Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin
Abstract
1. Introduction
2. Results
2.1. Effects of Different Substrates on Sperm Capacitation and mRNA Expression of Olfactory Receptor
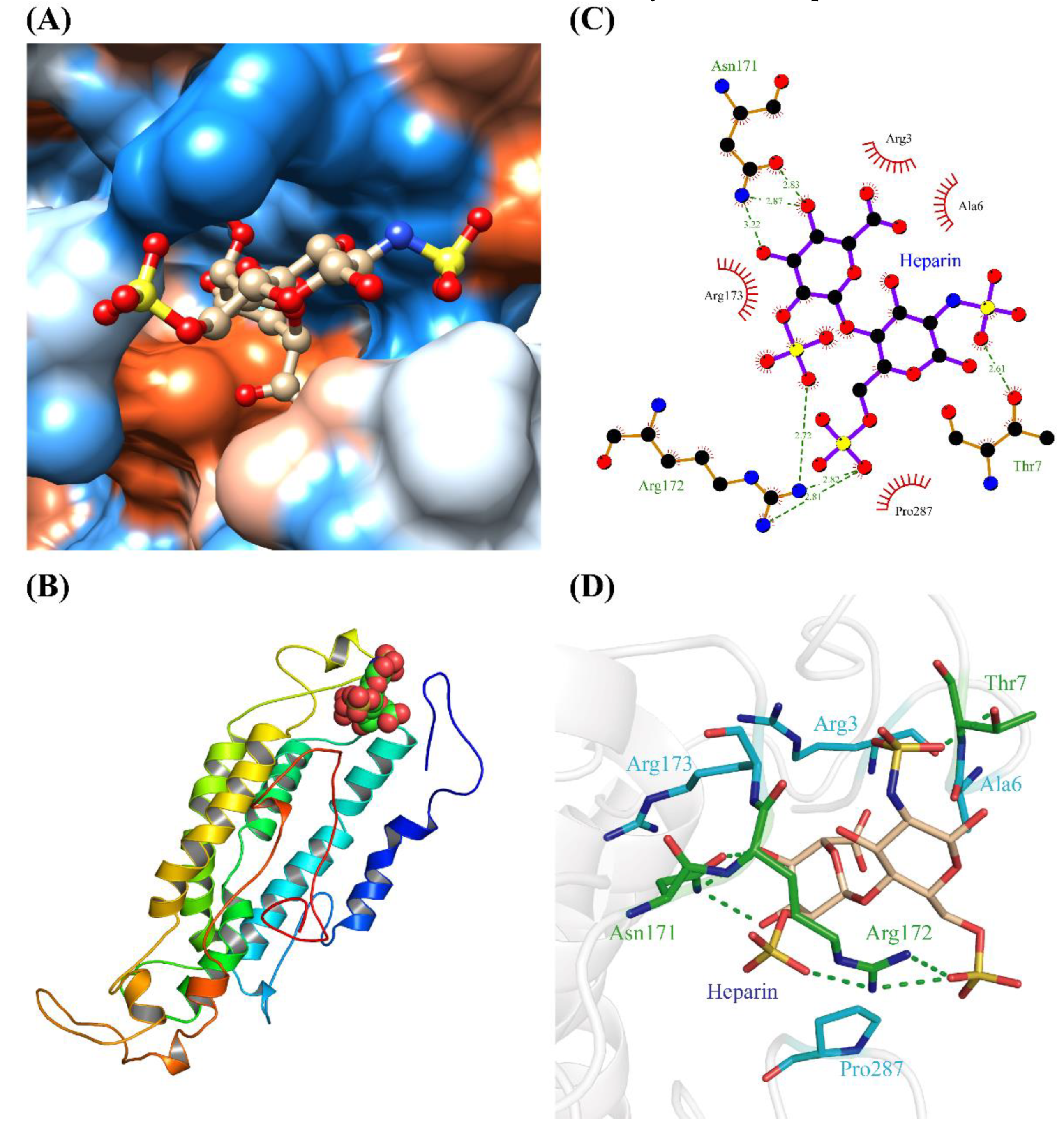
2.2. Homology Modelling and Molecular Docking of OR2C1 and Heparin
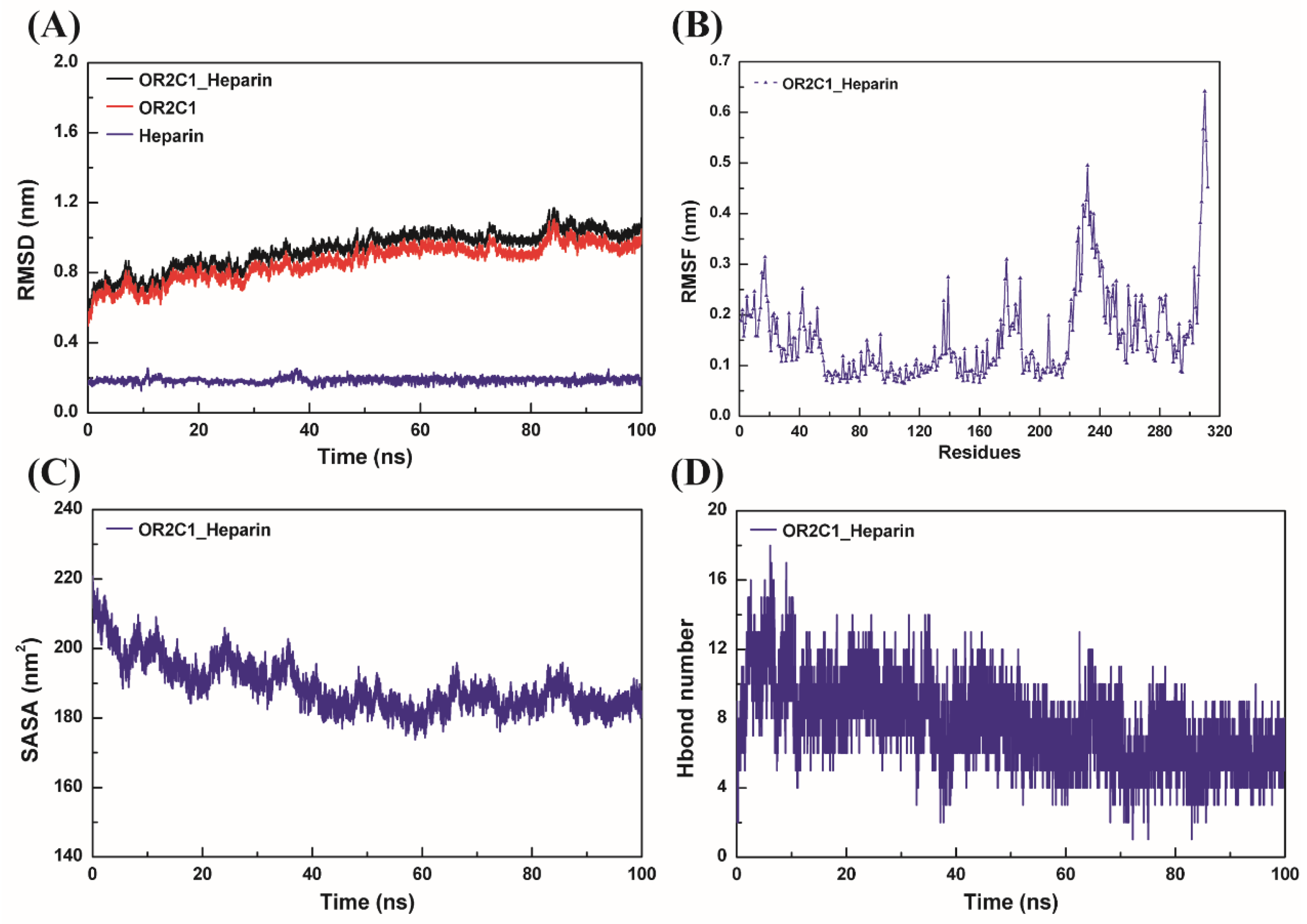
2.3. Molecular Dynamics Simulation for OR2C1 and Heparin
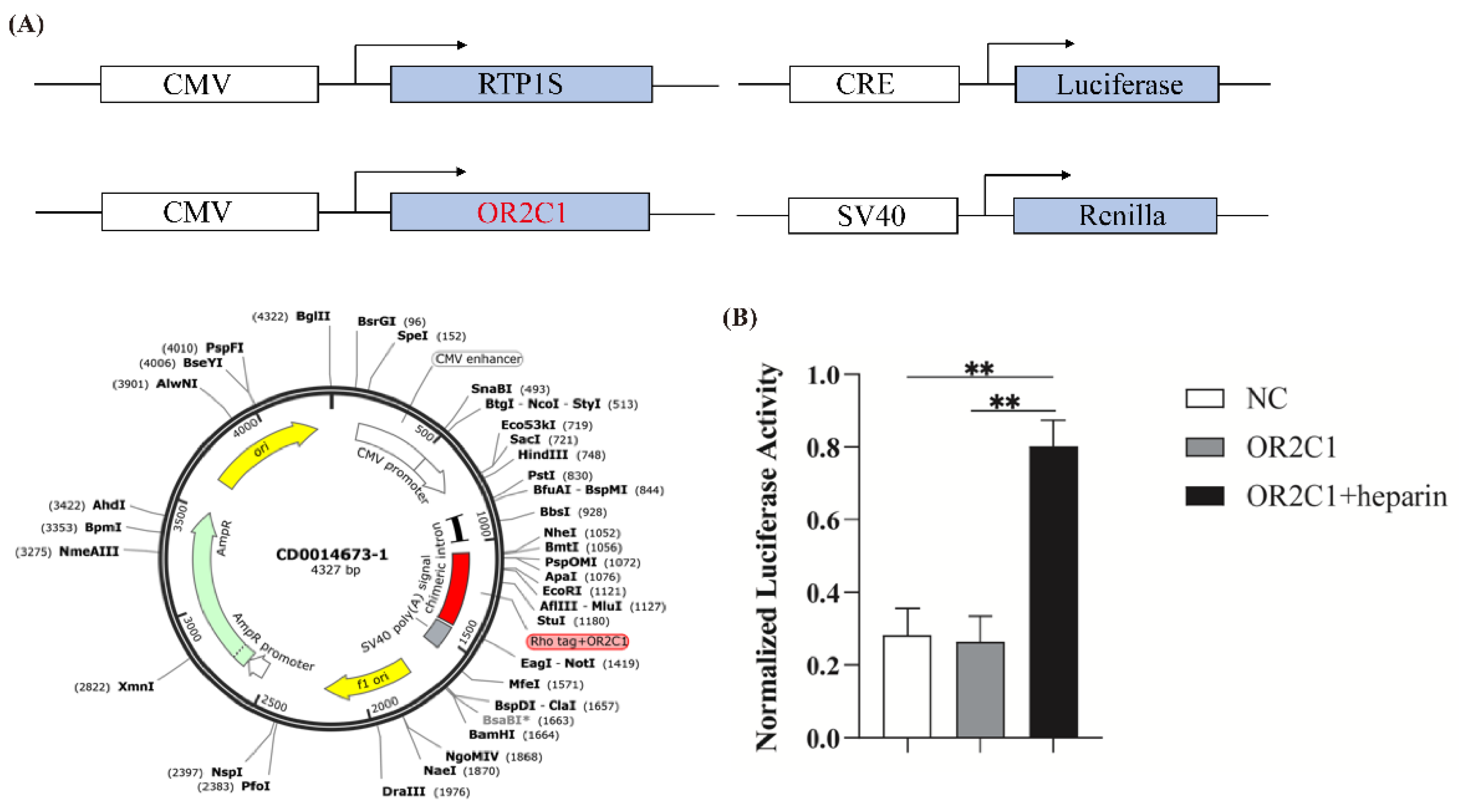
2.4. Activation of OR2C1 with Heparin
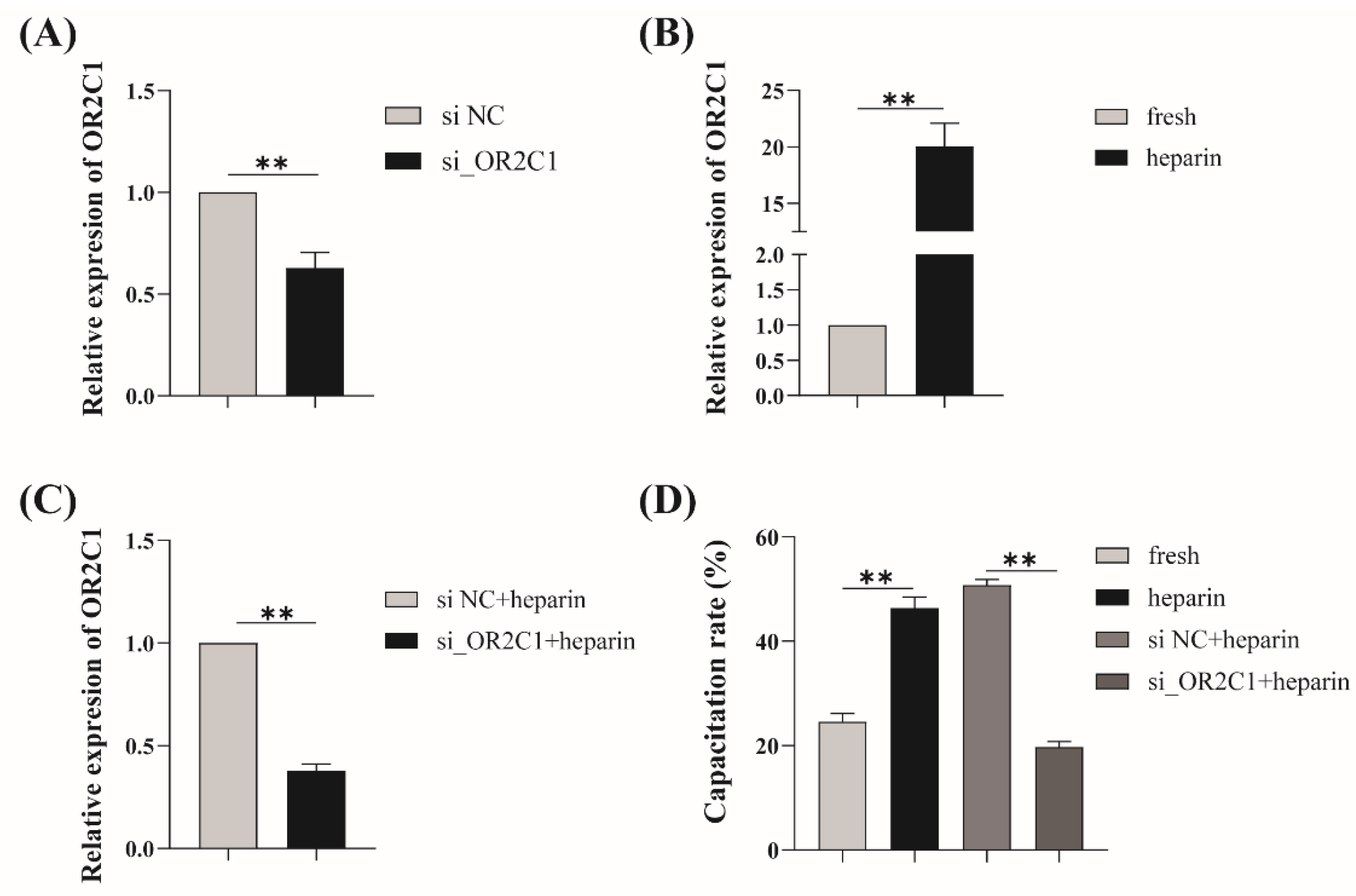
2.5. Regulation of Heparin-Activated OR2C1 in Sperm Capacitation
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Statement
4.2. Materials
4.3. Sperm Collection and Treatment
4.4. Assessment of Acrosomal Status and Capacitation Rate
4.5. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.6. Homology Model Building and Molecular Docking
4.7. Molecular Dynamic Simulations
4.8. Dual-Glo Luciferase Assay
4.9. Knock-Down of OR2C1
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pace, U.; Hanski, E.; Salomon, Y.; Lancet, D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature 1985, 316, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, R.S.; Wright, H.N.; Spiegel, A.M.; Levine, M.A.; Moses, A.M. Olfactory dysfunction in humans with deficient guanine nucleotide-binding protein. Nature 1986, 322, 635–636. [Google Scholar] [CrossRef]
- Jones, D.T.; Reed, R.R. Golf: An olfactory neuron specific-G protein involved in odorant signal transduction. Science 1989, 244, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Breer, H.; Boekhoff, I.; Tareilus, E. Rapid kinetics of second messenger formation in olfactory transduction. Nature 1990, 345, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Jovancevic, N.; Dendorfer, A.; Matzkies, M.; Kovarova, M.; Heckmann, J.C.; Osterloh, M.; Boehm, M.; Weber, L.; Nguemo, F.; Semmler, J.; et al. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res. Cardiol. 2017, 112, 13. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, B.; Schlimm, M.; Wojcik, S.; Philippou, S.; Maßberg, D.; Jansen, F.; Scholz, P.; Luebbert, H.; Ubrig, B.; Osterloh, S.; et al. Olfactory signaling components and olfactory receptors are expressed in tubule cells of the human kidney. Arch. Biochem. Biophys. 2016, 610, 8–15. [Google Scholar] [CrossRef]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J.; et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef]
- Dahmen, N.; Wang, H.L.; Margolis, F.L. Expression of olfactory receptors in Xenopus oocytes. J. Neurochem. 1992, 58, 1176–1179. [Google Scholar] [CrossRef]
- Walensky, L.D.; Roskams, A.J.; Lefkowitz, R.J.; Snyder, S.H.; Ronnett, G.V. Odorant receptors and desensitization proteins colocalize in mammalian sperm. Mol. Med. 1995, 1, 130–141. [Google Scholar] [CrossRef]
- González-Brusi, L.; Hamze, J.G.; Lamas-Toranzo, I.; Jiménez-Movilla, M.; Bermejo-Álvarez, P. The Sperm Olfactory Receptor OLFR601 is Dispensable for Mouse Fertilization. Front. Cell. Dev. Biol. 2022, 10, 854115. [Google Scholar] [CrossRef]
- Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.; Osthold, S.; Veitinger, S.; Becker, C.; Brockmeyer, N.H.; Muschol, M.; Wennemuth, G.; et al. Characterization of the Olfactory Receptors Expressed in Human Spermatozoa. Front. Mol. Biosci. 2015, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Makeyeva, Y.; Nicol, C.; Ledger, W.L.; Ryugo, D.K. Immunocytochemical Localization of Olfactory-signaling Molecules in Human and Rat Spermatozoa. J. Histochem. Cytochem. 2020, 68, 491–513. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Colussi, C.; Grande, G.; Vincenzoni, F.; Pierconti, F.; Mancini, F.; Baroni, S.; Castagnola, M.; Marana, R.; Pontecorvi, A. Olfactory Receptors in Semen and in the Male Tract: From Proteome to Proteins. Front. Endocrinol. 2017, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef]
- Kirton, K.T.; Hafs, H.D. Sperm capacitation by uterine fluid or beta-amylase in vitro. Science 1965, 150, 618–619. [Google Scholar] [CrossRef]
- Oh, S.A.; Park, Y.J.; You, Y.A.; Mohamed, E.A.; Pang, M.G. Capacitation status of stored boar spermatozoa is related to litter size of sows. Anim. Reprod. Sci. 2010, 121, 131–138. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; You, Y.A.; Pang, M.G. Improving litter size by boar spermatozoa: Application of combined H33258/CTC staining in field trial with artificial insemination. Andrology 2015, 3, 552–557. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Volker, G.; Brandt, H.; Töpfer-Petersen, E.; Waberski, D. Functional significance of responsiveness to capacitating conditions in boar spermatozoa. Theriogenology 2005, 64, 1766–1782. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhou, C.X.; Shi, Q.X.; Yuan, Y.Y.; Yu, M.K.; Ajonuma, L.C.; Ho, L.S.; Lo, P.S.; Tsang, L.L.; Liu, Y.; et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat. Cell Biol. 2003, 5, 902–906. [Google Scholar] [CrossRef]
- Davis, B.K. Timing of fertilization in mammals: Sperm cholesterol/phospholipid ratio as a determinant of the capacitation interval. Proc. Natl. Acad. Sci. USA 1981, 78, 7560–7564. [Google Scholar] [CrossRef] [PubMed]
- Working, P.K.; Meizel, S. Correlation of increased intraacrosomal pH with the hamster sperm acrosome reaction. J. Exp. Zool. 1983, 227, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shi, B.; Chou, K.C.; Oswalt, M.D.; Haug, A. Changes in intracellular calcium of porcine sperm during in vitro incubation with seminal plasma and a capacitating medium. Biochem. Biophys. Res. Commun. 1990, 172, 47–53. [Google Scholar] [CrossRef]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef]
- Leyton, L.; Saling, P. 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell 1989, 57, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Dapino, D.G.; Marini, P.E.; Cabada, M.O. Effect of heparin on in vitro capacitation of boar sperm. Biol. Res. 2006, 39, 631–639. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Lybaert, P.; Puga-Molina, L.C.; Santi, C.M. Conserved Mechanism of Bicarbonate-Induced Sensitization of CatSper Channels in Human and Mouse Sperm. Front. Cell. Dev. Biol. 2021, 9, 733653. [Google Scholar] [CrossRef]
- Bedu-Addo, K.; Lefièvre, L.; Moseley, F.L.; Barratt, C.L.; Publicover, S.J. Bicarbonate and bovine serum albumin reversibly ‘switch’ capacitation-induced events in human spermatozoa. Mol. Hum. Reprod. 2005, 11, 683–691. [Google Scholar] [CrossRef]
- Tardif, S.; Dubé, C.; Bailey, J.L. Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol. Reprod. 2003, 68, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Aspects Med. 2013, 34, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Galantino-Homer, H.L.; Althouse, G.C. Current concepts of molecular events during bovine and porcine spermatozoa capacitation. Arch. Androl. 2007, 53, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Go, K.J.; Wolf, D.P. Albumin-mediated changes in sperm sterol content during capacitation. Biol. Reprod. 1985, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Garénaux, E.; Kanagawa, M.; Tsuchiyama, T.; Hori, K.; Kanazawa, T.; Goshima, A.; Chiba, M.; Yasue, H.; Ikeda, A.; Yamaguchi, Y.; et al. Discovery, primary, and crystal structures and capacitation-related properties of a prostate-derived heparin-binding protein WGA16 from boar sperm. J. Biol. Chem. 2015, 290, 5484–5501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.H.; Ran, M.X.; Zhang, Y.; Liang, K.; Ren, Y.N.; He, W.C.; Zhang, M.; Zhou, G.B.; Qazi, I.H.; et al. High throughput small RNA and transcriptome sequencing reveal capacitation-related microRNAs and mRNA in boar sperm. BMC Genomics 2018, 19, 736. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.X.; Zhou, Y.M.; Liang, K.; Wang, W.C.; Zhang, Y.; Zhang, M.; Yang, J.D.; Zhou, G.B.; Wu, K.; Wang, C.D.; et al. Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca). Biomolecules 2019, 9, 432. [Google Scholar] [CrossRef]
- Mathuru, A.S.; Kibat, C.; Cheong, W.F.; Shui, G.; Wenk, M.R.; Friedrich, R.W.; Jesuthasan, S. Chondroitin fragments are odorants that trigger fear behavior in fish. Curr. Biol. 2012, 22, 538–544. [Google Scholar] [CrossRef]
- Clarris, H.J.; Rauch, U.; Key, B. Dynamic spatiotemporal expression patterns of neurocan and phosphacan indicate diverse roles in the developing and adult mouse olfactory system. J. Comp. Neurol. 2000, 423, 99–111. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Boyer-Boiteau, A.; Holbrook, E.H.; Jang, W.; Hahn, C.G.; Arnold, S.E.; Berretta, S. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr. Res. 2013, 150, 366–372. [Google Scholar] [CrossRef]
- Huang, T.W.; Li, S.T.; Wang, Y.H.; Young, T.H. Regulation of chitosan-mediated differentiation of human olfactory receptor neurons by insulin-like growth factor binding protein-2. Acta Biomater. 2019, 97, 399–408. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sugahara, K.; Faissner, A. Chondroitin sulfate "wobble motifs" modulate maintenance and differentiation of neural stem cells and their progeny. J. Biol. Chem. 2012, 287, 2935–2942. [Google Scholar] [CrossRef]
- Kim, N.H.; Day, B.N.; Lim, J.G.; Lee, H.T.; Chung, K.S. Effects of oviductal fluid and heparin on fertility and characteristics of porcine spermatozoa. Zygote 1997, 5, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Grandone, E.; Rezoagli, E.; Ageno, W. Efficacy of low molecular weight heparin in patients undergoing in vitro fertilization or intracytoplasmic sperm injection. J. Thromb. Haemost. 2011, 9, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Sekhavati, M.H.; Shadanloo, F.; Hosseini, M.S.; Tahmoorespur, M.; Nasiri, M.R.; Hajian, M.; Nasr-Esfahani, M.H. Improved bovine ICSI outcomes by sperm selected after combined heparin-glutathione treatment. Cell Reprogram. 2012, 14, 295–304. [Google Scholar] [CrossRef]
- Sekharan, S.; Ertem, M.Z.; Zhuang, H.; Block, E.; Matsunami, H.; Zhang, R.; Wei, J.N.; Pan, Y.; Batista, V.S. QM/MM model of the mouse olfactory receptor MOR244-3 validated by site-directed mutagenesis experiments. Biophys. J. 2014, 107, L5–Ll8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Zhang, Y.; Block, E.; Buehl, M.; Corr, M.J.; Cormanich, R.A.; Gundala, S.; Matsunami, H.; O’Hagan, D.; Ozbil, M.; et al. Molecular mechanism of activation of human musk receptors OR5AN1 and OR1A1 by (R)-muscone and diverse other musk-smelling compounds. Proc. Natl. Acad. Sci. USA 2018, 115, E3950–E3958. [Google Scholar] [CrossRef]
- Geithe, C.; Protze, J.; Kreuchwig, F.; Krause, G.; Krautwurst, D. Structural determinants of a conserved enantiomer-selective carvone binding pocket in the human odorant receptor OR1A1. Cell. Mol. Life Sci. 2017, 74, 4209–4229. [Google Scholar] [CrossRef] [PubMed]
- Liberda, J.; Manásková, P.; Prelovská, L.; Tichá, M.; Jonáková, V. Saccharide-mediated interactions of boar sperm surface proteins with components of the porcine oviduct. J. Reprod. Immunol. 2006, 71, 112–125. [Google Scholar] [CrossRef]
- Molina, L.C.P.; Pinto, N.A.; Torres, N.I.; González-Cota, A.L.; Luque, G.M.; Balestrini, P.A.; Romarowski, A.; Krapf, D.; Santi, C.M.; Treviño, C.L.; et al. CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC. J. Biol. Chem. 2018, 293, 9924–9936. [Google Scholar] [CrossRef]
- Ren, D.; Xia, J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology 2010, 25, 165–175. [Google Scholar] [CrossRef]
- Chen, W.Y.; Xu, W.M.; Chen, Z.H.; Ni, Y.; Yuan, Y.Y.; Zhou, S.C.; Zhou, W.W.; Tsang, L.L.; Chung, Y.W.; Höglund, P.; et al. Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: Involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR. Biol. Reprod. 2009, 80, 115–123. [Google Scholar] [CrossRef]
- Darszon, A.; Acevedo, J.J.; Galindo, B.E.; Hernández-González, E.O.; Nishigaki, T.; Treviño, C.L.; Wood, C.; Beltrán, C. Sperm channel diversity and functional multiplicity. Reproduction 2006, 131, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell. Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Galantino-Homer, H.L.; Florman, H.M.; Storey, B.T.; Dobrinski, I.; Kopf, G.S. Bovine sperm capacitation: Assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol. Reprod. Dev. 2004, 67, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Schwane, K.; Riffell, J.A.; Barbour, J.; Zimmer, R.K.; Neuhaus, E.M.; Hatt, H. Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J. Biol. Chem. 2004, 279, 40194–40203. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell. Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef]
- Hartmann, C.; Triller, A.; Spehr, M.; Dittrich, R.; Hatt, H.; Buettner, A. Sperm-Activating Odorous Substances in Human Follicular Fluid and Vaginal Secretion: Identification by Gas Chromatography-Olfactometry and Ca(2+) Imaging. Chempluschem 2013, 78, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Müller, A.; Kaupp, U.B.; Strünker, T. The CatSper channel: A polymodal chemosensor in human sperm. Embo J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Hong, C.Y.; Chiang, B.N.; Turner, P. Calcium ion is the key regulator of human sperm function. Lancet 1984, 2, 1449–1451. [Google Scholar] [CrossRef]
- Scott, A.M.; Zhang, Z.; Jia, L.; Li, K.; Zhang, Q.; Dexheimer, T.; Ellsworth, E.; Ren, J.; Chung-Davidson, Y.W.; Zu, Y.; et al. Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biol. 2019, 17, e3000332. [Google Scholar] [CrossRef]
- Saito, H.; Chi, Q.; Zhuang, H.; Matsunami, H.; Mainland, J.D. Odor coding by a Mammalian receptor repertoire. Sci. Signal. 2009, 2, ra9. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Block, E.; Li, Z.; Connelly, T.; Zhang, J.; Huang, Z.; Su, X.; Pan, Y.; Wu, L.; Chi, Q.; et al. Crucial role of copper in detection of metal-coordinating odorants. Proc. Natl. Acad. Sci. USA 2012, 109, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, J.A.; Del Mármol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Koo, J. Odorant G protein-coupled receptors as potential therapeutic targets for adult diffuse gliomas: A systematic analysis and review. BMB Rep. 2021, 54, 601–607. [Google Scholar] [CrossRef]
- Coy, P.; Cánovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; Matás, C.; Avilés, M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef]
- Mendoza, C.; Carreras, A.; Moos, J.; Tesarik, J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J. Reprod. Fertil. 1992, 95, 755–763. [Google Scholar] [CrossRef]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef]
- Biegert, A.; Mayer, C.; Remmert, M.; Söding, J.; Lupas, A.N. The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res. 2006, 34, W335–W339. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Molecular dynamics simulations of the unfolding of an alpha-helical analogue of ribonuclease A S-peptide in water. Biochemistry 1991, 30, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.A.; York, D.M.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.v.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Martoňák, R.; Laio, A.; Parrinello, M. Predicting crystal structures: The Parrinello-Rahman method revisited. Phys. Rev. Lett. 2003, 90, 075503. [Google Scholar] [CrossRef]


| Item | Energy (kcal/mol) | Delta |
|---|---|---|
| ΔGvdw (kcal/mol) | −35.293 | 3.934 |
| ΔGele (kcal/mol) | −318.485 | 41.848 |
| ΔGPB (kcal/mol) | 318.227 | 37.706 |
| ΔGnp (kcal/mol) | −5.193 | 0.349 |
| ΔGbind (kcal/mol) | −40.744 | 9.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Wang, Y.; Ali, M.A.; Qin, Z.; Guo, Z.; Zhang, Y.; Zhang, M.; Zhou, G.; Yang, J.; Chen, L.; et al. Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin. Int. J. Mol. Sci. 2023, 24, 1664. https://doi.org/10.3390/ijms24021664
Yuan X, Wang Y, Ali MA, Qin Z, Guo Z, Zhang Y, Zhang M, Zhou G, Yang J, Chen L, et al. Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin. International Journal of Molecular Sciences. 2023; 24(2):1664. https://doi.org/10.3390/ijms24021664
Chicago/Turabian StyleYuan, Xiang, Yihan Wang, Malik Ahsan Ali, Ziyue Qin, Zhihua Guo, Yan Zhang, Ming Zhang, Guangbin Zhou, Jiandong Yang, Lei Chen, and et al. 2023. "Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin" International Journal of Molecular Sciences 24, no. 2: 1664. https://doi.org/10.3390/ijms24021664
APA StyleYuan, X., Wang, Y., Ali, M. A., Qin, Z., Guo, Z., Zhang, Y., Zhang, M., Zhou, G., Yang, J., Chen, L., Shen, L., Zhu, L., & Zeng, C. (2023). Odorant Receptor OR2C1 Is an Essential Modulator of Boar Sperm Capacitation by Binding with Heparin. International Journal of Molecular Sciences, 24(2), 1664. https://doi.org/10.3390/ijms24021664









