The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond
Abstract
1. Introduction
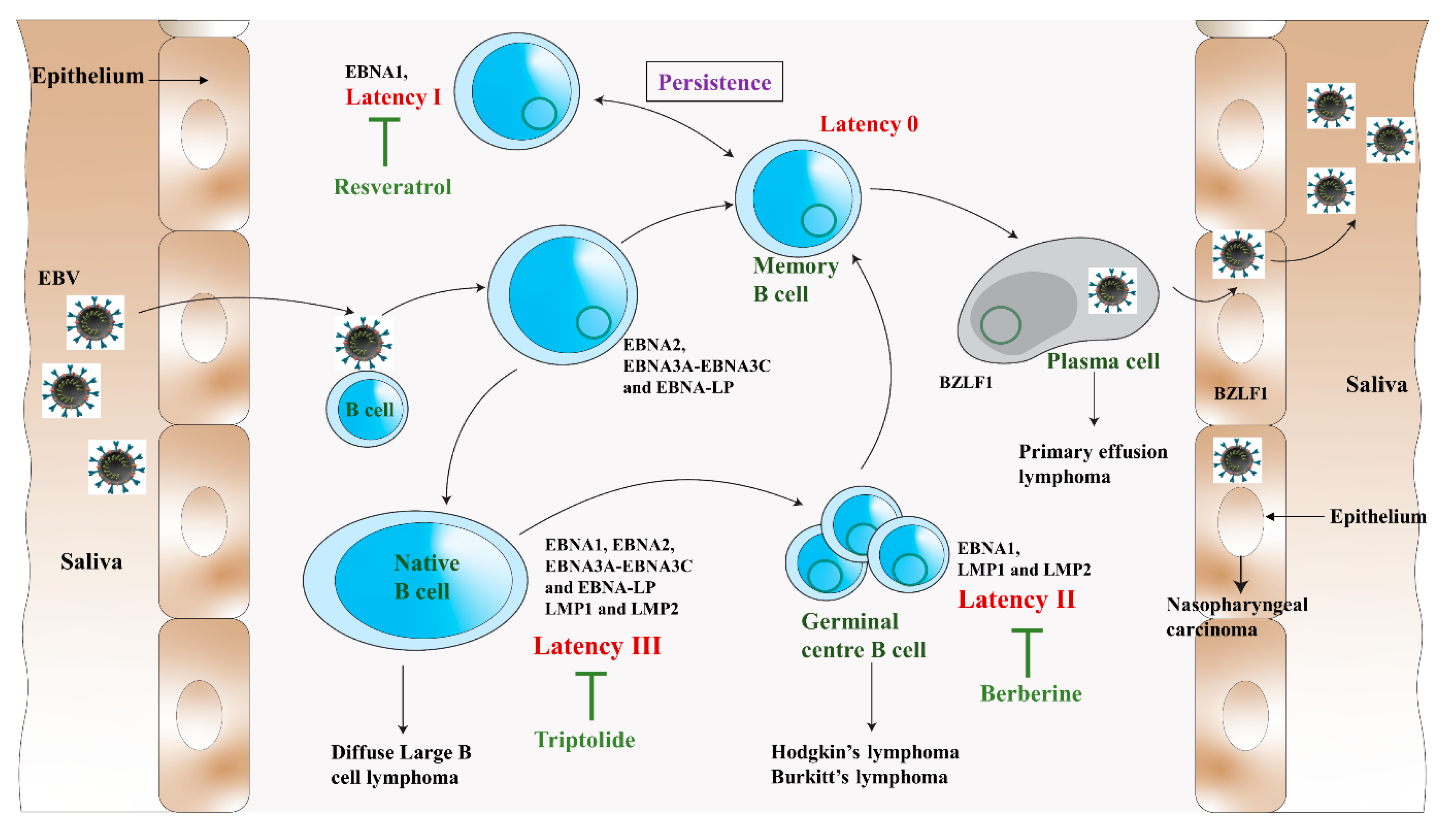
2. Epstein–Barr Virus
3. Gut Microbiota
3.1. Probiotics and EBV
3.2. Short-Chain Fatty Acids (SCFAs)
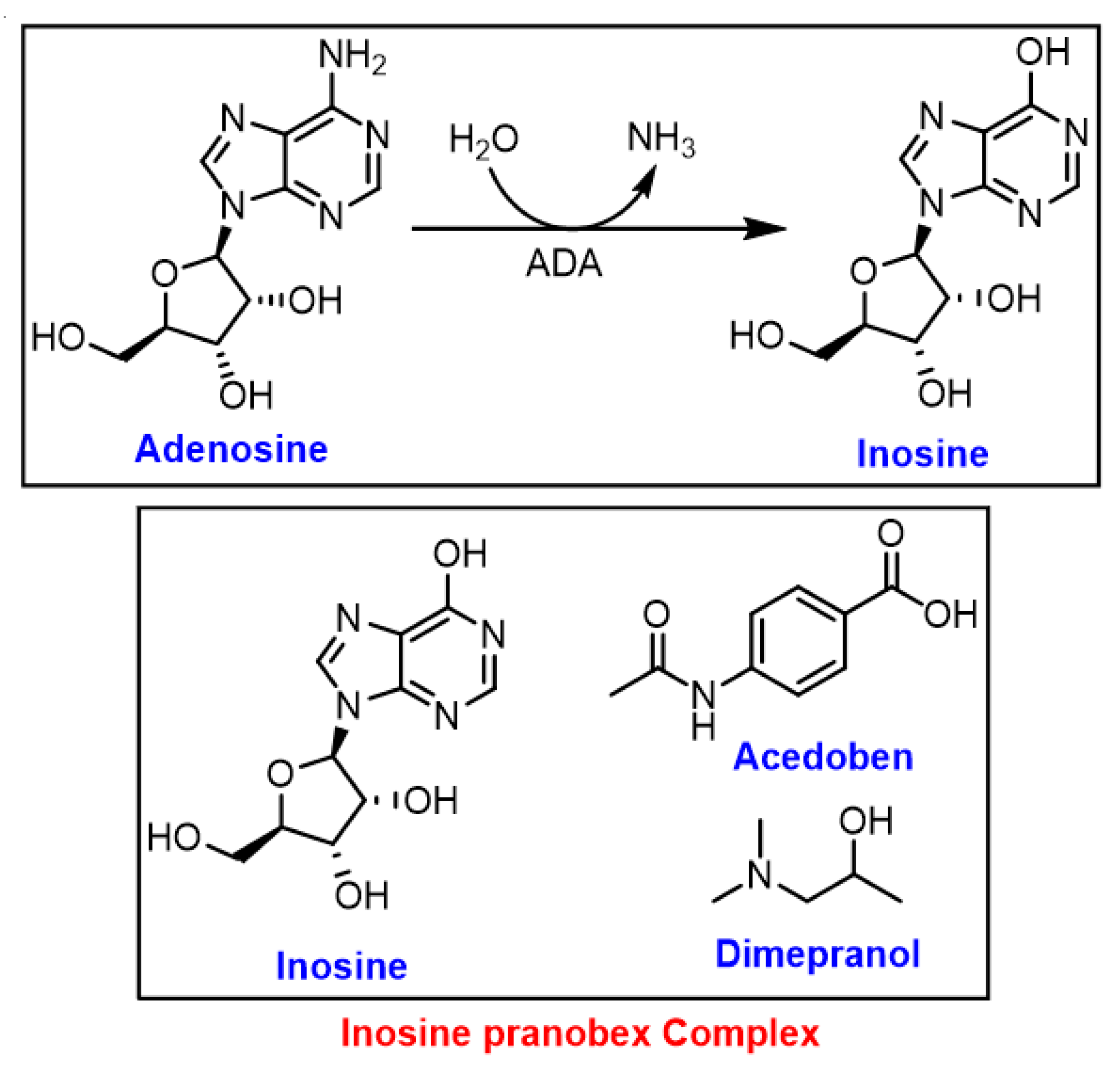
3.3. Inosine
| Infection | Type of Study | Main Findings | References |
|---|---|---|---|
| Epstein-Barr virus | In vitro | The protective immunomodulating effect of IP was investigated on EBV-transformed B lymphoid cells. IP was found to be an immunomodulating agent with an ability to enhance the response of sensitized peripheral blood mononuclear cells (PBL) to EBV-antigens. | [65] |
| Acute respiratory viral infections | Clinical: Randomised, double-blind, Phase 4 study (n = 231) with placebo (n = 232) | Faster improvement in subjects in the IP group versus those in the placebo group. | [66] |
| SARS-CoV-2 (COVID-19) | Clinical: The study was conducted during June-September 2020 in three nursing homes (in the Czech Republic) with 301 residents, 156 of whom (51.8%) tested positive for the SARS-CoV-2 virus (PCR test). | Demonstrated the positive effect of IP against COVID-19. The case-fatality rate in these three nursing homes at the end of the pandemic’s first wave was lower than similar nursing homes in the Czech Republic. | [67] |
3.4. Bacteriocins
4. Natural Products as Antiviral Agents against EBV
| Compound | Source | Cell Line/Animal | Mode of Action | References |
|---|---|---|---|---|
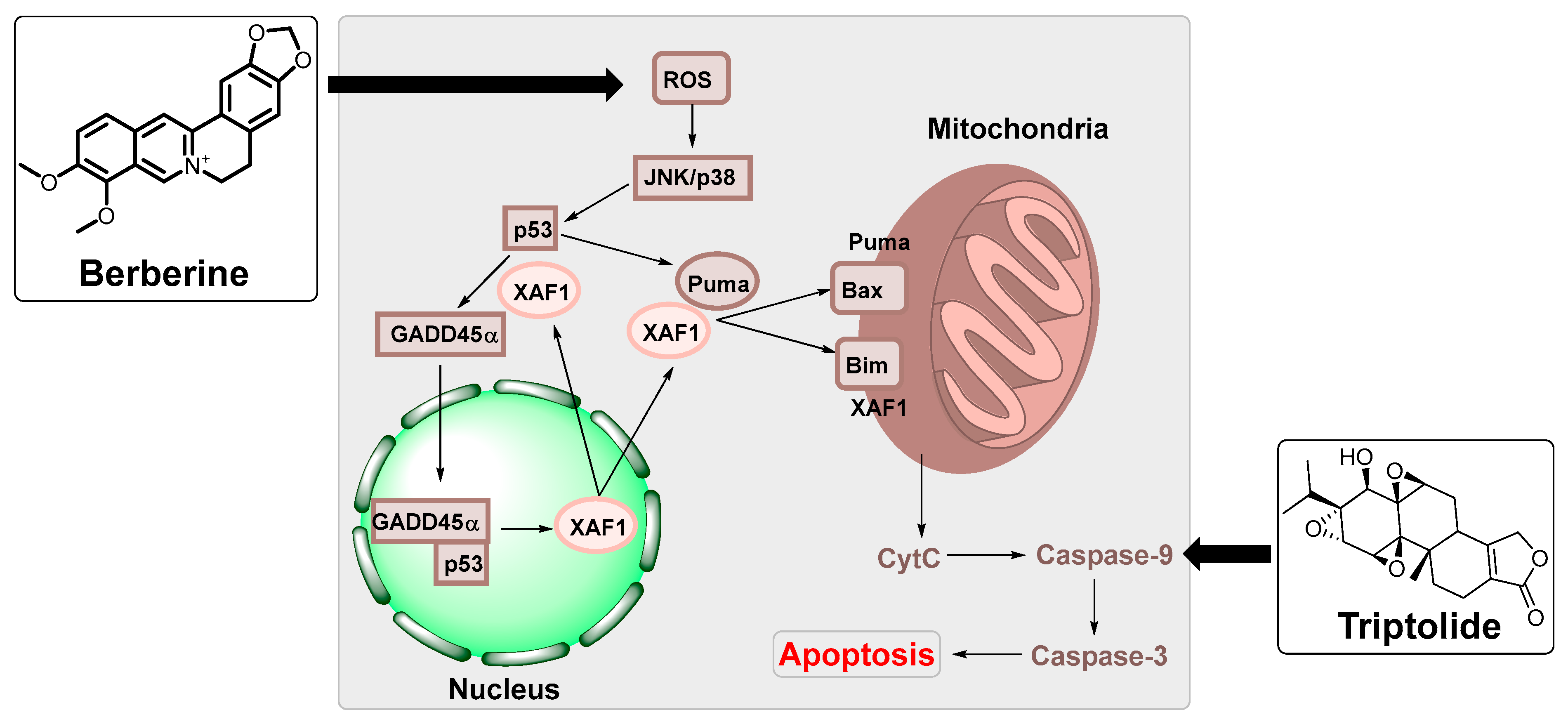
| Berberine | Berberis vulgaris | HK1 and HONE1 nasopharyngeal carcinoma cells and NOD/SCID mice | Suppressed the expression of EBNA1. | [88] |
| C666-1, HONE1, HK1 and NP460 nasopharyngeal carcinoma cells and nude mice | STAT3 activation was inhibited in the nasopharyngeal carcinoma cells. | [90] | ||
| CNE2 nasopharyngeal carcinoma cells and BALB/C-NU male mice | Regulated the expression of key proteins of the MAPK/ERK pathway. | [91] | ||
| IM-9 multiple myeloma cells | Upregulated XAF1 and GADD45a expression by MAPK and functional p53. | [89] | ||
| Resveratrol | Polygonum cuspidatum | Raji Burkitt lymphoma cells and female mice | Activation of TPA-induced EBV-early antigen was suppressed. | [76] |
| P3HR-1 Burkitt lymphoma cells | Decreased the growth and development of the virus. | [96] | ||
| Akata and Raji Burkitt lymphoma cells | Decreased protein synthesis and lowered reactive oxygen species (ROS) levels. Redox-sensitive transcription factors AP-1 and NF-kB were suppressed. | [97] | ||
| Akata and B95-8 Burkitt lymphoma cells | Antiapoptotic proteins Mcl-1 and survivin were downregulated. | [98] | ||
| Apigenin | Punica granatum | NA and HA nasopharyngeal carcinoma and P3HR1 Burkitt lymphoma cells | Suppressed the promoter activities of two viral IE genes and decreased the viral reactivation | [99] |
| (+)-Rutamarin | Rutu graveolens | P3HR-1 Burkitt lymphoma cells | Exhibited anti-EBV lytic DNA replication. | [100] |
| Wogonin | Scutellaria baicalensis | Raji Burkitt lymphoma cells and four-week-old male BALB/c nude mice. | The expression of NF-kB was downregulated through LMP1/miR155/NF-kB/PU.1 pathway. | [101] |
| Epigallocatechin gallate | Camellia sinensis | P3HR-1 Burkitt lymphoma cells | Inhibited the EBV lytic proteins expression. | [102] |
| CNE1-LMP1 nasopharyngeal carcinoma and B95-8 Burkitt lymphoma cells | The MEK/ERK1/2 and PI3K/AKT signalling were suppressed. | [103] | ||
| Downregulated LMP1. | [104] | |||
| Glycyrrhizin | Glycyrrhiza glabra | HEK (Human embryonic kidney) cells | Inhibited EBV infection. Targeted the first step of the SUMO (small ubiquitin-like modifier)ylation process resulting in limited cell growth and apoptosis. | [105,106] |
| Triptolide | Tripterygium wilfordii | HONE1/Akata, C666-1 nasopharyngeal carcinoma and P3HR-1 cells Burkitt lymphoma cells | Reduced the expression of LMP1 in EBV-positive B lymphocytes. | [93] |
| HEK and B95-8 Burkitt lymphoma cells, HeLa (cervical cancer cells and BALB/c male mice | Increased the mitochondrial apoptosis sensitivity in nasopharyngeal carcinoma cells. | [94] | ||
| HEK, B95-8 and P3HR-1 Burkitt lymphoma cells | Targeted and downregulated the translation factors SP1 and c-Myc. | [107] | ||
| Phytol | Lindernia crustacea | P3HR-1 Burkitt lymphoma cells | RTA expression was inhibited. | [108] |
| Aloe-emodin | Lindernia crustacea | EBV lytic cycle was inhibited. | [108] | |
| Cis/trans-martynoside & Cis/trans-isomartynoside | Lindernia crustacea | EBV lytic cycle was affected. | [108] | |
| Emodin | Polygonum cuspidatum | P3HR-1 Burkitt lymphoma cells | EBV lytic cycle was affected. Inhibited the transcription of EBV immediate early genes, the expression of EBV lytic proteins, and reduced EBV DNA replication. | [84] |
| NA, HA, HONE-1 and TW01 nasopharyngeal carcinoma cells | EBV reactivation and nasopharyngeal carcinoma recurrence were decreased. | [109] | ||
| (+)-Hyperjaponicol B & Hyperjaponicol D | Hypericum japonicum | B95-8 Burkitt lymphoma cells | Suppressed EBV DNA replication. | [110] |
| Hyperjaponicol H | Hypericum japonicum | B95-8 Burkitt lymphoma cells | Moderately inhibited EBV lytic DNA replication. | [111] |
| Grifolin | Albatrellus confluens & Boletus pseudocalopus | CNE1-LMP1 nasopharyngeal carcinoma cells | Targeted DNMT1 to block aerobic glycolysis. Blocked DNMT1 localization to restore OXPHOS | [112] |
| Quercetin | Glycyrrhizia uralensis or G. glabra | SNU719 gastric carcinoma cells | Induced cell cycle arrest and strong early apoptosis and necrosis/late apoptosis. Inhibited infection of EBV from lymphocytes to gastric adenocarcinoma cells. | [113] |
| Manassantin B | Saururus chinensis | P3HR-1 Burkitt lymphoma cells | Blocked lytic replication and virion production by inhibiting mTORC2 activity and blocking the mTORC2-PKC/AKT-signalling pathway. | [114] |
| Protoapigenone | Thelypteris torresiana | P3HR-1 Burkitt lymphoma cells | ZTA transactivation important for lytic cycle activation was inhibited. | [115] |
| Curcumin | Curcuma longa | HONE1 and HK1-EBV nasopharyngeal carcinoma cells | Induced cell cycle arrest and apoptosis via the mitochondria- and death receptor-mediated pathways. | [74] |
| HeLa cervical cancer cells | Inhibited the transcription level of EBNA1. | [74] | ||
| Andrographolide | Andrographis paniculata | P3HR1 Burkitt lymphoma cells | Inhibited the transcription of IE genes that encoded Rta and Zta | [75] |
| Thirteen ent-rosane-type diterpenoids | Euphorbia milii | P3HR-1 Burkitt lymphoma cells | Inhibited lytic replication. | [87] |
| Twenty-six neo-clerodane diterpenoids | Scutellaria barbata | P3HR-1 Burkitt lymphoma cells | Inhibited lytic replication. | [86] |
| Moronic acid | Rhus chinensis | HEK, P3HR-1 Burkitt lymphoma cells | Inhibited the expression of Rta, and Zta, and interfered with the function of Rta. | [116] |
| 28 lignans | Saururus chinensis | P3HR-1 Burkitt lymphoma cells | Inhibited lytic replication. | [117] |
| Polysaccharide | Astragalus membranaceus | Raji Burkitt lymphoma cells | Suppressed the expression of the IE protein, Rta, Zta, and EA-D. | [118] |
| Sulphated polysaccharides | Microalgae Ankistrodesmus convolutus, Synechococcus elongatus, and Spirulina platensis | Akata, B95-8, and P3HR- Burkitt lymphoma cells | Reduced cell-free EBV DNA. | [119] |
| Angelicin | Psoralea corylifolia | BC-3 and BCBL-1 lymphoma, and B95-8 and Raji Burkitt lymphoma cells | Inhibited lytic replication. | [120] |
| Lawsone (2-hydroxy-1,4-naphthoquinone) | Lawsonia inermis (henna leaf) | In vivo two-stage mouse skin model | Reduced EBV-early antigen activation Reduced skin carcinogenesis in a mouse model. | [121] |
| Luteolin (3,4,5,7-tetrahydroxyflavone) | Fruits and vegetables | NA, HA nasopharyngeal carcinoma, and P3HR-1 Burkitt lymphoma cells | Suppressed the activities of Zta and Rta by deregulating its Sp1 binding. | [122] |
4.1. Antiviral Agents from Food Sources
4.2. Structural Modifications of Natural Products for Enhanced Activity
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef]
- Fatima, I.; Kanwal, S.; Mahmood, T. Natural Products Mediated Targeting of Virally Infected Cancer. Dose-Response 2019, 17, 1559325818813227. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H.; de Villiers, E.M. Cancer: “causation” by infections--individual contributions and synergistic networks. Semin. Oncol. 2014, 41, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Butel, J.S. Viral carcinogenesis: Revelation of molecular mechanisms and etiology of human disease. Carcinog. 2000, 21, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Moore, P.S.; Weiss, R.A. Human oncogenic viruses: Nature and discovery. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160264. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulos, D. Infections and human cancer: Cancer surveys, volume 33; microbes and malignancy: Infection as a cause of human cancers. BMJ 1999, 319, 1207. [Google Scholar] [CrossRef]
- Foulkes, W.D. Inherited Susceptibility to Common Cancers. N. Engl. J. Med. 2008, 359, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.E.; Offit, K. Hereditary Cancer Predisposition Syndromes. J. Clin. Oncol. 2005, 23, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72 (Suppl. 3), 962–970. [Google Scholar] [CrossRef]
- Morales-Sánchez, A.; Fuentes-Pananá, E.M. Human Viruses and Cancer. Viruses 2014, 6, 4047–4079. [Google Scholar] [CrossRef]
- Khidr, L.; Chen, P.-L. RB, the conductor that orchestrates life, death and differentiation. Oncogene 2006, 25, 5210–5219. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Graham, M.E.; Lovrecz, G.O.; Bache, N.; Robinson, P.J.; Reddel, R.R. Protein Composition of Catalytically Active Human Telomerase from Immortal Cells. Science 2007, 315, 1850–1853. [Google Scholar] [CrossRef]
- Terrin, L.; Dal Col, J.; Rampazzo, E.; Zancai, P.; Pedrotti, M.; Ammirabile, G.; Bergamin, S.; Rizzo, S.; Dolcetti, R.; De Rossi, A. Latent Membrane Protein 1 of Epstein-Barr Virus Activates the hTERT Promoter and Enhances Telomerase Activity in B Lymphocytes. J. Virol. 2008, 82, 10175–10187. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Marquitz, A.R.; Raab-Traub, N. The role of miRNAs and EBV BARTs in NPC. Semin. Cancer Biol. 2012, 22, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Ramírez, G.K.; Espinoza-Sánchez, N.A.; Fuentes-Pananá, E.M. Protumor Activities of the Immune Response: Insights in the Mechanisms of Immunological Shift, Oncotraining, and Oncopromotion. J. Oncol. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Chadburn, A.; Abdul-Nabi, A.M.; Teruya, B.S.; Lo, A.A. Lymphoid Proliferations Associated With Human Immunodeficiency Virus Infection. Arch. Pathol. Lab. Med. 2013, 137, 360–370. [Google Scholar] [CrossRef]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The Story of Human Cytomegalovirus and Cancer: Increasing Evidence and Open Questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Trompet, E.; Snoeck, R. Novel therapeutics for Epstein–Barr virus. Molecules 2019, 24, 997. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Jin, Y.; Sun, H.; Wang, W.; Zhan, L. Difference between Acyclovir and Ganciclovir in the Treatment of Children with Epstein–Barr Virus-Associated Infectious Mononucleosis. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A. Natural Products as Antiviral Agents. Stud. Nat. Prod. Chem. 2000, 24, 473–572. [Google Scholar] [CrossRef]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Bhuyan, D.J. The complex interplay of gut microbiota with the five most common cancer types: From carcinogenesis to therapeutics to prognoses. Crit. Rev. Oncol. 2021, 165, 103429. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Epstein–Barr Virus Infection and Multiple Sclerosis: A Review. J. Neuroimmune Pharmacol. 2010, 5, 271–277. [Google Scholar] [CrossRef]
- Murata, T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef]
- Roderburg, C.; Krieg, S.; Krieg, A.; Luedde, T.; Kostev, K.; Loosen, S.H. The Association between Infectious Mononucleosis and Cancer: A Cohort Study of 24,190 Outpatients in Germany. Cancers 2022, 14, 5837. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.C.; Mertz, J.E. Regulation of the latent-lytic switch in Epstein–Barr virus. Semin. Cancer Biol. 2014, 26, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Genet. 2019, 17, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human Viral Oncogenesis: A Cancer Hallmarks Analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Thompson, M.P.; Kurzrock, R. Epstein-Barr virus and cancer. Clin. Cancer Res. 2004, 10, 803–821. [Google Scholar] [CrossRef]
- Niedobitek, G.; Young, L.; Herbst, H. Epstein-Barr virus infection and the pathogenesis of malignant lymphomas. Cancer Surv. 1997, 30, 143–162. [Google Scholar]
- Babcock, G.J.; Hochberg, D.; Thorley-Lawson, D.A. The Expression Pattern of Epstein-Barr Virus Latent Genes In Vivo Is Dependent upon the Differentiation Stage of the Infected B Cell. Immunity 2000, 13, 497–506. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Masucci, M.G. Regulation of Telomere Homeostasis during Epstein-Barr virus Infection and Immortalization. Viruses 2017, 9, 217. [Google Scholar] [CrossRef]
- Portis, T.; Longnecker, R. Epstein–Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene 2004, 23, 8619–8628. [Google Scholar] [CrossRef]
- Sbih-Lammali, F.; Djennaoui, D.; Belaoui, H.; Bouguermouh, A.; Decaussin, G.; Ooka, T. Transcriptional expression of Epstein-Barr virus genes and proto-oncogenes in north African nasopharyngeal carcinoma. J. Med. Virol. 1996, 49, 7–14. [Google Scholar] [CrossRef]
- zur Hausen, H.; O’Neill, F.J.; Freese, U.K.; Hecker, E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature 1978, 272, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2022, 21, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Sliva, J.; Pantzartzi, C.N.; Votava, M. Inosine Pranobex: A Key Player in the Game Against a Wide Range of Viral Infections and Non-Infectious Diseases. Adv. Ther. 2019, 36, 1878–1905. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.; Hill, C.; Ross, R. Bacteriocins: Developing innate immunity for food. Nat. Rev. Genet. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Chaiyasut, C. The Antiviral Potential of Probiotics—A Review on Scientific Outcomes. Appl. Sci. 2021, 11, 8687. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Struszczak, L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: A placebo-controlled, randomized trial. Eur. J. Appl. Physiol. 2016, 116, 1555–1563. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short-and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: A Closer Look at the Lungs. Infect. Immun. 2021, 89, e00188-21. [Google Scholar] [CrossRef] [PubMed]
- Gorres, K.L.; Daigle, D.; Mohanram, S.; Miller, G. Activation and repression of Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus lytic cycles by short-and medium-chain fatty acids. J. Virol. 2014, 88, 8028–8044. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Sumbria, D.; Berber, E.; Rouse, B.T. Supplementing the Diet with Sodium Propionate Suppresses the Severity of Viral Immuno-inflammatory Lesions. J. Virol. 2021, 95, e02056-20. [Google Scholar] [CrossRef]
- Li, J.; Richards, E.M.; Handberg, E.M.; Pepine, C.J.; Raizada, M.K. Butyrate Regulates COVID-19–Relevant Genes in Gut Epithelial Organoids From Normotensive Rats. Hypertension 2021, 77, e13–e16. [Google Scholar] [CrossRef]
- Srinivasan, S.; Torres, A.; de Pouplana, L.R. Inosine in Biology and Disease. Genes 2021, 12, 600. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Lasek, W.; Janyst, M.; Wolny, R.; Zapała, Ł.; Bocian, K.; Drela, N. Immunomodulatory effects of inosine pranobex on cytokine production by human lymphocytes. Acta Pharm. 2015, 65, 171–180. [Google Scholar] [CrossRef]
- Tsang, K.Y.; Fudenberg, H.H.; Pan, J.F.; Gnagy, M.J.; Bristow, C.B. An in vitro study on the effects of isoprinosine on immune responses in cancer patients. Int. J. Immunopharmacol. 1983, 5, 481–490. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Newman, A.S.; O’Daly, J.; Duffy, S.; Grafton, G.; Brady, C.A.; Curnow, S.J.; Barnes, N.M.; Gordon, J. Inosine Acedoben Dimepranol promotes an early and sustained increase in the natural killer cell component of circulating lymphocytes: A clinical trial supporting anti-viral indications. Int. Immunopharmacol. 2017, 42, 108–114. [Google Scholar] [CrossRef]
- Bekesi, J.; Tsang, P.; Wallace, J.; Roboz, J. Immunorestorative properties of isoprinosine in the treatment of patients at high risk of developing ARC or AIDS. J. Clin. Lab. Immunol. 1987, 24, 155–161. [Google Scholar]
- Tsang, K.Y.; Pan, J.F.; Swanger, D.; Fudenberg, H. In vitro restoration of immune responses in aging humans by isoprinosine. Int. J. Immunopharmacol. 1985, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Janíčková, O.; Ančicová, L.; Briestenská, K.; Mistríková, J. The effect of Isoprinosine treatment on persistent infection of Balb/c mice infected with murine gammaherpesvirus 68. Acta Virol. 2017, 61, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.K.; Barile, G.; Menezes, J. Isoprinosine enhances the activation of sensitized lymphocytes by Epstein-Barr virus antigens. Int. J. Immunopharmacol. 1985, 7, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Šalapová, E.; Špajdel, M. Inosine pranobex is safe and effective for the treatment of subjects with confirmed acute respiratory viral infections: Analysis and subgroup analysis from a Phase 4, randomised, placebo-controlled, double-blind study. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Beran, J.; Špajdel, M.; Katzerová, V.; Holoušová, A.; Malyš, J.; Rousková, J.F.; Slíva, J. Inosine pranobex significantly decreased the case-fatality rate among PCR positive elderly with SARS-CoV-2 at three nursing homes in the Czech Republic. Pathogens 2020, 9, 1055. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Petrova, M.I.; Andrei, G.; Huskens, D.; Hoorelbeke, B.; Snoeck, R.; Vanderleyden, J.; Balzarini, J.; Bartoschek, S.; Brönstrup, M.; et al. The Lantibiotic Peptide Labyrinthopeptin A1 Demonstrates Broad Anti-HIV and Anti-HSV Activity with Potential for Microbicidal Applications. PLoS ONE 2013, 8, e64010. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob. 2013, 5, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Ji, W.; Wang, C. Curcumin Inhibits Proliferation of Epstein–Barr Virus-Associated Human Nasopharyngeal Carcinoma Cells by Inhibiting EBV Nuclear Antigen 1 Expression. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Lin, T.-P.; Chen, S.-Y.; Duh, P.-D.; Chang, L.-K.; Liu, Y.-N. Inhibition of the Epstein-Barr Virus Lytic Cycle by Andrographolide. Biol. Pharm. Bull. 2008, 31, 2018–2023. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T.; Konoshima, T.; Nishino, H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein–Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002, 45, 499–505. [Google Scholar] [CrossRef]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Ruchawapol, C.; Yuan, M.; Wang, S.-M.; Fu, W.-W.; Xu, H.-X. Natural Products and Their Derivatives against Human Herpesvirus Infection. Molecules 2021, 26, 6290. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Kwok, S.-T.; Zhang, Q.-W.; Chan, S.-W. A Review of the Pharmacological Effects of the Dried Root ofPolygonum cuspidatum(Hu Zhang) and Its Constituents . Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Yiu, C.-Y.; Chen, S.-Y.; Chen, Y.-P.; Lin, T.-P. Inhibition of the ethanolic extract from Polygonum cuspidatum root on the functions of Epstein-Barr Virus latent membrane protein 1. J. Food Drug Anal. 2013, 21, 20–26. [Google Scholar]
- Yiu, C.-Y.; Chen, S.-Y.; Huang, C.-W.; Yeh, D.-B.; Lin, T.-P. Inhibitory effects of Polygonum cuspidatum on the Epstein-Barr virus lytic cycle. J. Food Drug Anal. 2011, 19, 3. [Google Scholar] [CrossRef]
- Yiu, C.-Y.; Chen, S.-Y.; Yang, T.-H.; Chang, C.-J.; Yeh, D.-B.; Chen, Y.-J.; Lin, T.-P. Inhibition of Epstein-Barr Virus Lytic Cycle by an Ethyl Acetate Subfraction Separated from Polygonum cuspidatum Root and Its Major Component, Emodin. Molecules 2014, 19, 1258–1272. [Google Scholar] [CrossRef]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A review of the ethnomedicinal uses, biological activities, and triterpenoids of Euphorbia species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, Q.; Jiang, C.; Morris-Natschke, S.L.; Cui, H.; Wang, Y.; Yan, Y.; Xu, J.; Lee, K.-H.; Gu, Q. neo-Clerodane Diterpenoids from Scutellaria barbata with Activity against Epstein–Barr Virus Lytic Replication. J. Nat. Prod. 2015, 78, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-N.; Hu, J.; Tan, S.H.; Wang, Q.; Xu, J.; Wang, Y.; Yuan, Y.; Gu, Q. ent-Rosane diterpenoids from Euphorbia milii showing an Epstein–Barr virus lytic replication assay. RSC Adv. 2017, 7, 46938–46947. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhang, Y.; Guo, W.; Long, C.; Wang, J.; Liu, L.; Sun, X. Berberine inhibits the proliferation of human nasopharyngeal carcinoma cells via an Epstein-Barr virus nuclear antigen 1-dependent mechanism. Oncol. Rep. 2017, 37, 2109–2120. [Google Scholar] [CrossRef]
- Park, G.B.; Park, S.H.; Kim, D.; Kim, Y.S.; Yoon, S.H.; Hur, D.Y. Berberine induces mitochondrial apoptosis of EBV-transformed B cells through p53-mediated regulation of XAF1 and GADD45α. Int. J. Oncol. 2016, 49, 411–421. [Google Scholar] [CrossRef]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.-Y.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.-P.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13, 619. [Google Scholar] [CrossRef]
- Zhou, F.; Hu, J.; Dai, N.; Song, L.; Lin, T.; Liu, J.; Li, K.; Peng, Z.; He, Y.; Liao, D.-F. Berberine and ginsenoside Rg3 act synergistically via the MAPK/ERK pathway in nasopharyngeal carcinoma cells. J. Funct. Foods 2020, 66, 103802. [Google Scholar] [CrossRef]
- Wong, K.-F.; Yuan, Y.; Luk, J. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin. Exp. Pharmacol. Physiol. 2012, 39, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guo, W.; Long, C.; Wang, H.; Wang, J.; Sun, X. Triptolide inhibits proliferation of Epstein–Barr virus-positive B lymphocytes by down-regulating expression of a viral protein LMP1. Biochem. Biophys. Res. Commun. 2015, 456, 815–820. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Wang, C.; Liu, L.; Wang, H.; Zhang, Y.; Long, C.; Sun, X. Triptolide inhibits Epstein-Barr nuclear antigen 1 expression by increasing sensitivity of mitochondria apoptosis of nasopharyngeal carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 192. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, D.H.; Schober, W.D.; McQueen, T.; Harris, D.; Estrov, Z.; Evans, R.L.; Andreeff, M. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood 2006, 108, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.-Y.; Chen, S.-Y.; Chang, L.-K.; Chiu, Y.-F.; Lin, T.-P. Inhibitory Effects of Resveratrol on the Epstein-Barr Virus Lytic Cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt’s lymphoma cells by affecting multiple molecular targets. Antivir. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Kato, S.; Nakao, S. Resveratrol Prevents EBV Transformation and Inhibits the Outgrowth of EBV-Immortalized Human B Cells. PLoS ONE 2012, 7, e51306. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Cheng, Y.-J.; Hsu, H.-Y.; Chou, S.-P.; Huang, S.-Y.; Tsai, C.-H.; Chen, J.-Y. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Q.; Gu, Q.; Zhang, H.; Jiang, C.; Hu, J.; Wang, Y.; Yan, Y.; Xu, J. Semisynthesis of (−)-Rutamarin Derivatives and Their Inhibitory Activity on Epstein–Barr Virus Lytic Replication. J. Nat. Prod. 2017, 80, 53–60. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Zhang, H.; Li, Y.; Salmani, J.M.M.; Wang, F.; Yang, K.; Fu, R.; Chen, Z.; Chen, B. Wogonin as a targeted therapeutic agent for EBV (+) lymphoma cells involved in LMP1/NF-κB/miR-155/PU. 1 pathway. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-K.; Wei, T.-T.; Chiu, Y.-F.; Tung, C.-P.; Chuang, J.-Y.; Hung, S.-K.; Li, C.; Liu, S.-T. Inhibition of Epstein–Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003, 301, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Chen, L.; Yang, L.; Li, L.; Tao, Y.; Li, W.; Li, Z.; Liu, H.; Tang, M. (-)-Epigallocatechin-3-gallate inhibition of Epstein–Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis 2013, 34, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Tang, M.; Cao, Y. (-)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves downregulation of latent membrane protein 1. Exp. Ther. Med. 2018, 15, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Cherng, J.-M.; Hung, M.-S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antivir. Res. 2008, 79, 6–11. [Google Scholar] [CrossRef]
- Bentz, G.L.; Lowrey, A.J.; Horne, D.C.; Nguyen, V.; Satterfield, A.R.; Ross, T.D.; Harrod, A.E.; Uchakina, O.N.; McKallip, R.J. Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency. PLoS ONE 2019, 14, e0217578. [Google Scholar] [CrossRef]
- Long, C.; Xu, Q.-B.; Ding, L.; Yang, L.; Ji, W.; Gao, F.; Ji, Y. Triptolide inhibits human telomerase reverse transcriptase by downregulating translation factors SP1 and c-Myc in Epstein-Barr virus-positive B lymphocytes. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Hohmann, J.; El-Shazly, M.; Chang, L.-K.; Dankó, B.; Kúsz, N.; Hsieh, C.-T.; Hunyadi, A.; Chang, F.-R. Bioactive constituents of Lindernia crustacea and its anti-EBV effect via Rta expression inhibition in the viral lytic cycle. J. Ethnopharmacol. 2020, 250, 112493. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, M.-S.; Cheng, Y.-J.; Ko, Y.-C.; Lin, S.-F.; Chiu, I.-M.; Chen, J.-Y. Emodin Inhibits EBV Reactivation and Represses NPC Tumorigenesis. Cancers 2019, 11, 1795. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Zhu, H.; Liu, J.; Li, H.; Li, X.-N.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y. Filicinic Acid Based Meroterpenoids with Anti-Epstein–Barr Virus Activities from Hypericum japonicum. Org. Lett. 2016, 18, 2272–2275. [Google Scholar] [CrossRef]
- Wu, R.; Le, Z.; Wang, Z.; Tian, S.; Xue, Y.; Chen, Y.; Hu, L.; Zhang, Y. Hyperjaponol H, A New Bioactive Filicinic Acid-Based Meroterpenoid from Hypericum japonicum Thunb. ex Murray . Molecules 2018, 23, 683. [Google Scholar] [CrossRef]
- Luo, X.; Hong, L.; Cheng, C.; Li, N.; Zhao, X.; Shi, F.; Liu, J.; Fan, J.; Zhou, J.; Bode, A.M.; et al. DNMT1 mediates metabolic reprogramming induced by Epstein–Barr virus latent membrane protein 1 and reversed by grifolin in nasopharyngeal carcinoma. Cell Death Dis. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Son, M.; Ryu, E.; Shin, Y.S.; Kim, J.G.; Kang, B.W.; Sung, G.-H.; Cho, H.; Kang, H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget 2015, 6, 12603–12624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, N.; Hu, J.; Wang, Y.; Xu, J.; Gu, Q.; Lieberman, P.M.; Yuan, Y. The mTOR inhibitor manassantin B reveals a crucial role of mTORC2 signaling in Epstein-Barr virus reactivation. J. Biol. Chem. 2020, 295, 7431–7441. [Google Scholar] [CrossRef]
- Tung, C.-P.; Chang, F.-R.; Wu, Y.-C.; Chuang, D.-W.; Hunyadi, A.; Liu, S.-T. Inhibition of the Epstein–Barr virus lytic cycle by protoapigenone. J. Gen. Virol. 2011, 92 Pt 8, 1760–1768. [Google Scholar] [CrossRef]
- Chang, F.-R.; Hsieh, Y.-C.; Chang, Y.-F.; Lee, K.-H.; Wu, Y.-C.; Chang, L.-K. Inhibition of the Epstein–Barr virus lytic cycle by moronic acid. Antivir. Res. 2010, 85, 490–495. [Google Scholar] [CrossRef]
- Cui, H.; Xu, B.; Wu, T.; Xu, J.; Yuan, Y.; Gu, Q. Potential Antiviral Lignans from the Roots of Saururus chinensis with Activity against Epstein–Barr Virus Lytic Replication. J. Nat. Prod. 2014, 77, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, X.; Zhang, Z.; Zhang, L.; Yao, G.; Li, F.; Yang, X.; Song, L.; Jiang, G. The effect of Astragalus polysaccharide on the Epstein-Barr virus lytic cycle. . Acta Virol. 2014, 58, 76–83. [Google Scholar] [CrossRef]
- Kok, Y.-Y.; Chu, W.-L.; Phang, S.-M.; Mohamed, S.M.; Naidu, R.; Lai, P.-J.; Ling, S.-N.; Mak, J.-W.; Lim, P.K.-C.; Balraj, P.; et al. Inhibitory activities of microalgal extracts against Epstein-Barr virus DNA release from lymphoblastoid cells. J. Zhejiang Univ. Sci. B 2011, 12, 335–345. [Google Scholar] [CrossRef]
- Cho, H.-J.; Jeong, S.-G.; Park, J.-E.; Han, J.-A.; Kang, H.-R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antivir. Res. 2013, 100, 75–83. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Rao, G.S.; Sridhar, R.; Ichiishi, E.; Takasaki, M.; Suzuki, N.; Konoshima, T.; Iida, A.; Tokuda, H. Chemoprevention of skin cancer: Effect of Lawsonia inermis L.(Henna) leaf powder and its pigment artifact, lawsone in the Epstein-Barr virus early antigen activation assay and in two-stage mouse skin carcinogenesis models. Anticancer Agents Med. Chem. 2013, 13, 1500–1507. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Hsu, H.-Y.; Chen, Y.-J.; Chou, S.-P.; Huang, S.-Y.; Cheng, Y.-J.; Lin, S.-F.; Chang, Y.; Tsai, C.-H.; et al. Luteolin inhibits Epstein-Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antivir. Res. 2016, 132, 99–110. [Google Scholar] [CrossRef]
- Wang, D.; Tan, G.T.; Van Hung, N.; Cuong, N.M.; Pezzuto, J.M.; Fong, H.H.; Soejarto, D.D.; Zhang, H. Litsea Species as Potential Antiviral Plant Sources. Am. J. Chin. Med. 2016, 44, 275–290. [Google Scholar] [CrossRef]
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.-H.; et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X. Targeting the signaling in Epstein–Barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal Transduct. Target Ther. 2021, 6, 1–33. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Arena, G.; Stecca, C.; Raciti, M.; Mattia, E. Resveratrol Inhibits Proliferation and Survival of Epstein Barr Virus–Infected Burkitt’s Lymphoma Cells Depending on Viral Latency Program. Mol. Cancer Res. 2011, 9, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Ramayanti, O.; Brinkkemper, M.; Verkuijlen, S.A.; Ritmaleni, L.; Go, M.L.; Middeldorp, J.M. Curcuminoids as EBV Lytic Activators for Adjuvant Treatment in EBV-Positive Carcinomas. Cancers 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Martins, A.; Danko, B.; Chang, F.-R.; Wu, Y.C. Protoflavones: A class of unusual flavonoids as promising novel anticancer agents. Phytochem. Rev. 2014, 13, 69–77. [Google Scholar] [CrossRef]
- Vágvölgyi, M.; Girst, G.; Kúsz, N.; Ötvös, S.B.; Fülöp, F.; Hohmann, J.; Servais, J.-Y.; Seguin-Devaux, C.; Chang, F.-R.; Chen, M.S.; et al. Less Cytotoxic Protoflavones as Antiviral Agents: Protoapigenone 1′-O-isopropyl ether Shows Improved Selectivity Against the Epstein–Barr Virus Lytic Cycle. Int. J. Mol. Sci. 2019, 20, 6269. [Google Scholar] [CrossRef]
- Stirpe, F.; Olsnes, S.; Pihl, A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J. Biol. Chem. 1980, 255, 6947–6953. [Google Scholar] [CrossRef]
- Shen, C.-L.; Huang, W.-H.; Hsu, H.-J.; Yang, J.-H.; Peng, C.-W. GAP31 from an ancient medicinal plant exhibits anti-viral activity through targeting to Epstein-Barr virus nuclear antigen 1. Antivir. Res. 2019, 164, 123–130. [Google Scholar] [CrossRef]


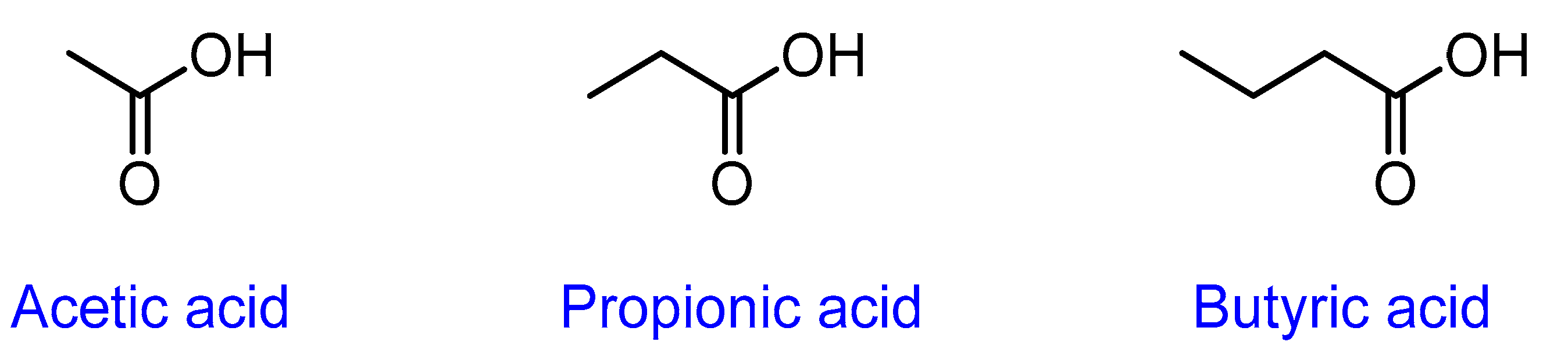
| SCFA | Viral Infection | Type of Study | Description and Outcome of the Study | References |
|---|---|---|---|---|
| Acetate | Respiratory syncytial virus (RSV) | In vitro | Pretreatment of the A549 cells with acetate resulted in a reduction of RSV infection and increased expressions of retinoic acid-inducible gene-I (RIG-I) and interferon-stimulated gene15 (ISG15). | [54] |
| In vivo | The intranasal treatment of acetate in mice reduced RSV infection and viral load and increased expression of RIG-I and Interferon β1 (IFNB1) in the lung tissue. | |||
| Ex vivo | Ex vivo treatment with acetate in RSV-positive infants showed reduced viral load and increased expression of ISG15, RIG-I, and MAVS (mitochondrial antiviral-signalling protein). | |||
| Clinical | Stools from 17 RSV-positive infants showed that the increased levels of acetate were associated with faster recovery, a shorter duration of fever, and higher oxygen saturation at admission. | |||
| Propionate | Herpes simplex virus 1 (HSV-1) | In vivo | Mice were given sodium propionate supplement in drinking water three weeks before induction of the corneal HSV-1 infection, the animals had fewer ocular lesions when compared to the control group. Lymphoid and corneal tissues showed lower levels of CD4 (T-helper) Th1 and Th17 cells, macrophages, and neutrophils and higher levels of regulatory T cells (Treg) when compared to the control. | [55] |
| Butyrate | SARS-CoV-2 (COVID-19) | Clinical | High throughput RNA sequencing of many genes related to SARS-CoV-2 infection (4526 upregulated genes and 3167 downregulated genes) in butyrate-treated organoids and showed that butyrate downregulates genes essential for SARS-CoV-2 infection, such as ACE2 and its associated genes. Butyrate was also found to upregulate toll-like receptors (TLR) and other antiviral pathways. | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eladwy, R.A.; Vu, H.T.; Shah, R.; Li, C.G.; Chang, D.; Bhuyan, D.J. The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. Int. J. Mol. Sci. 2023, 24, 1716. https://doi.org/10.3390/ijms24021716
Eladwy RA, Vu HT, Shah R, Li CG, Chang D, Bhuyan DJ. The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. International Journal of Molecular Sciences. 2023; 24(2):1716. https://doi.org/10.3390/ijms24021716
Chicago/Turabian StyleEladwy, Radwa A., Hang Thi Vu, Ravi Shah, Chun Guang Li, Dennis Chang, and Deep Jyoti Bhuyan. 2023. "The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond" International Journal of Molecular Sciences 24, no. 2: 1716. https://doi.org/10.3390/ijms24021716
APA StyleEladwy, R. A., Vu, H. T., Shah, R., Li, C. G., Chang, D., & Bhuyan, D. J. (2023). The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. International Journal of Molecular Sciences, 24(2), 1716. https://doi.org/10.3390/ijms24021716









