Vertebral Bone Marrow Clot towards the Routine Clinical Scenario in Spine Surgeries: What about the Antimicrobial Properties?
Abstract
1. Introduction
2. The Antimicrobial Activity of the vBM Clot
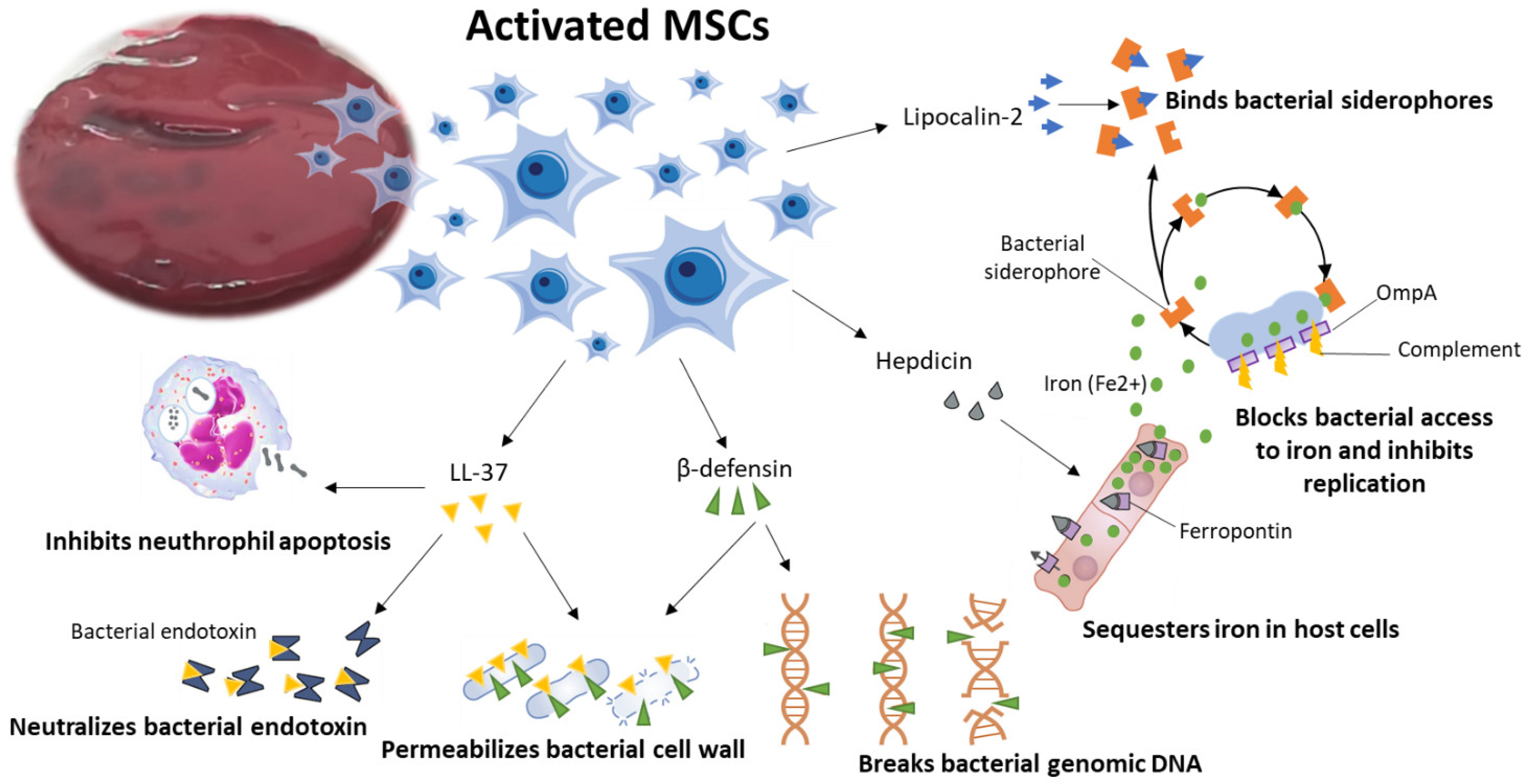
2.1. BMSCs Antimicrobial Activity
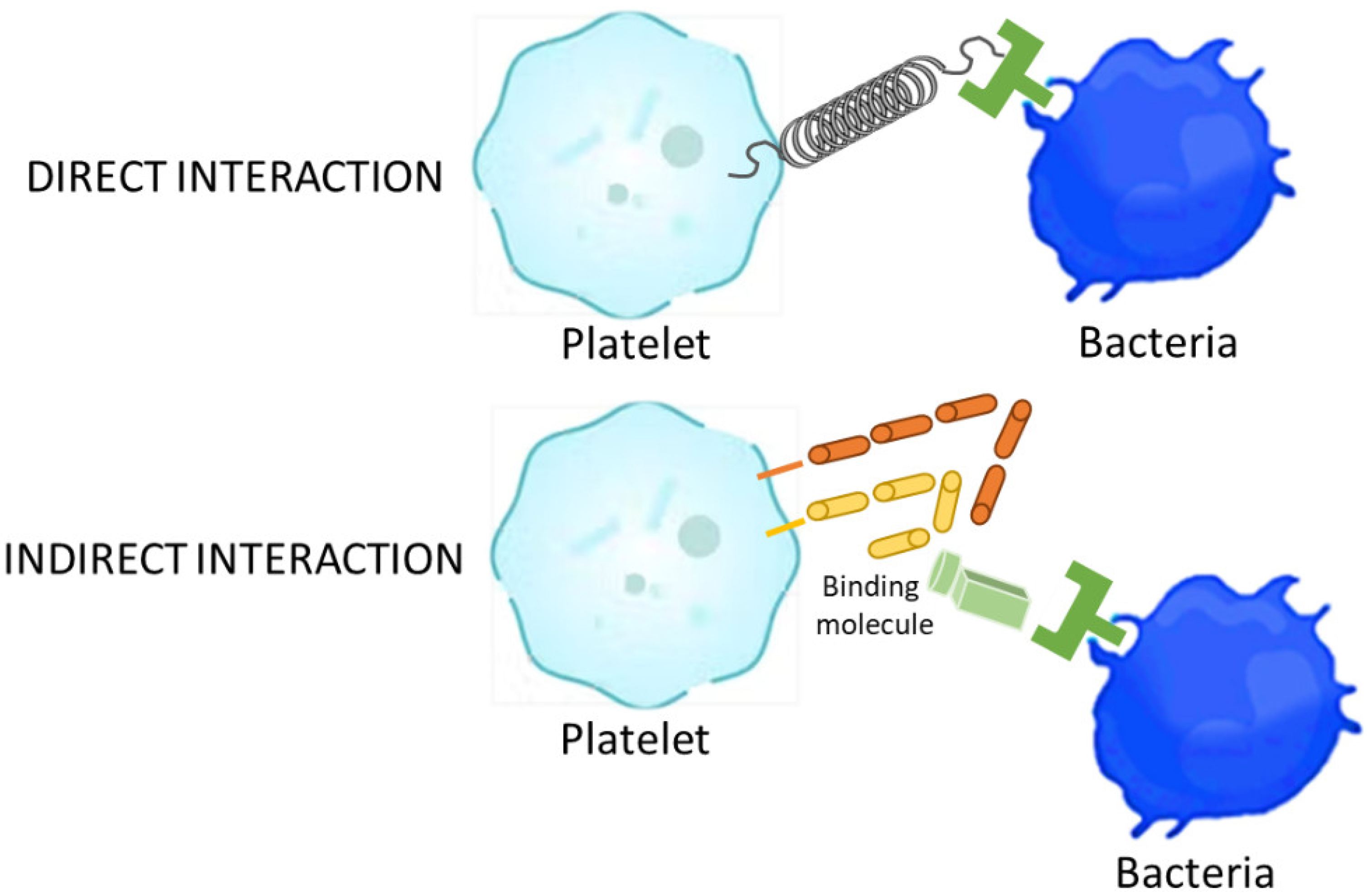
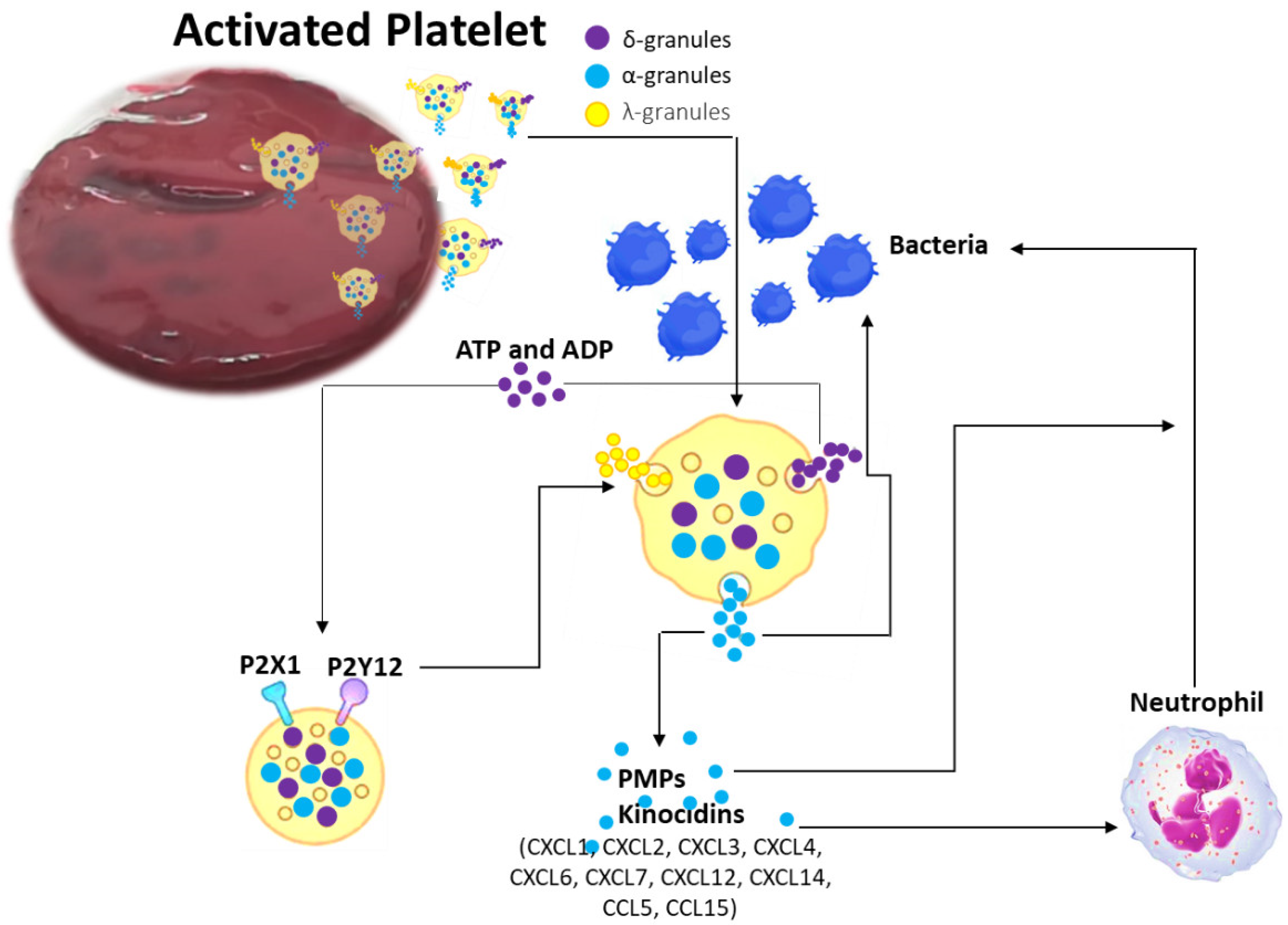
2.2. Coagulation Process: Platelets and Coagulation Factors of Antimicrobial Activity
2.3. Clinical Consequences of vBM Clot Antimicrobial Activity in Spine Surgery
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, R.C.; Mobbs, R.J.; Lu, V.M.; Xu, J.; Rao, P.J.; Phan, K. Posterolateral Fusion Versus Interbody Fusion for Degenerative Spondylolisthesis: Systematic Review and Meta-Analysis. Glob. Spine J. 2017, 7, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Pichelmann, M.A.; Lenke, L.G.; Bridwell, K.H.; Good, C.R.; O’Leary, P.T.; Sides, B.A. Revision rates following primary adult spinal deformity surgery: Six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine 2010, 35, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Contartese, D.; Nicoli Aldini, N.; Barbanti Brodano, G.; Griffoni, C.; Gasbarrini, A.; Fini, M. Bone marrow aspirate clot: A technical complication or a smart approach for musculoskeletal tissue regeneration? J. Cell. Physiol. 2018, 233, 2723–2732. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Giavaresi, G.; Sicuro, L.; Barbanti Brodano, G.; Gasbarrini, A.; Fini, M. A Rationale for the Use of Clotted Vertebral Bone Marrow to Aid Tissue Regeneration Following Spinal Surgery. Sci. Rep. 2020, 10, 4115. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Borsari, V.; Pagani, S.; Barbanti Brodano, G.; Griffoni, C.; Ricci, A.; Gasbarrini, A.; Fini, M. Two Hits for Bone Regeneration in Aged Patients: Vertebral Bone Marrow Clot as a Biological Scaffold and Powerful Source of Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2022, 9, 807679. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Knop, C.; Oeser, M.; Bastian, L.; Lange, U.; Zdichavsky, M.; Blauth, M. Entwicklung und Validierung des VAS-Wirbelsäulenscores [Development and validation of the Visual Analogue Scale (VAS) Spine Score]. Unfallchirurg 2001, 104, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, O.; Bulle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broadspectrum antimicrobial effector function mediated by indoleamine 2,3- dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef]
- Krasnodembskaya, A.; Samarani, G.; Song, Y.; Zhuo, H.; Su, X.; Lee, J.W.; Gupta, N.; Petrini, M.; Matthay, M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1003–13. [Google Scholar] [CrossRef]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via Toll-like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial properties of mesenchymal stem cells: Therapeutic potential for cystic fibrosis infection, and treatment. Stem Cells Int. 2016, 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Krasnodembskaya, A.; McKenna, D.H.; Song, Y.; Abbott, J.; Matthay, M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013, 187, 751–760. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Anderson, P.; Gonzalez, M.A.; Rico, L.; Buscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial activity of mesenchymal stem cells: Current status and new perspectives of antimicrobial peptide-based therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Martin, A.; Contreras, L.; Figueroa, F.E.; Khoury, M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res. Ther. 2015, 6, 199. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–540. [Google Scholar] [CrossRef]
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Shaw, T.D.; Krasnodembskaya, A.D.; Schroeder, G.N.; Zumla, A.; Maeurer, M.; O’Kane, C.M. Mesenchymal Stromal Cells: An Antimicrobial and Host-Directed Therapy for Complex Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e0006421. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Houamel, D.; Ducrot, N.; Lefebvre, T.; Daher, R.; Moulouel, B.; Sari, M.A.; Letteron, P.; Lyoumi, S.; Millot, S.; Tourret, J.; et al. Hepcidin as a Major Component of Renal Antibacterial Defenses against Uropathogenic Escherichia coli. J. Am. Soc. Nephrol. 2016, 27, 835–846. [Google Scholar] [CrossRef]
- Jiang, X.F.; Liu, Z.F.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Coordination of Bactericidal and Iron Regulatory Functions of Hepcidin in Innate Antimicrobial Immunity in a Zebrafish Model. Sci. Rep. 2017, 7, 4265. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Zinkernagel, A.S.; Datta, V.; Lauth, X.; Johnson, R.S.; Nizet, V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood 2006, 107, 3727–3732. [Google Scholar] [CrossRef]
- Ripley, D.A.; Morris, R.H.; Maddocks, S.E. Dual stimulation with bacterial and viral components increases the expression of hepcidin in human monocytes. FEMS Microbiol. Lett. 2014, 359, 161–165. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Cloutier, N.; Allaeys, I.; Marcoux, G.; Machlus, K.R.; Mailhot, B.; Zufferey, A.; Levesque, T.; Becker, Y.; Tessandier, N.; Melki, I.; et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl. Acad. Sci. USA 2018, 115, E1550–E1559. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell. Mol. Life Sci. 2010, 67, 525–544. [Google Scholar] [CrossRef]
- Cox, D.; Kerrigan, S.W.; Watson, S.P. Platelets and the innate immune system: Mechanisms of bacterial-induced platelet activation. J. Thromb. Haemost. 2011, 9, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. Bacterial-platelet interactions: Virulence meets host defense. Future Microbiol. 2010, 5, 471–506. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Li, Y.; Lang, S.; Yougbare, I.; Zhu, G.; Chen, P.; Ni, H. Crosstalk between platelets and the immune system: Old systems with new discoveries. Adv. Hematol. 2012, 2012, 384685. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C. Platelet-macrophage partnership in innate immunity and inflammation. Nat. Immunol. 2013, 14, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, A.; Campo, G.C.; Mari, M.; Pompei, G.; Pavasini, R.; Volta, C.A.; Spadaro, S. The Underestimated Role of Platelets in Severe Infection a Narrative Review. Cells 2022, 11, 424. [Google Scholar] [CrossRef]
- Krijgsveld, J.; Zaat, S.A.; Meeldijk, J.; van Veelen, P.A.; Fang, G.; Poolman, B.; Brandt, E.; Ehlert, J.E.; Kuijpers, A.J.; Engbers, G.H.; et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 2000, 275, 20374–20381. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets: At the nexus of antimicrobial defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef]
- Yount, N.Y.; Gank, K.D.; Xiong, Y.Q.; Bayer, A.S.; Pender, T.; Welch, W.H.; Yeaman, M.R. Platelet microbicidal protein 1: Structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 2004, 48, 4395–4404. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.; Waring, A.J.; Gank, K.D.; Kupferwasser, D.; Wiese, R.; Bayer, A.S.; Welch, W.H. Modular determinants of antimicrobial activity in platelet factor-4 family kinocidins. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Ganz, T.; Liese, A.M.; Burdick, M.D.; Liu, L.; Strieter, R.M. Cutting edge: IFN-inducible ELR−CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 2001, 167, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Hoover, D.M.; Staley, P.; Tucker, K.D.; Lubkowski, J.; Oppenheim, J.J. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J. Leukoc. Biol. 2003, 74, 448–455. [Google Scholar] [CrossRef]
- Aquino-Domínguez, A.S.; Romero-Tlalolini, M.L.A.; Torres-Aguilar, H.; Aguilar-Ruiz, S.R. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. Int. J. Mol. Sci. 2021, 22, 10230. [Google Scholar] [CrossRef]
- Yount, N.; Waring, A.J.; Gank, K.D.; Welch, W.H.; Kupferwasser, D.; Yeaman, M.R. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Linge, H.; Bjartell, A.; Giwercman, A.; Malm, J.; Egesten, A. Constitutive expression of the antibacterial CXC chemokine GCP-2/CXCL6 by epithelial cells of the male reproductive tract. J. Reprod. Immunol. 2008, 79, 37–43. [Google Scholar] [CrossRef]
- Jovic, S.; Linge, H.; Shikhagaie, M.M.; Olin, A.I.; Lannefors, L.; Erjefält, J.S.; Mörgelin, M.; Egesten, A. The neutrophil-recruiting chemokine GCP-2/CXCL6 is expressed in cystic fibrosis airways and retains its functional properties after binding to extracellular DNA. Mucosal. Immunol. 2015, 9, 112–123. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Krijgsveld, J.; de Boer, L.; Nguyen, L.T.; Boszhard, L.; Vreede, J.; Dekker, H.L.; Speijer, D.; Drijfhout, J.W.; te Velde, A.A.; et al. Native thrombocidin-1 and unfolded thrombocidin-1 exert antimicrobial activity via distinct structural elements. J. Biol. Chem. 2011, 286, 43506–43514. [Google Scholar] [CrossRef]
- Maerki, C.; Meuter, S.; Liebi, M.; Mühlemann, K.; Frederick, M.J.; Yawalkar, N.; Moser, B.; Wolf, M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J. Immunol. 2008, 182, 507–514. [Google Scholar] [CrossRef]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G.S. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Nolan, E.M. Human α-defensin 6: A small peptide that self-assembles and protects the host by entangling microbes. Acc. Chem. Res. 2017, 50, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Varoga, D.; Wruck, C.J.; Podschun, R.; Sachweh, B.H.; Bornemann, J.; Bovi, M.; Sönmez, T.T.; Slowik, A.D.; Houben, A.; et al. Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelets 2011, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Varoga, D.; Podschun, R.; Wruck, C.J.; Seekamp, A.; Brandenburg, L.O.; Pufe, T.; Lippross, S. Thrombocytes are effectors of the innate immune system releasing human beta defensin-3. Injury 2011, 42, 682–686. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.A.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Valle-Jiménez, X.; Ramírez-Cosmes, A.; Aquino-Domínguez, A.S.; Sánchez-Peña, F.; Bustos-Arriaga, J.; Romero-Tlalolini, M.D.; Torres-Aguilar, H.; Serafín-López, J.; Aguilar Ruíz, S.R. Human platelets and megakaryocytes express defensin alpha 1. Platelets 2019, 31, 344–354. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczynska, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Salamah, M.F.; Ravishankar, D.; Kodji, X.; Moraes, L.A.; Williams, H.F.; Vallance, T.M.; Albadawi, D.A.; Vaiyapuri, R.; Watson, K.; Gibbins, J.M.; et al. The endogenous antimicrobial cathelicidin LL37 induces platelet activation and augments thrombus formation. Blood Adv. 2018, 2, 2973–2985. [Google Scholar] [CrossRef]
- Horn, M.; Bertling, A.; Brodde, M.F.; Müller, A.; Roth, J.; van Aken, H.; Jurk, K.; Heilmann, C.; Peters, G.; Kehrel, B.E. Human neutrophil alpha-defensins induce formation of fibrinogen and thrombospondin-1 amyloid-like structures and activate platelets via glycoprotein IIb/IIIa. J. Thromb. Haemost. 2012, 10, 647–661. [Google Scholar] [CrossRef]
- Torres-Juarez, F.; Trejo-Martínez, L.A.; Layseca-Espinosa, E.; Leon-Contreras, J.C.; Enciso-Moreno, J.A.; Hernandez-Pando, R.; Rivas-Santiago, B. Platelets immune response against Mycobacterium tuberculosis infection. Microb. Pathog. 2021, 153, 104768. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; You, X. Coagulation factors: A novel class of endogenous host antimicrobial proteins against drug-resistant gram-negative bacteria. Signal Transduct. Target. Ther. 2019, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Li, L.; Zhang, T.; Zhang, Q.; Wu, F.; Wang, D.; Hu, H.; Tian, C.; Liao, D.; et al. Coagulation factors VII, IX and X are effective antibacterial proteins against drug-resistant Gram-negative bacteria. Cell Res. 2019, 29, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Gulzar, F. Postoperative infections of the spine. World Spinal Column. J. 2015, 1, 19–26. [Google Scholar]
- Anderson, P.A.; Savage, J.W.; Vaccaro, A.R.; Radcliff, K.; Arnold, P.M.; Lawrence, B.D.; Shamji, M.F. Prevention of surgical site infection in spine surgery. Neurosurgery 2017, 80, S114–S123. [Google Scholar] [CrossRef]
- Whitmore, R.G.; Stephen, J.; Stein, S.C.; Campbell, P.G.; Yadla, S.; Harrop, J.S.; Sharan, A.D.; Maltenfort, M.G.; Ratliff, J.K. Patient comorbidities and complications after spinal surgery: A societal-based cost analysis. Spine 2012, 37, 1065–1071. [Google Scholar] [CrossRef]
- Yeramaneni, S.; Robinson, C.; Hostin, R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr. Rev. Musculoskelet. Med. 2016, 9, 327–332. [Google Scholar] [CrossRef]
| Kinocidins | Target Microorganisms |
|---|---|
| CXCL1 | E. coli, S. aureus, S. typhimurium, C. albicans [43,45] |
| CXCL2 | E. coli, S. aureus [43] |
| CXCL3 | E. coli, S. aureus [43] |
| CXCL4 | E. coli, S. aureus, S. typhimurium, C. albicans [41,45] |
| CXCL6 | N. gonorrhoeae, E. faecalis, P. aeruginosa, S. pyogenes, S. dysgalactiae subsp, S. aureus, E. coli, B. subtilis [46,47] |
| CXCL7 | E. coli, S. aureus, C. neoformans [39] |
| CXCL7 (fragment TC-1) | E. coli, B. subtilis, C. neoformans, S. aureus [37,48] |
| CXCL7 (fragment TC-2) | E. coli, S. aureus, B. subtilis [37] |
| CXCL12 | E. coli, S. aureus [42] |
| CXCL14 | E. coli, S. aureus, E.coli, C. albicans [42,49] |
| CCL5 | E. coli, S. aureus, S. typhimurium [39,45] |
| CCL15 | E. coli, S. aureus [39] |
| CCL17 | E. coli, S. aureus [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contartese, D.; Sartori, M.; Tedesco, G.; Gasbarrini, A.; Giavaresi, G.; Salamanna, F. Vertebral Bone Marrow Clot towards the Routine Clinical Scenario in Spine Surgeries: What about the Antimicrobial Properties? Int. J. Mol. Sci. 2023, 24, 1744. https://doi.org/10.3390/ijms24021744
Contartese D, Sartori M, Tedesco G, Gasbarrini A, Giavaresi G, Salamanna F. Vertebral Bone Marrow Clot towards the Routine Clinical Scenario in Spine Surgeries: What about the Antimicrobial Properties? International Journal of Molecular Sciences. 2023; 24(2):1744. https://doi.org/10.3390/ijms24021744
Chicago/Turabian StyleContartese, Deyanira, Maria Sartori, Giuseppe Tedesco, Alessandro Gasbarrini, Gianluca Giavaresi, and Francesca Salamanna. 2023. "Vertebral Bone Marrow Clot towards the Routine Clinical Scenario in Spine Surgeries: What about the Antimicrobial Properties?" International Journal of Molecular Sciences 24, no. 2: 1744. https://doi.org/10.3390/ijms24021744
APA StyleContartese, D., Sartori, M., Tedesco, G., Gasbarrini, A., Giavaresi, G., & Salamanna, F. (2023). Vertebral Bone Marrow Clot towards the Routine Clinical Scenario in Spine Surgeries: What about the Antimicrobial Properties? International Journal of Molecular Sciences, 24(2), 1744. https://doi.org/10.3390/ijms24021744









