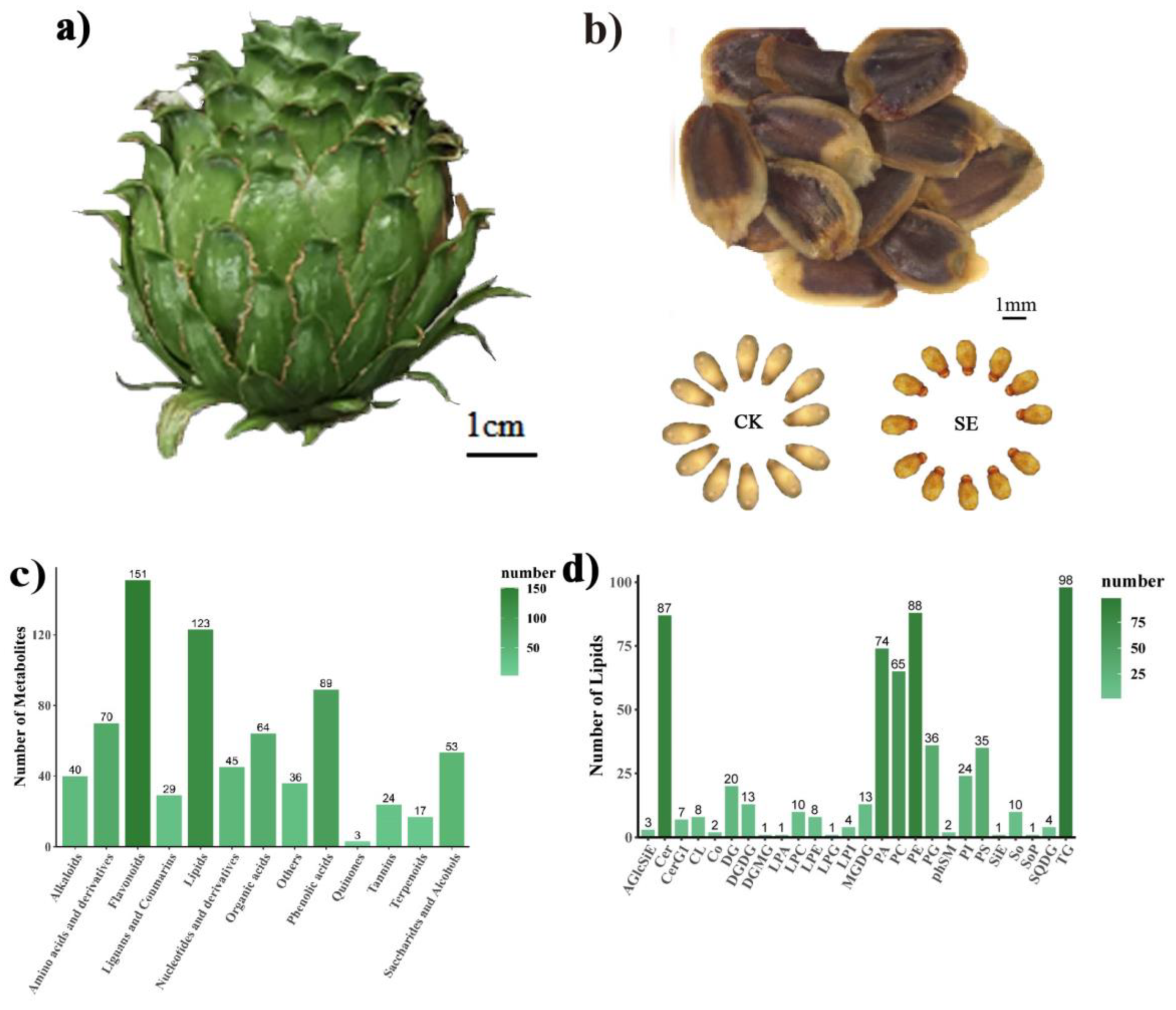
2.1. Sample Collection and Metabolite/Lipid Identification
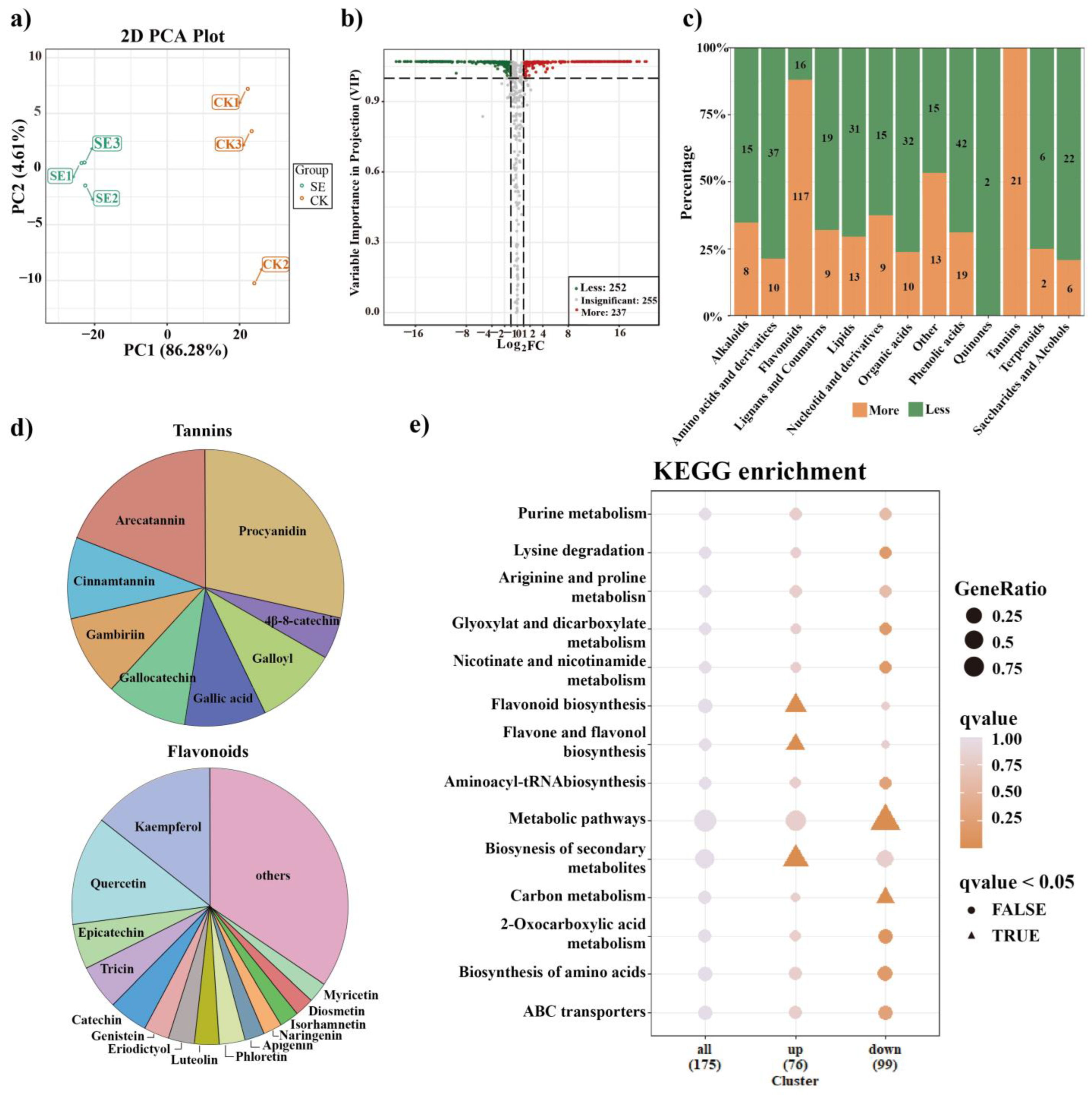
2.2. Global Metabolic Alterations in Astringent Seeds
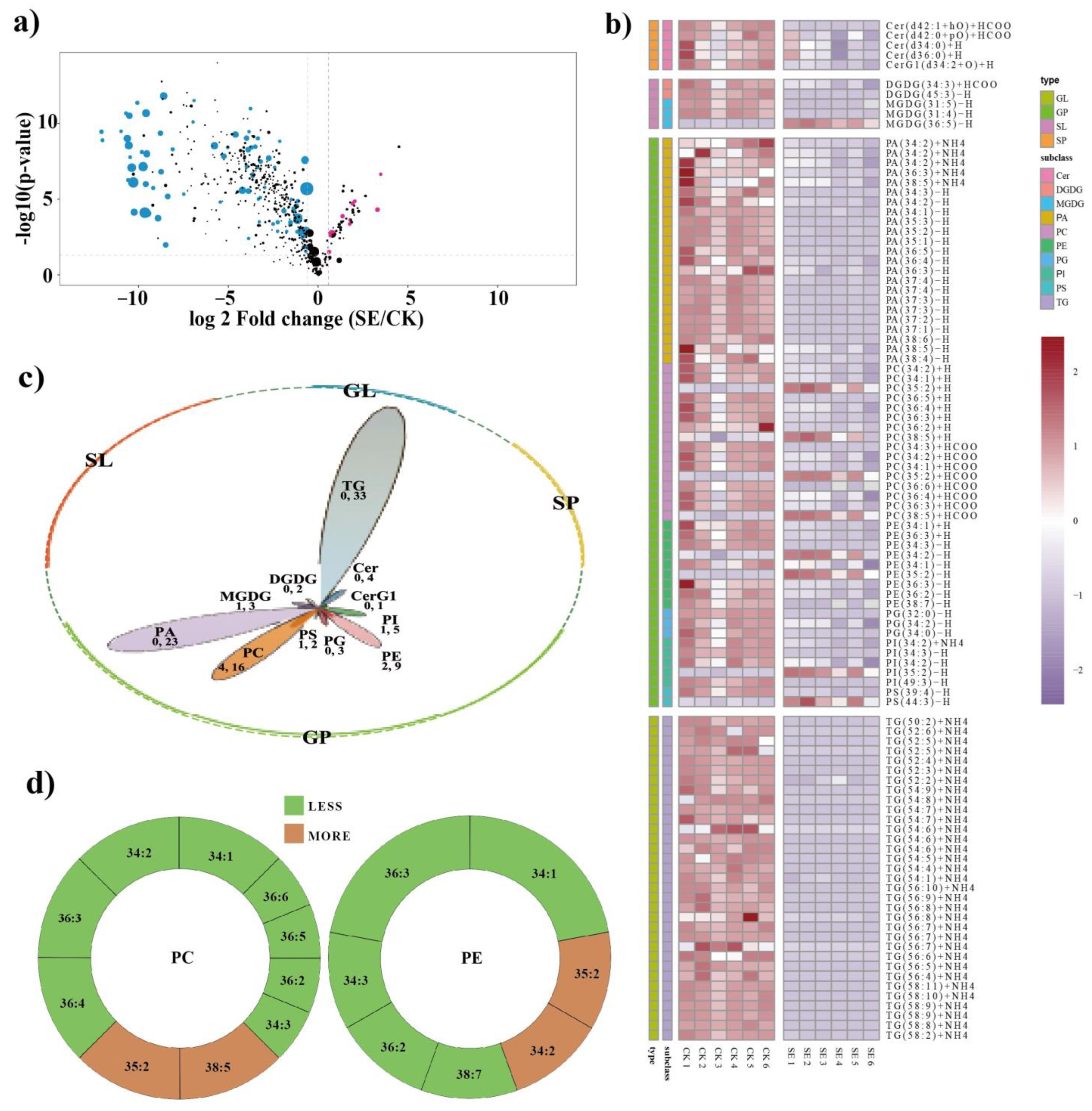
2.3. Global Lipidomic Alterations in Astringent Seeds
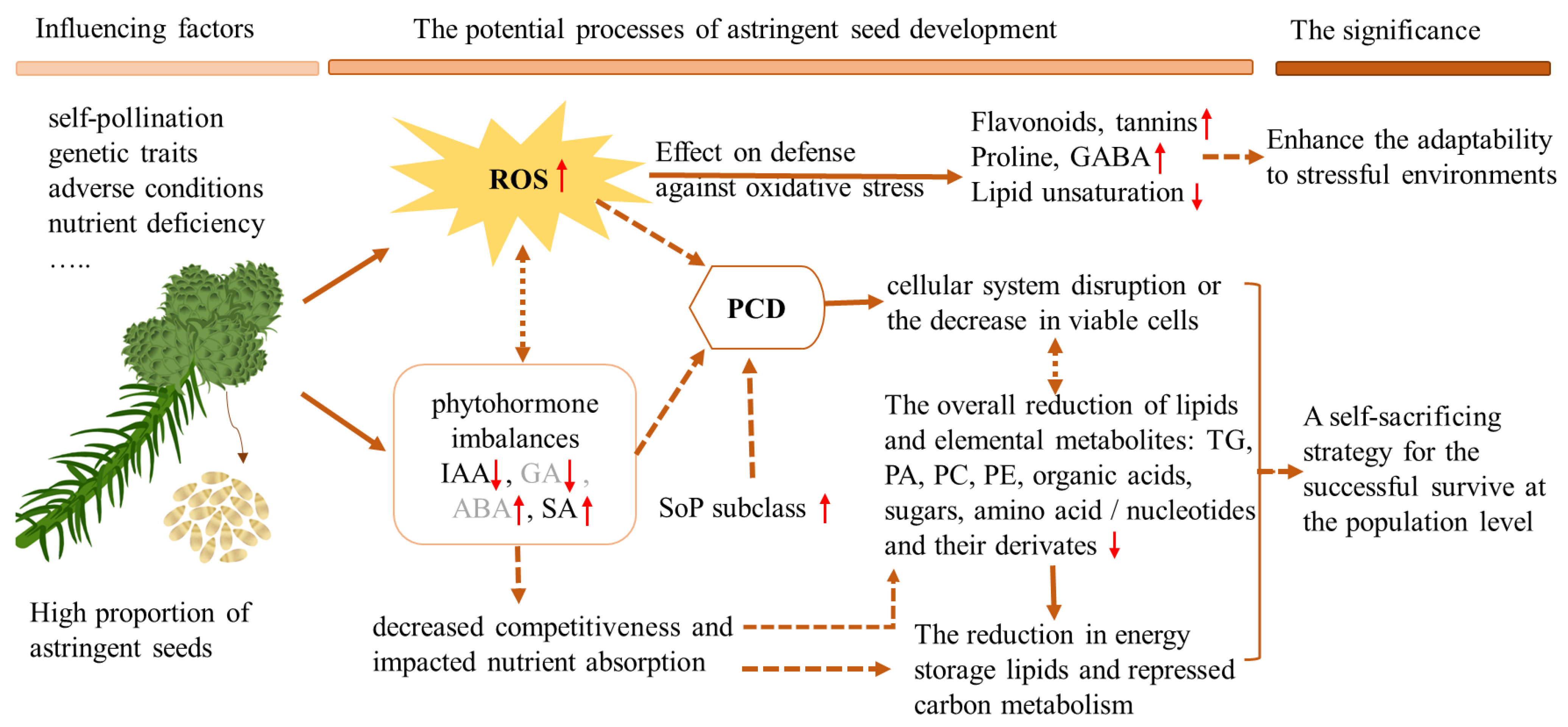
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Seed Collection
4.2. Metabolome Sequencing and Data Analysis
4.3. Lipidome Sequencing and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Wu, C.; Wu, Y.; Lin, S. Research progress on the occurrence mechanism of astringent seeds in Chinese fir. Subtrop. Agric. Res. 2020, 16, 247–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, D.; Chen, Y.; Xiong, S.; Wang, Z.; Lin, S. Anatomical observation of ovule of Cunninghamia lanceolata. J. For. Environ. 2017, 37, 142–147. [Google Scholar] [CrossRef]
- Guan, K. Comprehensive Analysison Causes for Hollow, Kernel and Woody Seeds of Chinese Fir Cones. J. Zhejiang For. Coll. 1997, 1, 90–95. [Google Scholar]
- Gao, Z.; Shi, Z.; Xu, Y.; Zhu, Z.; Shen, R.; Tang, X. Studies on the Seed Developmental Physiology, the Formation ofthe Abortive Seed and the Measures of Decreasing Abortionin Cunninghamia lanceolata. For. Res. 2000, 6, 659–666. [Google Scholar] [CrossRef]
- Huang, L.; Xie, Y.; Qi, Q.; Xie, J. Aborted Embryo, lts Physiology and Biochemistry of the Aborted Chinese Fir Seed. J. Fujian Coll. For. 1993, 4, 401–406. [Google Scholar]
- Yu, X.; Li, P.; He, F.; Lin, X.; Li, Y. Studies of The Abortive Seed and Its Constituents in Cunninghamia lanceolata (Lamb.) Hook. Acta Bot. Boreali-Occident. Sin. 1989, 4, 252–256. [Google Scholar]
- Zheng, Y.; Yu, X.; Luo, S.; Chen, J.; Zhang, F.; Wu, Z. Studies on the Relations between Physiological BiochemicalProperties of High Yield Chinese Fir Seeds Garden. J. Fujian Coll. For. 1993, 2, 105–112. [Google Scholar]
- Chen, Y.; Wu, Y.; Wu, C.; Lin, S. Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages. Forests 2020, 11, 1206. [Google Scholar] [CrossRef]
- Weng, C.; Qi, M.; Hua, C.; Li, Z.; Bao, X.; Luo, X. Genetic Analysis on Seed Sowing Ouality Traits of Chinese-fir and Causes Study for their Hollow, Kernel and Woody Seeds. J. Fujian For. Sci. Technol. 2016, 43, 160–165. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, Y.; Gao, Z.; Zhu, Z.; Shen, R.; Shen, Z. Variation of Nucleic Acids, Proteins and Lipids Contents in AbortiveSeed of Cunninghamia lanceolata. Plant Physiol. Commun. 1992, 3, 186–188. [Google Scholar] [CrossRef]
- Lin, S.-Z.; Chen, Y.; Wu, C.; Sun, W.-H.; Li, Z.; Chen, H.; Wang, J.; Ji, C.; Li, S.-B.; Wang, Z.-W. Chinese fir genome and the evolution of gymnosperms. bioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Lin, Y. Research of Hybridization Pollination Technology in Chinese fir. Anhui Agric. Sci. Bull. 2015, 21, 91–92. [Google Scholar] [CrossRef]
- Pei, S.-J.; Yang, Y.-M.; Wang, J. Economic Forest plants. In Plants China: A Companion Flora China; Cambridge University Press: Cambridge, UK, 2015; p. 309. [Google Scholar]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive oxygen species as mediators of gametophyte development and double fertilization in flowering plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef]
- Zhang, F.J.; Wang, Z.Q.; Dong, W.; Sun, C.Q.; Wang, H.B.; Song, A.P.; He, L.Z.; Fang, W.M.; Chen, F.D.; Teng, N.J. Transcriptomic and proteomic analysis reveals mechanisms of embryo abortion during chrysanthemum cross breeding. Sci. Rep. 2014, 4, 6536. [Google Scholar] [CrossRef] [PubMed]
- Zoong Lwe, Z.S.; Welti, R.; Anco, D.; Naveed, S.; Rustgi, S.; Narayanan, S. Heat stress elicits remodeling in the anther lipidome of peanut. Sci. Rep. 2020, 10, 22163. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Q.; Fu, H.; Chen, K.; Zhao, S.; Zhang, C.; Cai, T.; Wang, L.; Lu, W.; Dang, H.; et al. Identification of Key Gene Networks and Deciphering Transcriptional Regulators Associated with Peanut Embryo Abortion Mediated by Calcium Deficiency. Front. Plant Sci. 2022, 13, 814015. [Google Scholar] [CrossRef]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.-L. Cell wall invertase is essential for ovule development through sugar signaling rather than provision of carbon nutrients. Plant Physiol. 2020, 183, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Zhang, Z.; Li, S.; Hou, Y.; Yang, N. Studies on Different Family Seeds of Larix Kaenpferi from Chang Linggang Seed Orchard. Seed J. 2008, 10, 108–112. [Google Scholar] [CrossRef]
- Schauer, N.; Fernie, A.R. Plant metabolomics: Towards biological function and mechanism. Trends Plant Sci. 2006, 11, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gaquerel, E. Next-generation mass spectrometry metabolomics revives the functional analysis of plant metabolic diversity. Annu. Rev. Plant Biol. 2021, 72, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef]
- Kehelpannala, C.; Rupasinghe, T.; Hennessy, T.; Bradley, D.; Ebert, B.; Roessner, U. The state of the art in plant lipidomics. Mol. Omics 2021, 17, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid metabolism: Critical roles in male fertility and other aspects of reproductive development in plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Ma, D.K.; Zhang, Z.Y.; Wang, S.W.; Du, S.; Deng, X.P.; Yin, L.N. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, D.J.; Sun, C.L.; Li, Y.; Lu, H.; Wang, X. Comprehensive Lipidome and Metabolome Profiling Investigations of Panax quinquefolius and Application in Different Growing Regions Using Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 6710–6719. [Google Scholar] [CrossRef]
- Li, L.L.; Lu, X.; Zhao, J.Y.; Zhang, J.J.; Zhao, Y.N.; Zhao, C.X.; Xu, G.W. Lipidome and metabolome analysis of fresh tobacco leaves in different geographical regions using liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5009–5020. [Google Scholar] [CrossRef] [PubMed]
- Cheong, B.E.; Ho, W.W.H.; Biddulph, B.; Wallace, X.; Rathjen, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping reproductive stage chilling and frost tolerance in wheat using targeted metabolome and lipidome profiling. Metabolomics 2019, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Cheong, B.E.; Onyemaobi, O.; Ho, W.W.H.; Ben Biddulph, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling. Cells 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Zhang, L.; Lu, X.; Zhao, C.X.; Zhu, Z.; Wang, F.; Zhang, J.J.; Chen, S.L.; Zhao, Y.N.; Xu, G.W. A simultaneous extraction method for metabolome and lipidome and its application in cry1Ac and sck-transgenic rice leaf treated with insecticide based on LC-MS analysis. Metabolomics 2014, 10, 1197–1209. [Google Scholar] [CrossRef]
- Wang, L.; Shen, W.; Kazachkov, M.; Chen, G.; Chen, Q.; Carlsson, A.S.; Stymne, S.; Weselake, R.J.; Zou, J. Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 2012, 24, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Pressman, E.; Peet, M.M.; Pharr, D.M. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann. Bot. 2002, 90, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotechnol. 2018, 49, 191–198. [Google Scholar] [CrossRef]
- Kim, H.U. Lipid Metabolism in Plants. Plants 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Reszczynska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Gautam, C.; Kumar, R.; Tilgam, J.; Natta, S. Phenolic Biosynthesis and Metabolic Pathways to Alleviate Stresses in Plants. In Plant Phenolics in Abiotic Stress Management; Springer: Berlin/Heidelberg, Germany, 2023; pp. 63–87. [Google Scholar]
- Ghosh, U.; Islam, M.; Siddiqui, M.; Cao, X.; Khan, M. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of the γ-Aminobutyric Acid (GABA) in Plant Salt Stress Tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Yu, Y.C.; Kou, M.; Gao, Z.H.; Liu, Y.; Xuan, Y.; Liu, Y.J.; Tang, Z.H.; Cao, Q.H.; Li, Z.Y.; Sun, J. Involvement of Phosphatidylserine and Triacylglycerol in the Response of Sweet Potato Leaves to Salt Stress. Front. Plant Sci. 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Shishova, M.F.; Yemelyanov, V.V. Proteome and Lipidome of Plant Cell Membranes during Development. Russ. J. Plant Physiol. 2021, 68, 800–817. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Tauler, R.; Iriondo-Frias, G.; Jaumot, J. Untargeted lipidomic evaluation of hydric and heat stresses on rice growth. J. Chromatogr. B 2019, 1104, 148–156. [Google Scholar] [CrossRef]
- Radojičić, A.; Li, X.; Zhang, Y. Salicylic acid: A double-edged sword for programed cell death in plants. Front. Plant Sci. 2018, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiao, Y.; Zhang, C.; Dou, M.; Weng, K.; Wang, Y.; Xu, Y. VvHDZ28 positively regulate salicylic acid biosynthesis during seed abortion in Thompson Seedless. Plant Biotechnol. J. 2021, 19, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Zhao, G.; Fan, X.; Long, H.; Fan, Y.; Shi, M.; Tan, X.; Zhang, L. New insights of salicylic acid into stamen abortion of female flowers in tung tree (Vernicia fordii). Front. Genet. 2019, 10, 316. [Google Scholar] [CrossRef]
- Du, B.; Nie, X.; Long, T.; Liu, J.; Zhang, Q.; Xing, Y.; Cao, Q.; Qin, L.; Kefeng, F. Programmed Cell Death Is Responsible for Ovule Abortion in Castanea Mollissima. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4353617 (accessed on 1 September 2023).
- Zhao, Q.; Guan, X.; Zhou, L.; Asad, M.A.U.; Xu, Y.; Pan, G.; Cheng, F. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ. 2023, 46, 1453–1471. [Google Scholar] [CrossRef]
- Zakharova, E.V.; Khaliluev, M.R.; Kovaleva, L.V. Hormonal signaling in the progamic phase of fertilization in plants. Horticulturae 2022, 8, 365. [Google Scholar] [CrossRef]
- Kitatani, K.; Taniguchi, M.; Okazaki, T. Cell death/survival signal by ceramide and sphingosine-1-phosphate. In Glycoscience Pro-Tocols (GlycoPODv2) [Internet]; Japan Consortium for Glycobiology and Glycotechnology: Saitama, Japan, 2022. [Google Scholar]
- Chipuk, J.E.; McStay, G.P.; Bharti, A.; Kuwana, T.; Clarke, C.J.; Siskind, L.J.; Obeid, L.M.; Green, D.R. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 2012, 148, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Z.; Qu, L.-J.; Dresselhaus, T. Stigmatic ROS: Regulator of compatible pollen tube perception? Trends Plant Sci. 2021, 26, 993–995. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
| Subclass | Full Name | Lipid Types | Mean_SE | Mean_CK | log2FC | p-Value | Changes |
|---|---|---|---|---|---|---|---|
| TG | Triglyceride | Glycerolipids | 2,418,466,213.00 | 398,000,000,000.00 | −7.36 | 0.000 | less |
| SiE | Sitosterol ester | SterolEsters | 63,066.94 | 3,338,359.46 | −5.73 | 0.000 | less |
| DG | Diglyceride | Glycerolipids | 49,875,524.58 | 1,829,902,611.00 | −5.20 | 0.000 | less |
| PG | Phosphatidylglycerol | Glycerophospholipids | 88,987,548.34 | 2,634,724,739.00 | −4.89 | 0.000 | less |
| AGlcSiE | Acylglcsitosterol ester | SterolEsters | 8,772,664.30 | 111,780,732.70 | −3.67 | 0.000 | less |
| LPE | Lysophosphatidylethanolamine | Glycerophospholipids | 2,533,033.93 | 25,965,487.90 | −3.36 | 0.000 | less |
| Co | Coenzyme | Others | 4,810,648.04 | 45,922,404.57 | −3.25 | 0.000 | less |
| LPC | Lysophosphatidylcholine | Glycerophospholipids | 12,033,231.72 | 79,404,329.43 | −2.72 | 0.000 | less |
| SQDG | Sulfoquinovosyldiacylglycerol | Glyceroglycolipids | 4,349,611.59 | 25,803,596.25 | −2.57 | 0.000 | less |
| PA | Phosphatidic acid | Glycerophospholipids | 1,000,871,191.00 | 5,444,599,004.00 | −2.44 | 0.000 | less |
| MGDG | Monogalactosyldiacylglycerol | Glyceroglycolipids | 98,227,212.47 | 461,038,998.00 | −2.23 | 0.000 | less |
| PI | Phosphatidylinositol | Glycerophospholipids | 328,142,065.30 | 1,296,038,926.00 | −1.98 | 0.000 | less |
| DGDG | Digalactosyldiacylglycerol | Glyceroglycolipids | 69,555,932.89 | 252,982,297.10 | −1.86 | 0.000 | less |
| PS | Phosphatidylserine | Glycerophospholipids | 106,284,549.40 | 359,065,750.00 | −1.76 | 0.000 | less |
| PC | Phosphatidylcholine | Glycerophospholipids | 2,090,068,304.00 | 6,189,833,094.00 | −1.57 | 0.000 | less |
| PE | Phosphatidylethanolamine | Glycerophospholipids | 703,590,741.40 | 1,819,163,636.00 | −1.37 | 0.000 | less |
| phSM | Sphingomyelin (phytosphingosine) | Sphingolipids | 15,154,103.72 | 37,533,428.50 | −1.31 | 0.000 | less |
| CerG1 | Simple Glc series | Sphingolipids | 105,905,712.10 | 249,572,929.00 | −1.24 | 0.000 | less |
| CL | Cardiolipin | Glycerophospholipids | 4,877,980.67 | 9,895,607.47 | −1.02 | 0.003 | less |
| SoP | Sphingosine phosphate | Sphingolipids | 9,236,615.28 | 4,034,788.16 | 1.19 | 0.004 | more |
| LPA | Lysophosphatidic acid | Glycerophospholipids | 874,176.31 | 2,832,812.07 | −1.70 | 0.073 | not sig |
| LPI | Lysophosphatidylinositol | Glycerophospholipids | 1,780,832.34 | 5,315,378.73 | −1.58 | 0.169 | not sig |
| Cer | Ceramides | Sphingolipids | 1,341,801,269.00 | 1,882,630,305.00 | −0.49 | 0.002 | not sig |
| So | Sphingosine | Sphingolipids | 122,697,762.80 | 132,845,014.30 | −0.11 | 0.330 | not sig |
| DGMG | Digalactosylmonoacylglycerol | Glyceroglycolipids | 789,186.10 | 803,985.60 | −0.03 | 0.957 | not sig |
| LPG | lysophosphatidylglycerol | Glycerophospholipids | 769,702.94 | 692,126.93 | 0.15 | 0.765 | not sig |
| Formula | Compounds | Class I | Class II | VIP | Log2FC |
|---|---|---|---|---|---|
| C45H38O19 | Gallocatechin-catechin-catechin | Tannins | Proanthocyanidins | 1.07 | 20.27 |
| C45H38O18 | Procyanidin C1 | Tannins | Proanthocyanidins | 1.07 | 20.05 |
| C21H24O11 | Sieboldin | Flavonoids | Chalcones | 1.07 | 19.17 |
| C45H38O18 | Arecatannin C1 | Tannins | Tannin | 1.07 | 18.80 |
| C45H38O20 | Gallocatechin-gallocatechin-catechin | Tannins | Proanthocyanidins | 1.07 | 18.79 |
| C27H30O15 | 5,7-Dihydroxy-4-methoxyflavone-3-O-xylose-(1-6)-glucose | Flavonoids | Flavonoid | 1.07 | 17.81 |
| C60H50O24 | Cinnamtannin B2 | Tannins | Tannin | 1.07 | 17.74 |
| C15H12O8 | Dihydromyricetin (Ampelopsin) | Flavonoids | Dihydroflavonol | 1.07 | 17.67 |
| C21H20O11 | Luteolin-7-O-glucoside (Cynaroside) | Flavonoids | Flavonoid | 1.07 | 17.63 |
| C22H22O13 | Patuletin-3-O-glucoside | Flavonoids | Flavonoid | 1.07 | 17.44 |
| C60H50O24 | Arecatannin A1 | Tannins | Tannin | 1.07 | 17.31 |
| C7H6O4 | 2,4-Dihydroxybenzoic acid | Phenolic acids | Phenolic acids | 1.07 | 17.31 |
| C60H50O24 | Arecatannin A2 | Tannins | Tannin | 1.07 | 17.27 |
| C22H22O13 | Laricitrin-3-O-glucoside | Flavonoids | Flavonoid | 1.07 | 17.25 |
| C7H6O4 | 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | Phenolic acids | Phenolic acids | 1.07 | 17.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Shen, M.; Liu, R.; Cai, X.; Lin, J.; Wang, L.; Chen, Y.; Chen, G.; Cao, S.; Qin, Y. Revealing Further Insights into Astringent Seeds of Chinese Fir by Integrated Metabolomic and Lipidomic Analyses. Int. J. Mol. Sci. 2023, 24, 15103. https://doi.org/10.3390/ijms242015103
Zheng P, Shen M, Liu R, Cai X, Lin J, Wang L, Chen Y, Chen G, Cao S, Qin Y. Revealing Further Insights into Astringent Seeds of Chinese Fir by Integrated Metabolomic and Lipidomic Analyses. International Journal of Molecular Sciences. 2023; 24(20):15103. https://doi.org/10.3390/ijms242015103
Chicago/Turabian StyleZheng, Ping, Mengqian Shen, Ruoyu Liu, Xinkai Cai, Jinting Lin, Lulu Wang, Yu Chen, Guangwei Chen, Shijiang Cao, and Yuan Qin. 2023. "Revealing Further Insights into Astringent Seeds of Chinese Fir by Integrated Metabolomic and Lipidomic Analyses" International Journal of Molecular Sciences 24, no. 20: 15103. https://doi.org/10.3390/ijms242015103
APA StyleZheng, P., Shen, M., Liu, R., Cai, X., Lin, J., Wang, L., Chen, Y., Chen, G., Cao, S., & Qin, Y. (2023). Revealing Further Insights into Astringent Seeds of Chinese Fir by Integrated Metabolomic and Lipidomic Analyses. International Journal of Molecular Sciences, 24(20), 15103. https://doi.org/10.3390/ijms242015103








