Abstract
Susceptibility to upper respiratory infections (URIs) may be influenced by host, microbial, and environmental factors. We hypothesized that multi-omic analyses of molecular factors in infant saliva would identify complex host-environment interactions associated with URI frequency. A cohort study involving 146 infants was used to assess URI frequency in the first year of life. Saliva was collected at 6 months for high-throughput multi-omic measurement of cytokines, microRNAs, transcripts, and microbial RNA. Regression analysis identified environmental (daycare attendance, atmospheric pollution, breastfeeding duration), microbial (Verrucomicrobia, Streptococcus phage), and host factors (miR-22-5p) associated with URI frequency (p < 0.05). These results provide pathophysiologic clues about molecular factors that influence URI susceptibility. Validation of these findings in a larger cohort could one day yield novel approaches to detecting and managing URI susceptibility in infants.
1. Introduction
Upper respiratory infections (URIs) are the most common cause of healthcare visits worldwide [1]. URIs are a major cause of chronic respiratory disease exacerbations [1]. Infants are particularly susceptible to URIs and URI-related hospitalization [2]. Enhancing our understanding of how environmental and biologic factors interact to influence URI risk could provide opportunities to develop novel interventions and therapeutics, reducing the burden of URIs on individuals and society.
Infant predisposition to URIs is influenced by environmental risk factors [3]. Infants who attend day care, live in large households, or require recurrent healthcare visits are at risk for experiencing a high number of URIs [4]. Additionally, secondhand smoking exposure, low birth weight, and malnutrition have been associated with increased frequency of URIs in infants [4]. Genetic risk factors have also been identified [5]. Genes affecting interferon (IFN) signaling, interleukins, angiotensin-converting enzymes, and transmembrane serine protease-2 are critical for maturation of the developing immune system [5]. Polymorphisms in immune regulatory genes have been associated with sub-optimal immune responses and elevated risk of infection [6,7]. One mechanism by which infant genetics regulates immune responsivity is through the production and release of cytokines [7].
Cytokines, such as C-X-C Motif Chemokine Ligand 10 (CXCL10), interleukin 18 (IL-18), interleukin 6 (IL-6), and interleukin 8 (IL-8) are involved in the early stages of inflammation associated with viral URIs [8,9]. There is evidence indicating that the risk of acquiring URIs and the risk of developing severe complications are mediated through cytokine activity, which is regulated by immune system genes [10]. Aberrant cytokine production may contribute to increased airway inflammation associated with more severe symptoms [10]. Inter-individual variability in cytokine responses may stem from genetic, epigenetic, or epi-transcriptional factors, such as micro-ribonucleic acids (miRNAs) [11,12].
miRNAs are short, non-coding nucleic acids that modulate protein expression by binding and sequestering messenger RNAs for degradation [13]. Intriguingly, host miRNAs also interact with viral pathogens [14]. These miRNA-virus interactions can promote or inhibit the virus life cycle. Studies have revealed that specific miRNAs are over-expressed in response to viral replication [15], and that miRNAs can down-regulate mRNA targets with protective anti-viral function [15,16]. This down-regulation may permit virus replication, maturation, and release [15,16]. Alternatively, viral gene products may also influence miRNA expression to regulate their own replication, promoting evasion of the host immune response, and increasing viral latency [17]. Therefore, two-way communication between viruses and miRNAs may profoundly impact URI frequency and duration [14,15,16,17].
The microbiome has also been implicated in viral URIs [18]. Changes in the respiratory microbiome have been reported in patients with viral URIs [19]. Similar observations have been reported in infantile wheezing episodes [20]. It remains unclear, however, if changes in the composition of the gut or respiratory microbiota (microbial dysbiosis) predisposes infants to URIs. Given the potential importance of host-microbial interactions in URI pathogenesis, the correlations between oral microbes and the host immune response, and the ability to measure genetic, cytokine, and miRNA factors in the oropharynx, the current study harnessed infant saliva samples to interrogate mult-omic networks with potential involvement in URI pathogenesis.
Although various molecular factors have been implicated in immune system maturation and URI predisposition [14,15,16], to our knowledge, few studies have employed a multi-omic approach to explore their interactions in developing infants. Such information could enhance our knowledge about the pathophysiologic mechanisms underlying URIs, leading to novel approaches for prevention and treatment. This study tested the hypothesis that interactions between specific microbes, miRNAs, mRNAs, and cytokines could modulate URI frequency (Figure 1). This hypothesis was tested using a longitudinal cohort study of 146 infants from birth through 12 months of age.
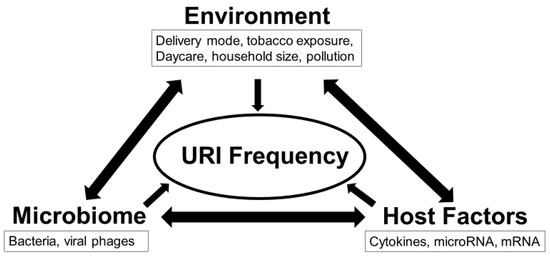
Figure 1.
Interactions between environment, microbiome, and host immune factors determine frequency of upper respiratory infection (URI) in the first 12 months. This study utilized a multi-omics approach to test the overarching hypothesis that interactions between the exposome, microbiome, and inflammasome dictate URI frequency.
2. Results
2.1. Participants
The majority of the 146 participating infants were female (82, 56%), white race (121, 82%), and non-Hispanic (132, 90%; Table 1). The average gestational age at delivery was 39 (±1) weeks, and the average birth weight was 3376 (±445) grams. Most (111, 76%) lacked a family history of asthma. Most infants were delivered vaginally (117, 80%). The majority did not attend daycare (90, 61%), and were not exposed to passive tobacco use (130, 89%), or atmospheric pollution (91, 62%). The average duration of exclusive breastfeeding was 5 months (±2; range = 0–12), and the average household size was 3 persons (±1; range = 2–9).

Table 1.
Participant Characteristics.
2.2. URI Characteristics
The average number of URIs reported in the first 12 months was 2 (±2; range = 0–12). The first URI episode occurred, on average, 1 (±1) month after birth. No participants were hospitalized for bronchiolitis, two received a diagnosis of pneumonia, two received a prescription for albuterol, and three were prescribed oral steroids.
2.3. Multi-Omic Analysis
Step-wise linear regression identified eight omic features that accurately (R2 = 0.39, AIC = 593, RMSE = 1.72, p < 0.001) modeled the number of URIs in the first 12 months (Table 2).

Table 2.
Linear regression utilizing environmental and multi-omic factors models the number of upper respiratory infections in the first 12 months.
Higher number of URIs were associated with daycare attendance (F = 52.8, p < 0.001), atmospheric pollution (F = 7.76, p = 0.006), and longer duration of exclusive breastfeeding (F = 4.27, p = 0.041) (Figure 2).
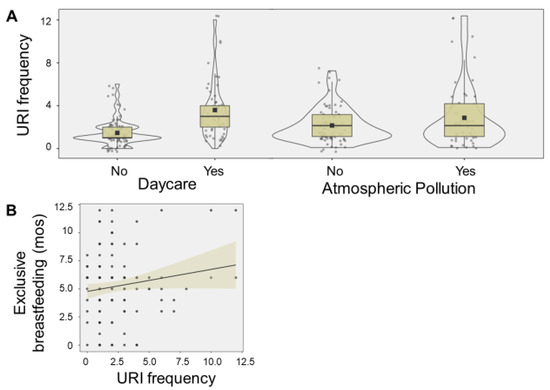
Figure 2.
Exposome relationship with URI frequency. The violin plots display number of upper respiratory infections (URIs) associated with daycare attendance (F = 52.8, p < 0.001), and atmospheric pollution (F = 7.76, p = 0.006) (A). The boxes denote 95% confidence intervals, along with mean (square) and median values (black line). The scatter plot display the relationship between number of URIs and the duration of duration (months (mos)) of exclusive breastfeeding (F = 4.27, p = 0.041) (B). A trend line and 95% confidence interval (shaded area) are displayed.
Salivary levels of Verrucomicrobia (F = 7.77, p = 0.006) and Streptococcus phage SpSL1 (F = 6.57, p = 0.011) were directly associated with the number of URIs, whereas levels of miR-22-5p (F = 6.93, p = 0.009) were inversely related. Haemophilus virus HP1 (F = 2.56, 0.11), and TMPRSS2 (F = 2.24, p = 0.13) met criteria for model inclusion (F > 2.0), but did not display significant relationships with URI frequency (Figure 3).
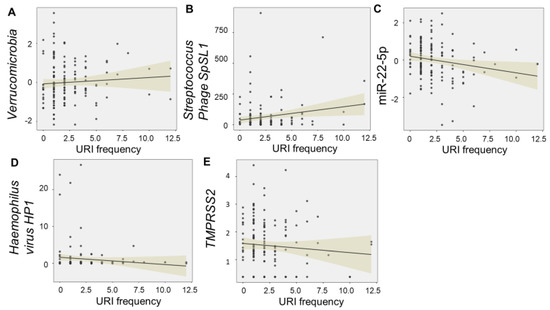
Figure 3.
Multi–omic relationships with URI frequency. The scatter plots display significant relationships between frequency of upper respiratory infections (URIs) in the first 12 months and levels of salivary “omic” factors. Levels of Verrucomicrobia (F = 7.77, p = 0.006) (A) and Streptococcus phage SpSL1 (F = 6.57, p = 0.011) (B) were directly associated with the number of URIs, whereas levels of miR-22-5p (F = 6.93, p = 0.009) (C) were inversely related. Haemophilus virus HP1 (F = 2.56, 0.11) (D), and TMPRSS2 (F = 2.24, p = 0.13) (E) met criteria for model inclusion (F > 2.0), but did not display significant relationships with URI frequency. Trend lines with 95% confidence intervals are displayed for each relationship.
Notably, no cytokine components met criteria for inclusion in the model, although levels of CXCL10 were modestly correlated with the number of URIs (R = 0.20, p = 0.024). There were significant interactions between daycare attendance and levels of Streptococcus phage SpSL1, as well as Verrucomicrobia levels and atmospheric pollution. Streptococcus phage SpSL1 potentiated the number of URIs among infants who attended daycare (F = 12.1, p < 0.001), whereas Verrucomicrobia potentiated URI frequency in infants without atmospheric pollution exposure (F = 3.21, p = 0.009; Figure 4). A significant interaction effect was also observed between levels of miR-22-5p and duration of exclusive breastfeeding (F = 10.8, p = 0.001). High levels of infant miR-22-5p had a protective effect on URI frequency among infants with longer breastfeeding duration.
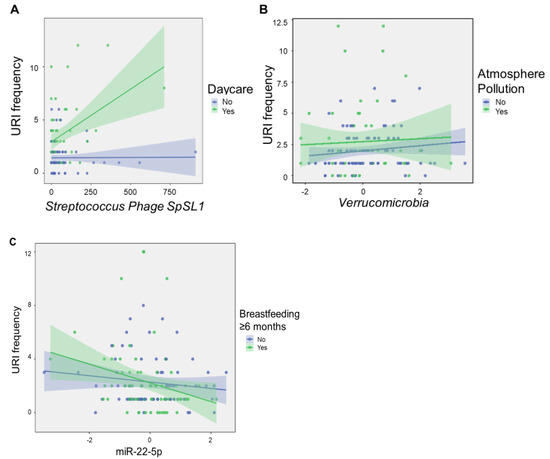
Figure 4.
Microbial factors modulate environmental impacts on URI frequency. The scatter plots show molecular features of interest that displayed significant interaction effects with environmental exposures on linear regression analysis. Streptococcus phage SpSL1 potentiated the number of URIs among infants who attended daycare (F = 12.1, p < 0.001) (A), whereas Verrucomicrobia potentiated URI frequency in infants without atmospheric pollution exposure (F = 3.21, p = 0.009) (B), and miR-22-5p levels potentiated the protective effects of breastfeeding ≥ 6 months (F = 10.8, p = 0.001) (C). Normalized, scaled read counts are displayed for Streptococcus phage SpSL1, Verrucomicrobia, and miR-22-5p. URI frequency represents the number of URIs reported in the first 12 months after birth. Shaded areas represent 95% confidence intervals.
3. Discussion
In this cohort of 146 infants, we identified three environmental factors (daycare attendance, atmospheric pollution, and duration of exclusive breastfeeding), one microbe (Verrucomicrobia), and one viral phage (Streptococcus phage SpSL1) that were directly associated with URI frequency in the first 12 months. One miRNA (miR-22-5p) was inversely correlated with URI frequency. Abundance of specific microbes in the infant oropharynx modulated the impacts of environmental exposures on URI frequency.
Several “omic” features in this study have been described in previous studies of viral pathogenesis. For example, miR-22 has been shown to inhibit adenovirus replication within lung fibroblasts by targeting retinoblastoma (Rb) dependent cell cycle genes [21,22]. Rb inactivation may increase pro-inflammatory signaling by stimulating the interleukin-6/STAT3 pathway [23]. In this study miR-22-5p was associated with less frequent URI infections which supports its protective role in suppressing viral replication and proliferation. TMPRSS2 also aided modeling of URI frequency. Prior studies have found that TMPRSS2 promotes viral propagation by activating the spike protein and enhancing viral entry into host cells in severe acute respiratory syndrome-related coronavirus (SARS-CoV) and Middle East respiratory syndrome-related coronavirus (MERS-CoV) [24]. It is possible that the infant upper respiratory tract responds to recurrent viral URI infections by down-regulating TMPRSS2 expression in an effort to reduce URI frequency. Additionally, these immunoregulatory mediators have been demonstrated to influence the pro-inflammatory shifts in influenza A and respiratory syncytial virus (RSV) mediated URIs [25,26].
Host immunologic factors may be driven by microbial flora in the oropharynx [27]. For example, elevated levels of Haemophilus, Corynebacterium, Dolosigranulum, and Streptococcus in saliva have been associated with perturbations in host immunity that are linked to activation of innate immune mechanisms involved with URIs and airway remodeling [27,28,29]. One study found that aberrant presence of Verrucomicrobia in the gut can induce pro-inflammatory chemokines implicated in neuroinflammation [30]. Bacteriophages, such as Streptococcus phage SpSL1 may also induce inflammatory perturbations in the oral microbiome [29,31]. In this study, levels of Verrucomicrobia and Streptococcus phage SpSL1 were higher in the saliva of infants with more frequent URIs, supporting their pro-inflammatory role in viral infections.
Intriguingly, Streptococcus phage SpSL1 potentiated the number of URIs among infants who attended daycare, whereas Verrucomicrobia potentiated URI frequency in infants without atmospheric pollution exposure. These results are consistent with prior studies demonstrating that air pollution and daycare exposure may interact with host or microbial factors to influence infection susceptibility [32,33,34]. Exposure to air pollution during infancy has been shown to alter the presence of symbiotic microbes that impact regulatory T cell function in reactive airway disease [31], and cause intestinal microbiome shifts critical to the lung-gut microbiome axis [34]. Daycare exposure may also impact individual microbiome variability [35], which can, in turn, influence host miRNAs involved in host immune responses [36,37,38].
Based on the findings of this study, we propose a conceptual framework for URI pathogenesis involving microbial perturbations, miR-22-5p expression, immune dysregulation, and environmental exposures (Figure 5). In this conceptual model, miR-22 inhibits viral replication through inactivation of Rb and enhancement of pro-inflammatory signaling (i.e., interleukin-6/STAT3 pathway) [23]. Cytokine shifts may perturb microbial diversity through complex interactions between lipopolysaccharide (LPS), Toll-Like Receptor 4 (TLR4), TMPRSS2, and miRNA regulation [21,22,23,24,27,28,29,30,31]. Environmental exposures such as daycare attendance and air pollution may also influence microbial composition and blunt immune responses that predispose infants to frequent URIs [32,33,34,35]. Intriguingly, miR-22-5p levels appear to potentiate the protective effect of sustained breastfeeding. This is consistent with prior studies suggesting that maternal-infant transfer of miRNAs in breastmilk may impact immune development [39]. It also supports prior studies implicating miR-22 expression in upper respiratory infections, pneumonia, and asthma [40,41].
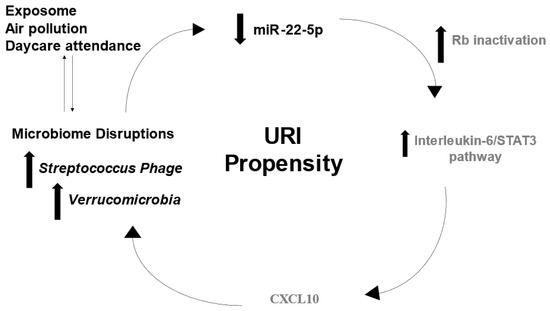
Figure 5.
Putative multi-omic mechanism for URI susceptibility. The concept diagram displays mechanisms through which molecular observations in this cohort may contribute to URI propensity. Decreased levels of miR-22-5p lead to retinoblastoma (Rb) inactivation, which may increase pro-inflammatory signaling by stimulating the interleukin-6/STAT3 pathway. Microbial diversity may be influenced by cytokine signaling or environmental exposures. Increased activity of Verrucomicrobia and Streptococcus Phage could influence epi-transcriptional regulators of the immune response (i.e., miR-22-5p) that increase URI risk. Black text denotes factors identified in this cohort, whereas gray text denotes interactions reported in the scientific literature.
There are several limitations of this study. URIs were identified through validated surveys and review of the medical record. However, surveys could introduce recall bias, and it is possible that the medical record might miss URIs that were diagnosed at an outside facility. We do note that participants were followed at our outpatient pediatric clinic for routine well-child check-ups, at which time recent urgent care or outpatient visits were typically recorded in the medical record. Although the vast majority of infant URIs are viral in nature, the precise pathogen responsible for each infection episode was not confirmed through laboratory testing. It is possible that some infectious episodes involved bacterial pathogens. Finally, the primary outcome of the study was URI frequency. Although details regarding severity were recorded, the study was not powered to compare multi-omic profiles between infants with severe and non-severe URI episodes. This should be explored in future studies.
The results of this study support the premise that multi-omic analysis of infant saliva may provide insight into pathophysiologic mechanisms that underlie URIs. Such knowledge could eventually aid in novel diagnostic, preventative, or therapeutic measures. Validation of these results in a larger cohort with ancestral and geographic diversity is required.
4. Materials and Methods
4.1. Ethics
This study was approved by the Institutional Review Board at the Penn State College of Medicine (STUDY00008657). Parents/guardians provided written, informed consent.
4.2. Participants
This study involved a subset of infants from a prospective longitudinal cohort of 221 mother-infant dyads [39]. Eligibility criteria were term delivery (37–42 weeks gestation) and plan to breastfeed ≥ four months. Exclusion criteria were factors affecting long-term follow up (e.g., plan for primary pediatric care at an outside hospital, plan for infant adoption), inability to complete electronic surveys (e.g., non-English speaking), and medical conditions that could impact breastfeeding ability (e.g., maternal drug addiction, human immunodeficiency virus infection, infant cleft lip/palate, NICU admission > 7 days). A convenience sample was enrolled within seven days of delivery. Enrollment occurred between 20 April 2018 and 5 October 2020 at the newborn nursery or the outpatient pediatrics clinics affiliated with our academic medical center. Among the 2487 infants screened for eligibility, there were 359 who met criteria, and 221 consented to participate. Of these, 146 completed the study (Figure 6). The primary medical outcome was number of URIs in the first 12 months after birth, as reported by parents/guardians on the International Study of Asthma and Allergies in Childhood–Wheezing Questionnaire (ISAAC-WQ) [42]. Review of medical records by research staff was used to confirm presence/absence of acute appointments for URI symptoms for all participants.
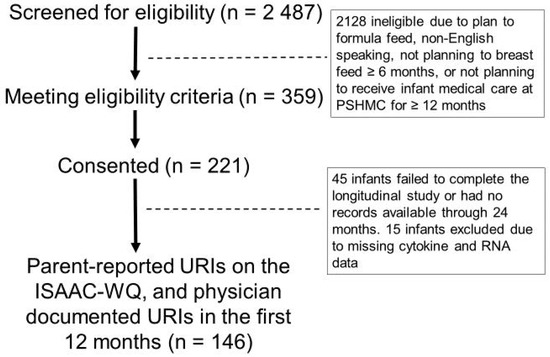
Figure 6.
Participant CONSORT diagram. The CONSORT diagram displays the number of participants who were screened (2487), eligible (359), consented (221), and completed the 12-month study (146).
4.3. Survey Collection
Electronic surveys administered by research staff were used to obtain medical and demographic characteristics at the time of enrollment. Survey responses were confirmed through review of the electronic medical record. The following medical and demographic characteristics were collected: biological sex, race, ethnicity, gestational age (weeks), birth weight (g), and family history of asthma. The National Survey of Lead and Allergens in Housing (NSLAH) [43] was administered at 1 month of age to assess the infant “exposome”: delivery mode (vaginal, cesarean section), passive tobacco exposure, duration of exclusive breastfeeding (months), daycare attendance, number of people living in the household, and atmospheric pollution. Details about URIs were collected using the ISAAC-WQ, administered at 12 months: age of first upper respiratory infection, hospitalization for bronchiolitis, diagnosis of pneumonia, prescription for albuterol, prescription of oral steroids.
4.4. Saliva Collection
Two non-fasting saliva samples were collected from each infant at 6 months of age. One swab contained nucleic acid stabilizer (DNA Genotek, Ottowa, ON, Canada), and was used for analysis of the miRnome (miRNAs), the transcriptome (mRNAs), the microbiome (microbial RNAs), and the virome (viral RNAs). The second swab contained no stabilizing reagents (Oasis Diagnostics, Vancouver, WA, USA), and was used for analysis of the inflammasome (cytokines). Saliva was collected from the sub-lingual and parotid regions by swabbing the oropharynx for 10–20 s. Samples were stored at −80 ℃ prior to molecular analysis.
4.5. Inflammasome Analysis
Cytokines were measured using the ELLA SimplePlex immunoassays (Protein Simple, San Jose, CA, USA). The ELLA instrument performs automated, fluorescent-labeled antibody detection of cytokines using a microfluidic cartridge. Immunoassays were used to measure levels of four salivary cytokines: C-X-C Motif Chemokine Ligand 10 (CXCL10), interleukin 18 (IL-18), interleukin 6 (IL-6), and interleukin 8 (IL-8). These cytokines were selected based on their bioavailability in saliva, as well as their relevance to inflammation and URIs [44,45,46,47,48]. As we have previously described, saliva samples were centrifuged for 4 min at 16,000× g, and 70 µL of sample was mixed with 70 µL of diluent [49]. The solutions were centrifuged for 2 min at 1000× g, then 50 µL of diluted samples were loaded on a custom cartridge and processed on an ELLA Simplex instrument per manufacturer instructions.
4.6. RNA Analysis
RNA was extracted from each sample using the miRNeasy Kit (Qiagen, Inc., Germantown, MD, USA), and RNA quality was assessed with an Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). Downstream RNA processing was performed as we have previously described [50]. Briefly, RNA sequencing was completed at the SUNY Molecular Analysis Core using the Illumina TruSeq Small RNA Prep protocol and a NextSeq500 instrument (Illumina; San Diego, CA, USA) at a targeted depth of ten million, 50 base, paired-end reads per sample. Reads were aligned to the hg38 build of the human genome using Partek Flow (Partek; St. Louis, MO, USA) and the Bowtie2 aligner. Quantification of messenger RNAs (mRNAs) was performed using Ensembl annotation, and quantification of mature microRNAs was performed using miRBase 22 annotation. RNA reads that were unaligned to the human genome were aligned to the NCBI RefSeq genome using Kraken. Bacteria were aligned at the phylum level and viral phages were aligned at the species level. We chose to measure microbial RNA in order to streamline the nucleic acid extraction and analyses steps. Quality of RNA sequencing results was verified through read quality score and total read count. The RNA features with consistent detection (raw read counts ≥10 in ≥100% of samples) in each category (mRNAs, miRNAs, bacteria, virus) were quantile normalized and scaled prior to statistical analysis. Downstream analysis involved 8 transcripts (TMPRSS2, IL1RN, IL6ST, CCL2, TNFAIP3, TLR4, TAB2, NFKBIA), 8 miRNAs (miR-140-3p, miR-22-5p, miR-29c-5p, miR-34c-5p, miR-125a-5p, miR-27b-3p, miR-203a-3p, miR-155-5p), 8 viral phages (Streptococcus phage SpSL1, Pseudomonas phage PPpW-3, Proteus virus PM135, Streptococcus phage phiARI0131-1, Mycobacterium virus Cooper, Bacillus virus Mater, Haemophilus virus HP1, Klebsiella phage vB_KpV477), and 8 microbial phyla (Actinobacteria, Bacteroidetes, Candidatus Saccharibacteria, Firmicutes, Fusobacteria, Proteobacteria, Verrucomicrobia), plus a measure of microbial diversity (Simpson alpha index). These features were selected based on their biologic relevance to viral upper respiratory infections and their abundance in infant saliva [25,26,51,52,53,54]. A maximum of 8 features were permitted in each category based on an a priori power analysis demonstrating that the sample size (n = 146) provided > 95% power to detect an effect size ≥ 2.0 for 8 predictors, with alpha set at 0.05 (assuming a non-central distribution).
4.7. Statistical Analysis
Descriptive statistics were used to summarize the medical and demographic characteristics of participating infants. The relationship between the primary medical outcome (number of URIs in the first 12 months) and molecular features from the 6 “omic” categories (exposome, inflammasome, transcriptome, miRnome, microbiome, and virome) were assess using linear regression. Number of URIs was the dependent variable, and omic features served as independent variables. Variables were introduced in a feed-forward, step-wise procedure. Only features with F ≥ 2.0 were retained, and a maximum of 8 features was permitted in the final model. For variables with large effect sizes (F > 5.0), interactions were explored. Overall model statistics (Akaike Information Criteria (AIC), R2, and Root-mean-square-deviation (RMSE)) and individual factor statistics (ANOVA Omnibus F-value, p-value, estimate) were reported. A Durbin-Watson test for autocorrelation was performed, and Q-Q plots were used to visualize residuals. All analyses were completed using Jamovi Software (v2.3) [55].
5. Conclusions
The findings in this study suggest that multi-omic analysis of infant saliva may provide insight into pathophysiologic mechanisms that underlie upper respiratory infections, especially interactions between host immunity, microbial activity, and epi-transcriptomic. The measurement of these multi-omic factors may aid the identification of infants at risk for future respiratory infections, allowing clinicians to provide personalized anticipatory guidance and treatment. If a sufficiently accurate tool were developed, the results might be used to guide preventative measures, which could decrease emergency visits and the need for hospitalizations due to severe upper respiratory infections.
Author Contributions
S.D.H. conceived of the study and oversaw data/sample collection. E.S.H. and S.D.H. guided sample analysis. Data interpretation was performed by S.D.H. and R.B. All authors contributed to drafting and revising the manuscript and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
Support for this study was provided by a grant from the Gerber Foundation (#5295) to S.D.H. and the Children’s Miracle Network (#106016) to E.S.H. and S.D.H.
Institutional Review Board Statement
This study was approved by the Institutional Review Board at the Penn State College of Medicine (STUDY00008657). All participants provided written, informed consent.
Informed Consent Statement
Mothers provided written, informed consent for infant participation.
Data Availability Statement
FASTQ files from RNA sequencing involved in this project have been deposited into the NIH GEO database (GSE192543). GEO respository link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE192543 (accessed on 25 December 2022).
Acknowledgments
The authors would like to thank Alle Confair for enrolling the participants and the sample collection and Kaitlyn Warren for the processing of the samples.
Conflicts of Interest
S.D.H. is a consultant and advisory board member for Quadrant Biosciences and Spectrum Solutions, who played no role in the current study. The other authors have no financial relationships to disclose. The study does not discuss an investigative use of a commercial product.
References
- Shahan, B.; Barstow, C.; Mahowald, M. Respiratory Conditions: Upper Respiratory Tract Infections. FP Essent. 2019, 486, 11–18. [Google Scholar] [PubMed]
- Mally, P.V.; Agathis, N.T.; Bailey, S.M. Early term infants are at increased risk of requiring neonatal intensive care. World J. Pediatr. 2016, 12, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Forbester, J.L.; Humphreys, I.R. Genetic influences on viral-induced cytokine responses in the lung. Mucosal Immunol. 2021, 14, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Bouvier, N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef]
- Yildirim, Z.; Sahin, O.S.; Yazar, S.; Bozok Cetintas, V. Genetic and epigenetic factors associated with increased severity of Covid-19. Cell Biol. Int. 2021, 45, 1158–1174. [Google Scholar] [CrossRef]
- Kubistova, Z.; Mrazek, F.; Petrek, M. Polymorphisms of the immune response genes: Selected biological, methodical and medical aspects. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2009, 153, 93–102. [Google Scholar] [CrossRef]
- Newport, M.J. The genetic regulation of infant immune responses to vaccination. Front. Immunol. 2015, 6, 18. [Google Scholar] [CrossRef]
- Vecchié, A.; Bonaventura, A.; Toldo, S.; Dagna, L.; Dinarello, C.A.; Abbate, A. IL-18 and infections: Is there a role for targeted therapies? J. Cell Physiol. 2021, 236, 1638–1657. [Google Scholar] [CrossRef]
- König, B.; Krusat, T.; Streckert, H.J.; König, W. IL-8 release from human neutrophils by the respiratory syncytial virus is independent of viral replication. J. Leukoc. Biol. 1996, 60, 253–260. [Google Scholar] [CrossRef]
- Hsu, R.J.; Yu, W.C.; Peng, G.R.; Ye, C.H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.C.; Yu, S.H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef]
- Cox, A.J.; Gleeson, M.; Pyne, D.B.; Callister, R.; Fricker, P.A.; Scott, R.J. Cytokine gene polymorphisms and risk for upper respiratory symptoms in highly-trained athletes. Exerc. Immunol. Rev. 2010, 16, 8–21. [Google Scholar] [PubMed]
- Jung, N.; Schenten, V.; Bueb, J.L.; Tolle, F.; Bréchard, S. miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils. Int. J. Mol. Sci. 2019, 20, 5699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Mackowiak, B.; Gao, B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Plaisance-Bonstaff, K.; Renne, R. Viral miRNAs. Methods Mol. Biol. 2011, 721, 43–66. [Google Scholar] [PubMed]
- Girardi, E.; López, P.; Pfeffer, S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Cairns, M.J. Identifying miRNAs, targets and functions. Brief. Bioinform. 2014, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Couto, L.B.; High, K.A. Viral vector-mediated RNA interference. Curr. Opin. Pharmacol. 2010, 10, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- Wu, L.; Shen, C.; Chen, Y.; Yang, X.; Luo, X.; Hang, C.; Yan, L.; Xu, X. Follow-up study of airway microbiota in children with persistent wheezing. Respir. Res. 2021, 22, 213. [Google Scholar] [CrossRef]
- Tahamtan, A.; Inchley, C.S.; Marzban, M.; Tavakoli-Yaraki, M.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. The role of microRNAs in respiratory viral infection: Friend or foe? Rev. Med. Virol. 2016, 26, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, O.; Razquin, N.; Zaratiegui, M.; Narvaiza, I.; Fortes, P. Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J. Virol. 2006, 80, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Takahashi, C. Intersection of retinoblastoma tumor suppressor function, stem cells, metabolism, and inflammation. Cancer Sci. 2017, 108, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, I.S.; Jarrar, Y.B. Targeting the intestinal TMPRSS2 protease to prevent SARS-CoV-2 entry into enterocytes-prospects and challenges. Mol. Biol. Rep. 2021, 48, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Moheimani, F.; Koops, J.; Williams, T.; Reid, A.T.; Hansbro, P.M.; Wark, P.A.; Knight, D.A. Influenza A virus infection dysregulates the expression of microRNA-22 and its targets; CD147 and HDAC4, in epithelium of asthmatics. Respir. Res. 2018, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Inchley, C.S.; Sonerud, T.; Fjærli, H.O.; Nakstad, B. Nasal mucosal microRNA expression in children with respiratory syncytial virus infection. BMC Infect. Dis. 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- de Steenhuijsen Piters, W.A.A.; Watson, R.L.; de Koff, E.M.; Hasrat, R.; Arp, K.; Chu, M.L.J.N.; de Groot, P.C.M.; van Houten, M.A.; Sanders, E.A.M.; Bogaert, D. Early-life viral infections are associated with disadvantageous immune and microbiota profiles and recurrent respiratory infections. Nat. Microbiol. 2022, 7, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 2019, 16, 129. [Google Scholar] [CrossRef]
- McShan, W.M.; McCullor, K.A.; Nguyen, S.V. The Bacteriophages of Streptococcus pyogenes. Microbiol Spectr. 2019, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dasgupta, S.; Mishra, P.K.; Chaudhury, K. Air pollution-induced epigenetic changes: Disease development and a possible link with hypersensitivity pneumonitis. Environ. Sci. Pollut. Res. Int. 2021, 28, 55981–56002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zhang, B.; Zhang, K.; Lv, X.; Wang, Q.; Bai, X. The Impact of Air Pollution on Intestinal Microbiome of Asthmatic Children: A Panel Study. Biomed. Res. Int. 2020, 2020, 5753427. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; Erez-Granat, O.; Braun, T.; Sosnovski, K.; Hadar, R.; BenShoshan, M.; Heiman, S.; Abbas-Egbariya, H.; Glick Saar, E.; Efroni, G.; et al. Gut microbiome development in early childhood is affected by day care attendance. NPJ Biofilms Microbiomes 2022, 8, 2. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Newton, A.H.; Cardani, A.; Braciale, T.J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 2016, 38, 471–482. [Google Scholar] [CrossRef]
- Holla, S.; Balaji, K.N. Epigenetics and miRNA during bacteria-induced host immune responses. Epigenomics 2015, 7, 1197–1212. [Google Scholar] [CrossRef]
- Hicks, S.D.; Beheshti, R.; Chandran, D.; Warren, K.; Confair, A. Infant consumption of microRNA miR-375 in human milk lipids is associated with protection from atopy. Am. J. Clin. Nutr. 2022, 116, 1654–1662. [Google Scholar] [CrossRef]
- Sun, J.; Mao, S.; Ji, W. LncRNA H19 activates cell pyroptosis via the miR-22-3p/NLRP3 axis in pneumonia. Am. J. Transl. Res. 2021, 13, 11384. [Google Scholar]
- Taka, S.; Tzani-Tzanopoulou, P.; Wanstall, H.; Papadopoulos, N.G. MicroRNAs in asthma and respiratory infections: Identifying common pathways. Allergy Asthma Immunol. Res. 2020, 12, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Clickner, R.P.; Marker, D.; Viet, S.M.; Rogers, J.; Broene, P. National Survey of Lead. Final Report, Vol. I and Allergens in Housing. Analysis of Lead Hazards (WESTAT, 2001). Available online: https://www.niehs.nih.gov/research/clinical/studies/nslah/index.cfm (accessed on 22 September 2022).
- Chung, H.L.; Shin, J.Y.; Ju, M.; Kim, W.T.; Kim, S.G. Decreased interleukin-18 response in asthmatic children with severe Mycoplasma pneumoniae pneumonia. Cytokine 2011, 54, 218–221. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.S.; Flanagan, B.F.; Hart, C.A.; Smyth, R.L. Production of Chemokines in the Lungs of Infants with Severe Respiratory Syncytial Virus Bronchiolitis. J. Infect. Dis. 2005, 191, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Koren, H.S.; Henke, D.C. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1, and interleukin-6. Am. J. Respir. Cell Mol. Biol. 1993, 8, 20–27. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Rotondi, M. Interleukin-6, CXCL10 and infiltrating macrophages in COVID-19-related cytokine storm: Not one for all but all for one! Front. Immunol. 2021, 12, 668507. [Google Scholar] [CrossRef]
- Little, F.F.; Delgado, D.M.; Wexler, P.J.; Oppenheim, F.G.; Mitchell, P.; Feldman, J.A.; Walt, D.R.; Peng, R.D.; Matsui, E.C. Salivary inflammatory mediator profiling and correlation to clinical disease markers in asthma. PLoS ONE 2014, 9, e84449. [Google Scholar] [CrossRef]
- Beheshti, R.; Halstead, E.S.; McKeone, D.; Hicks, S.D. Multi-omic factors associated with future wheezing in infants. Pediatr. Res. 2022, 27, 1–7. [Google Scholar] [CrossRef]
- Beheshti, R.; Halstead, S.; McKeone, D.; Hicks, S.D. Understanding immunological origins of atopic dermatitis through multi-omic analysis. Pediatr. Allergy Immunol. 2022, 33, e13817. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Noroozi, R.; Vafaee, R.; Branicki, W.; Poṡpiech, E.; Pyrc, K.; Łabaj, P.P.; Omrani, M.D.; Taheri, M.; Sanak, M. Effects of host genetic variations on response to, susceptibility and severity of respiratory infections. Biomed. Pharmacother. 2020, 128, 110296. [Google Scholar] [CrossRef]
- Moschos, S.A.; Williams, A.E.; Perry, M.M.; Birrell, M.A.; Belvisi, M.G.; Lindsay, M.A. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genom. 2007, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. The oral microbiome: Its role in health and in oral and systemic infections. Clin. Microbiol. Newsl. 2010, 35, 163–169. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.; Aybek, E. Jamovi: An easy to use statistical software for the social scientists. Int. J. Assess. Tools Educ. 2019, 6, 670–692. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).