Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice
Abstract
1. Introduction
2. Results
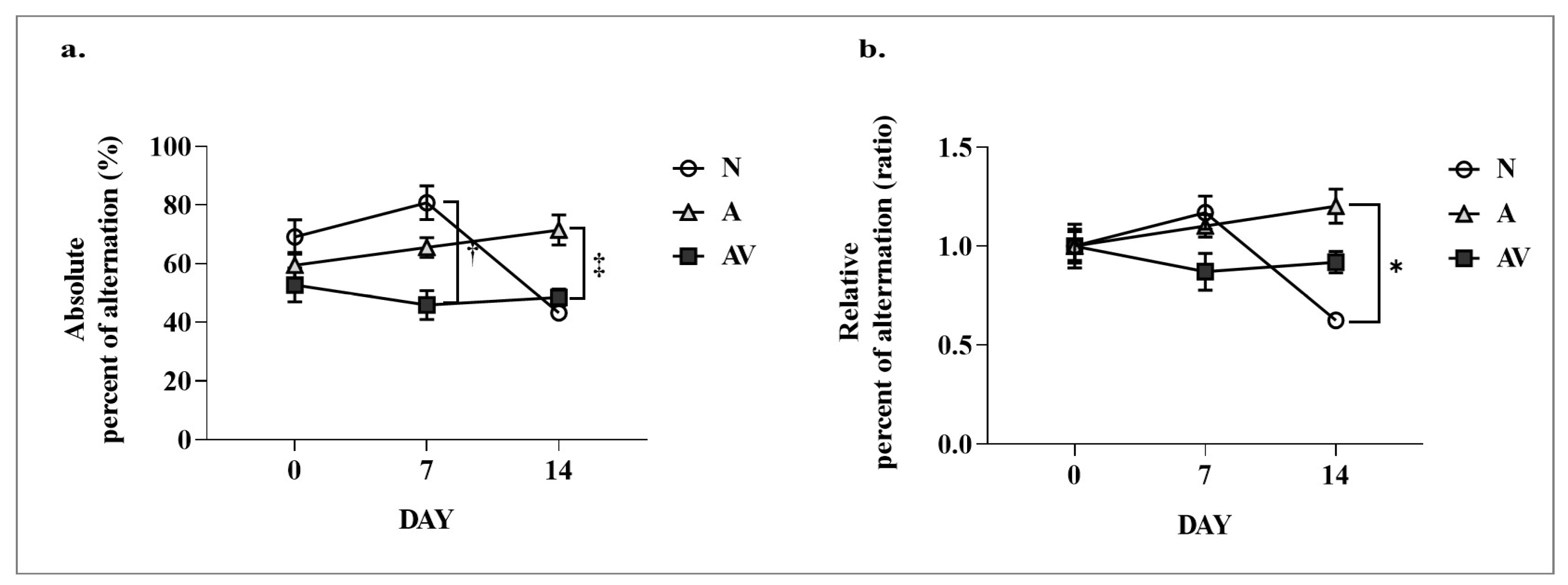
2.1. Improvement in Cognitive Function of ApoE4 KI Mice after Sensory Stimulation
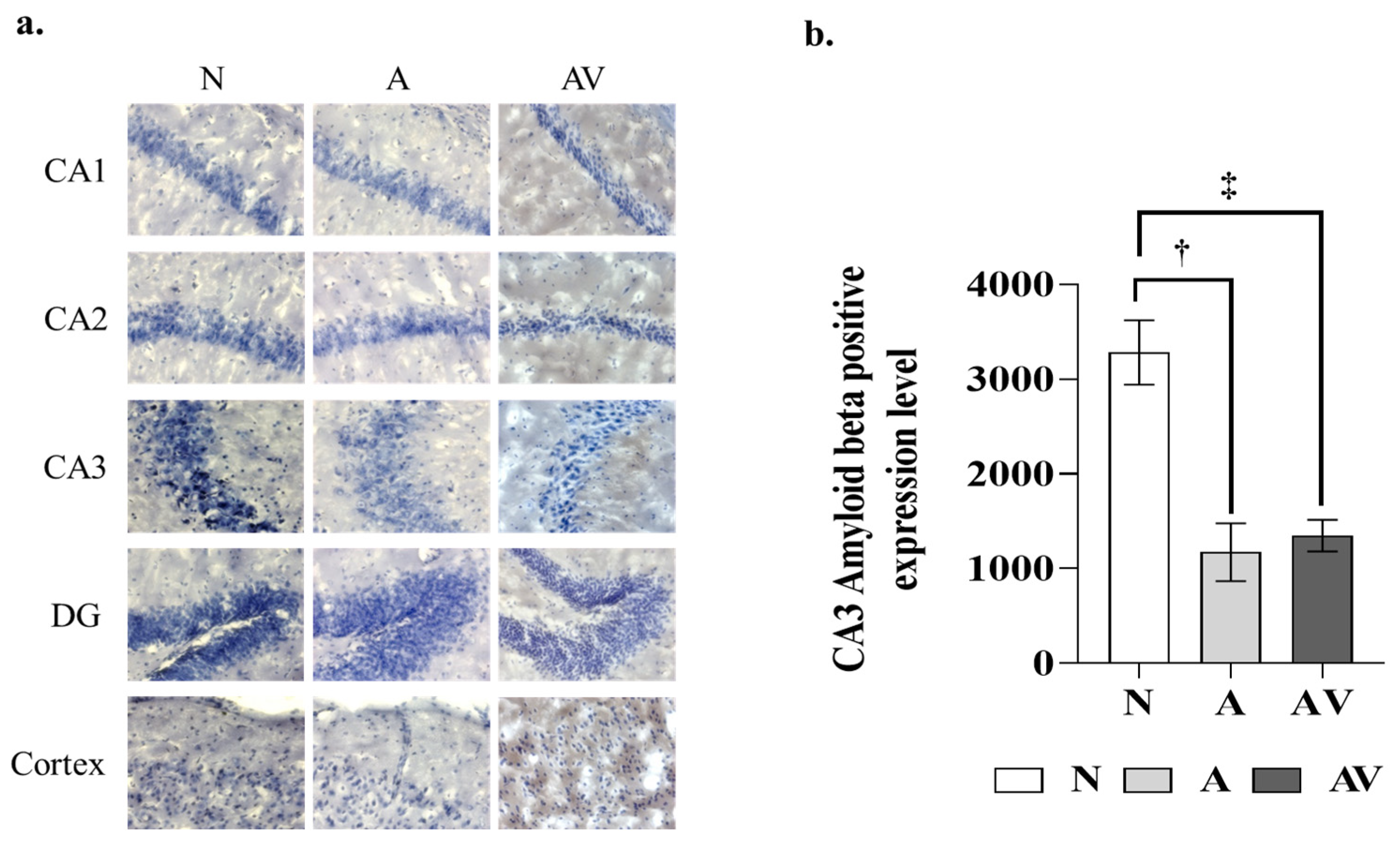
2.2. Reduction in Aβ Deposition in the Hippocampus of ApoE4 KI Mice after Sensory Stimulation
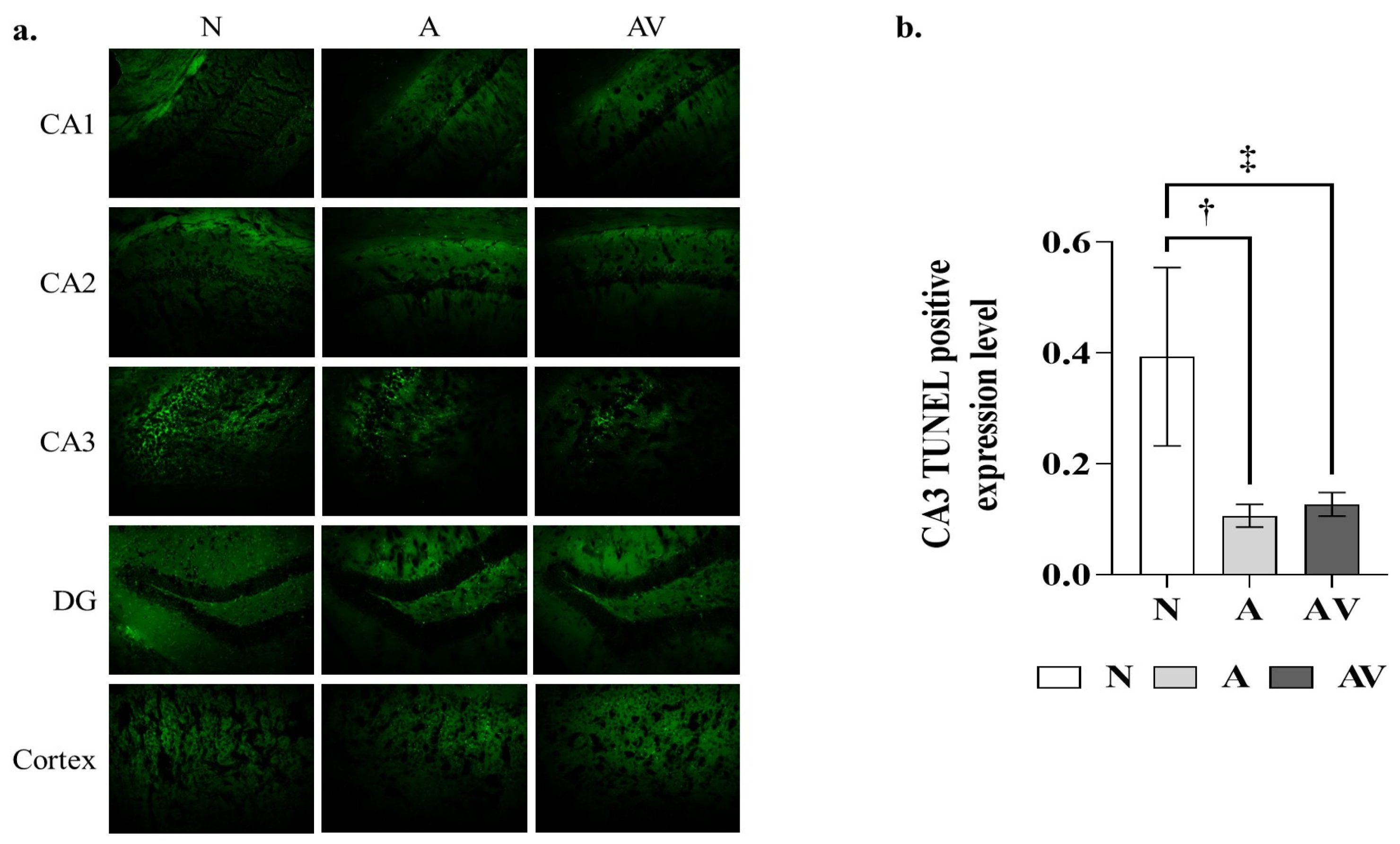
2.3. Reduction in Apoptosis in the Hippocampus of ApoE4 KI Mice after Sensory Stimulation
2.4. Changes in Brain Acetylcholine Levels in ApoE4 KI Mice after Sensory Stimulation
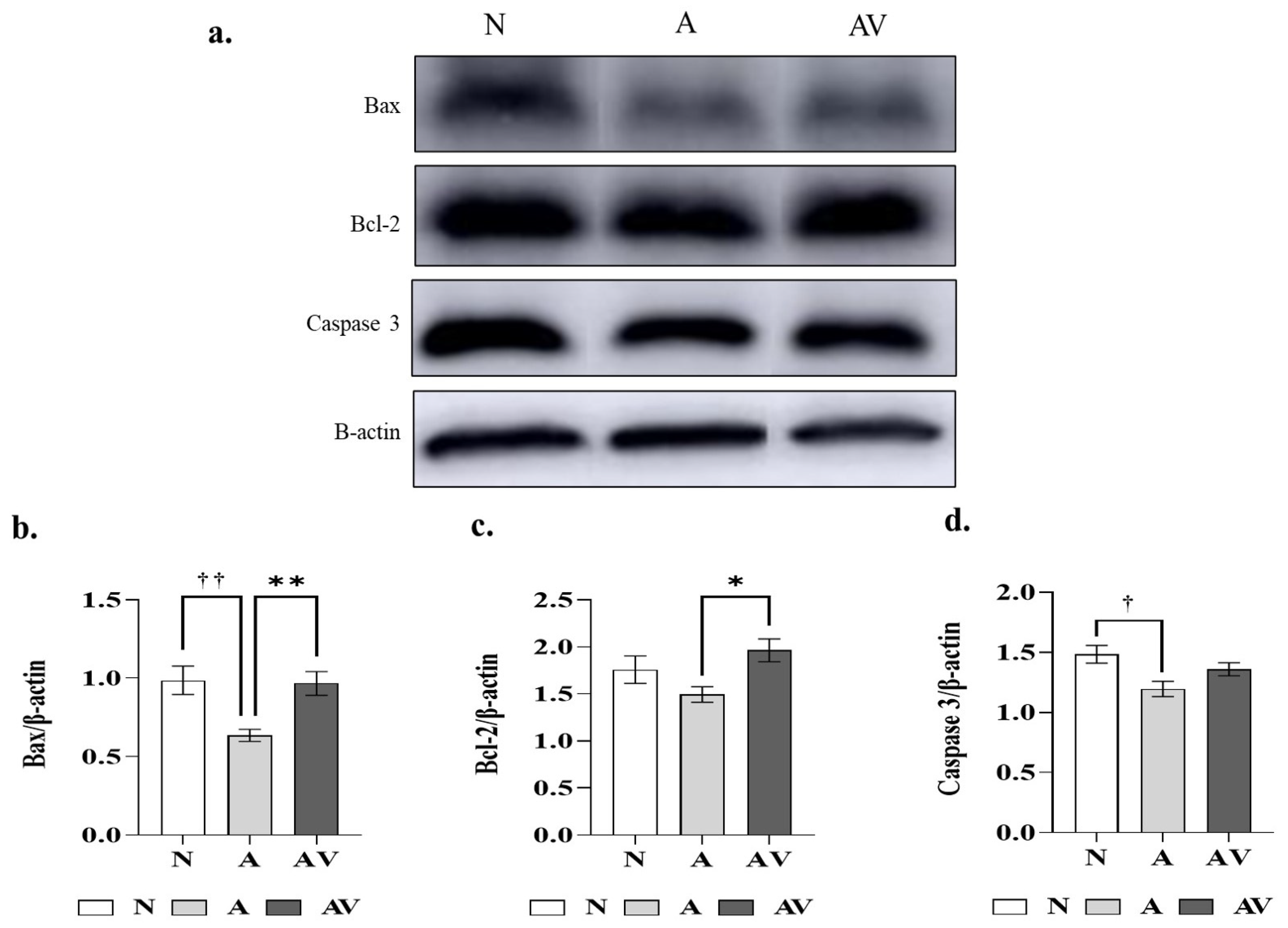
2.5. Changes in Apoptosis-Related Proteins in ApoE4 KI Mice after Sensory Stimulation
3. Discussion
4. Materials and Methods
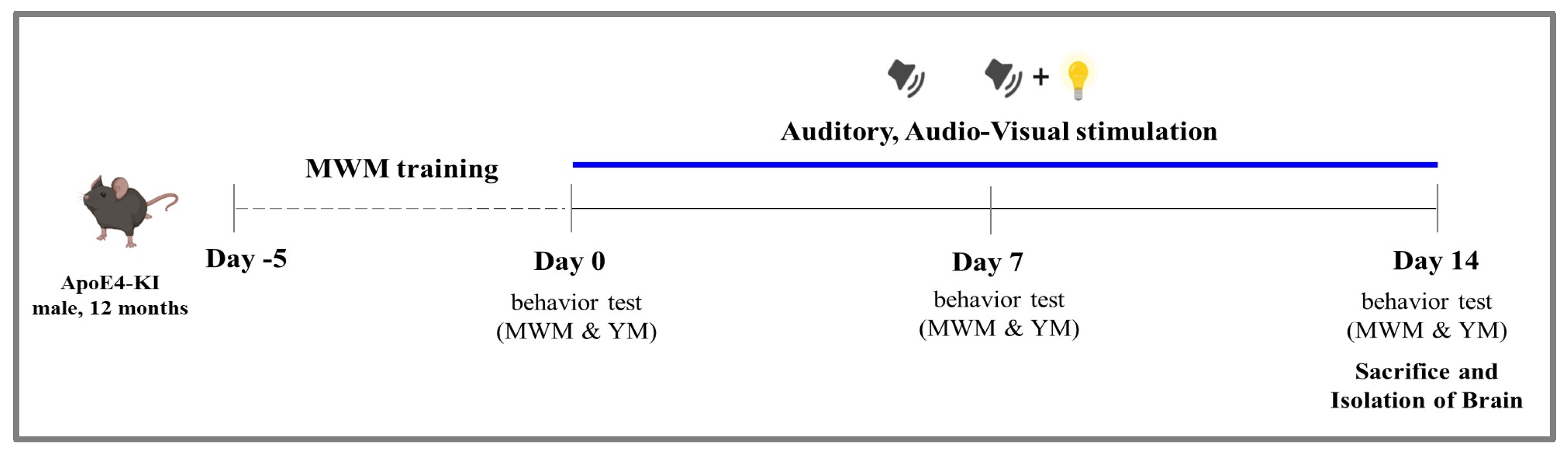
4.1. Experimental Animals and Timeline
4.2. Sensory Stimulation Protocol
4.3. Behavioral Tests
4.3.1. Morris Water Maze Test
4.3.2. The Y Maze Test
4.4. Histological Analysis
4.4.1. Immunohistochemistry
4.4.2. Terminal-Deoxynucleotidyl Transferase Mediated Nick end Labeling (TUNEL) Assay
4.5. Biochemical Analyses
4.5.1. Acetylcholine Assay
4.5.2. Apoptotic Protein Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Linskens, E.J.; MacDonald, R.; Silverman, P.C.; McCarten, J.R.; Talley, K.M.C.; Forte, M.L.; Desai, P.J.; Nelson, V.A.; Miller, M.A.; et al. Benefits and Harms of Prescription Drugs and Supplements for Treatment of Clinical Alzheimer-Type Dementia. Ann. Intern. Med. 2020, 172, 656–668. [Google Scholar] [CrossRef]
- Cronin-Golomb, A.; Corkin, S.; Rizzo, J.F.; Cohen, J.; Growdon, J.H.; Banks, K.S. Visual dysfunction in Alzheimer’s disease: Relation to normal aging. Ann. Neurol. 1991, 29, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Cobb, J.L.; Linn, R.T.; Rees, T.; Wolf, P.A.; D’Agostino, R.B. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch. Otolaryngol. Head. Neck Surg. 1996, 122, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C. Loss of olfactory function in dementing disease. Physiol. Behav. 1999, 66, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Beiser, A.; Rees, T.S.; D’Agostino, R.B.; Wolf, P.A. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J. Am. Geriatr. Soc. 2002, 50, 482–488. [Google Scholar] [CrossRef]
- Uhlmann, R.F.; Larson, E.B.; Rees, T.S.; Koepsell, T.D.; Duckert, L.G. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989, 261, 1916–1919. [Google Scholar] [CrossRef]
- Rizzo, M.; Anderson, S.W.; Dawson, J.; Nawrot, M. Vision and cognition in Alzheimer’s disease. Neuropsychologia 2000, 38, 1157–1169. [Google Scholar] [CrossRef]
- Rahayel, S.; Frasnelli, J.; Joubert, S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: A meta-analysis. Behav. Brain Res. 2012, 231, 60–74. [Google Scholar] [CrossRef]
- Ford, A.H.; Hankey, G.J.; Yeap, B.B.; Golledge, J.; Flicker, L.; Almeida, O.P. Hearing loss and the risk of dementia in later life. Maturitas 2018, 112, 1–11. [Google Scholar] [CrossRef]
- Thomson, R.S.; Auduong, P.; Miller, A.T.; Gurgel, R.K. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig. Otolaryngol. 2017, 2, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Heywood, R.; Gao, Q.; Nyunt, M.S.Z.; Feng, L.; Chong, M.S.; Lim, W.S.; Yap, P.; Lee, T.-S.; Yap, K.B.; Wee, S.L.; et al. Hearing Loss and Risk of Mild Cognitive Impairment and Dementia: Findings from the Singapore Longitudinal Ageing Study. Dement. Geriatr. Cogn. Disord. 2017, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Luchsinger, J.A.; Manly, J.J.; Stern, Y.; Mayeux, R.; Schupf, N. Observed Hearing Loss and Incident Dementia in a Multiethnic Cohort. J. Am. Geriatr. Soc. 2017, 65, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, R.K.; Ward, P.D.; Schwartz, S.; Norton, M.C.; Foster, N.L.; Tschanz, J.T. Relationship of hearing loss and dementia: A prospective, population-based study. Otol. Neuroto 2014, 35, 775–781. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L.; Metter, E.J.; An, Y.; Zonderman, A.B.; Resnick, S.M. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011, 25, 763–770. [Google Scholar] [CrossRef]
- Liu, L.; Shen, P.; He, T.; Chang, Y.; Shi, L.; Tao, S.; Li, X.; Xun, Q.; Guo, X.; Yu, Z.; et al. Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci. Rep. 2016, 6, 20374. [Google Scholar] [CrossRef]
- Kraus, K.S.; Mitra, S.; Jimenez, Z.; Hinduja, S.; Ding, D.; Jiang, H.; Gray, L.; Lobarinas, E.; Sun, W.; Salvi, R.J. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 2010, 167, 1216–1226. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.J.; Lee, S.; Lee, H.S.; Jeong, Y.J.; Son, Y.; Kim, J.M.; Lee, Y.J.; Park, M.H. Conductive Hearing Loss Aggravates Memory Decline in Alzheimer Model Mice. Front. Neurosci. 2020, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G. Treatment Development for Alzheimer’s Disease: How Are We Doing? Adv. Exp. Med. Biol. 2020, 1195, 19. [Google Scholar] [PubMed]
- Yu, T.-W.; Lane, H.-Y.; Lin, C.-H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef] [PubMed]
- Adaikkan, C.; Tsai, L.H. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends Neurosci. 2020, 43, 24–41. [Google Scholar] [CrossRef]
- Chang, C.H.; Lane, H.Y.; Lin, C.H. Brain Stimulation in Alzheimer’s Disease. Front. Psychiatry 2018, 9, 201. [Google Scholar] [CrossRef]
- Yang, H.; Luo, Y.; Hu, Q.; Tian, X.; Wen, H. Benefits in Alzheimer’s Disease of Sensory and Multisensory Stimulation. J. Alzheimers Dis. 2021, 82, 463–484. [Google Scholar] [CrossRef]
- Rajji, T.K. Transcranial Magnetic and Electrical Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment: A Review of Randomized Controlled Trials. Clin. Pharm. 2019, 106, 776–780. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, W.J.; Shan, P.Y.; Lu, M.; Wang, T.; Li, R.H.; Zhang, N.; Ma, L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Neurol. Sci. 2019, 398, 184–191. [Google Scholar] [CrossRef]
- Pellicciari, M.C.; Miniussi, C. Transcranial Direct Current Stimulation in Neurodegenerative Disorders. J. ECT 2018, 34, 193–202. [Google Scholar] [CrossRef]
- van Deursen, J.A.; Vuurman, E.F.; van Kranen-Mastenbroek, V.H.; Verhey, F.R.; Riedel, W.J. 40-Hz steady state response in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2011, 32, 24–30. [Google Scholar] [CrossRef]
- Clements-Cortes, A.; Ahonen, H.; Evans, M.; Freedman, M.; Bartel, L. Short-Term Effects of Rhythmic Sensory Stimulation in Alzheimer’s Disease: An Exploratory Pilot Study. J. Alzheimers Dis. 2016, 52, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, S.; Kim, H.J.; Jung, J.; Lee, B.; Kim, T. 40 Hz acoustic stimulation decreases amyloid beta and modulates brain rhythms in a mouse model of Alzheimer’s disease. bioRxiv 2018. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Myers, A.; McGonigle, P. Overview of Transgenic Mouse Models for Alzheimer’s Disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef]
- Foidl, B.M.; Humpel, C. Can mouse models mimic sporadic Alzheimer’s disease? Neural Regen. Res. 2020, 15, 401–406. [Google Scholar]
- Youmans, K.L.; Tai, L.M.; Nwabuisi-Heath, E.; Jungbauer, L.; Kanekiyo, T.; Gan, M.; Kim, J.; Eimer, W.A.; Estus, S.; Rebeck, G.W.; et al. APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2012, 287, 41774–41786. [Google Scholar] [CrossRef]
- Lewandowski, C.T.; Maldonado Weng, J.; LaDu, M.J. Alzheimer’s disease pathology in APOE transgenic mouse models: The Who, What, When, Where, Why, and How. Neurobiol. Dis. 2020, 139, 104811. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Monteiro, F.; Sotiropoulos, I.; Carvalho, Ó.; Sousa, N.; Silva, F.S. Multi-mechanical waves against Alzheimer’s disease pathology: A systematic review. Transl. Neurodegener 2021, 10, 36. [Google Scholar] [CrossRef]
- Papalambros, N.A.; Weintraub, S.; Chen, T.; Grimaldi, D.; Santostasi, G.; Paller, K.A.; Zee, P.C.; Malkani, R.G. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann. Clin. Transl. Neurol. 2019, 6, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Papalambros, N.A.; Santostasi, G.; Malkani, R.G.; Braun, R.; Weintraub, S.; Paller, K.A.; Zee, P.C. Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Front. Hum. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.E.; Hewes, A.A.; Garceau, D.T.; Kotredes, K.P.; Carter, G.W.; Sasner, M.; Howell, G.R. The APOE (ε3/ε4) Genotype Drives Distinct Gene Signatures in the Cortex of Young Mice. Front. Aging Neurosci. 2022, 14, 838436. [Google Scholar] [CrossRef] [PubMed]
- Colurso, G.J.; Nilson, J.E.; Vervoort, L.G. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer’s disease. Life Sci. 2003, 73, 1795–1803. [Google Scholar] [CrossRef]
- Rad, S.K.; Arya, A.; Karimian, H.; Madhavan, P.; Rizwan, F.; Koshy, S.; Prabhu, G. Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: Link between type 2 diabetes and Alzheimer’s disease. Drug Des. Dev. Ther. 2018, 12, 3999–4021. [Google Scholar]
- Xu, C.; Xiao, Z.; Wu, H.; Zhou, G.; He, D.; Chang, Y.; Li, Y.; Wang, G.; Xie, M. BDMC protects AD in vitro via AMPK and SIRT1. Transl. Neurosci. 2020, 11, 319–327. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Adaikkan, C.; Middleton, S.J.; Marco, A.; Pao, P.C.; Mathys, H.; Kim, D.N.; Gao, F.; Young, J.Z.; Suk, H.J.; Boyden, E.S.; et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron 2019, 102, 929–943.e8. [Google Scholar] [CrossRef] [PubMed]
- Traikapi, A.; Konstantinou, N. Gamma Oscillations in Alzheimer’s Disease and Their Potential Therapeutic Role. Front. Syst. Neurosci. 2021, 15, 782399. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Colon-Motas, K.M.; Pybus, A.F.; Piendel, L.; Seppa, J.K.; Walker, M.L.; Manzanares, C.M.; Qiu, D.; Miocinovic, S.; Wood, L.B.; et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement 2021, 7, e12178. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.T.; Meredith, M.A.; Stein, B.E. Multisensory integration in the superior colliculus of the alert cat. J. Neurophysiol. 1998, 80, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.A.; Nemitz, J.W.; Stein, B.E. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J. Neurosci. 1987, 7, 3215–3229. [Google Scholar] [CrossRef]
- King, A.J. Multisensory integration: Strategies for synchronization. Curr. Biol. 2005, 15, R339–R341. [Google Scholar] [CrossRef]
- Sugita, Y.; Suzuki, Y. Audiovisual perception: Implicit estimation of sound-arrival time. Nature 2003, 421, 911. [Google Scholar] [CrossRef]
- Kopinska, A.; Harris, L.R. Simultaneity constancy. Perception 2004, 33, 1049–1060. [Google Scholar] [CrossRef]
- Alais, D.; Carlile, S. Synchronizing to real events: Subjective audiovisual alignment scales with perceived auditory depth and speed of sound. Proc. Natl. Acad. Sci. USA 2005, 102, 2244–2247. [Google Scholar] [CrossRef]
- Strelnikov, K.; Hervault, M.; Laurent, L.; Barone, P. When two is worse than one: The deleterious impact of multisensory stimulation on response inhibition. PLoS ONE 2021, 16, e0251739. [Google Scholar] [CrossRef]
- Dean, C.L.; Eggleston, B.A.; Gibney, K.D.; Aligbe, E.; Blackwell, M.; Kwakye, L.D. Auditory and visual distractors disrupt multisensory temporal acuity in the crossmodal temporal order judgment task. PLoS ONE 2017, 12, e0179564. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Turner, G.H.; Coons, S.W.; Maalouf, M.; Reiman, E.M.; Shi, J. Association of amyloid burden, brain atrophy and memory deficits in aged apolipoprotein ε4 mice. Curr. Alzheimer Res. 2014, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Andrews-Zwilling, Y.; Yoon, S.Y.; Jain, S.; Ring, K.; Dai, J.; Wang, M.M.; Tong, L.; Walker, D.; Huang, Y. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS ONE 2012, 7, e53569. [Google Scholar] [CrossRef]
- Bour, A.; Grootendorst, J.; Vogel, E.; Kelche, C.; Dodart, J.C.; Bales, K.; Moreau, P.H.; Sullivan, P.M.; Mathis, C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008, 193, 174–182. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Mavroudis, I.; Fotiou, D.; Baloyannis, S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr. Danub. 2012, 24, 152–158. [Google Scholar] [PubMed]
- Cruz-Sánchez, F.F.; Gironès, X.; Ortega, A.; Alameda, F.; Lafuente, J.V. Oxidative stress in Alzheimer’s disease hippocampus: A topographical study. J. Neurol. Sci. 2010, 299, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Kesner, R.P. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007, 14, 771–781. [Google Scholar] [CrossRef]
- Florian, C.; Roullet, P. Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav. Brain Res. 2004, 154, 365–374. [Google Scholar] [CrossRef]
- Prieur, E.A.K.; Jadavji, N.M. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio Protoc. 2019, 9, e3162. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar]
- Pinto, J.O.; Dores, A.R.; Geraldo, A.; Peixoto, B.; Barbosa, F. Sensory stimulation programs in dementia: A systematic review of methods and effectiveness. Expert Rev. Neurother. 2020, 21, 1229–1247. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Suk, H.-J.; Jackson, B.L.; Milman, N.P.; Stark, D.; Klerman, E.B.; Kitchener, E.; Avalos, V.S.F.; de Weck, G.; Banerjee, A.; et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS ONE 2022, 17, e0278412. [Google Scholar] [CrossRef] [PubMed]
- Clements-Cortes, A.; Bartel, L. Long-Term Multi-Sensory Gamma Stimulation of Dementia Patients: A Case Series Report. Int. J. Environ. Res. Public Health 2022, 19, 15553. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, E.; Wittevrongel, B.; Reinartz, M.; Dauwe, I.; Carrette, E.; Meurs, A.; Van Roost, D.; Boon, P.; Van Hulle, M.M. Cognitive tasks propagate the neural entrainment in response to a visual 40 Hz stimulation in humans. Front. Aging Neurosci. 2022, 14, 1010765. [Google Scholar] [CrossRef] [PubMed]

| N | A | AV | p-Value | |||
|---|---|---|---|---|---|---|
| Between Times (F Value) | Between Groups (F Value) | Interactions (F Value) | ||||
| Absolute alternation (%) in YM | ||||||
| Day 0 | 69.09 ± 5.09 | 59.45 ± 6.93 | 52.74 ± 9.21 | <0.001 * (11.59) | ns (2.111) | <0.001 * (4.116) |
| Day 7 | 80.77 ± 5.00 † | 65.51 ± 5.34 | 45.9 ± 7.78 † | |||
| Day 14 | 43.17 ± 1.67 | 71.46 ± 8.10 ‡ | 48.45 ± 4.53 ‡ | |||
| Relative alternation (ratio) in YM | ||||||
| Day 0 | 1.00 ± 0.07 | 1.00 ± 0.12 | 1.00 ± 0.17 | <0.05 * (4.055) | ns (1.228) | <0.05 * (3.071) |
| Day 7 | 1.17 ± 0.07 | 1.10 ± 0.09 | 0.87 ± 0.15 | |||
| Day 14 | 0.62 ± 0.02 # | 1.20 ± 0.14 # | 0.92 ± 0.09 | |||
| N | A | AV | p-Value | |
|---|---|---|---|---|
| CA1 | 51.60 ± 23.96 | 314.10 ± 155.14 | 384.70 ± 110.97 | ns |
| CA2 | 113.60 ± 86.41 | 269.10 ± 99.82 | 251.30 ± 76.63 | ns |
| CA3 | 3282.00 ± 338.95 | 1174.67 ± 457.19 † | 1348.00 ± 263.17 ‡ | <0.001 * |
| DG | 93.00 ± 56.88 | 217.20 ± 90.11 | 128.40 ± 39.66 | ns |
| Cortex | 322.20 ± 145.70 | 651.10 ± 227.3 | 441.40 ± 139.60 | ns |
| N | A | AV | p-Value | |
|---|---|---|---|---|
| CA1 | 0.10 ± 0.04 | 0.05 ± 0.01 | 0.07 ± 0.03 | ns |
| CA2 | 0.07 ± 0.03 | 0.05 ± 0.02 | 0.12 ± 0.11 | ns |
| CA3 | 0.39 ± 0.16 | 0.11 ± 0.03 † | 0.13 ± 0.03 ‡ | <0.01 * |
| DG | 0.11 ± 0.06 | 0.04 ± 0.01 | 0.04 ± 0.01 | ns |
| Cortex | 0.11 ± 0.06 | 0.05 ± 0.02 | 0.07 ± 0.02 | ns |
| N | A | AV | p-Value | |
|---|---|---|---|---|
| Total choline (nmol/mg) | 149.31 ± 18.79 | 422.72 ± 28.58 † | 465.66 ± 51.82 ‡ | <0.001 * |
| Free choline (nmol/mg) | 7.47 ± 0.94 | 21.14 ± 1.43 † | 23.28 ± 2.59 ‡ | <0.001 * |
| Acetylcholine (nmol/mg) | 31.01 ± 3.14 | 44.77 ± 2.46 † | 48.02 ± 2.30 ‡ | <0.001 * |
| Apoptosis-Related Proteins | N | A | AV | p-Value |
|---|---|---|---|---|
| Bax/β-actin | 0.99 ± 0.10 †† | 0.64 ± 0.06 ††,## | 0.97 ± 0.11 ## | <0.01 * |
| Bcl-2/β-actin | 1.76 ± 0.16 | 1.49 ± 0.13 # | 1.96 ± 0.19 # | <0.05 * |
| Caspase-3/β-actin | 1.48 ± 0.08 † | 1.20 ± 0.10 † | 1.36 ± 0.09 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Lee, Y.; Lee, S.-H.; Sohn, J.-H. Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice. Int. J. Mol. Sci. 2023, 24, 938. https://doi.org/10.3390/ijms24020938
Jung H, Lee Y, Lee S-H, Sohn J-H. Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice. International Journal of Molecular Sciences. 2023; 24(2):938. https://doi.org/10.3390/ijms24020938
Chicago/Turabian StyleJung, Harry, Yeonkyeong Lee, Sang-Hwa Lee, and Jong-Hee Sohn. 2023. "Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice" International Journal of Molecular Sciences 24, no. 2: 938. https://doi.org/10.3390/ijms24020938
APA StyleJung, H., Lee, Y., Lee, S.-H., & Sohn, J.-H. (2023). Auditory or Audiovisual Stimulation Ameliorates Cognitive Impairment and Neuropathology in ApoE4 Knock-In Mice. International Journal of Molecular Sciences, 24(2), 938. https://doi.org/10.3390/ijms24020938





