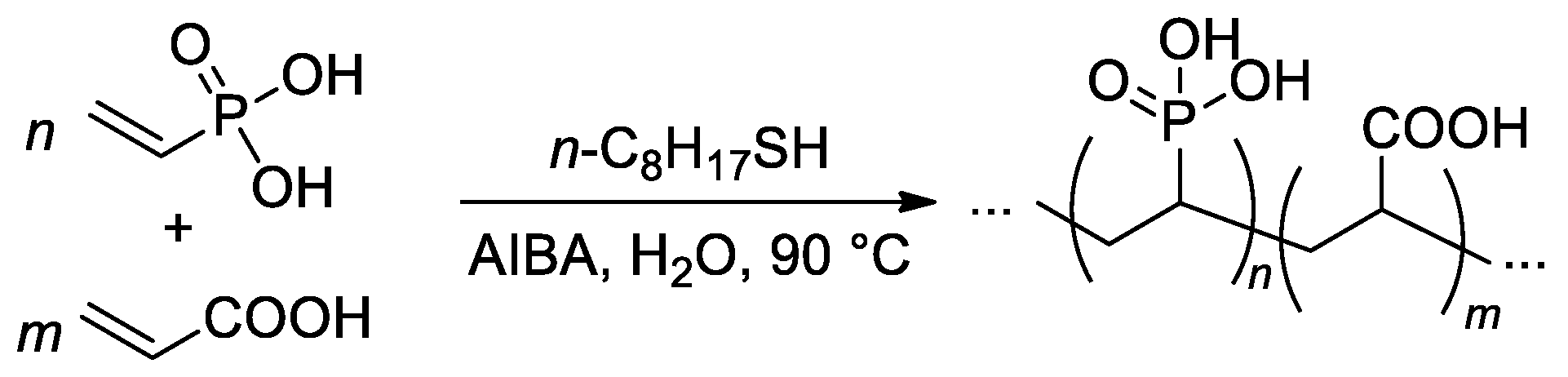Abstract
Macromolecules containing acidic fragments in side-groups—polyacids—occupy a special place among synthetic polymers. Properties and applications of polyacids are directly related to the chemical structure of macromolecules: the nature of the acidic groups, polymer backbone, and spacers between the main chain and acidic groups. The chemical nature of the phosphorus results in the diversity of acidic >P(O)OH fragments in sidechain phosphorus-containing polyacids (PCPAs) that can be derivatives of phosphoric or phosphinic acids. Sidechain PCPAs have many similarities with other polyacids. However, due to the relatively high acidity of –P(O)(OH)2 fragment, bone and mineral affinity, and biocompatibility, sidechain PCPAs have immense potential for diverse applications. Synthetic approaches to sidechain PCPAs also have their own specifics. All these issues are discussed in the present review.
1. Introduction
Polymers containing multiple acidic (–C(O)OH, S(O)2OH, –P(O)(OH)2, etc.) fragments distributed throughout the polymer backbone—polyacids, more commonly named anionic polyelectrolytes—constantly attract the researchers’ attention [1,2,3]. Enhanced hydrophilicity, proton conductivity, ability to complexation with metal ions and organic bases, and biocompatibility are occasionally shown by this type of polymer—this is not a complete list of attractive properties of polyacids that determine a variety of their applications. Phosphorus-containing polyacids (PCPAs) represent a separate group of polyanionic electrolytes due to the relatively high acidity of the –P(O)(OH)2 group, higher biocompatibility, and bone and mineral affinity of phosphates. PCPAs can be classified into main-chain PCPAs (polyphosphodiesters, discussed in Part 1 of the review) and sidechain PCPAs that represent macromolecules containing acidic phosphate or phosphonate fragments as substituents distributed throughout the polymer backbone. Sidechain PCPAs have more ‘degrees of freedom’ in polymer designs when compared with polyphosphodiesters; however, not all possible synthetic approaches to sidechain PCPAs have been realized to date.
Some issues related to chemistry [4,5,6,7,8,9,10] and applications [6,11,12,13,14] of the sidechain PCPAs have been addressed fragmentary in previously published reviews. In the present work, we tried to cover the issues of the synthesis and actual applications of sidechain PCPAs more exhaustively.
2. Design and Synthesis of Sidechain PCPAs
2.1. Synthetic Approaches to Sidechain PCPAs: An Overview
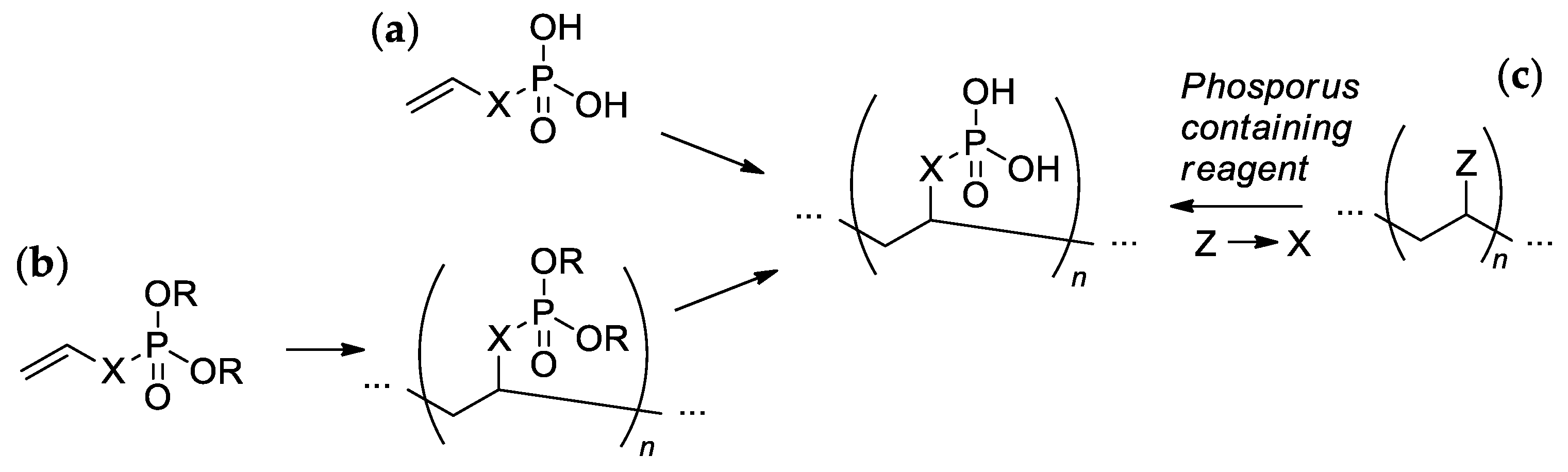
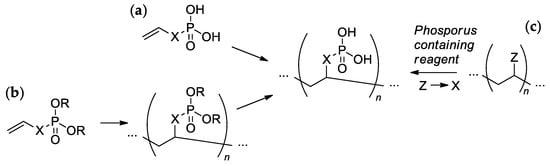
Most of the synthetic approaches to sidechain PCPAs use (co)polymerization of the phosphorus-containing vinyl monomers (Scheme 1a,b). The alternative approach is based on the post-modification of the (co)polymers (Scheme 1c). These main approaches are reviewed and discussed in more detail in Section 2.3 and Section 2.4, respectively. Several other specific methods (e.g., acyclic diene metathesis (ADMET) polycondensation and ring-opening metathesis polymerization (ROMP)) that found their application in particular cases are discussed in Section 2.5.
Scheme 1.
The common synthetic approaches to sidechain PCPAs: (a) polymerization of unsaturated phosphoric or phosphinic acids; (b) polymerization of unsaturated phosphoesters followed by hydrolysis; (c) phosphorylation of polymers.
2.2. Phosphorus Containing Vinyl Monomers
Vinyl monomers, containing phosphonate or phosphate fragments, are definitely starting compounds suitable for the synthesis of sidechain PCPAs, and that was the reason why we chose to devote a separate section to these compounds. The synthesis and chemistry of the phosphorus-containing vinyl monomers were discussed previously in the review of Macarie and Ilia [7], outlining poly(vinylphosphonic acid) and its derivatives, and in the review of David and Coll. [10], devoted to phosphonate vinyl monomers and polymers. Monomers and PCPAs containing fluoro substituents in the main polymer chain were mentioned in the review of Améduri and coll. [9]. A representative list of vinyl monomers suitable for the preparation of adhesive (co)polymers for dentistry was presented in the work of Moszner, Salz, and Zimmermann [5].
2.2.1. Vinylphosphonic Acid and Related Compounds
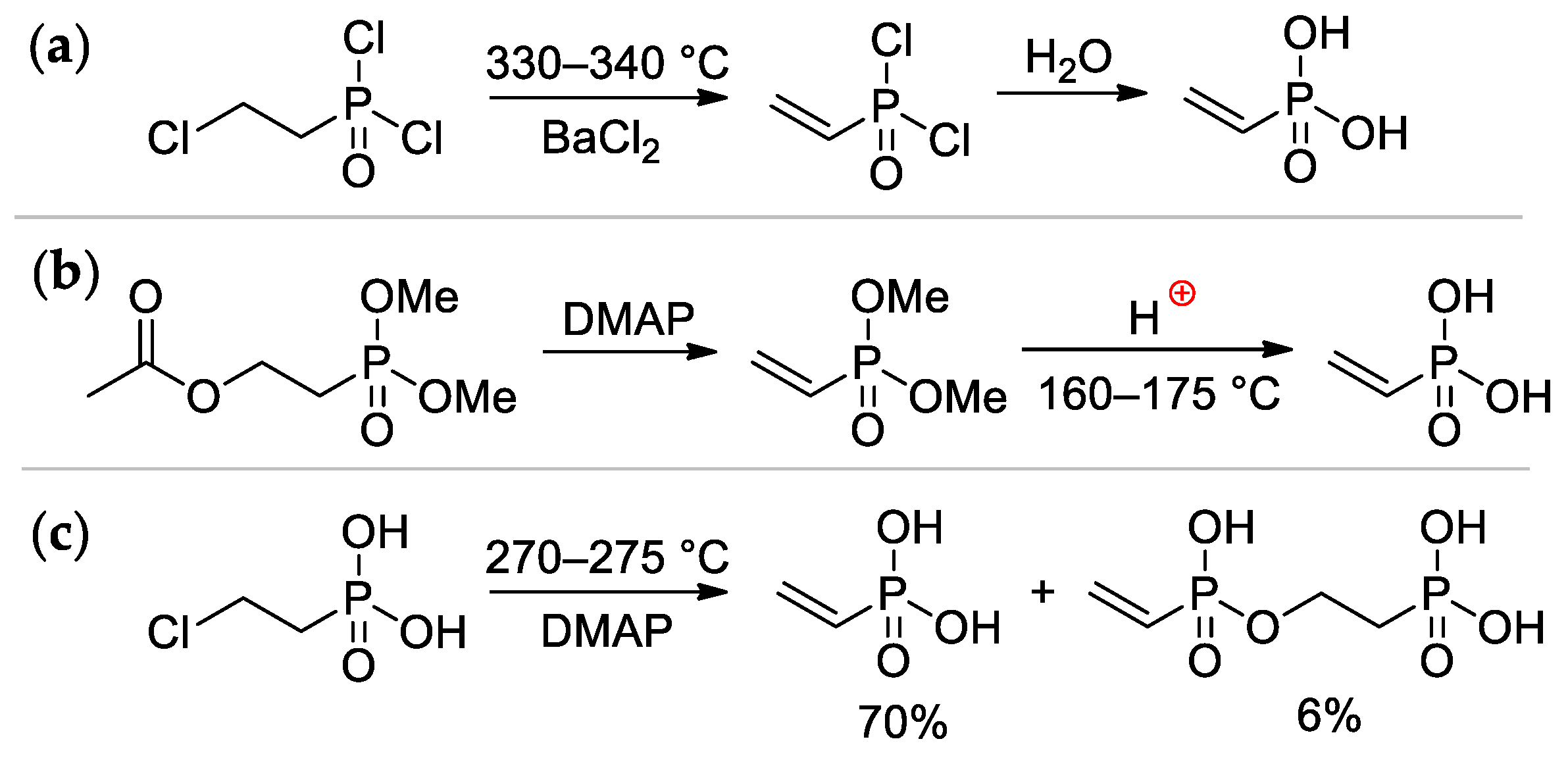
Vinylphosphonic acid (VPA) CH2=CHP(O)(OH)2 represents the simplest monomer for the synthesis of sidechain PCPAs [7]. It was obtained for the first time by Kabachnik and Medved in 1959 via dehydrochlorination of ClCH2CH2P(O)Cl2 (330–340 °C, BaCl2) followed by hydrolysis of CH2=CHP(O)Cl2 intermediate [15] (Scheme 2a); in turn, ClCH2CH2P(O)Cl2 was prepared by the reaction of PCl3 with oxirane, rearrangement of the resulting P(OCH2CH2Cl)3 to ClCH2CH2P(O)(OCH2CH2Cl)2, followed by its reaction with thionyl chloride [16]. Another method was based on the reaction of AcOCH2CH2P(O)(OMe)2 with DMAP, followed by acidic hydrolysis of the CH2=CHP(O)(OMe)2 intermediate [17]. (Scheme 2b). The cost-effective synthetic approach was based on a readily available starting compound ClCH2CH2P(O)(OH)2 (used as a growth regulator for plants), its pyrolysis was accompanied by the formation of the bis(phosphonate) side product [18] (Scheme 2c). The use of microwave irradiation in the pyrolysis of ClCH2CH2P(O)(OH)2 proved ineffective [19]. Vinylphosphonic acid is a colorless, low-melting solid (M.p. 36 °C) [20].
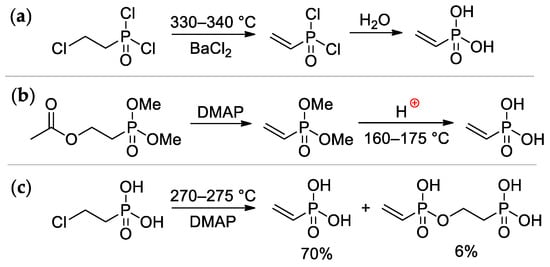
Scheme 2.
The synthesis of vinylphosphonic acid: (a) from (2-chloroethyl)dichlorophopshonate [15]; (b) from AcOCH2CH2P(O)(OMe)2 [17]; (c) based on commercially available(2-chloroethyl) phopshonic acid [18].
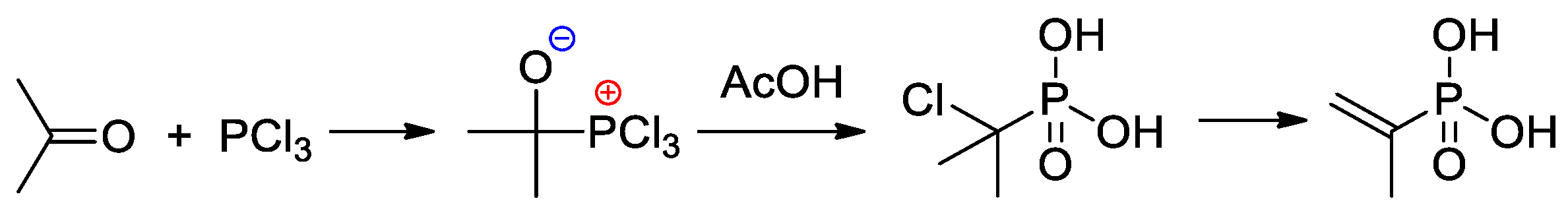
An efficient method of the synthesis of 2-propenylphosphonic acid involves the nucleophilic addition of PCl3 to acetone followed by the reaction with AcOH and subsequent dehydrochlorination [21] (Scheme 3).
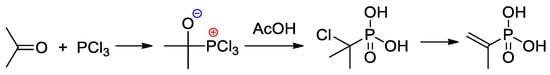
Scheme 3.
The synthesis of 2-propenylphosphonic acid [21].
2.2.2. Phosphorylated Acrylates and Related Compounds
The high reactivity of the esters of acrylic and methacrylic acids in polymerization and their greater synthetic availability in comparison with VPA and VPA esters are the reasons for researchers’ continued interest in vinyl monomers of this type. Among others, derivatives of (2-hydroxyethyl)methacrylate (HEMA) deserve special mention due to their synthetic availability.
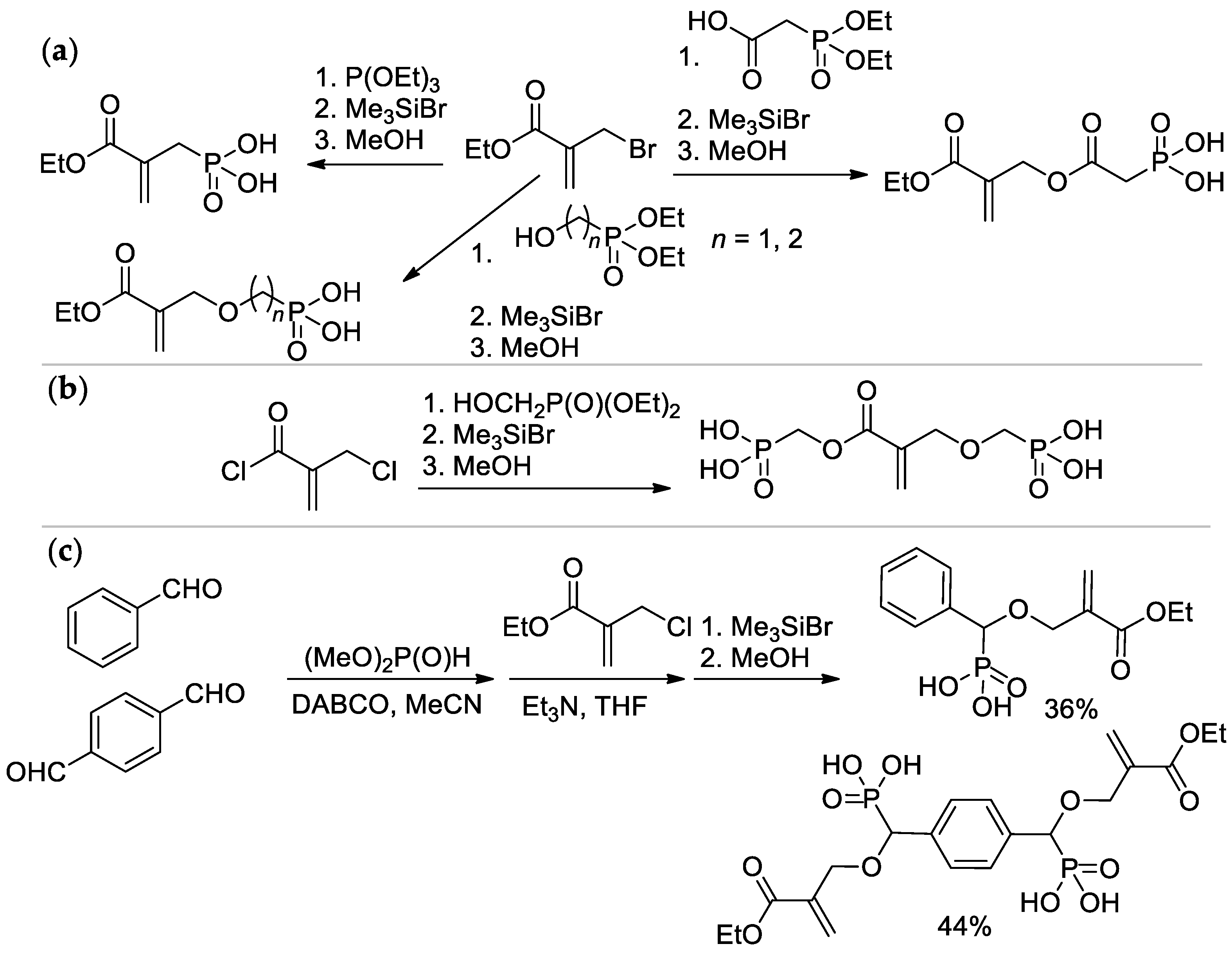
Different synthetic approaches to phosphate- and phosphonate-functionalized acrylates are presented and discussed below. Evidently, phosphate or phosphonate groups can be introduced as a part of the alkoxy fragment of (meth)acrylate (the easiest and most affordable way, Scheme 4), but functionalization of the methyl group of methacrylate also leads to prospective monomers (Scheme 5).
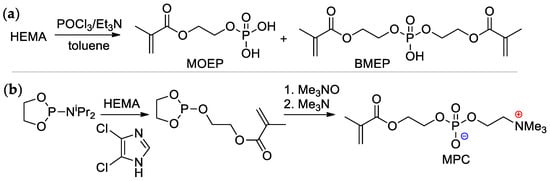
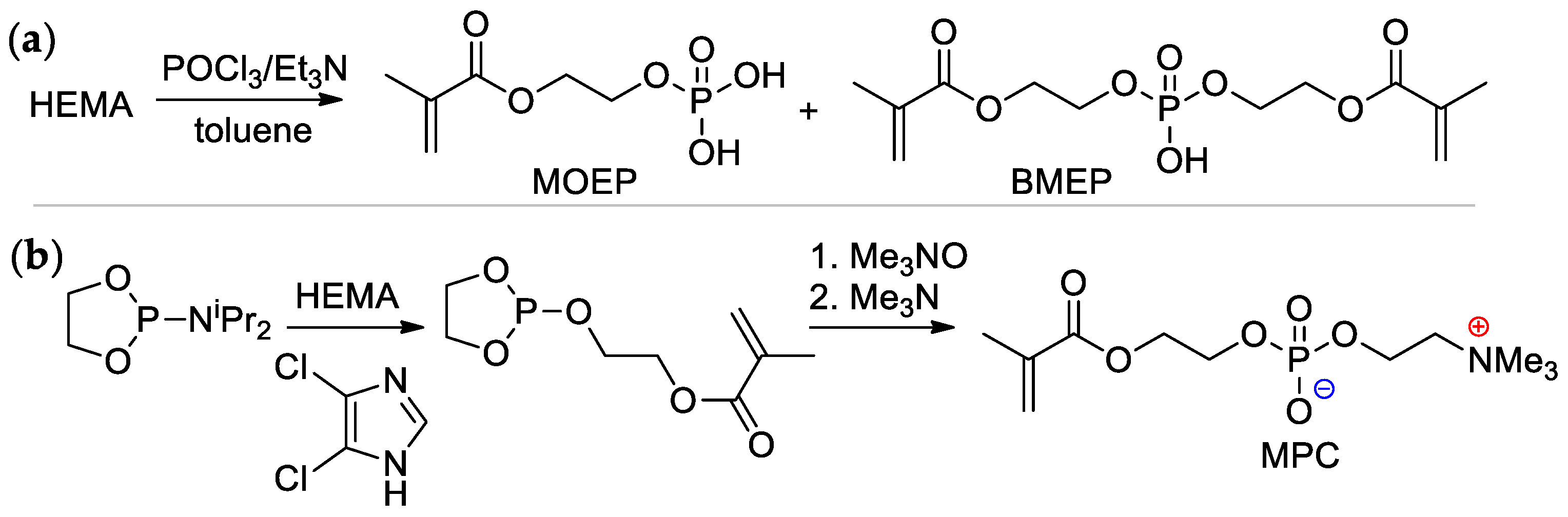
Scheme 4.
Synthesis of (meth)acrylates containing –P(O)(OH)2 group in alkoxy fragment: (a) phosphoryl chloride based approach; (b) 1,3,2-dioxaphospholan-2-amine based approach [22,23,24].
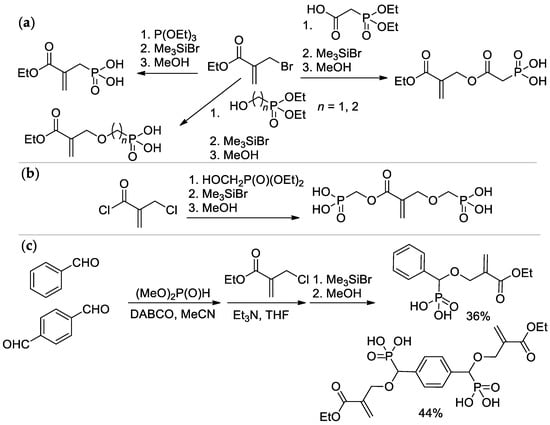
Scheme 5.
Synthesis of phosphonated methacrylates via functionalization of the methyl group: (a) based on ethyl 2-(bromomethyl)acrylate; (b) from 2-(chloromethyl)acryloyl chloride; (c) via condensation of aldehydes with dimethyl phosphonate.
2-(Methacryloyloxy)ethyl phosphate (MOEP) and bis[2-(methacryloyloxy)ethyl]phosphate (BMEP) were synthesized by the reaction of HEMA with POCl3, followed by hydrolysis, the yields of the products depended on the HEMA/POCl3 ratio [22,23] (Scheme 4a). Direct condensation of HEMA with phosphorylcholine is accompanied by polymerization, and the synthesis of methacryloyl phosphorylcholine (MPC) was carried out using the ‘phosphoramidite’ route with an overall yield of 37% [24] (Scheme 4b).
The Michaelis–Arbuzov reaction of α-bromo ethyl methacrylate with P(OEt)3 with subsequent hydrolysis resulted in the formation of (2-(ethoxycarbonyl)allyl)phosphonic acid [22,25], while its acylation by 2-(diethoxyphosphoryl)acetic acid and hydrolysis allowed to obtain (2-((2-(ethoxycarbonyl)allyl)oxy)-2-oxoethyl)phosphonic acid [26]; alkylation and hydrolysis lead to ether-linked acrylic phosphonates [27] (Scheme 5a). Interaction of 2-(chloromethyl)acryloyl chloride with hydroxymethyl diethyl phosphonate afforded bis(phosphinic acid) [25] (Scheme 5b). Synthesis of aromatic acrylic phosphonates was carried out by the Pudovik reaction, starting from benzaldehyde or terephthaldialdehyde [22,27] (Scheme 5c).
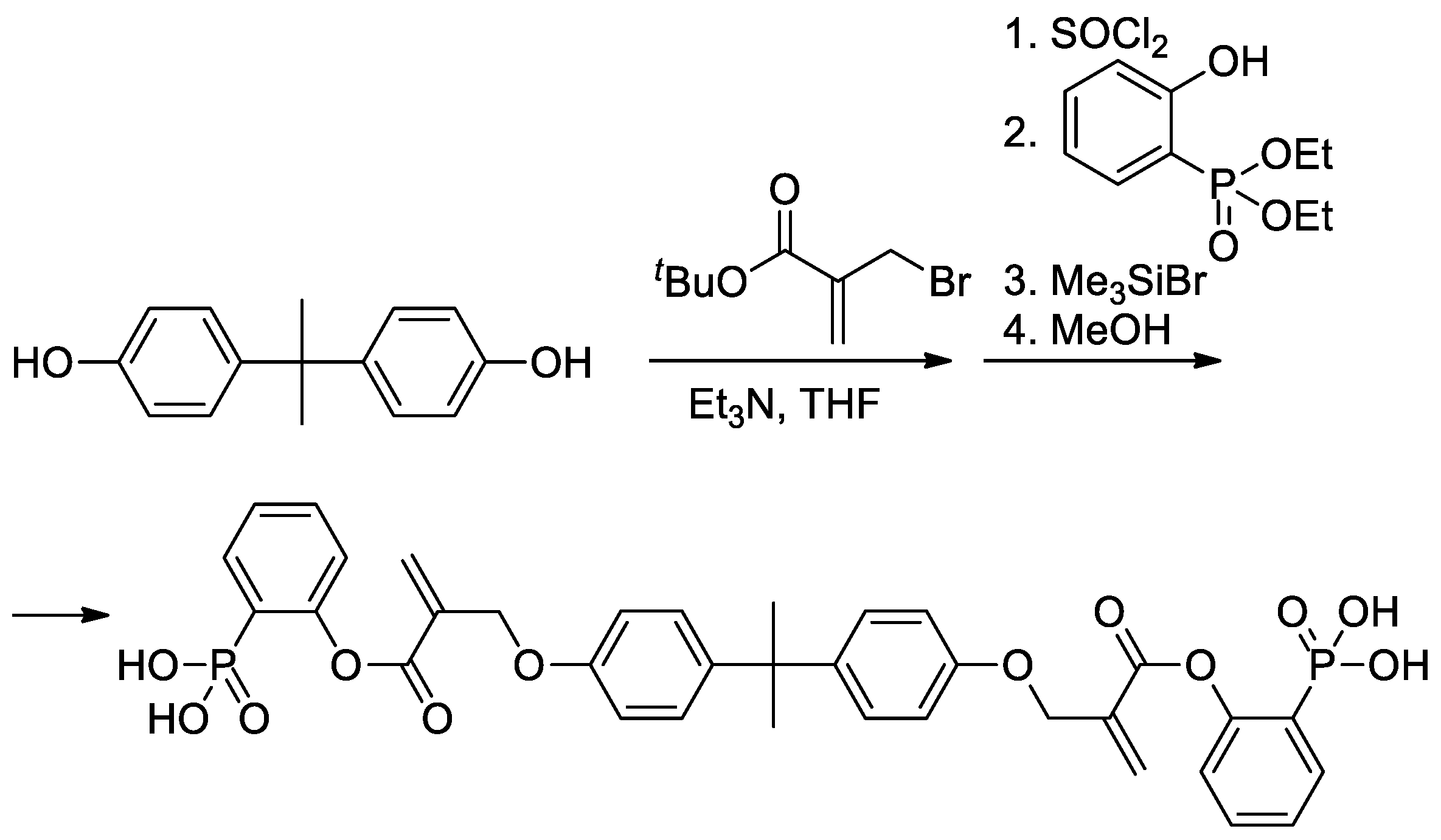
As can be seen in Scheme 5b, both alkoxy and methyl groups of methacrylate can be affected by modification. The applicability of this approach was also demonstrated by Avci and coll et al., who used the example of the synthesis of Bisphenol A-based bis(phosphonic acid) (Scheme 6) [28].
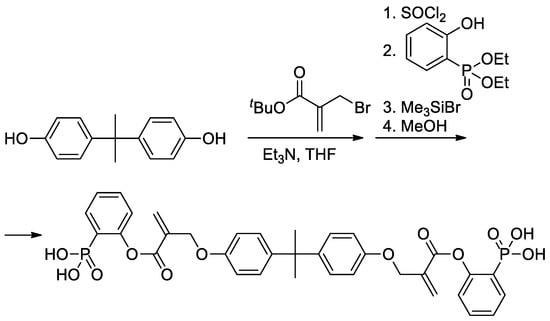
Scheme 6.
Synthesis of bis(phosphonic acid) from Bisphenol A [28].
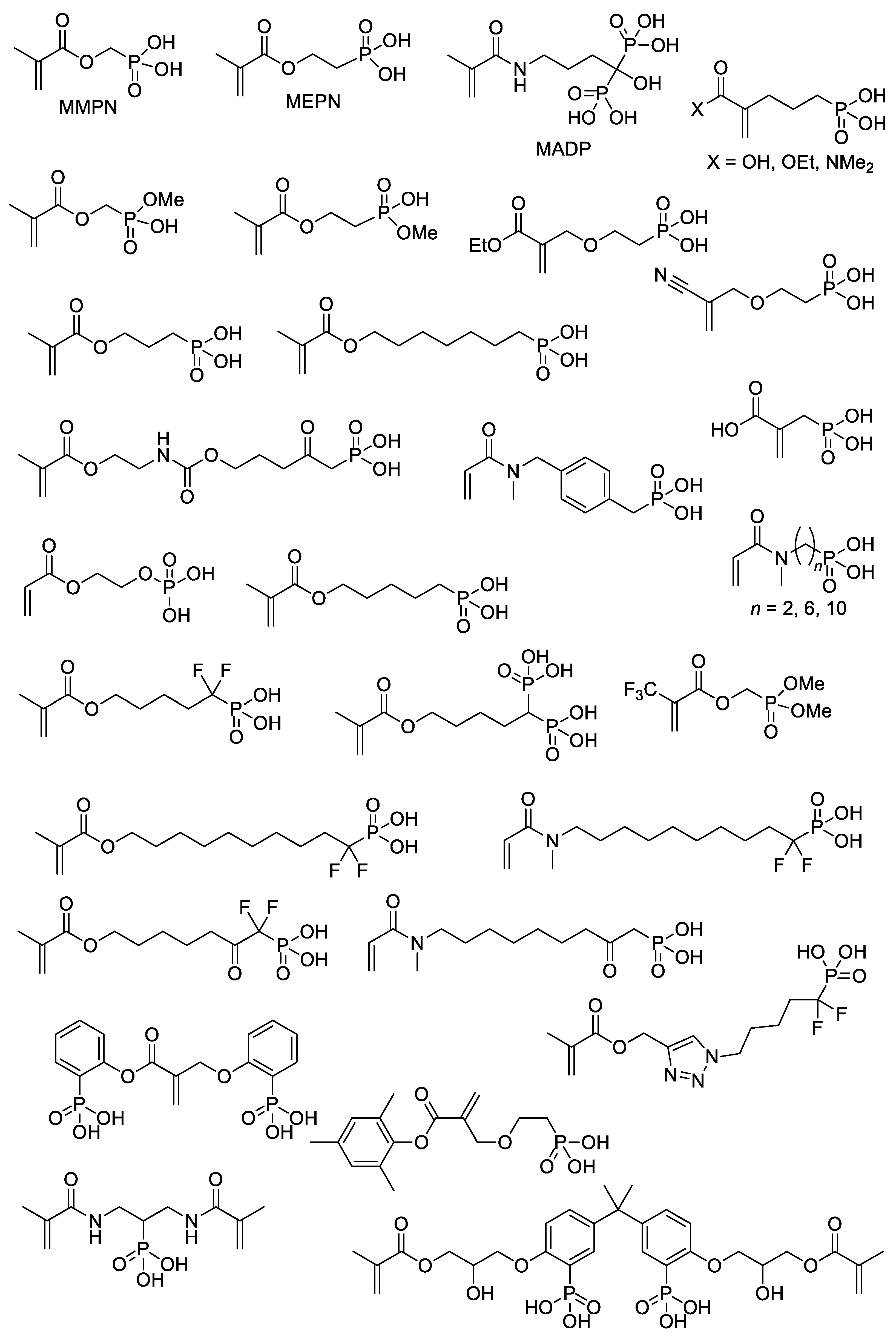
With the use of common synthetic approaches (Scheme 4 and Scheme 5) and other equally simple reactions, dozens of –P(O)(OH)2-functionalized acrylates were synthesized [22,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] (Scheme 7). An important note is that an incomplete list of acrylates, presented in Scheme 7, can be further expanded by introducing dialkyl phosphonate since deprotection with the formation of PCPAs can be easily carried out after polymerization [44,45]. However, a number of acrylate monomers containing –P(O)(OR)2 groups and polymers obtained on their basis were not studied in the synthesis of PCPAs [46,47].
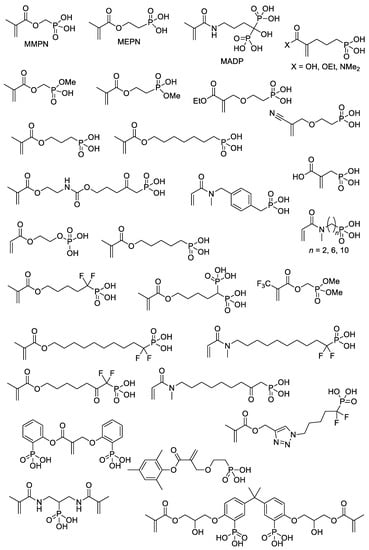
Scheme 7.
The structures of the (meth)acrylates containing –P(O)(OH)2 fragments [22,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
2.2.3. Other Phosphorus Containing Monomers
Fluorinated polymers represent a separate group of materials due to specific surface characteristics and high thermal and chemical stability. In a recent review by Améduri et al. [9], different synthetic strategies for fluorinated (co)polymers containing phosphorus groups are discussed, with the consideration of –P(O)(OH)2-functionalized monomers and polymers. However, the synthesis of such type compounds was described in a single article by Yamabe et al. who developed a complex and time-consuming method for the synthesis of perfuorovinyloxy-substituted perfuoroalkylphosphonic acid derivatives CF2=CFO(CF2)3P(O)(OMe)2 [48].
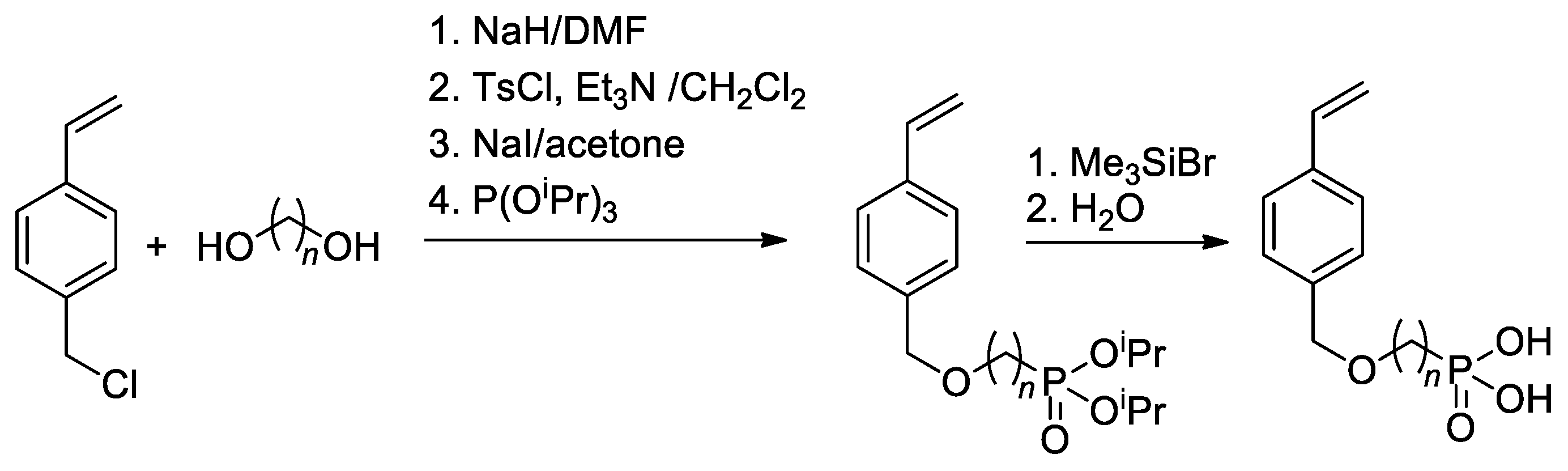
Since substituted styrenes are highly active in all types of polymerizations, the idea of using –P(O)(OH)2-functionalized styrenes in polymerization is certainly feasible. Based on 4-(chloromethyl)-styrene, Yoon and Coll. [49] have synthesized styryl phosphonic acids with different lengths of the alkanediyl spacers between the benzene ring and –P(O)(OH)2 group (Scheme 8). To introduce CH2CF2 linkage between styrene and phosphonate fragments, Alter and Hoge used the reaction of 4-(iodomethyl)styrene with LiCF2P(O)(OEt)2 [50].
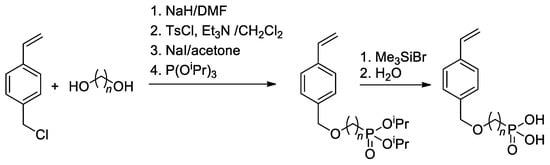
Scheme 8.
Synthesis of styrene-derivative monomers containing the phosphonic acid [49].
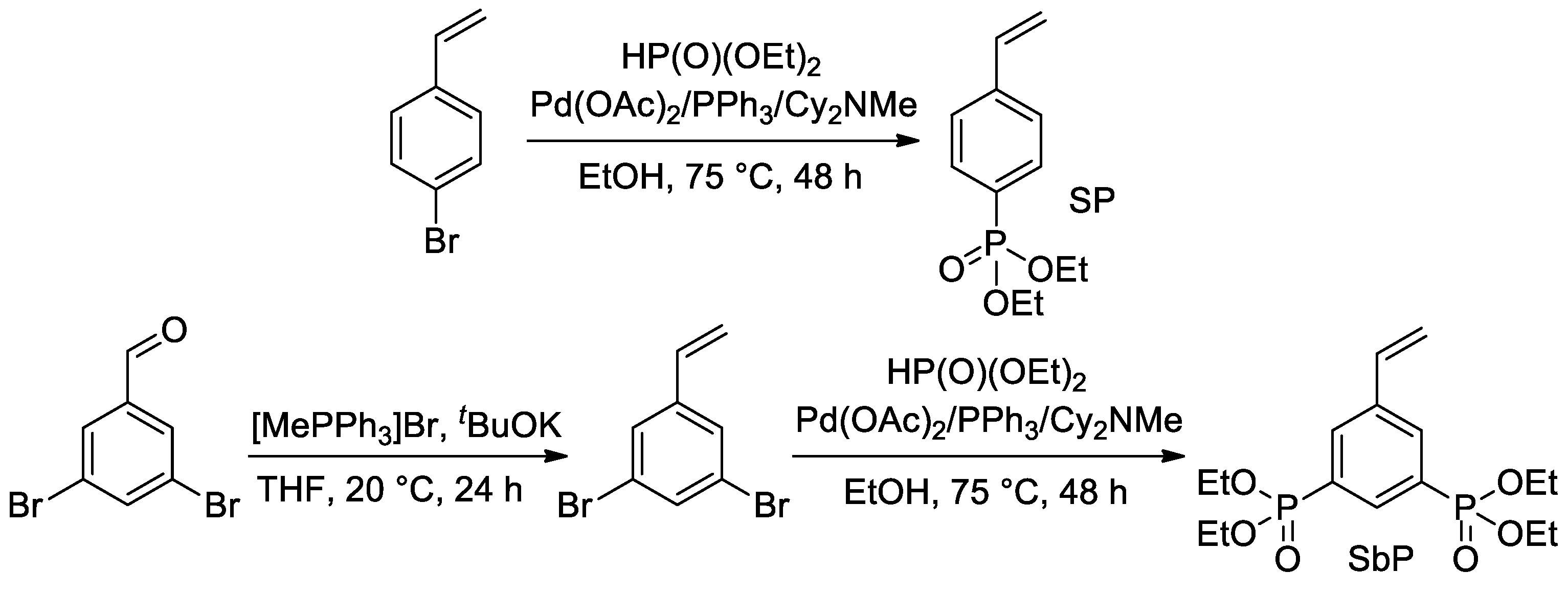
Later, Park et al. proposed the use of Pd-catalyzed cross-coupling for the direct introduction of one or two –P(O)(OEt)2 fragments into an aromatic ring with a formation of styryl phosphonate and bis(phosphonate) (SP and SbP, respectively) [51] (Scheme 9). Hydrolytic cleavage of the diethyl phosphonate fragments was planned and realized after polymerization (see Section 2.3.4).
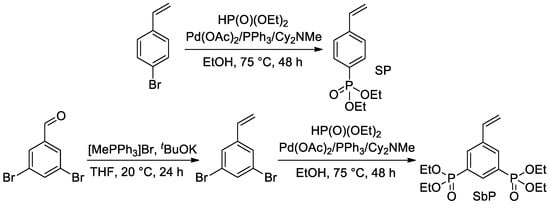
Scheme 9.
Synthesis of styrenes with one or two –P(O)(OEt)2 substituents [51].
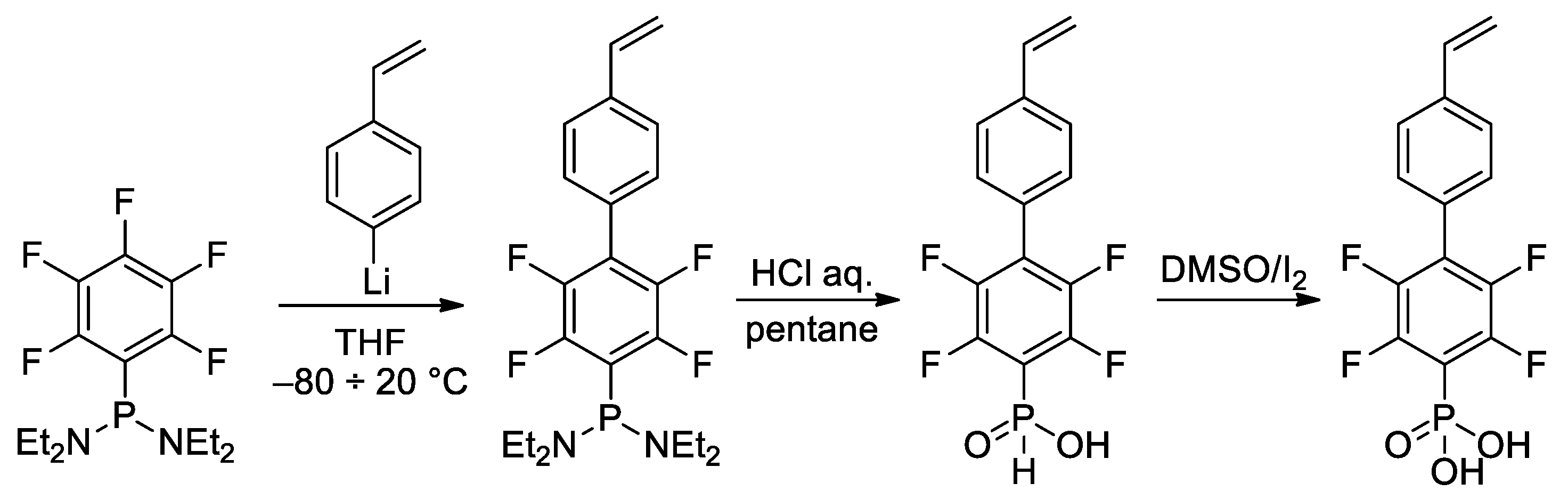
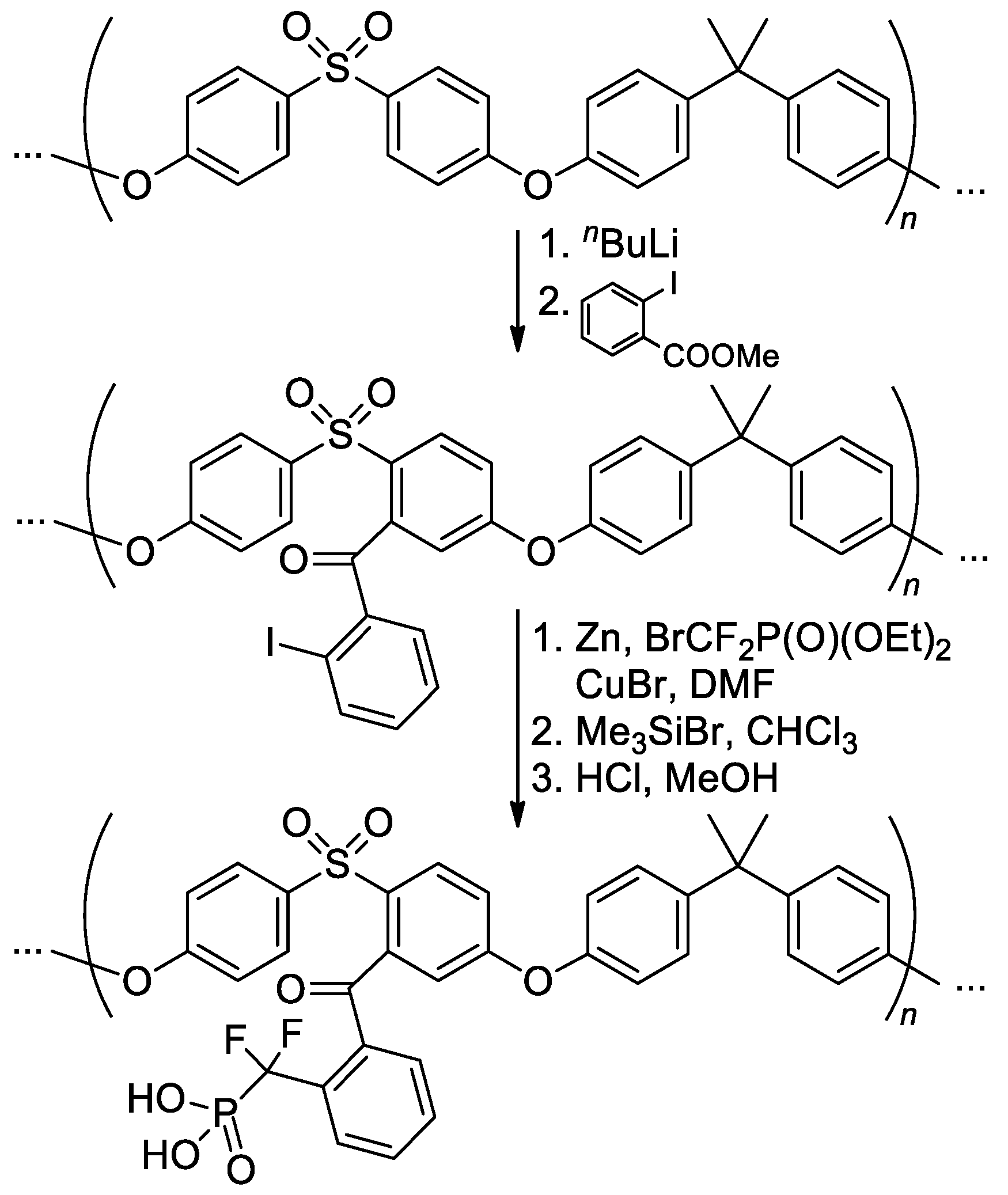
In the study of Hoge et al. [52], the idea of the use of styrene-derived phosphonates was further developed. Considering the high para-selectivity of nucleophilic substitution in N,N,N′,N′-tetraethyl-1-(perfluorophenyl)phosphinediamine, they synthesized vinyl monomer having 1,4-perfluorophenylene linkage between styryl and –P(O)(OH)2 fragments (Scheme 10). Note that intermediate phosphinic acid is virtually the only phosphorus-containing ‘monomer’ with a proven molecular structure (Figure 1).
Scheme 10.
Synthesis of phosphonic acid with 1,4-perfluorophenylene linkage between styryl and –P(O)(OH)2 fragments [52].
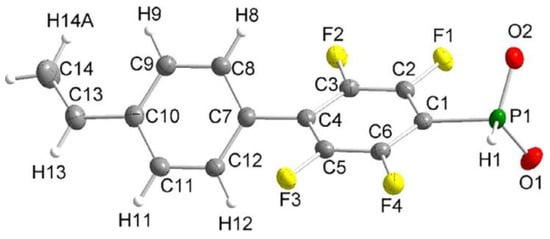
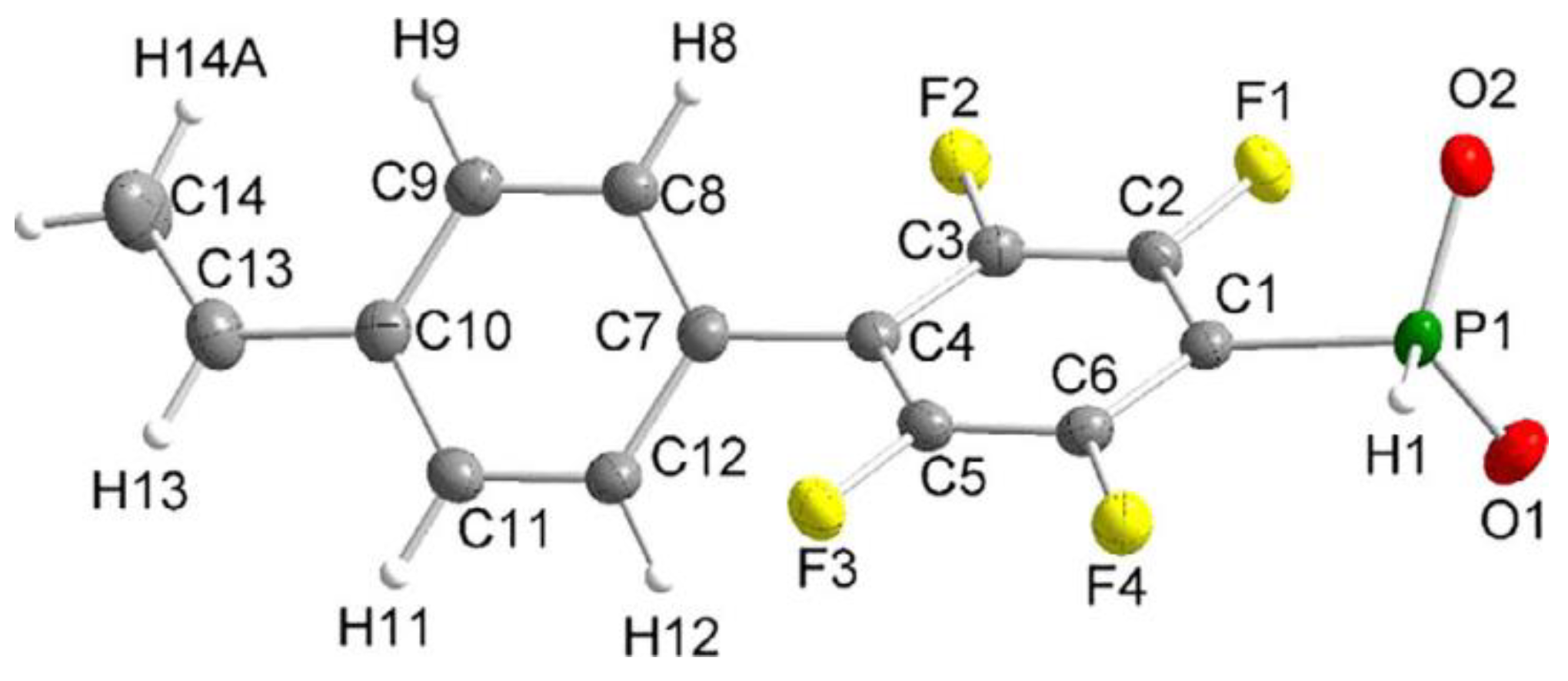
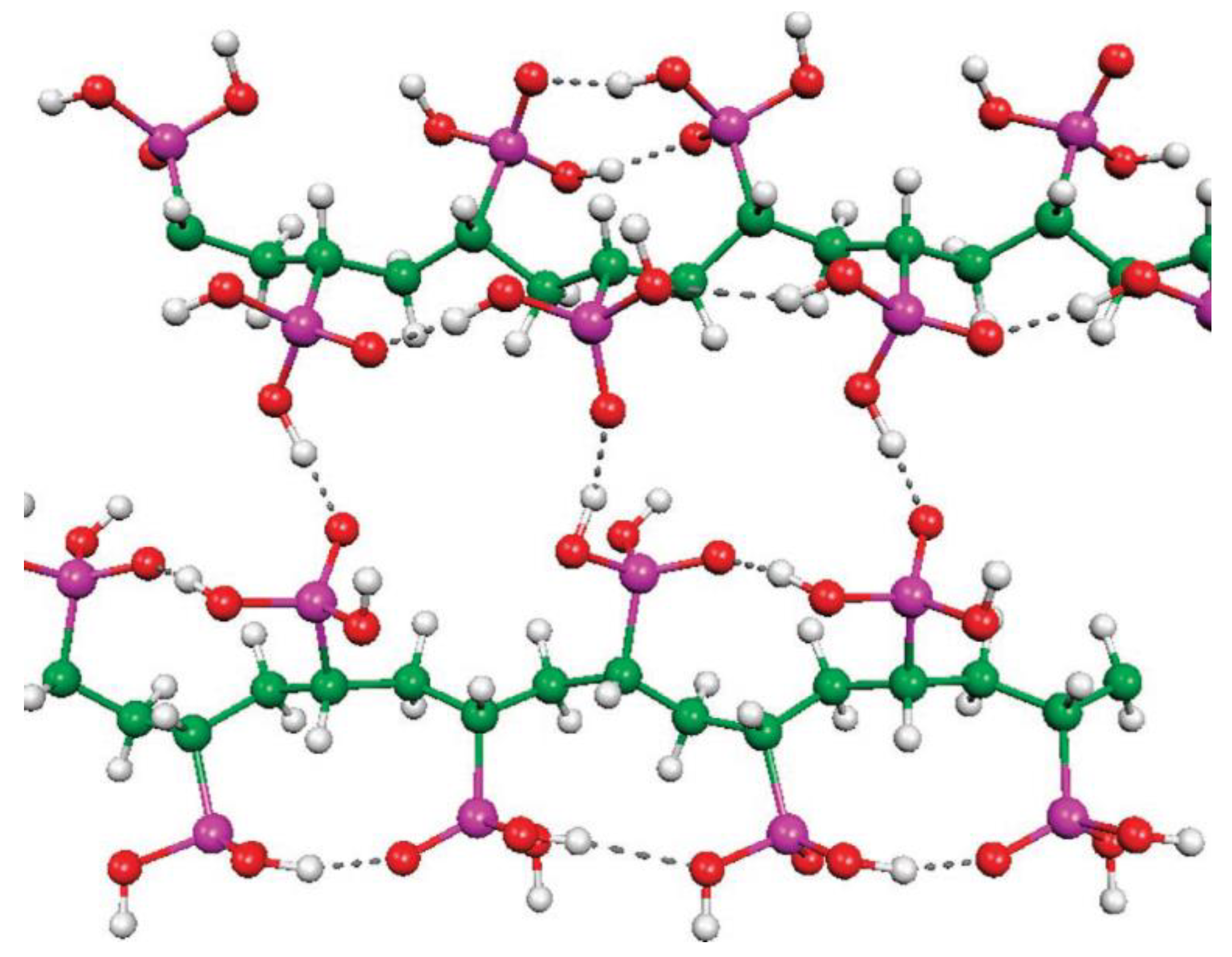
Figure 1.
Molecular structure of the anion of the diethyl ammonium salt of phosphinic acid with 1,4-perfluorophenylene linkage between styryl and –PO2H− fragments (thermal ellipsoids are set to 50% probability; cation omitted.). Reprinted with permission from [52]. Copyright (2020) Wiley-VCH GmbH.
Dialkyl(alkenyl)phosphates, readily available by the reaction of enolates with ClP(O)(OR)2 [53,54], can also be considered as monomers for the synthesis of sidechain PCPAs; however, their formation is complicated by the rearrangement to β-ketophosphonates [55,56].
2.3. Homopolymerization and Copolymerization of the Phosphorus Containing Vinyl Monomers
2.3.1. Homopolymerization of VPA, Its Derivatives and Analogs
Since poly(vinylphosphonic acid) (PVPA) continues to attract the researchers’ attention based on new fields of PVPA can be synthesized via radical polymerization of vinylphosphonic acid in the presence of initiators and optionally chain transfer agents (CTAs) with different functionality and reactivity.
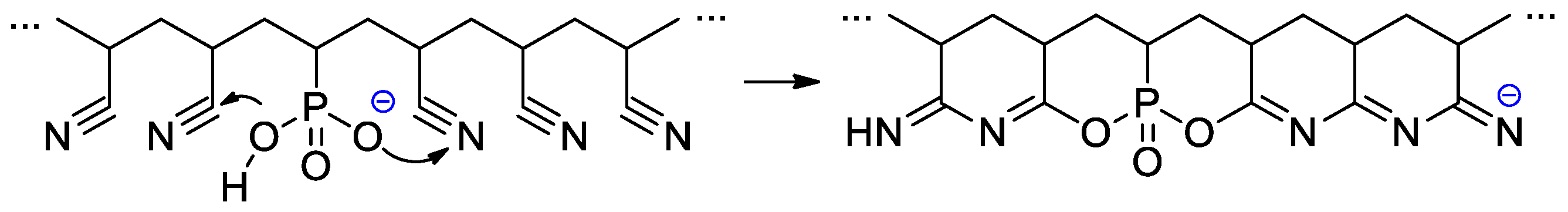
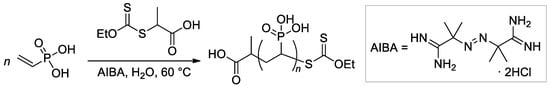
Wegner et al. [57] conducted a series of experiments on VPA polymerization in aqueous media using α,α′-azodiisobutyramidine dihydrochloride (AIBA) as an initiator. In contrast to free-radical polymerization of other polar vinyl monomers, polymerization of VPA occurred with low regioselectivity, and a probability of head-to-head and tail-to-tail links over regular head-to-tail links of 23.5% was obtained. When using AIBN as an initiator and ethyl acetate as a solvent, PVPA containing up to 17% head-to-head fragments was obtained [58]. To explain the low regioselectivity of VPA polymerization, Wegner et al. [57] suggested that the process may occur according to two different mechanisms, i.e., a classical head-to-tail radical polymerization and a cyclopolymerization of the VPA anhydride formed in situ (Scheme 11). Further studies on VPA polymerization in Ac2O media, conducted by Voit and Coll. [59], have shown that the intermediate formation of anhydride results in an acceleration of the reaction due to higher reactivity of the VPA anhydride with a formation of cyclic side-products (Scheme 11). The formation of anhydride fragments (up to 19 wt%) was also detected when the polymerization of VPA was conducted in ethyl acetate with the use of a benzoyl peroxide initiator [60]. AIBA-initiated homopolymerization of VPA was used in the preparation of PVPA samples suitable for composition with different inorganic compounds [61,62,63].
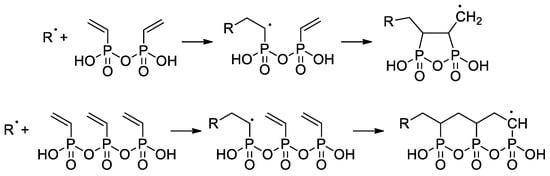
Scheme 11.
Formation of the side products during polymerization of VPA resulting from intermediate formation of the VPA anhydrides [57,59].
The use CTAs in the radical polymerization of VPA was reported for the first time by David and Coll. [64]. Only CBrCl3 successfully served as CTA in the synthesis of telechelic VPA oligomers with yields of ~70% and DPn = 6–60. High-MW PVPA (Mn 42.2 kDa) was obtained in aqueous media at 80 °C when using a 620:1 VPA/AIBA ratio [65].
Prospective results were received when using n-C6F13I as a CTA that exhibited CF2I functionalization of the oligomer obtained. MALDI-TOF MS and NMR analysis indicated that conventional radical initiation and termination do not occur and that the oligomers were obtained from transfer reactions [66].
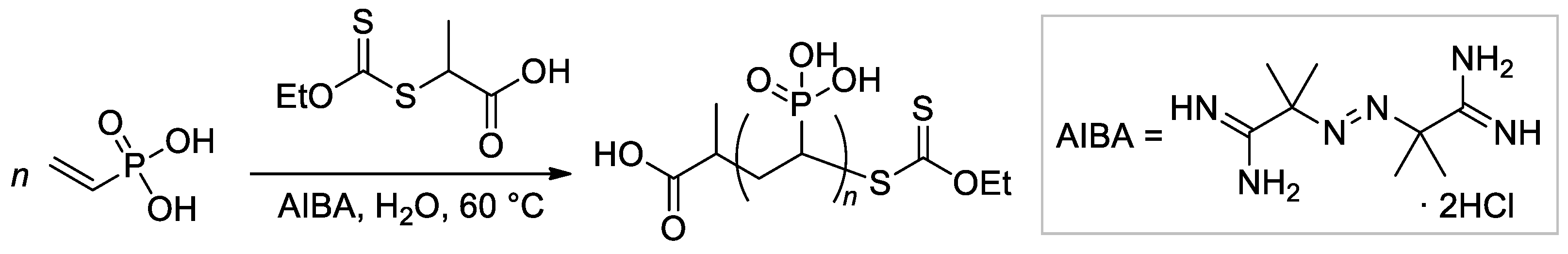
The applicability of reversible addition-fragmentation chain transfer/macromolecular design via the interchange of xanthate (RAFT/MADIX) approach to radical polymerization of VPA was studied by Destarac and Coll. [67]. They demonstrated that at 65 °C in water carboxy functionalized O-ethyl xanthate reacts rapidly and quantitatively with VPA oligoradicals to yield low-MW O-ethyl xanthate-capped PVPAs (Scheme 12), and later, reversible transfer of the xanthate terminal group leads to an increase of Mn during polymerization. AIBA was used as a water-soluble initiator of radical polymerization. A follow-up study by the same group has shown that in the presence of 0.25-0.75, molar equivalents of NaOH, the rate and the final conversion of both conventional and RAFT polymerizations were increased, and the fastest rates of polymerization were obtained in the presence of 0.5 equivalents of NaOH [68].
Scheme 12.
Aqueous RAFT/MADIX polymerisation of VPA mediated by a carboxy-functional xanthate [67].
The other possibility to obtain PVPA consists in the polymerization of vinylphosphonic acid derivatives CH2=CHP(O)Cl2 or CH2=CHP(O)(OR)2 followed by hydrolysis. As was demonstrated by Ellis and Wilson, PVPA can also be obtained by free-radical polymerization of CH2=CHP(O)Cl2 with the use of an AIBN initiator, followed by hydrolysis [69]. However, to provide acceptable mechanical characteristics of PVPA-based materials, the relatively low-MW polymer thus obtained was forcedly subjected to the treatment with cross-linking reagents [70].
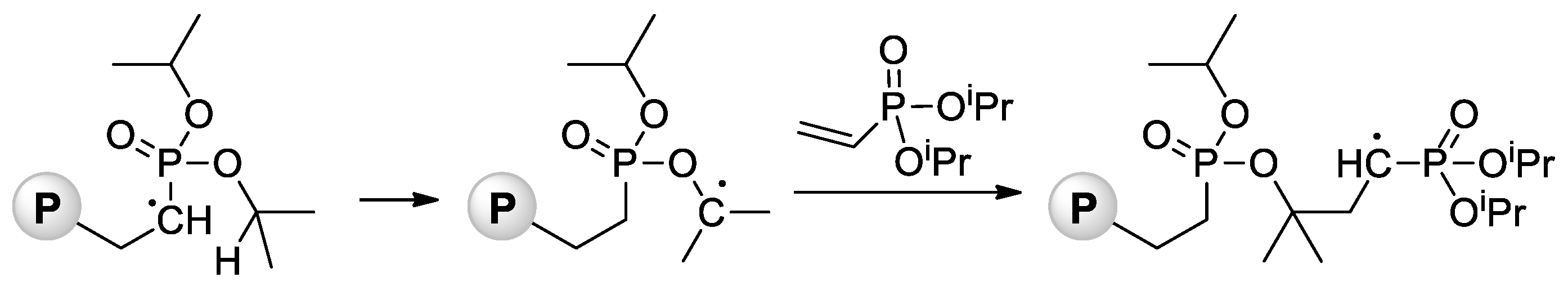
Jin and Gonsalves reported a relatively low Mn value of 8 kDa for homopolymer formed during AIBN-initiated bulk polymerization of CH2=CHP(O)(OMe)2 [71], the microstructure of the polymer was not determined. During the study of AIBA-initiated polymerization of CH2=CHP(O)(OMe)2, Wegner et al. [57] showed higher regioselectivity of this reaction in comparison with VPA homopolymerization. For the transformation of the polymer obtained into PVPA, it reacted with an excess of aq. HBr at 110 °C for 8 h. Note that radical polymerization of CH2=CHP(O)(OiPr)2 can be complicated by intramolecular hydrogen transfer from the backbone to the side chain [72] (Scheme 13).
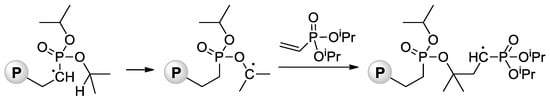
Scheme 13.
Intramolecular hydrogen transfer from the backbone to the side chain with formation of P–O–C bonds during radical polymerization of diisopropyl vinyl phosphonate (P = polymer) [72].
The study of UV-induced copolymerization of VPA with CH2=CHP(O)(OMe)2 [73] deserves to be mentioned: in the presence of 3 wt% Darocur 4265 photoinitiator (that represents a mixture of (diphenylphosphoryl)(mesityl)methanone and 2-hydroxy-2-methyl-1-phenylpropan-1-one), VPA or VPA/phosphonate mixtures were laid on PTFE plates and exposed to UV irradiation. PVPA with Mn = 27.75 kDa (ÐM = 1.23) was obtained; for copolymers, Mn values increased from 8.78 to 16.12 kDa with an increase in the VPA/CH2=CHP(O)(OMe)2 ratio from 1:1 to 4:1. ATRP was also used in the synthesis of core-shell materials with the use of oligo-(CMe2Br) substituted polyaromatics and CH2=CHP(O)(OEt)2 [74].
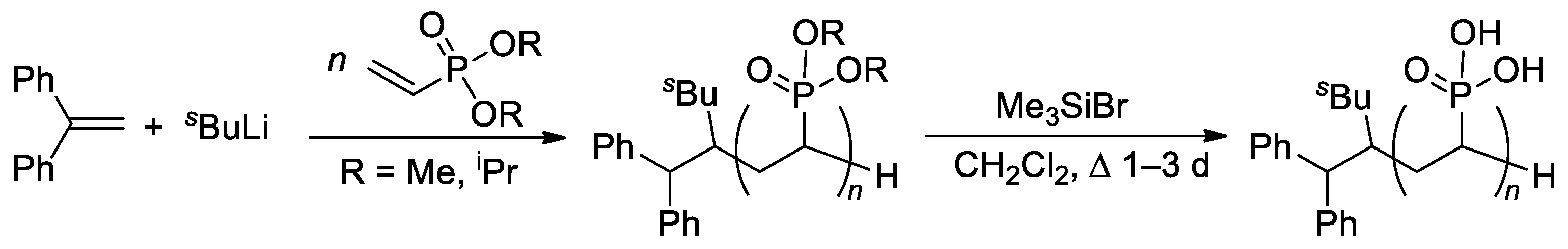
The relatively low reactivity of CH2=CHP(O)(OR)2 in free-radical polymerization has heavily conditioned the search for alternative methods of polymerization of dialkyl vinyl phosphonates. Meyer and Coll. proposed the use of sBuLi and Ph2C=CH2 as an initiator of the anionic polymerization of CH2=CHP(O)(OR)2 (R = Me, iPr) in THF [75]. High yields of the polymers were obtained when using diisopropyl vinyl phosphonate, while CH2=CHP(O)(OMe)2 gave only low yields due to the precipitation of the polymer already in low conversions. Anionic polymerization proceeded regioselectively, but with low stereoselectivity. Given that mild and quantitative hydrolysis of dialkyl phosphonates can be performed via the intermediate formation of silyl esters [76], polyesters were refluxed with Me3SiBr in CH2Cl2 to yield PVPA samples of different Mw (4.2–814 kDa) and ĐM of 2.1–3.9 (Scheme 14).
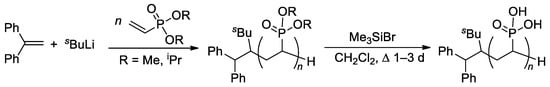
Scheme 14.
Synthesis of PVPA by anionic polymerization of dialkyl vinyl phosphonate, followed by the cleavage using Me3SiBr [75].
A similar approach to PVPA via anionic polymerization of CH2=CHP(O)(OMe)2 with subsequent hydrolysis was implemented by Takeichi et al. [77] with the use of tBuLi or tBuLi/nBu3Al (1:5 mol/mol) as an initiator at the first stage (polymerization was conducted in toluene media), and conc. HCl with 12 h of reflux at the second stage. When polymerization was initiated by tBuLi/nBu3Al instead of tBuLi alone, a threefold increase in monomer conversion was detected and, more significantly, ÐM decreased by half, from 2.65 to 1.30, and isotacticity increased. The water solutions of the PVPAs were cast on a glass plate and allowed to stand at room temperature for seven days. After drying, the PVPA (Mn = 5.0 kDa, m = 52%), prepared through the radical polymerization, became viscous liquids, whereas PVPA (Mn = 5.5 kDa, m = 67%), prepared through the anionic process, formed self-standing transparent film. In this way, the quality of the PVPA obtained at –78 °C with the use of tBuLi/nBu3Al initiator is clearly—in the proper sense of the word—illustrated by Figure 2.

Figure 2.
Photograph of a PVPA film derived from PDMVP prepared with tBuLi/nBu3Al at −78 °C. Reprinted with permission from [77]. Copyright (2010) Wiley-VCH GmbH.
Both free-radical and anionic polymerization of CH2=CHP(O)(OR)2 are usually not able to provide high monomer conversions and desirable molecular weight characteristics and microstructure of the polymer. In polyolefin chemistry, a similar problem was effectively solved by the use of coordination catalysis. However, conventional Ziegler-Natta and single-site catalysts, suitable for α-olefin polymerization, cannot be applied in the coordination polymerization of phosphonates due to the high electron donation ability of the oxygen atom of →P=O fragment. To date, only rare earth metal complexes were used successfully to polymerize dialkyl vinyl phosphonates.
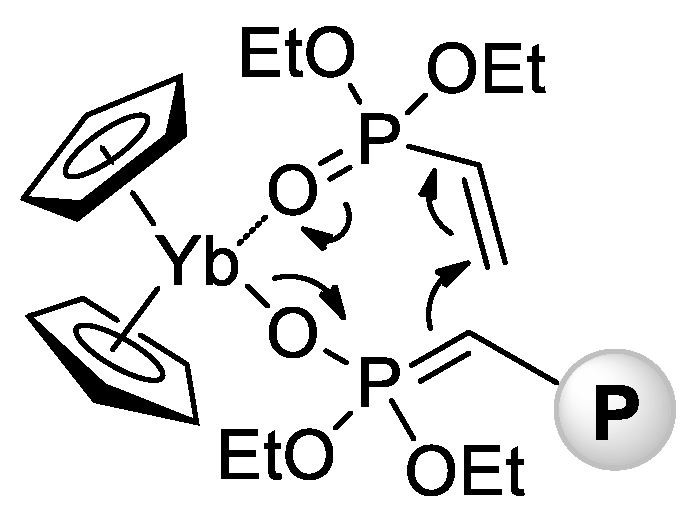
In 2010 [78], Rabe and Coll. reported that rare earth metal amides of the formula [N(SiHMe2)2]3(THF)2 (Ln = La, Nd, Sm) catalyze polymerization of CH2=CHP(O)(OEt)2 with a formation of high-MW isotactic polymers (Mn = 65–84 kDa, ÐM = 3.1–3.6, mm = 66–79%). A corresponding Y complex was found to be inactive in polymerization, forming a stable adduct [N(SiHMe2)2]3[CH2=CHP(O)(OEt)2]2. In the same year, Rieger and Coll. described the first example of metallocene-catalyzed polymerization of CH2=CHP(O)(OEt)2 [79]. The complexes (η5-C5H5)2YbX (Cp2YbX, X = Cl, Me) have demonstrated high activities in toluene reaction media, polymerization had the ‘living’ character, and Mw up to 1280 kDa were achieved. Already in this first work, Rieger et al. proposed a group-transfer mechanism of polymerization (Scheme 15) that was confirmed and discussed in subsequent research articles [80,81,82,83,84,85,86] and reviews [87,88] of this scientific group.
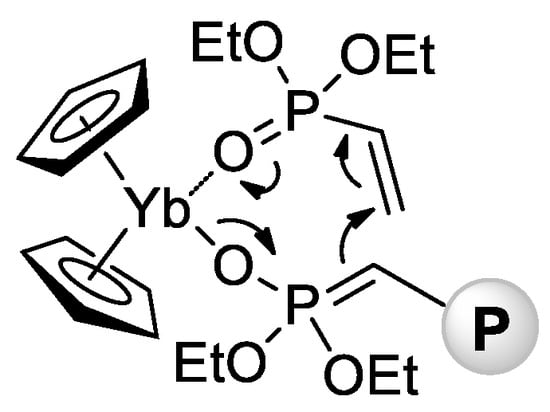
Scheme 15.
Postulated intermediate in the group-transfer mechanism of CH2=CHP(O)(OEt)2 polymerization, catalyzed by Cp2YbX. [79].
When using Cp3Ln complexes (Ln = Lu, Yb, Tm, Er, Ho, Dy) as coordination catalysts of CH2=CHP(O)(OEt)2 polymerization, C5H5 fragment acted as an initiator of the chain growth (Scheme 16) [80]. Polymerization had the ‘living’ character, polymers with given Mn (60–710 kDa depending on monomer-to-catalyst ratio) and ÐM = 1.05–1.36 were obtained. Corresponding PVPAs were then prepared for the reaction with Me3SiBr, followed by mild hydrolysis (HCl/MeOH). Dimethyl and diisopropyl vinyl phosphonates were also polymerized. DCS studies have shown that CH2=CHP(O)(OR)2-based polymers decompose at 280–340 °C (R = Et) and 245–270 °C (R = iPr) with olefin elimination and formation of PVPA. When using Cp3Yb catalyst, highly statistic copolymers of CH2=CHP(O)(OR)2 (R = Me, Et, nPr) were obtained [81].
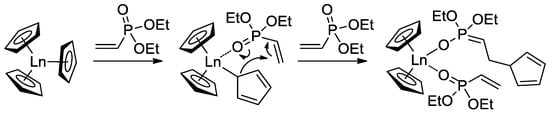
Scheme 16.
Initiation of the Cp3Ln-induced polymerization of CH2=CHP(O)(OEt)2 [80].
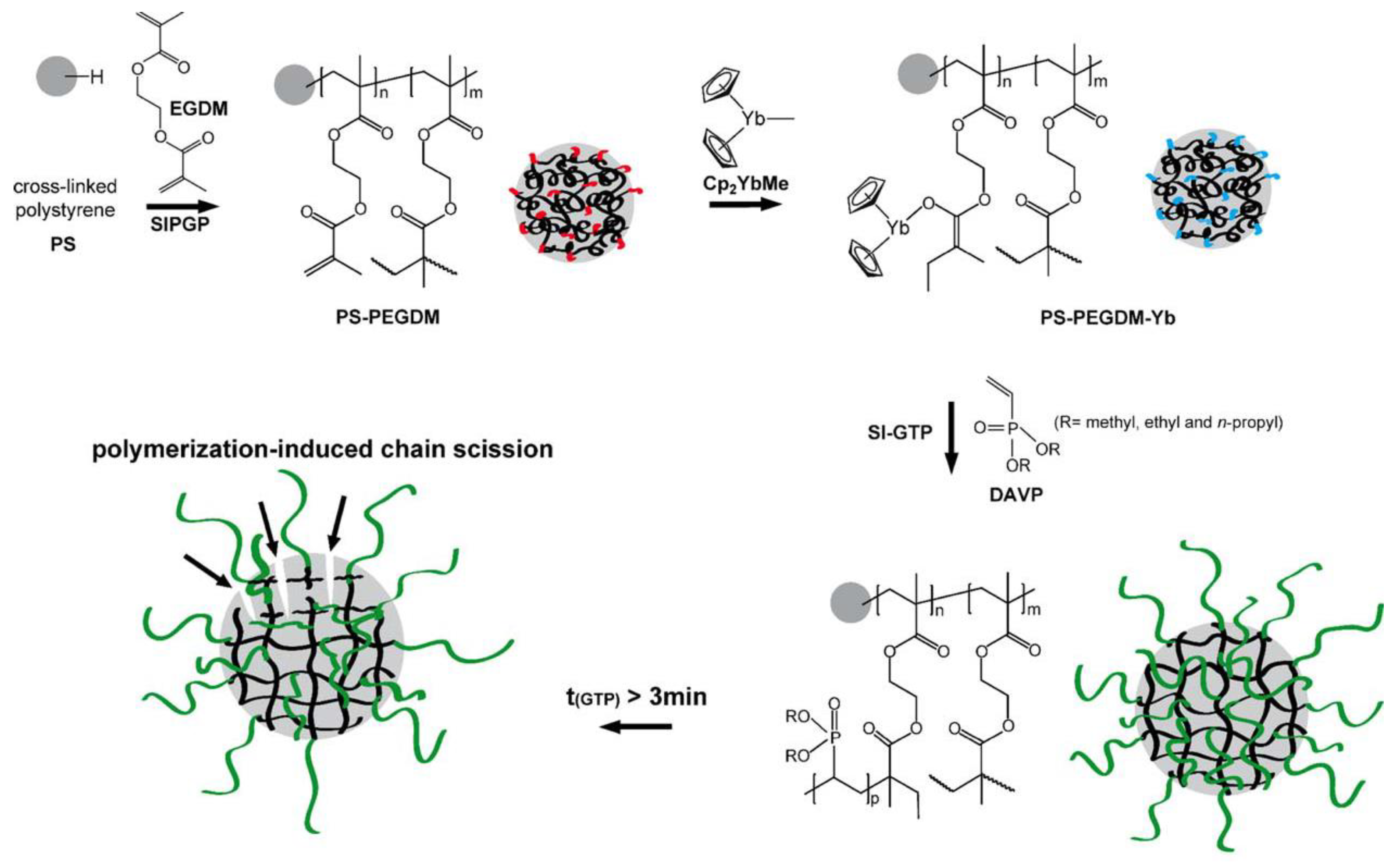
Since Cp2YbX-catalyzed polymerization of acrylates and CH2=CHP(O)(OEt)2 proceed according to similar group-transfer mechanisms, a surface-initiated group transfer polymerization (SI-GTP), which allows the covalent modification of solids with dense poly(vinylphosphonate) brushes, was carried out [89]. Silicon surfaces were modified with methacrylate functionalities, either via a self-assembled monolayer of 3–(trimethoxysilyl)propyl methacrylate or via a poly(ethylene glycol dimethacrylate) film (prepared by self-initiated photografting and photopolymerization), the surfaces were treated with Cp2YbMe, and then by vinyl phosphonate monomer. After Me3SiBr and Me3SiBr treatment, hydrophilic PVPA surfaces formed. A similar approach was realized using cross-linked polystyrene microspheres [90] (Figure 3).
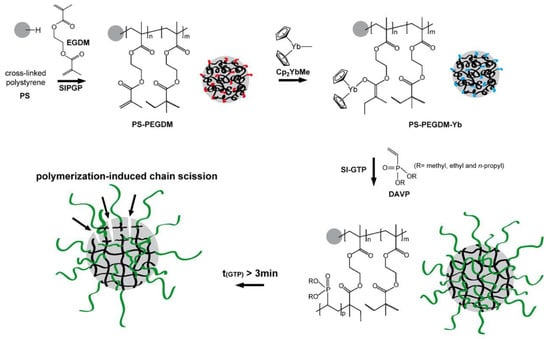
Figure 3.
Schematic illustration of the covalent grafting of a PEGDM on a cross-linked polystyrene microsphere and subsequent immobilization of the Cp2YbMe. In the next step, SI-GTP of dialkyl vinyl phosphonate leads to the formation of poly(dialkyl vinyl phosphonate)-functionalized microspheres. Reprinted with permission from [90]. Copyright (2014) Wiley-VCH GmbH.
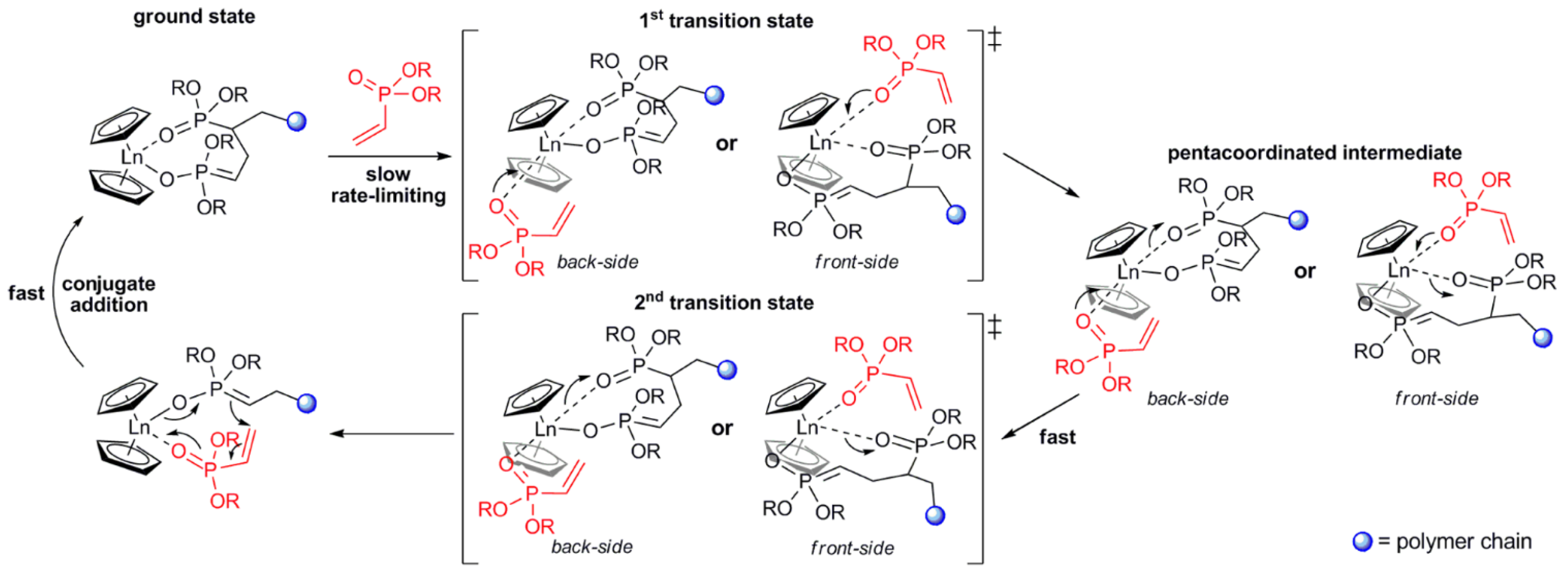
As a result of the mechanistic studies of polymerization of CH2=CHP(O)(OR)2 [82] it was demonstrated that initiation by Cp2LnX follows a complex reaction pathway depending on the nature of X. For X = Me, CH2SiMe3 initiation occurs via the abstraction of the acidic α-CH of the vinylphosphonate, and for X = Cp, SR initiation occurs via the nucleophilic transfer of X to a coordinated monomer. For X = Cl, OR a monomer-induced ligand-exchange reaction with a formation of active Cp3Ln catalyst was observed. The further elemental steps of the reaction are presented in Figure 4 [82].
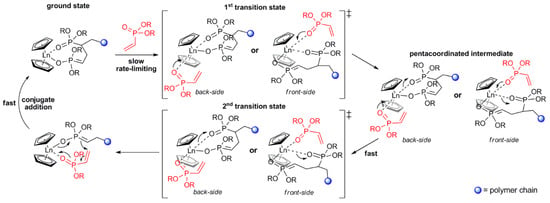
Figure 4.
Elemental steps of rare earth-mediated group transfer polymerization of vinyl phosphonates. The rate-limiting step is an SN2-type associative displacement of the polymer phosphonate ester by a vinylphosphonate monomer, presumably via a pentacoordinated intermediate. Reprinted with permission from [82]. Copyright (2013) American Chemical Society.
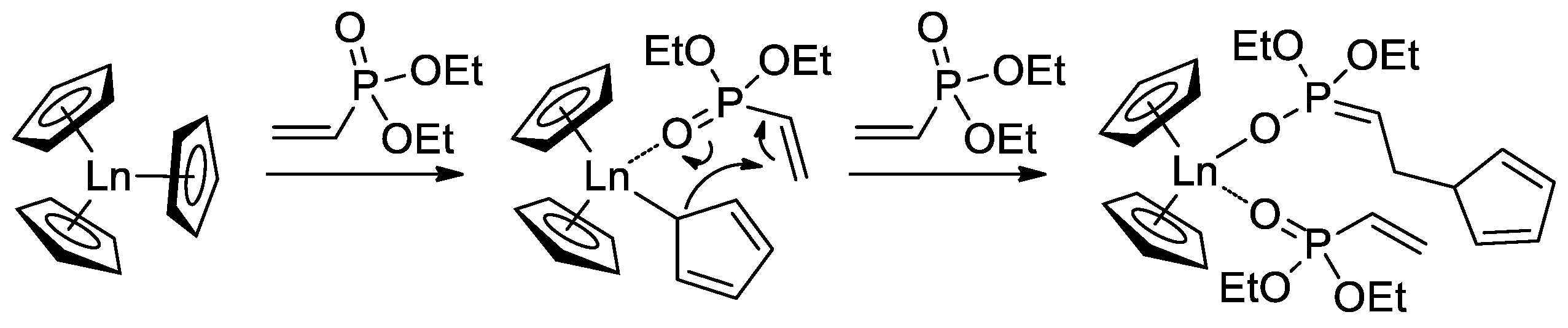
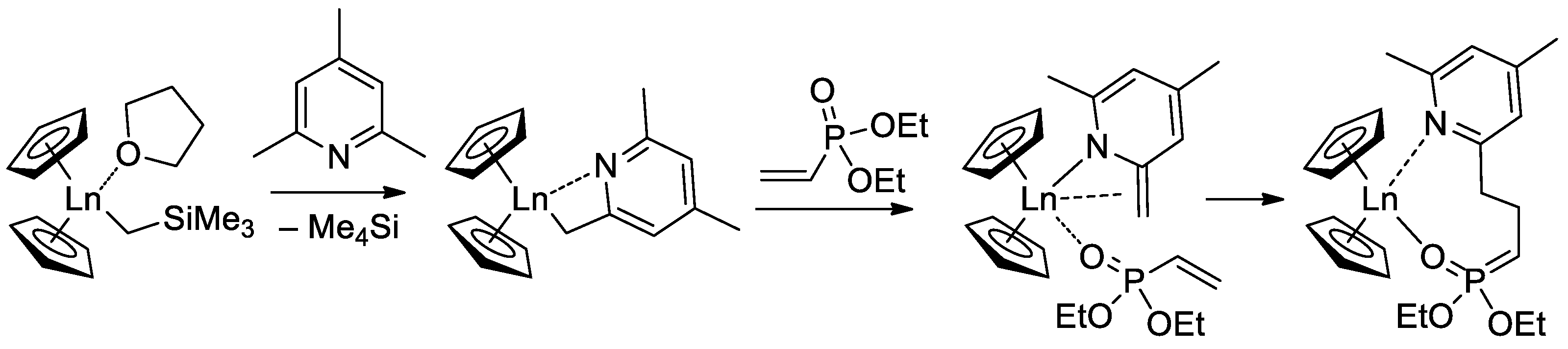
Since the initiation stage of Cp2LnX-mediated polymerization of CH2=CHP(O)(OR)2, this stage is significantly slower than propagation stages (leading to the broadening of MWD and partial loss of the polymer chain control), subsequent studies were focused on the development of highly efficient initiators. In that capacity, Cp2Ln derivatives of 2,4,6-trimethylpyridine were proposed [83]. The first stage of the process is presented in Scheme 17. Polymerization experiments have demonstrated the absence of the induction period when using this new initiator.
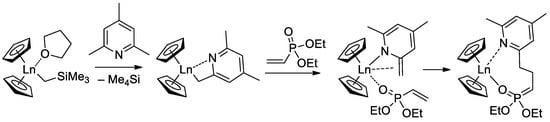
Scheme 17.
Synthesis of Cp2Ln(CH2(C5H2Me2N)) via C−H bond activation by σ-bond metathesis and initiation step of the CH2=CHP(O)(OEt)2 polymerization [83].
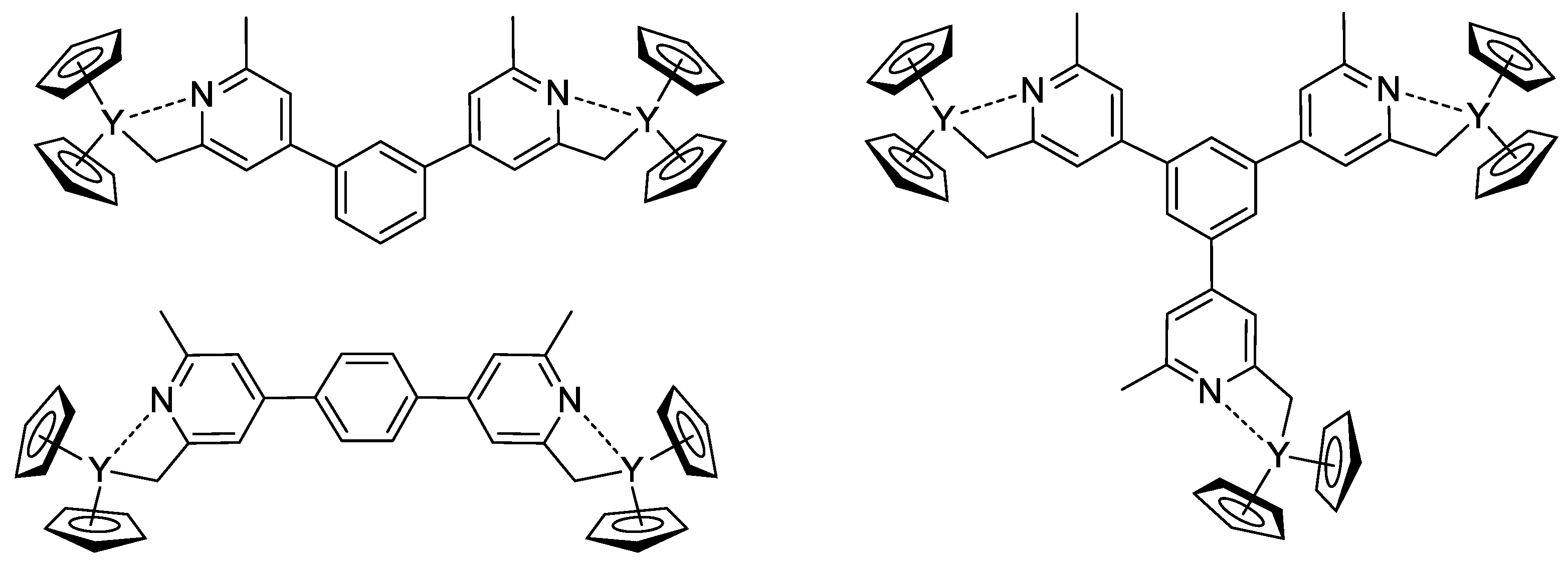
The concept of the efficient initiator of rare earth metal-mediated group transfer polymerization was beautifully realized by the example of the core-first polymer synthesis [85]. Based on 1,3- (or 1,4-bis-) and 1,3,5-tris(2,6-dimethylpyridin-4-yl)benzene, Cp2Y complexes (Scheme 18) were obtained and used as initiators of the group transfer polymerization of CH2=CHP(O)(OEt)2. When using dinuclear complexes, bimodal polymer distribution differing in molecular weight by a factor of two was detected. In the case of the trinuclear complex, trimodal molecular weight distribution was observed.
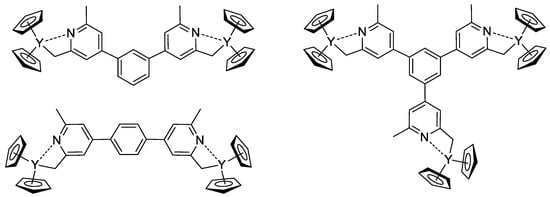
Scheme 18.
Efficient initiators for two- and three-pronged polymerization of CH2=CHP(O)(OEt)2 [85].
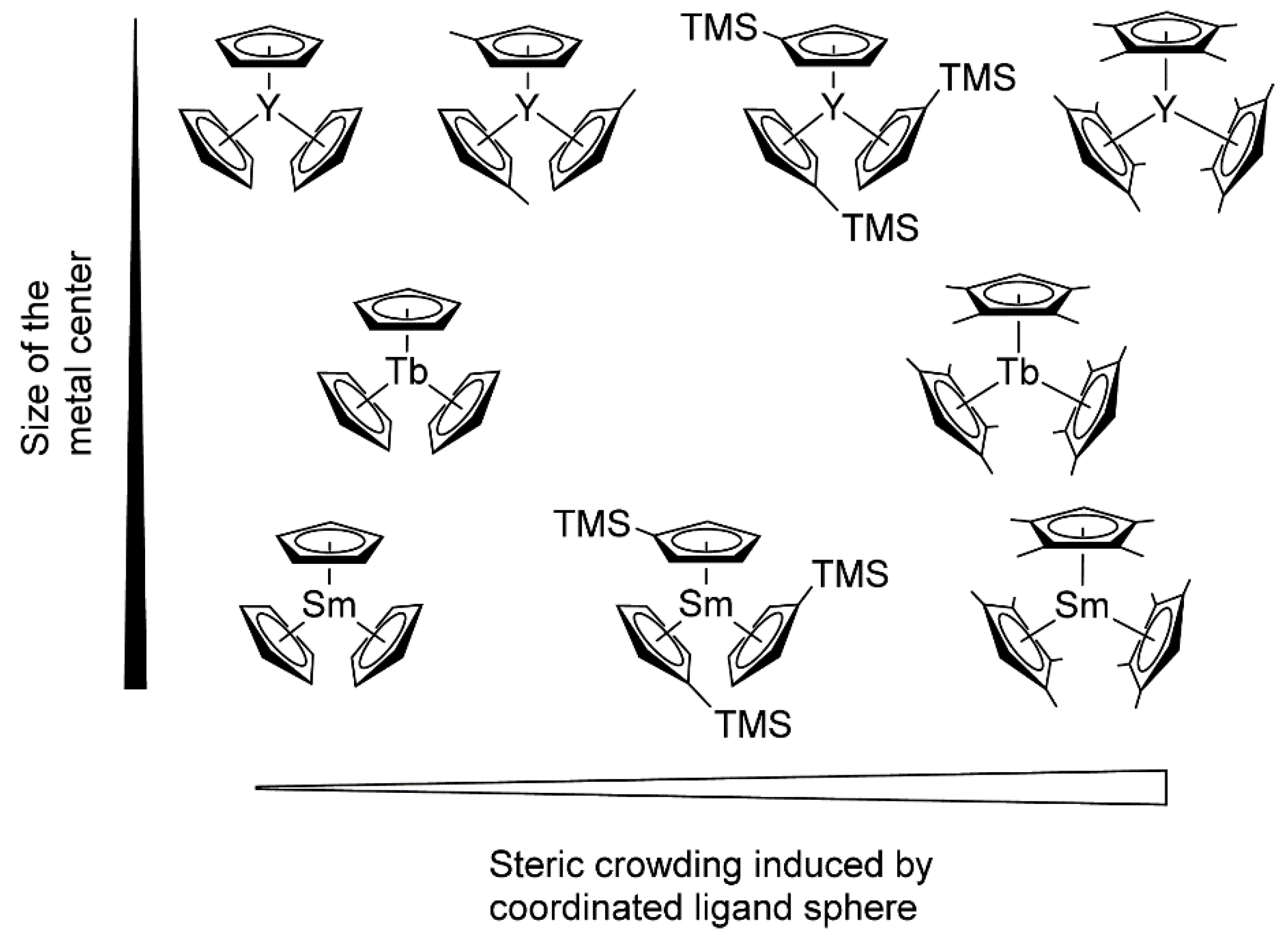
Rieger and Coll. also drew attention to another important aspect of lanthanidocene-initiated polymerization of CH2=CHP(O)(OR)2, namely, steric factors (Figure 5) [84]. As a result of the metal−ligand interactions, the studied catalysts varied in their properties, ranging from inert (Cp3Ln, Ln = Tb, Sm) to highly active ((C5H4Me)3Y. (C5Me4H)3Sm was the first example of the active ‘early’ lanthanidocenes, and in general, the activities accelerated with decreasing cation size. Furthermore, the polymerization behaviors of (C5Me4H)3Ln complexes differed from unsubstituted or monosubstituted metallocenes. The pentacoordinate intermediates of these complexes exhibited lengthened metal-monomer bond lengths, resulting in a higher enthalpy ΔH≠ term, and are partly compensated by lower ΔS≠ contributions to the activation barrier.
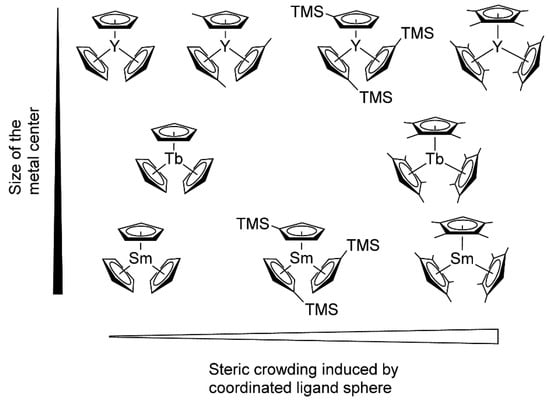
Figure 5.
Molecular structures of monosubstituted and tetramethyl-substituted Cp3Ln catalysts studied in polymerization of CH2=CHP(O)(OEt)2. Reprinted with permission from [84]. Copyright (2016) American Chemical Society.
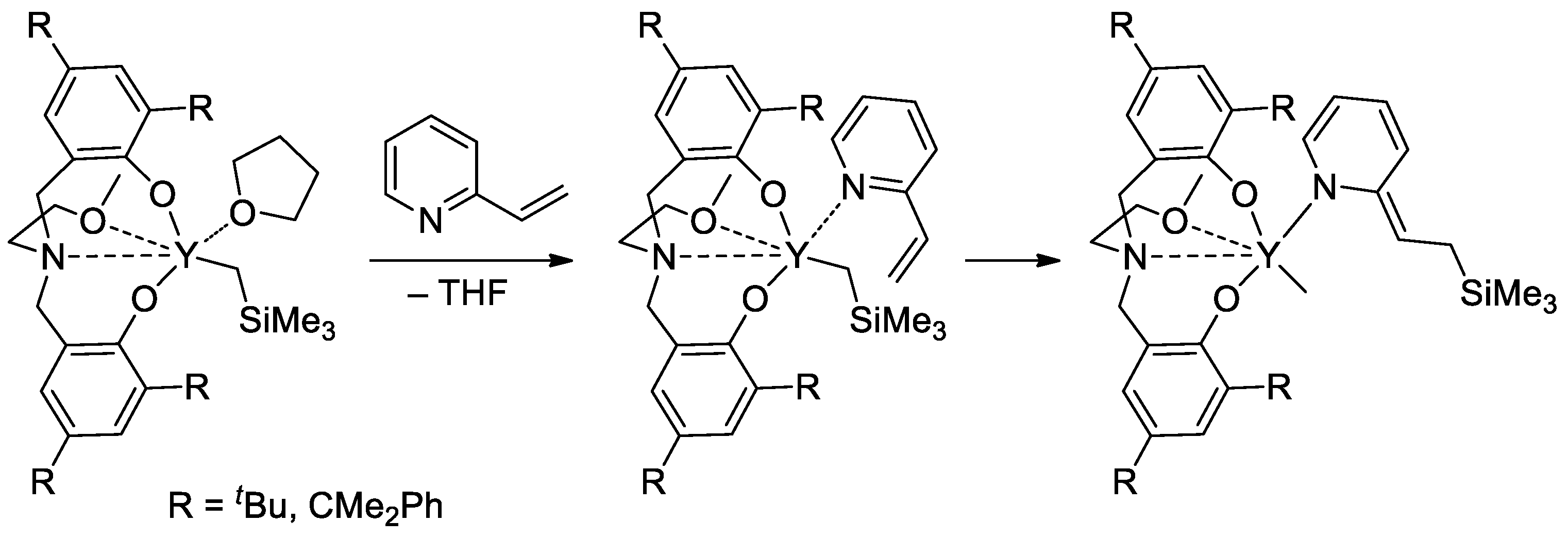
Rare-earth metal-based catalysts of the polymerization of vinyl phosphonates are not limited by sandwich lanthanide complexes. The high catalytic activity of the yttrium bis(phenolate) in the polymerization of CH2=CHP(O)(OR)2 (R = Et, iPr) was demonstrated [91], 2-vinylpyridine efficiently activated the pre-catalyst at the initiation stage (Scheme 19).
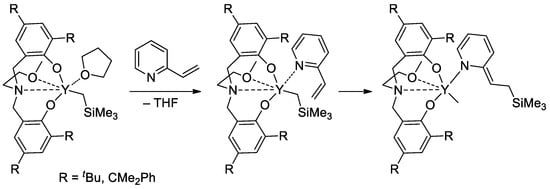
Scheme 19.
Six-membered initiation mechanism for 2-vinylpyridine and Y chelate complexes in polymerization of CH2=CHP(O)(OR)2 (R = Et, iPr) [91].
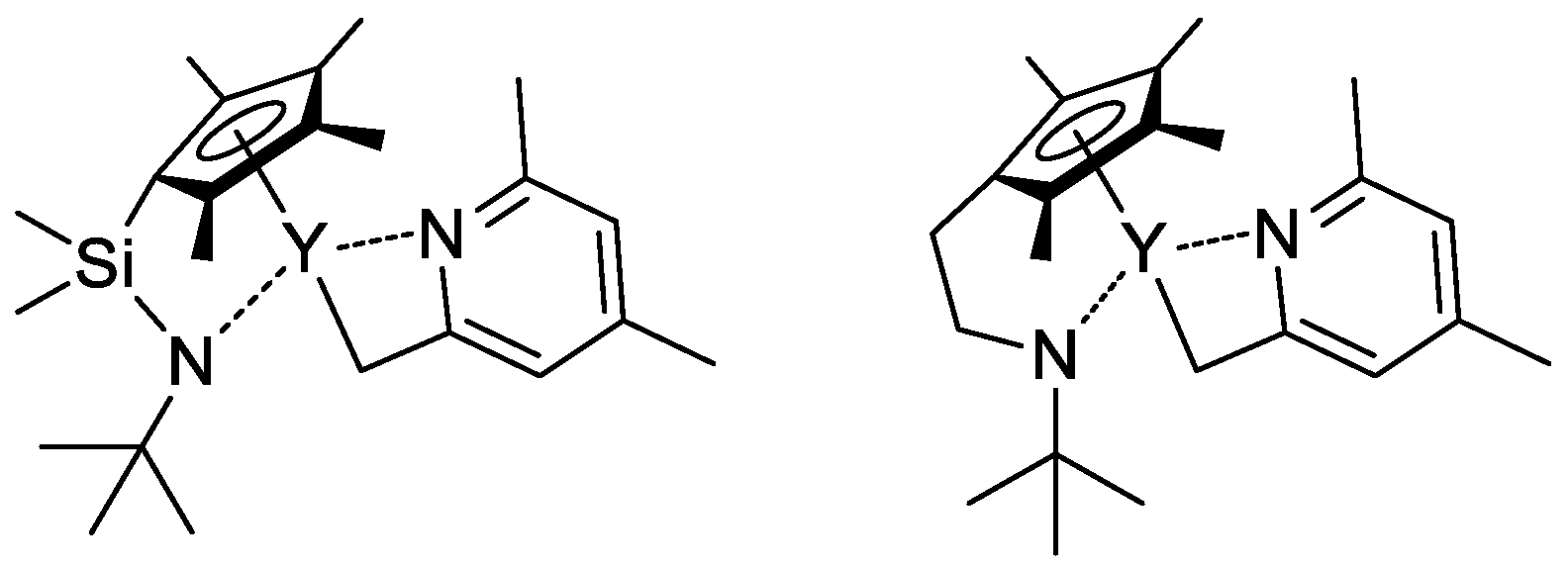
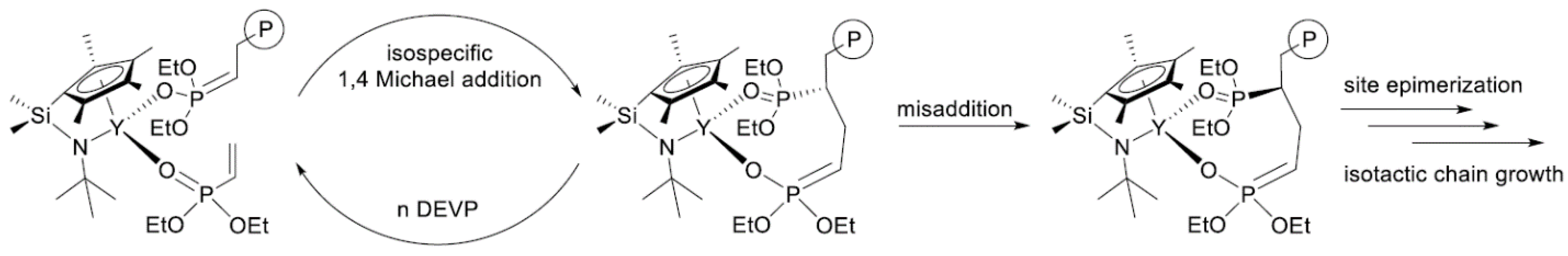
In their recent publication, Rieger and Coll. described the synthesis and catalytic behavior of half-sandwich ‘constrained geometry’ complexes of Y and Al in the polymerization of CH2=CHP(O)(OEt)2 [86]. The complexes of Y (Scheme 20) have demonstrated high activity even at −78 °C, under these conditions highly isotactic polymer (mmm > 98%) was obtained. The proposed stereocontrol mechanism is presented in Figure 6.
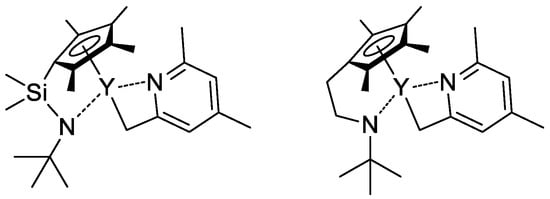
Scheme 20.
Efficient constrained-geometry catalysts for ‘living’ polymerization of vinyl phosphonates [86].
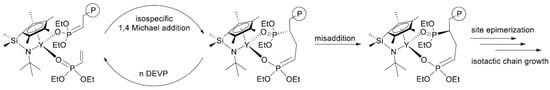
Figure 6.
Proposed stereocontrol mechanism and formation of stereoerrors for the isospecific polymerization of CH2=CHP(O)(OEt)2. Reprinted with permission from [86]. Copyright (2019) American Chemical Society.
Note that in 2013 [92], Ni and Coll. have demonstrated high catalytic activity of Ln(BH4)3(THF)3 (Ln = Y, La, Nd, Sm, Gd, Dy, Lu) in group transfer polymerization of CH2=CHP(O)(OEt)2. ‘Living’ character of the reaction was confirmed by the termination of the polymerization with the use of PhCH2Cl or EtI, however, obtained polymers were characterized by broadened MWD (ÐM = 1.63–1.81).
In conclusion, it is important to notice that the treatment with Me3SiBr, followed by mild hydrolysis, or multi-hour reflux in HCl is not the only method for transforming poly(phosphonates) to PVPA. High efficiency of the use of CF3SO3H or highly acidic cationites in ‘wet’ (hydrolysis) and ‘dry’ (olefin elimination) transformation of dialkyl phosphonates to phosphonic acids was demonstrated by Han and Coll. [93]. Different synthetic approaches to the hydrolysis of phosphinates and phosphonates have been reviewed very recently [94], and nothing prevents researchers from using these methods in the synthesis of PVPA and other sidechain PCPAs.
2.3.2. Copolymerization of VPA and VPA Derivatives with Other Vinyl Monomers
Since VPA homopolymers have the prospective but limited potential of applications, valuable works on the synthesis of copolymers containing –CH2CHP(O)(OH)2– fragments were published even at the end of the last century.
The free-radical copolymerization of acrylic acid and VPA was conducted by Budd and Coll. in aqueous media (Scheme 21), AIBA was used as an initiator [95]. As the VPA content in the feed was increased, the monomer conversion and yield of the copolymers showed a general decrease. The reactivity ratios of acrylic acid (r1) and VPA (r2) were 4.09 and 0.042, respectively. Such a high difference in r1 and r2 values may give rise to composition drift, where the composition of the copolymer changes as the polymerization proceeds. Where the VPA content in the feed was below 50%, a chain transfer agent was introduced into the polymerization to restrict the molecular weight. Using this method, a range of copolymer compositions were produced with consistent molecular weights (Mw = 150–200 kDa) up to a VPA content of 59 mol%. At higher VPA contents, high-MW polymers were not obtained, and the highest Mw for VPA homopolymer was 29 kDa.
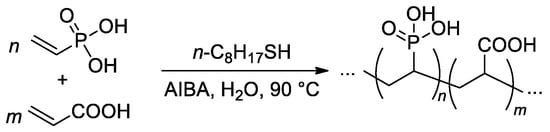
Scheme 21.
Free-radical copolymerization of acrylic acid and VPA [95].
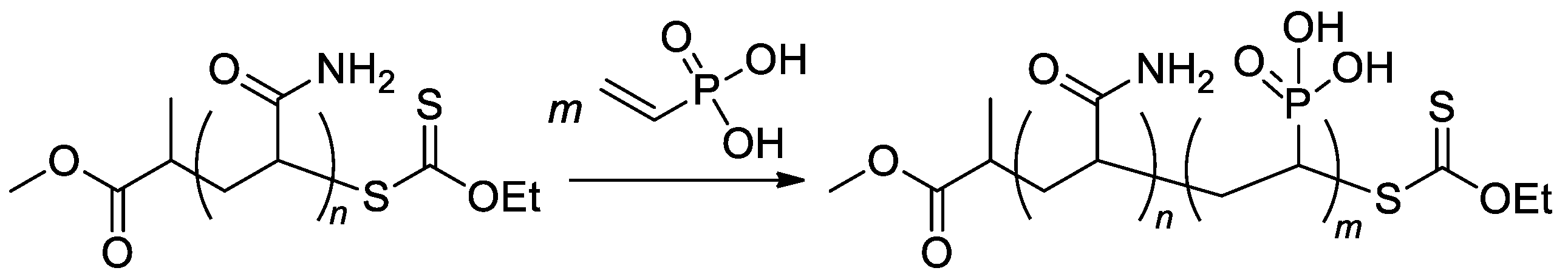
RAFT/MADIX approach was used in the synthesis of poly(acrylamide)-b-PVPA copolymers (Scheme 22) [96] with a given length of poly(acrylamide) block and varied length of PVPA fragment, copolymers with Mn 5.5–11.4 kDa were obtained.
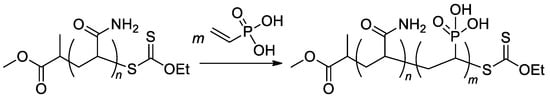
Scheme 22.
Aqueous RAFT/MADIX polymerization of VPA via chain extension of a polyacrylamide macroxanthate [96].
When using ethylene glycol diacrylate, cross-linked copolymers of acrylic acid and VPA were obtained [97]. Copolymers of VPA and 1-vinyl-1,2,4-triazole with 1:1, 1:2, and 2:1 comonomer ratios were obtained in DMF solution at 85 °C with AIBN initiator, the Mn values ranged from 6.0 to 8.2 kDa, the dispersity ÐM was ~2 [98]. Copolymerization of VPA and methacrylic ester of mPEG 475 was carried out in ethyl acetate with the use of AIBN as an initiator, side reactions and gel formation were detected [99].
UV-initiated copolymerization of VPA and acrylamide at the oxidized silicon surface in the presence of 2,2-dimethoxy-2-phenylacetophenone (DMPA) was described in [100], the binding of the copolymer with the surface was confirmed by photoelectron spectroscopy.
The synthesis of CH2=CHP(O)(OMe)2, CH2=CF2, and CH2=CHSi(OEt)3 terpolymers was described in [101]. The best results were obtained when using 2,5-dimethyl-2,5-di(tert-butylperoxy)hexane as an initiator of the reaction that was conducted in dimethyl carbonate at 115 °C. Si–O–Si cross-linking was accompanied by partial hydrolysis of methyl phosphonate fragments.
Poly(styrene)-b-PVPA was obtained by nBuLi-initiated sequential anionic polymerization of styrene and CH2=CHP(O)(OiPr)2 in THF media with subsequent hydrolysis [75].
The syntheses of VPA copolymers with 2-deoxy-2-methacrylamido-D-glucose, 4-acryloylmorpholine, or acrylamide, were the subject of the recent study of Nazarova and Coll. [102]. The reactions were conducted in DMF or MeOH (AIBN initiator) or aqueous media (AIBA initiator). VPA has demonstrated values of reactivity comparable with 4-acryloylmorpholine and acrylamide, thus confirming the preference for the use of VPA instead of VPA esters in copolymerization.
With the use of (NH4)2S2O8 initiated free-radical polymerization in aqueous media, random terpolymers of VPA, acrylonitrile, and methyl acrylate were synthesized [103]. Poly(vinylphosphonic acid-co-styrene-co-maleic anhydride) was obtained using AIBN as an initiator and DMSO as a solvent [104]. Water-soluble initiator was used in the random copolymerization of VPA with 5-(methacryloylamido)tetrazole [105].
As a final note, the use of rare-earth metal complexes in the synthesis of copolymers of vinyl phosphonates was the subject of the recent review of Rieger et al. [88].
2.3.3. Homopolymerization and Copolymerization of Phosphorylated Acrylates
Derivatives of acrylic and methacrylic acid, containing –OP(O)(OH)2 and –P(O)(OH)2 fragments, have been evidently regarded as prospective monomers for the synthesis of sidechain PCPAs. There is extensive literature on this subject, and in this section, we have tried to present the most interesting and recent works in this field.
Evidently, one of the common methods of the polymerization of acrylates—anionic polymerization—is not applicable for derivatives of phosphoric and phosphinic acids, and the vast majority of research has used free-radical polymerization in one way or another. Trivial methods of free-radical polymerization (solution process, azo compounds, peroxides or UV exposure as initiators, no CTAs or other additives) are fully applicable to phosphorylated and phosphonated acrylates. This is particularly true in the case of acrylates, containing the phosphorus atom in an alkoxy fragment of the acrylate molecule (Scheme 4), due to the high reactivity of these monomers, and a number of examples of the homo- and co-polymerization of phosphorylated and phosphonated (meth)acrylates can be cited [22,23,25,26,28,30,31,37,38,39,106,107,108]. Note that the studies of AIBN-initiated copolymerization of methyl methacrylate and CH2=C(Me)C(O)OCH2P(O)(OMe)OH showed r1 and r2 values of 0.98 and 1.03, respectively, thus confirming close reactivity of ‘conventional’ and phosphonated acrylates [30].
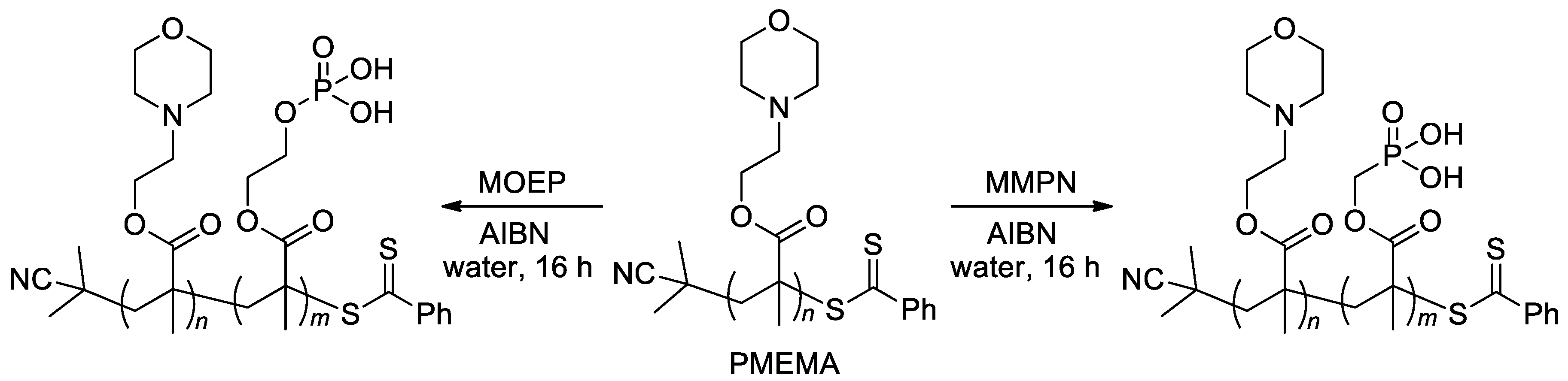
On the other hand, RAFT homo- and co-polymerization were successfully applied to similar acrylate monomers [29,33,109]. In particular, zwitterionic diblock copolymers were synthesized by copolymerization of the macro-RAFT agent, PMEMA (Scheme 23) with methacrylate monomers MOEP and MMPN via RAFT polymerization [29].
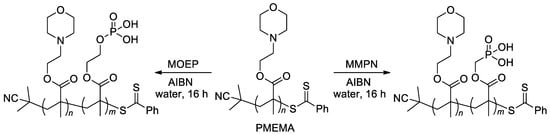
Scheme 23.
Synthesis of diblock copolymers via RAFT polymerization [29].
Copolymers of MPC and methyl acrylate with (4-formylphenyl) fragment via –(OCH2CH2)9– spacer, were synthesized by RAFT polymerization [110]. AIBN-initiated copolymerization of MPC, n-butyl methacrylate, MADP (optionally) and rhodamine-linked methacrylate (optionally, for the pharmacokinetic studies) resulted in copolymers with Mn = 14–43 kDa and ÐM ~2 [40] (Scheme 24).
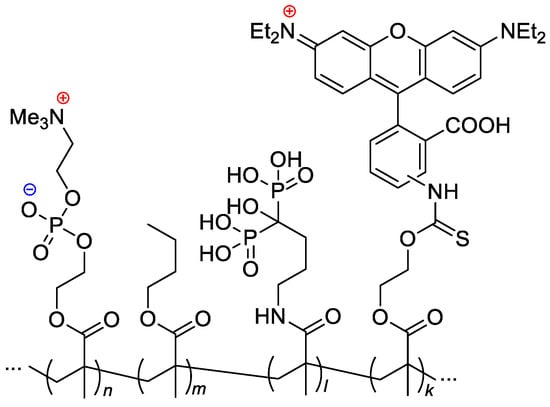
Scheme 24.
Formulation of the bone-targeting phospholipid copolymers [40].
The hyper-crosslinked polymer was obtained by AIBN-initiated free-radical polymerization of (CH2=C(Me)C(O)OCH2CH2O)2P(O)OH (BMEP) in DMF [111].
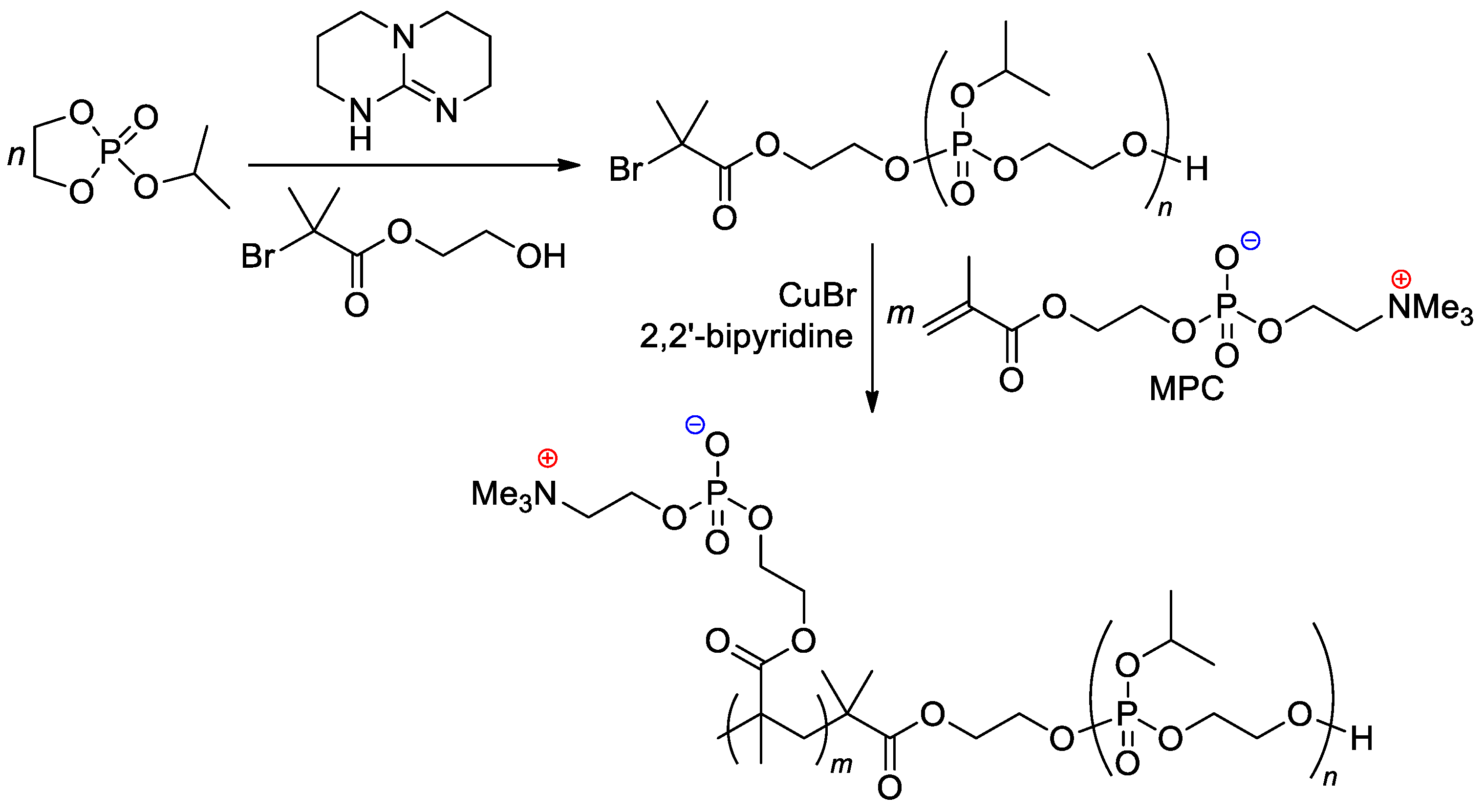
The zwitterionic block copolymer was synthesized by sequential carrying out of organocatalytic ROP of 2-isopropoxy-1,3,2-dioxaphospholane 2-oxide (iPrOEP) with the use of BrCMe2C(O)O(CH2)2OH as an initiator, and atom transfer radical polymerization (ATRP) of the phosphorylcholine-substituted methacrylate (MPC) in the presence of CuBr and 2,2′-bipyridine (Scheme 25) [112]. Note that MOEP-based polymer brushes were obtained with the use of the ATRP approach, based on an SH-substituted –CMe2Br initiator bonded with gold nanoparticles [113].
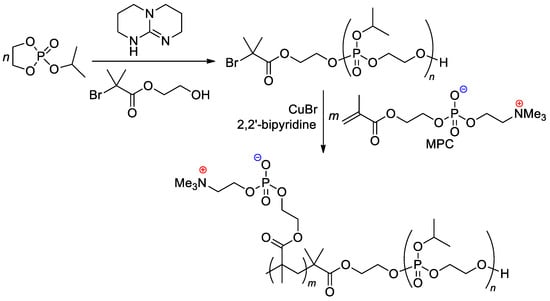
Scheme 25.
Synthetic route to block copolymer containing both main-chain and sidechain phosphate fragments [112].
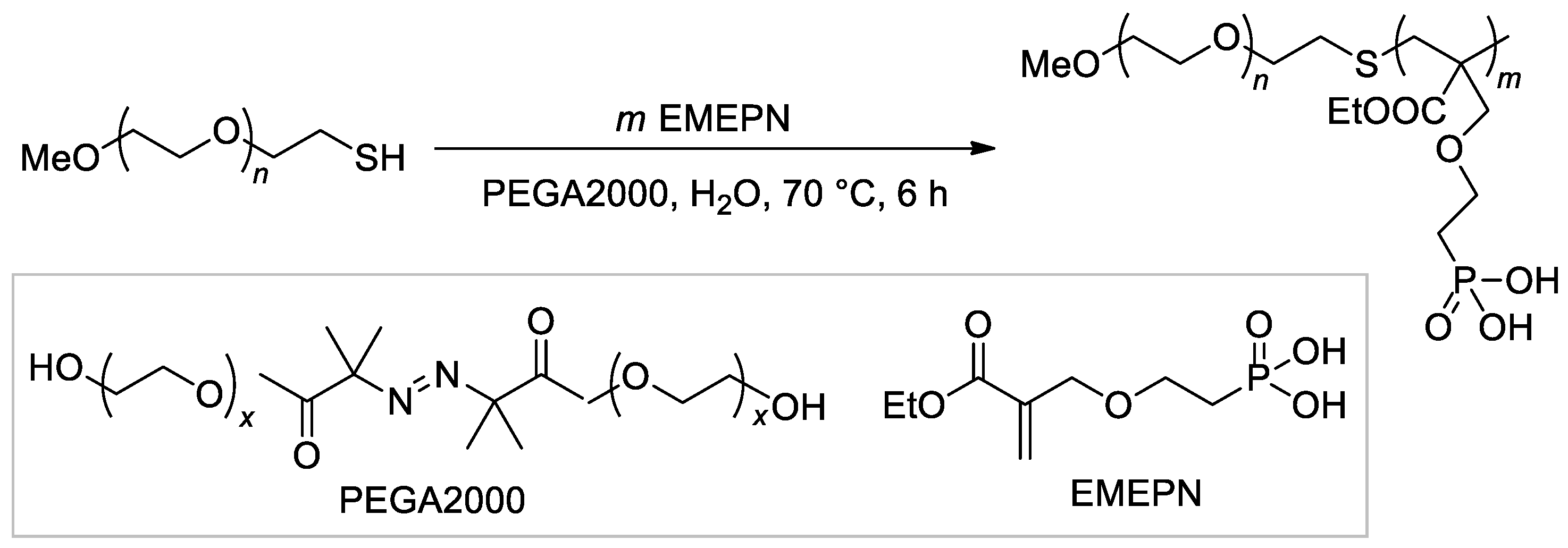
The synthesis of the diblock copolymer, containing mPEG-5000 fragment and polymer of (2-((2-(ethoxycarbonyl)allyl)oxy)ethyl)phosphonic acid as the second block [114], is of particular interest due to the chosen method, ATRP with thiol CTA and macroinitiator PEGA2000 (Scheme 26).
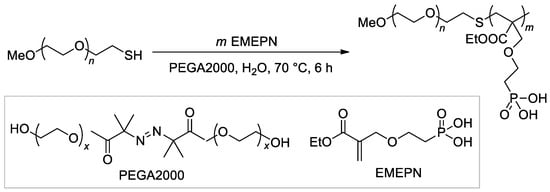
Scheme 26.
The use of ATRP with PEGA2000 initiator in the synnthesis of hydrophilic diblock-copolymer [114].
2.3.4. Homopolymerization and Copolymerization of Other Vinyl Monomers
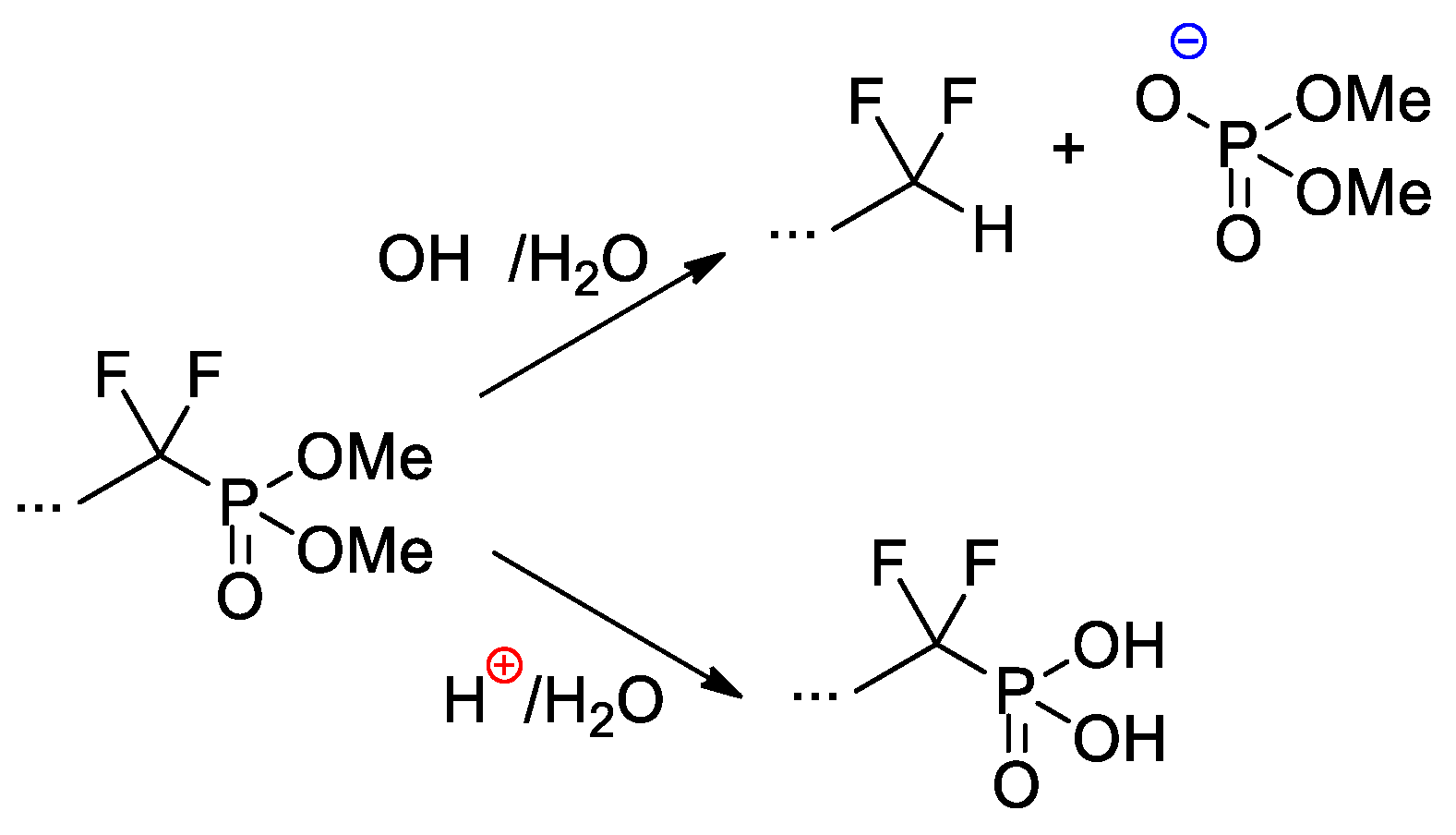
Copolymers of CF2=CF2, CF2=CF(C3F7) and CF2=CFO(CF2)3P(O)(OMe)2 were obtained with the use of AIBN-initiated reaction in 1,1,2-trichloro-1,2,2-trifuoroethane (R-113) media [48]. These copolymers as such have not demonstrated outstanding characteristics, but, nevertheless, a very significant chemical aspect should be mentioned here. Whereas hydrolysis of –P(O)(OR)2-functionalized polymers is not accompanied by noteworthy side reactions, hydrolytic cleavage of the –CF2P(O)(OMe)2 fragment is not so straightforward (Scheme 27). Under basic conditions, the cleavage of the C–P bond was observed, but acidic hydrolysis resulted in the formation of the phosphinic acid.
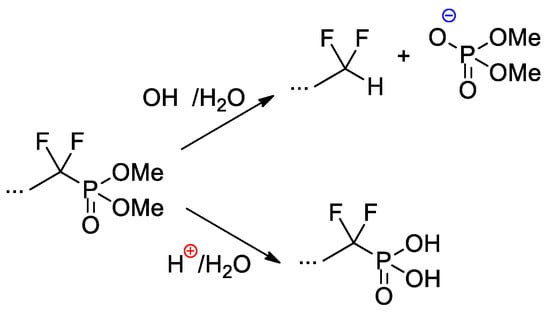
Scheme 27.
Hydrolysis of perfluoroalkyl phosphonate fragment [48].
For the synthesis of homopolymers of phosphonated styrenes SP and SbP (Scheme 9) and their block copolymers with poly(isobutylene) Park and Coll. used ATRP in toluene media with CuCl/N,N,N′,N″,N″-pentamethyldiethylenetriamine catalytic system [51]. Corresponding PCPAs were obtained by treatment with Me3SiBr/CHCl3 (36 h at 40 °C) followed by 8 h of methanolysis. Alter and Hoge [50] have synthesized –CH2CF2P(O)(OH)2-functionalized polystyrene (Mn = 12.1 kDa, ÐM = 2.42) by AIBN-initiated polymerization of styrene containing—CH2CF2P(O)(OEt)2 substituent in the position 4, followed by two-stage deprotection (reaction with Me3SiBr/CH2Cl2 and methanolysis).
2.3.5. Cyclopolymerization
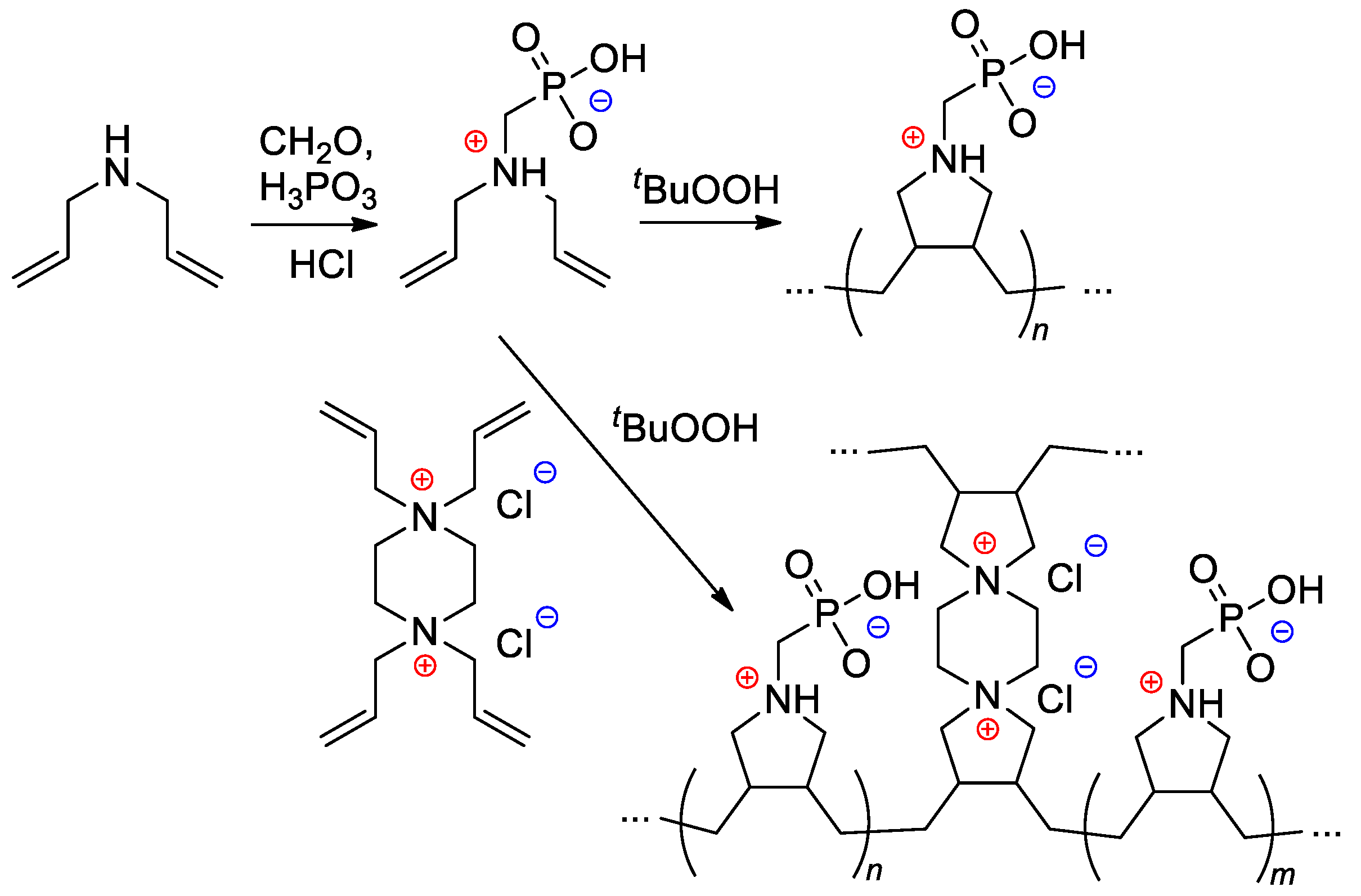
Starting from diallylamine, both linear and cross-linked PCPAs were synthesized by Al Hamouz and Ali [115] by free-radical diene cyclopolymerization approach (Scheme 28). Treatment of the polymers by NaOH resulted in anionic polyelectrolytes that have an excellent adsorption capacity for metal ions (see Section 3.3).
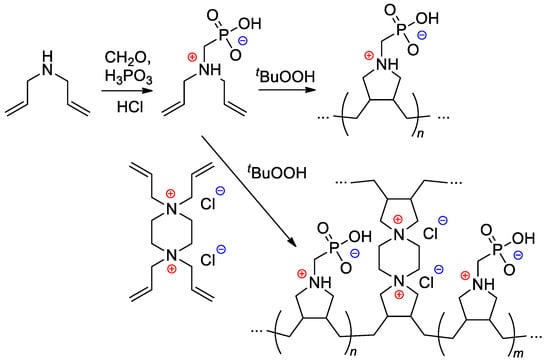
Scheme 28.
Synthesis of phosphonate-containing diene monomer and its free-radical diene cyclopolymerization [115].
2.4. Phosphonation and Phosphorylation of the Polymers
2.4.1. Direct Phosphonation of ‘Saturated’ Polymers
The reaction of saturated hydrocarbons with PCl3 and molecular oxygen results in the phosphonation of some of the carbon atoms in paraffin [116]. This reaction is also applicable to polyolefins [117]. To a first approximation, saturated hydrocarbons form alkylphosphonyl chlorides by the overall reaction, presented in Equation (1),
and the R–P(O)Cl2 can further be hydrolyzed to the corresponding phosphonic acid. This reaction requires an excess of PCl3 and is difficult to control. Bulk phosphonation negatively affects the mechanical characteristics of the polymers [117]. Twenty years ago, Allan and Coll. reported the results of the study of surface phosphonation of low-density polyethylene. The most uniform surface treatment was achieved in the gas phase at 25 °C within 30 min [118]. Further studies in this field have not been conducted, apparently, due to the low environmental friendliness of the process and the complexity of controlling the properties of the materials obtained.
R–H + 2PCl3 + O2 → R–P(O)Cl2 + POCl3 + HCl
2.4.2. Modification of the Reactive –OH Groups in the Polymer Backbone
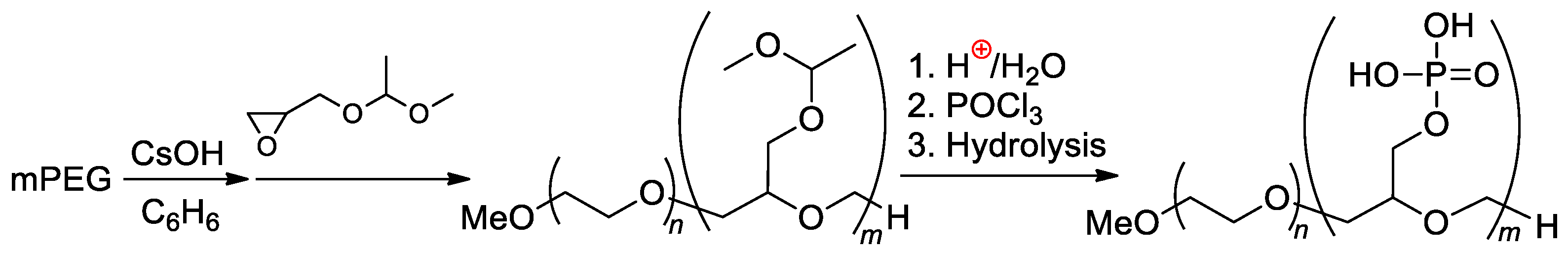
Double-hydrophilic copolymers containing nonionic PEG fragments and ionic phosphorylated polyglycidol blocks were synthesized by Penczek and Coll. [119,120] by the phosphorylation of the polymer obtained previously by mPEG-initiated polymerization of CH2=CHOMe-protected glycidol [121,122] (Scheme 29).
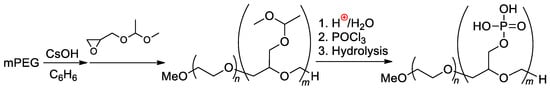
Scheme 29.
Synthesis and hydroboration of PEG-b-poly(buta-1,3-diene) to PEG-b-poly((2-hydroxyethyl)ethylene), followed by phosphorylation [122]. Original reaction scheme presented in [122], was corrected.
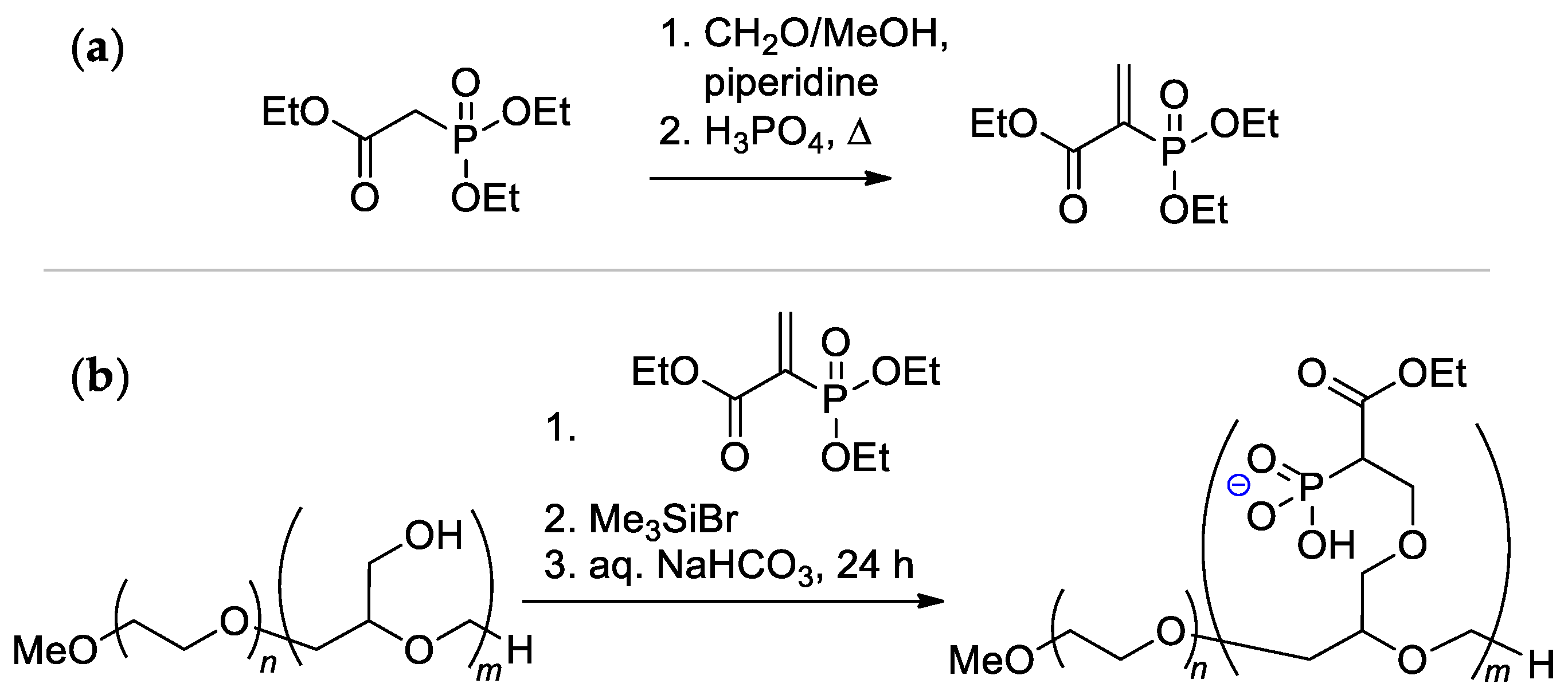
The alternative method of functionalization of polyglycidol block is based on the reaction with ethyl 2-(diethoxyphosphoryl)acrylate, followed by hydrolysis (Scheme 30) [123]. The reaction of the –OH groups with ethyl 2-(diethoxyphosphoryl)acrylate did not require catalysts and was completed at 25 °C in THF or CH2Cl2 after 1 day. Such an approach has an advantage due to the hydrolytic stability of the C–P bond, but the issue of the toxicity of copolymer remains open.
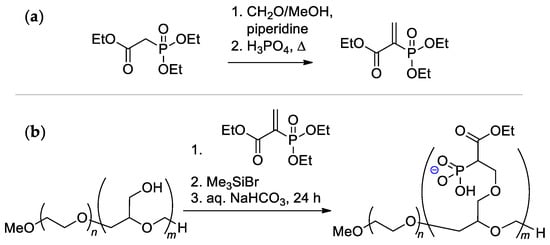
Scheme 30.
(a) Synthesis of ethyl 2-(diethoxyphosphoryl)acrylate; (b) Functionalization of the polyglycidol block [123].
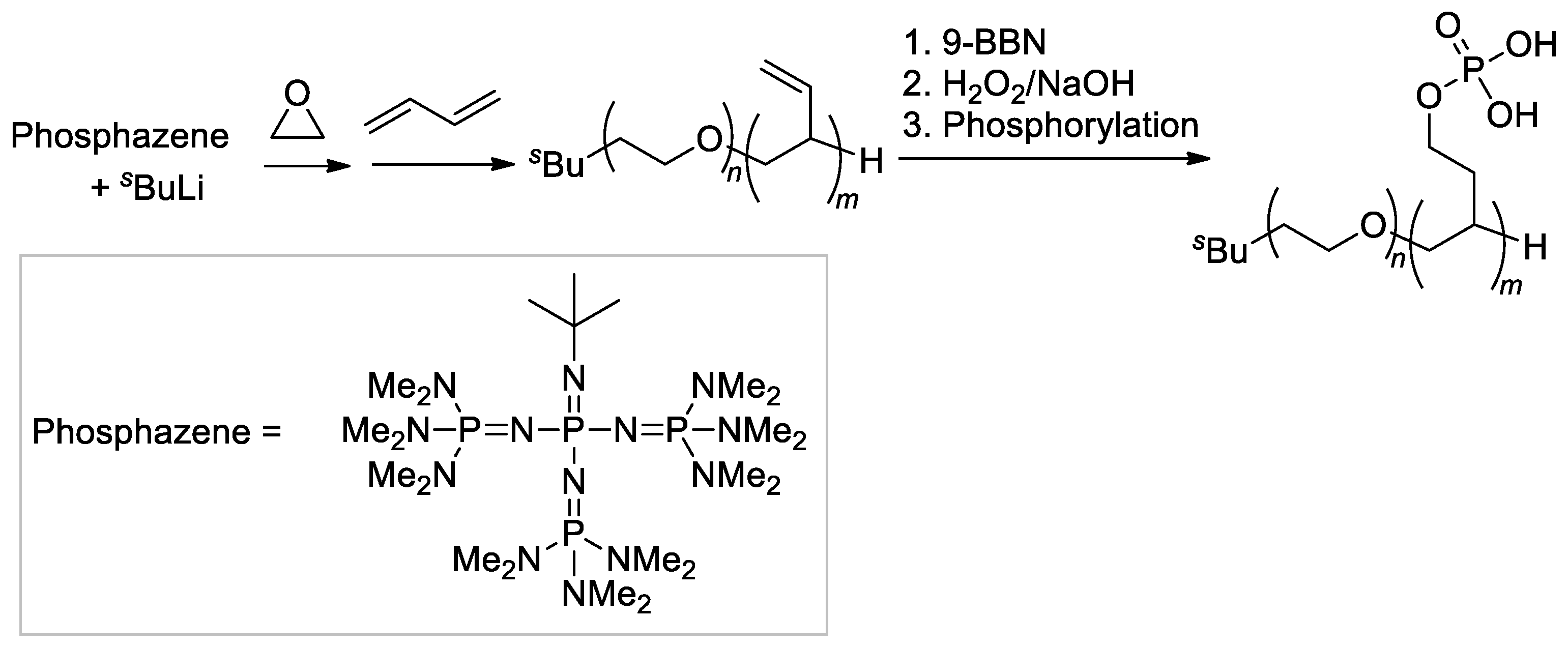
Another example of double-hydrophilic block copolymers, containing PEG fragment and hydrocarbon chain with –CH2CH2OP(O)(OH)2 substituents, was also synthesized by Penczek’s group [122] by post-modification of the block copolymer of oxirane and buta-1,3-diene (Scheme 31), obtained previously using sBuLi/phosphazene initiator [124]. Note that this strategy was reported later by Dimova and Coll. [125] who described the same method for the synthesis of block copolymers.
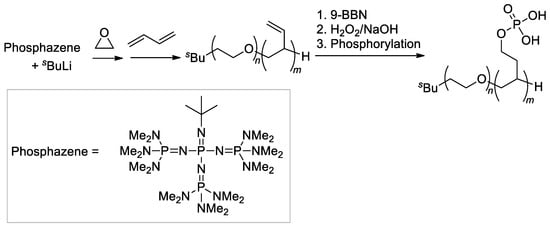
Scheme 31.
Synthesis and hydroboration of PEG-b-poly(buta-1,3-diene) to PEG-b-poly((2-hydroxyethyl)ethylene), followed by phosphorylation [122]. Original reaction scheme, presented in [122], was corrected.
However, one should not forget about an even simpler and more accessible polymer, poly(vinyl alcohol). Its phosphorylation was carried out by the reaction of poly(vinyl alcohol) (Mw = 76 kDa) with ~60% aq. H3PO4 (1 h, reflux), the product was separated using precipitation in MeOH [126]. Degree of phosphorylation was not determined in this work.
2.4.3. Modification of the Reactive –COOH Groups in the Polymer Backbone
Since (co)polymers of acrylic and methacrylic acid are synthetically available, their post-modification via chemical binding of –OP(O)(OH)2 and –P(O)(OH)2 groups with carboxylate fragment seems feasible and promising. Efficient transformation of –COOH to –C(OH)[P(O)(OH)2]2 fragment under the action of PCl3/H3PO3, followed by hydrolysis, was proposed by Kieczykowski et al. in 1995 [127]. This approach was used in the synthesis of partially phosphorylated PEG-b-poly(methacrylic acid) (PEG-b-PMMA-PO3H2(1%)) [128].
2.4.4. Modification Based on Michaelis–Arbuzov Reaction
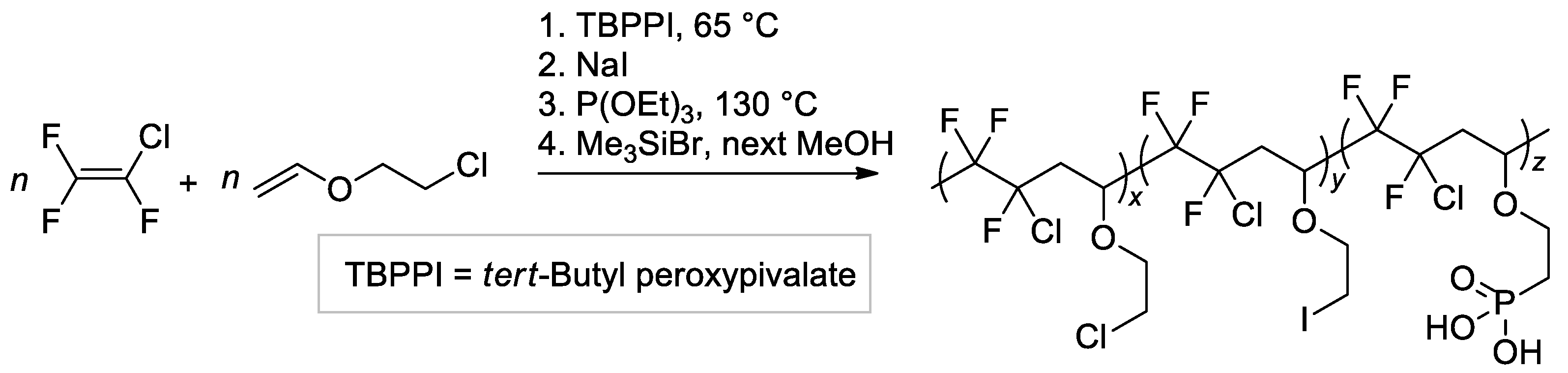
The copolymer of CF2=CFCl with 2-chloroethyl vinyl ether of the altering microstructure was obtained by free-radical copolymerization and modified with the use of the Michaelis–Arbuzov reaction [129] (Scheme 32). Five copolymers with different phosphonic acid content were thus synthesized.
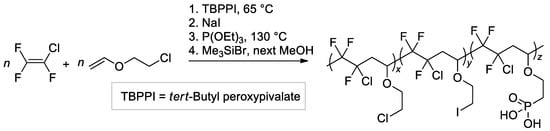
Scheme 32.
Synthetic pathway to graft phosphonic acid groups onto fluorinated copolymers obtained by radical copolymerization of CF2=CFCl with 2-chloroethyl vinyl ether [129].
Functionalization of poly(styrene-ethylene/butylene-styrene) block copolymer (Mn = 89 kDa, ÐM < 1.06) was carried out in two stages, by chloromethylation and the subsequent Michaelis–Arbuzov reaction with P(OEt)3 followed by acidic hydrolysis [130]. Copolymer samples with 9, 24, 42, and 54% degrees of chloromethylation/phosphonation were obtained for subsequent preparation of the proton conducting membranes (see Section 3.4).
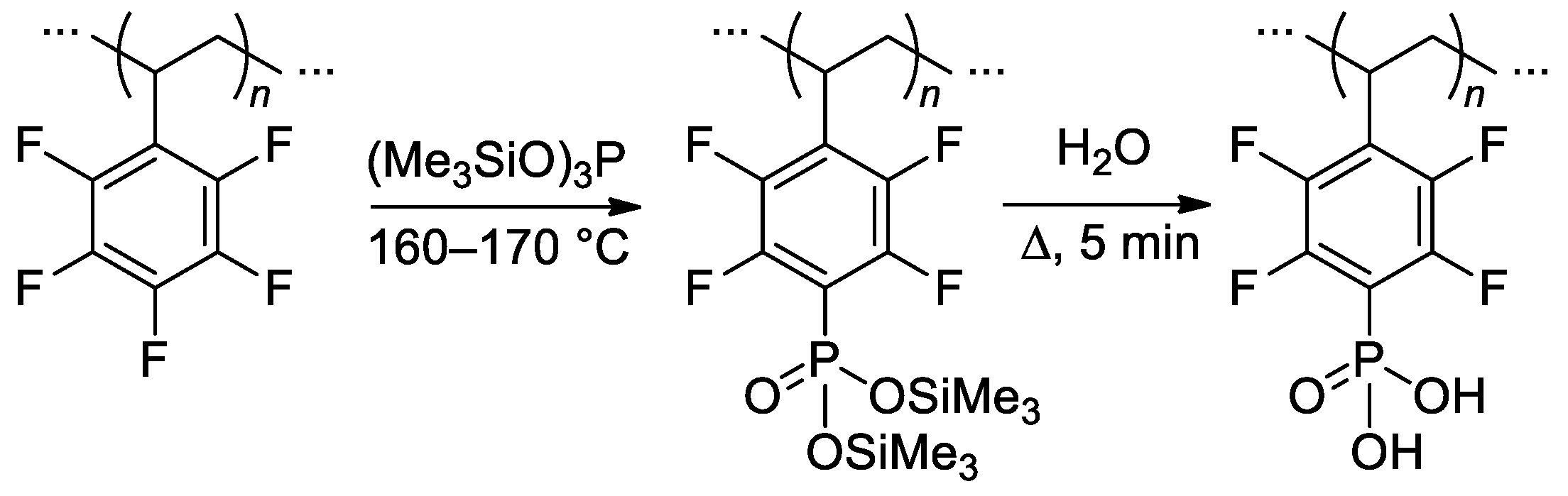
Efficient (up to 90%) post-phosphonation of poly(pentafluorostyrene) (Mn = 30.2 kDa, ÐM = 1.92) was achieved via the Michaelis–Arbuzov reaction with (Me3SiO)3P (2.4–3 equiv.) in bulk at 160–170 °C, followed by hydrolysis [131] (Scheme 33). In [132], the phosphonation protocol was modified for achieving a lower degree of phosphonation (lower (Me3SiO)3P to poly(pentafluorostyrene) ratio, 100 °C, homogenization by the use of N,N-dimethylacetamide). The materials obtained allowed us to make significant progress in the development of high-temperature fuel cells [133,134,135] (see Section 3.4).
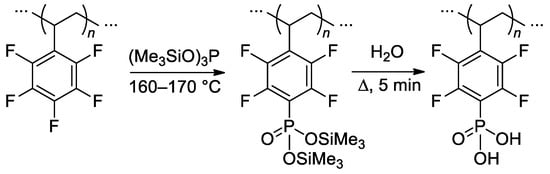
Scheme 33.
Direct selective phophonation of poly(pentafluorostyrene) [131].
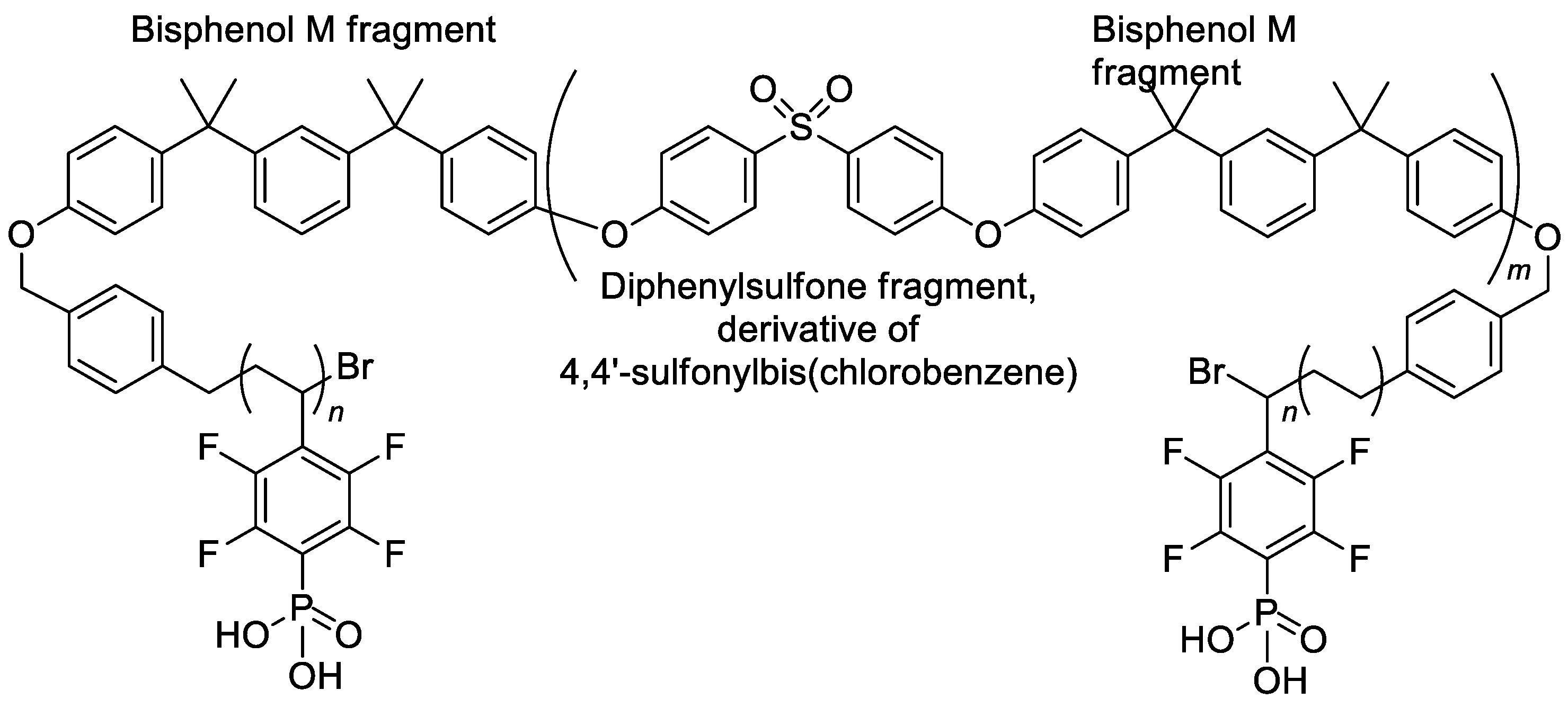
The reaction, presented in Scheme 33, was also used for post-modification of the complex triblock copolymer, the product of the (i) polycondensation of Bisphenol M with 4,4′-sulfonylbis(chlorobenzene); (ii) treatment with 1,4-bis(bromomethyl)benzene; (iii) ATRP with pentafluorostyrene [136]. The structural formula of the copolymer thus obtained is presented in Scheme 34.
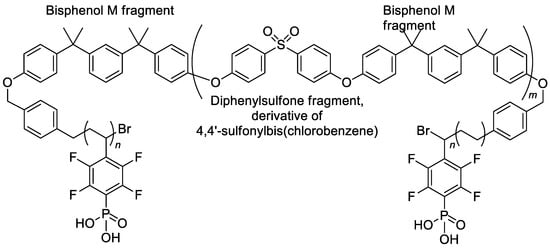
Scheme 34.
Structure of phosphonated triblock copolymers [136].
2.4.5. Other Methods
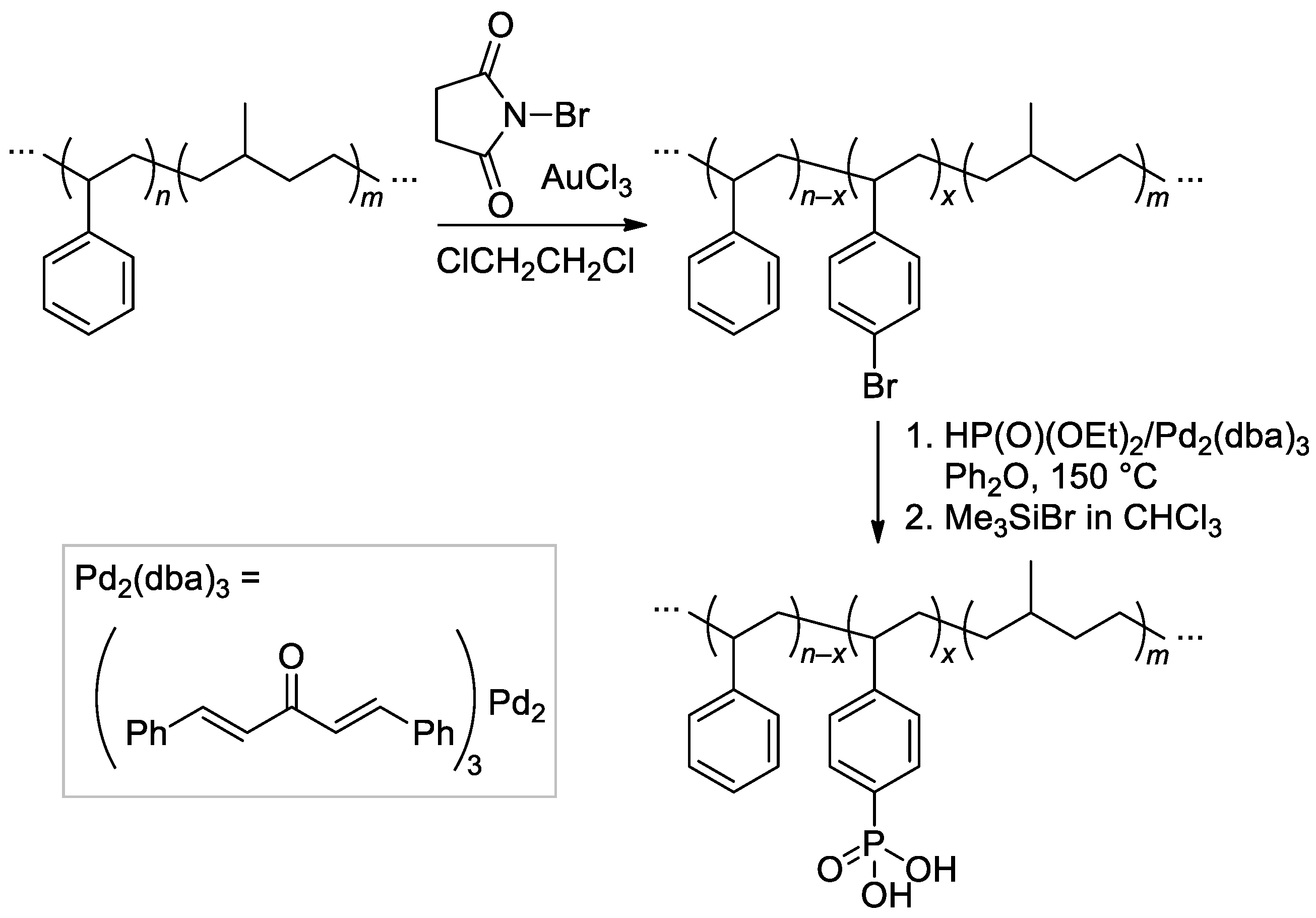
Poly(styrene-b-methylbutylene)s were synthesized by sequential anionic polymerization of styrene and isoprene, followed by selective hydrogenation of aliphatic C=C bonds. Bromination, cross-coupling reaction with HP(O)(OEt)2, and two-stage cleavage (Scheme 35) resulted in phosphonated copolymers with different content of the –P(O)(OH)2 groups [137,138].
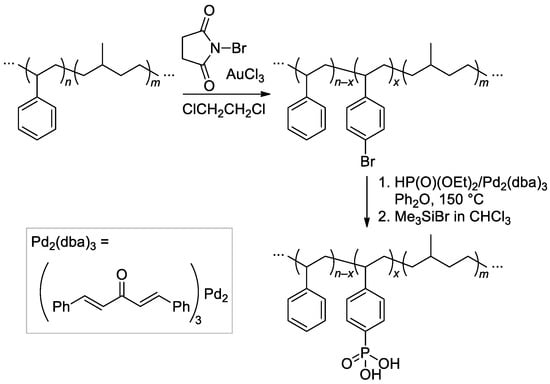
Scheme 35.
Synthetic procedure for phosphonated poly(styrene-b-methylbutylene)s [137,138].
Cross-coupling approach was also used in the post-modification of the Bisphenol A/4,4′-sulfonylbis(chlorobenzene) polycondensation product [139] (Scheme 36).
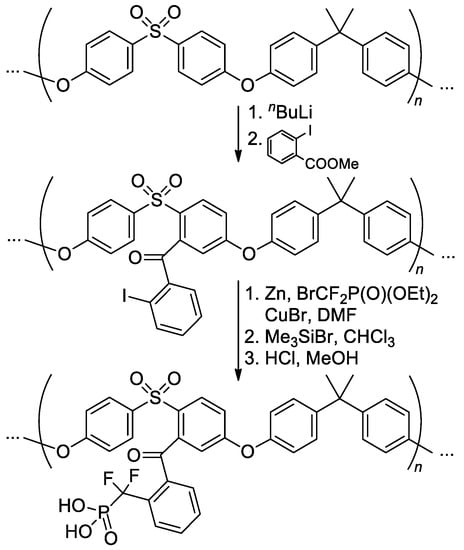
Scheme 36.
Synthetic pathway to o-benzoyl(difluoromethylenephosphonic acid)-functionalized polymer [139].
The original approach to cross-linked copolymers, derivatives of poly(vinylidene difluoride), proposed by Sinirlioglu et al. [140], is based on dehydrofluorination of –(CH2CF2)n– with a formation of unsaturated fragments, free radical-initiated graft copolymerization with glycidyl methacrylate, and the reaction of the oxirane fragments with PVPA at the final stage. Polymer membranes were obtained by evaporation of the polymer solutions in DMF.
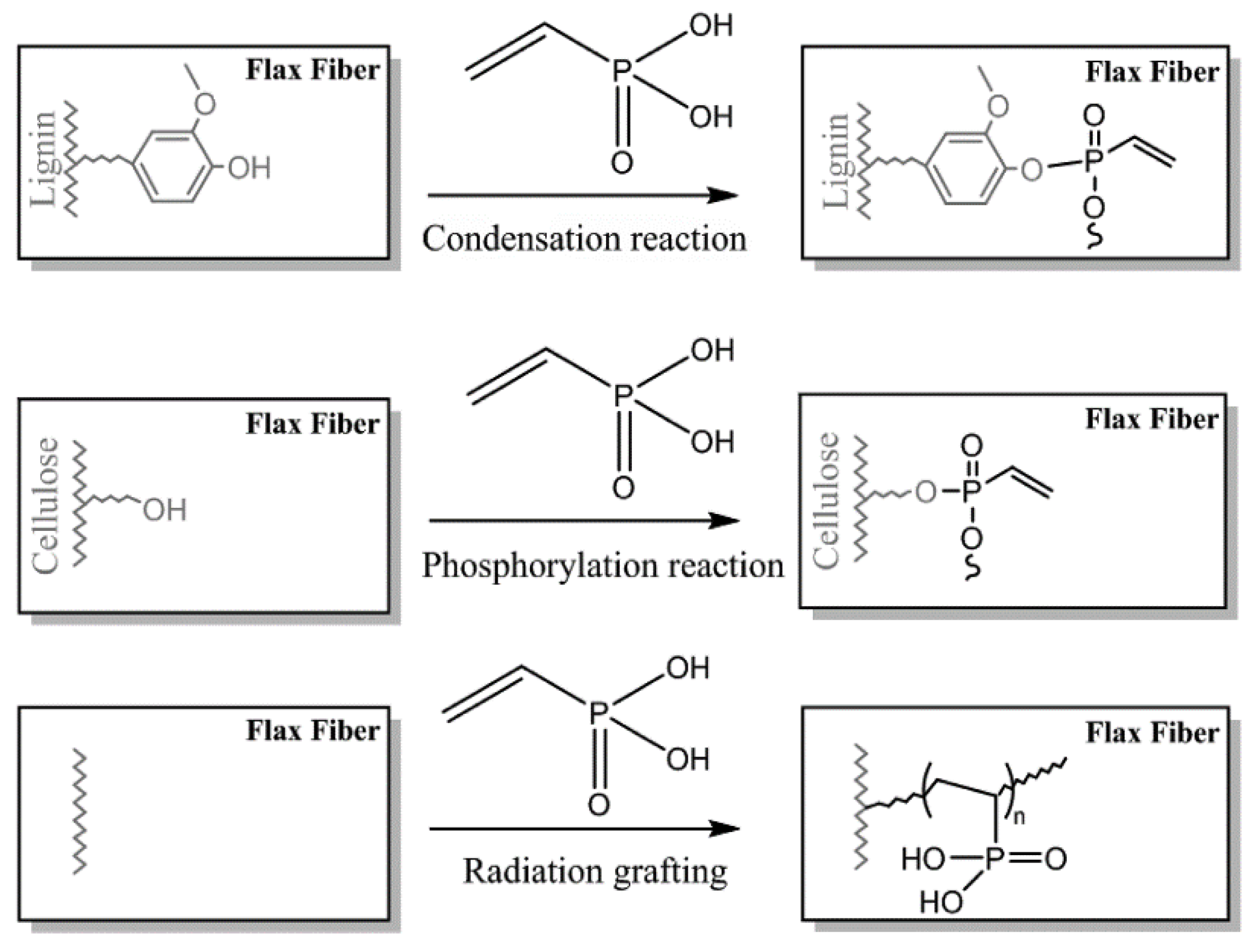
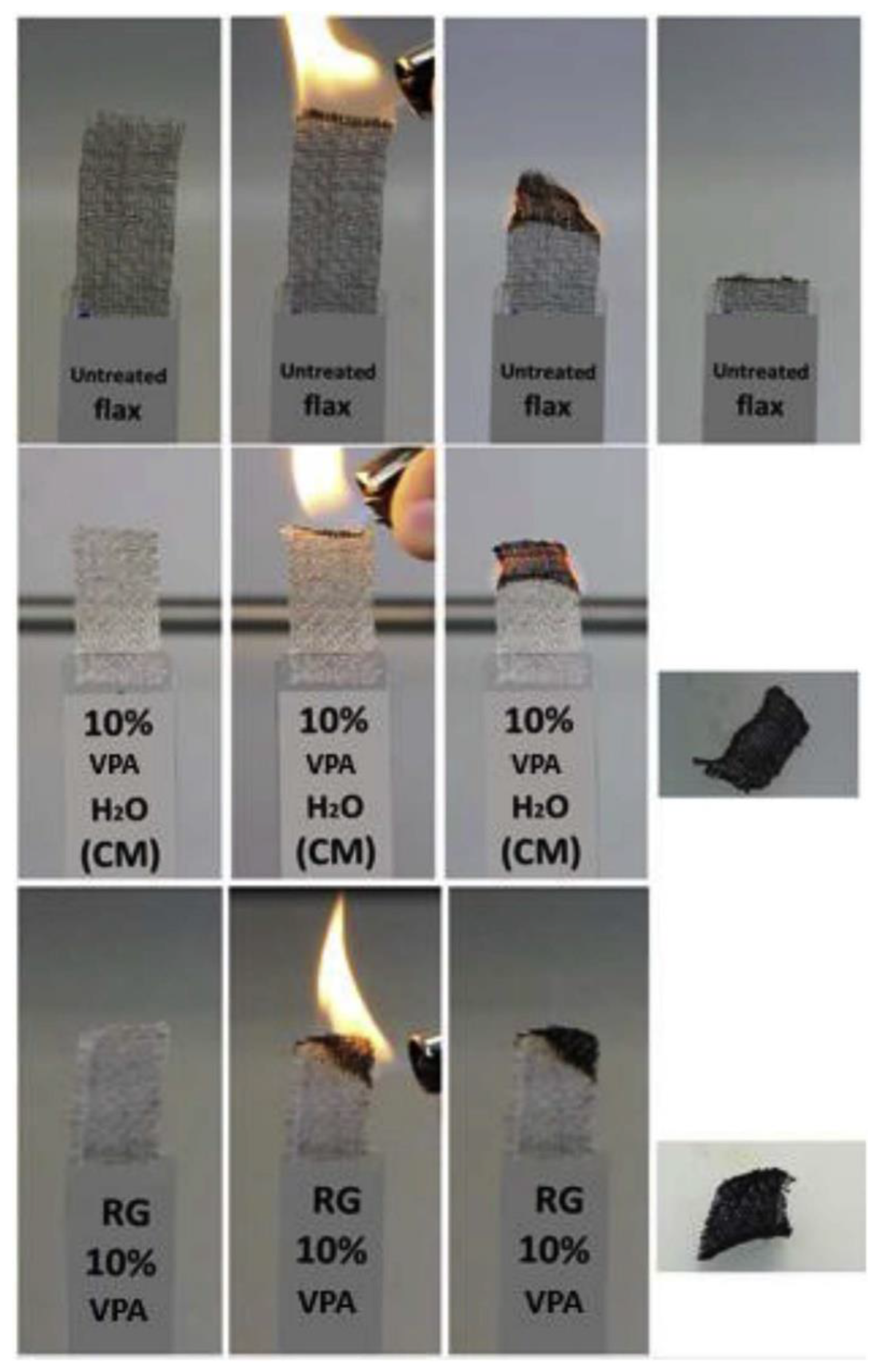
Two routes for grafting of the VPA on flax fabrics (81 wt% of cellulose, 13 wt% of hemicelluloses, and 2.7 wt% of lignin) were studied in [141], namely, radiation grafting and chemical modification (Figure 7). These approaches were studied in order to improve flame retardant characteristics of the fibers, qualitatively different results were obtained for the methods used (see Section 3.5.1).
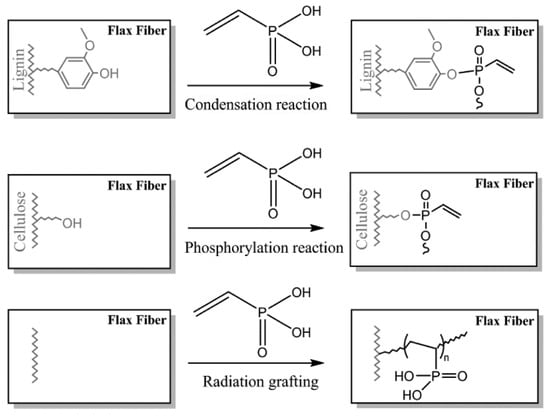
Figure 7.
VPA grafting reactions on flax fibers through chemical modification in soft conditions (top), chemical modification in severe conditions (middle), and radiation grafting (bottom). Reprinted with permission from [141]. Copyright (2018) Elsevier B. V.
Hydrolyzed polyvinyl alcohols (72 and 145 kDa) were phosphorylated using reactions with H3PO4/urea, with POCl3 and subsequent hydrolysis, and with (MeO)3P/I2 [142]. In the latter case, PCPAs were obtained by the reactions with NaI in N-methyl-2-pyrrolidone or via conventional silylation/methanolysis.
Efficient mechanochemical phosphorylation of cellulose, lignin, PEG, poly(vinyl alcohol), and poly(vinyl chloride) using P2O5 was described in [143]. Phosphate loadings of ~0.2 kg∙mol−1 were achieved for PEG and lignin, whereas for cellulose, poly(vinyl alcohol), and poly(vinyl chloride) phosphate loadings were 3.3, 4.4, and 2.2 kg∙mol−1, respectively.
2.5. Other Synthetic Approaches to SideChain PCPAs
2.5.1. Metathesis Polycondensation
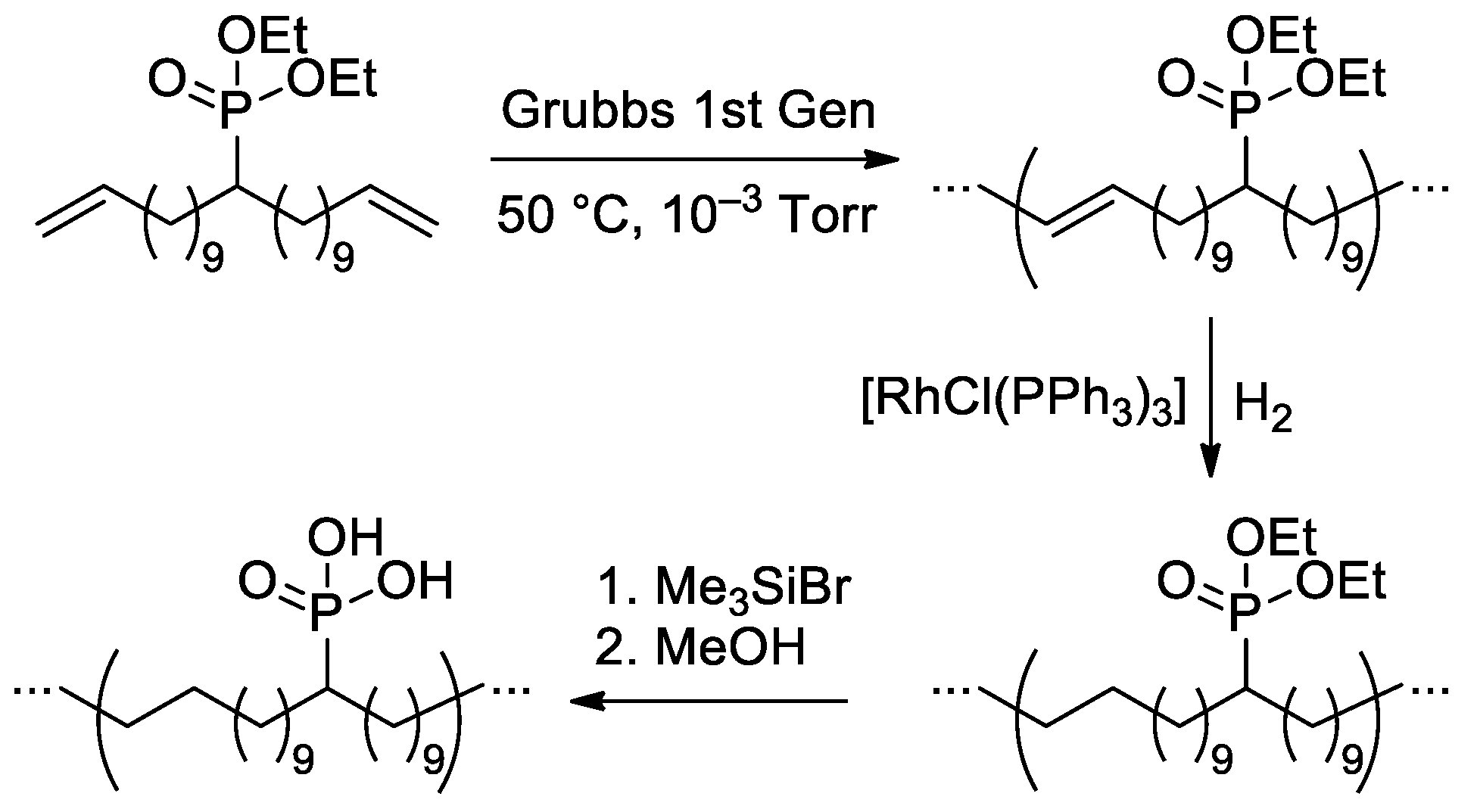
Polyethylene’ containing one –P(O)(OH)2 substituent in 21 carbon atoms, was synthesized in three steps by Wagener and Coll. [144] (Scheme 37). The authors noted that compared to the typical oxidative phosphorylation reactions on polyethylene [118], the proposed method offers complete control of the microstructure, which will lead to a systematic understanding of how microstructure and resulting morphology dictate particular properties. However, the properties of the material obtained were not studied in depth due to the low solubility of the –P(O)(OH)2 substituted PE (before deprotection, the Mn was 19.5 kDa, and ÐM was 1.7).
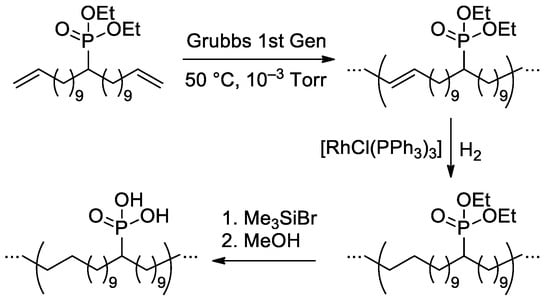
Scheme 37.
Synthetic scheme of precisely functionalized polyethylene with phosphonic acid on every 21st carbon [144].
Later, the Wagener group expanded a range of diene monomers by varying the methylene units between phosphonate and –CH=CH2 fragments and a number of –P(O)(OR)2 groups at the central carbon atom [145]. As a result, a series of diethyl phosphonate substituted ‘polyethylenes’ with different lengths of (CH2)n spacers between –CHP(O)(OEt)2– or –C[P(O)(OEt)2]2– fragments were obtained, without further transformation to PCPAs.
2.5.2. Ring-Opening Metathesis Polymerization
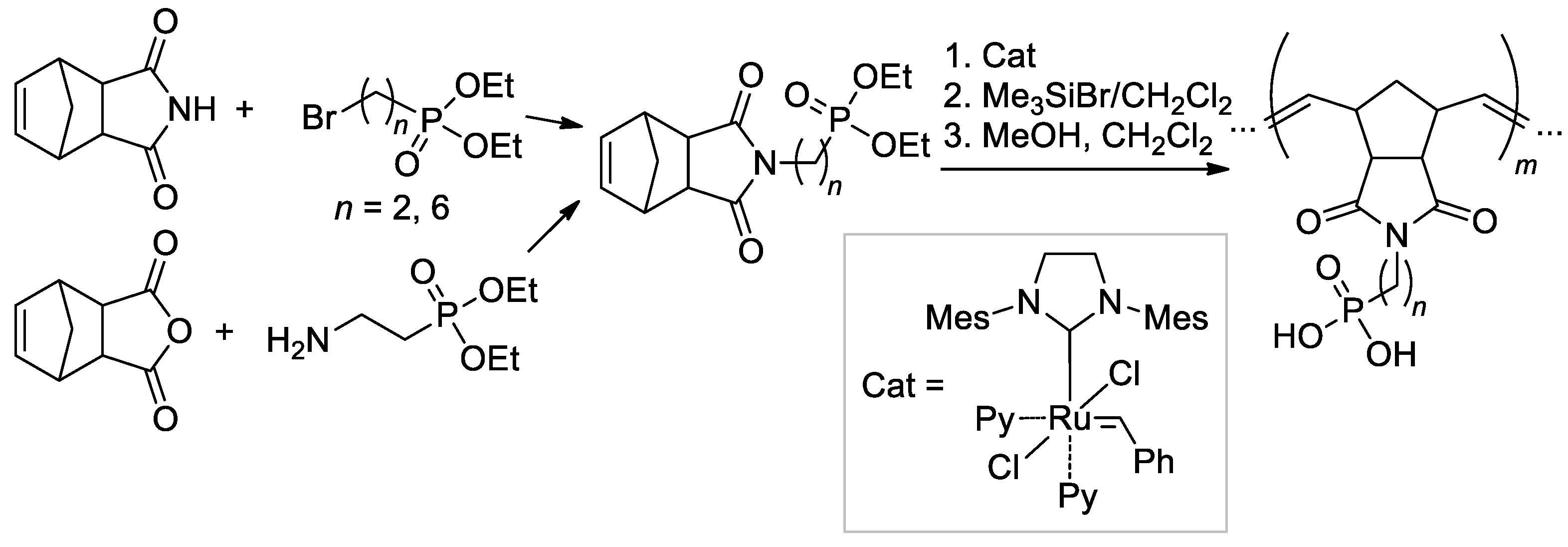
An efficient method of the synthesis of the phosphonated polymers with a well-defined MWD, composition, and architecture was proposed by Bingöl and Coll. [146] based on synthetically available adducts of cyclopenta-1,3-diene with maleimide or maleic anhydride (Scheme 38). Block- and stat-copolymers were also obtained with the use of n-Bu substituted imide. Note that –P(O)(OH)2-functionalized polymers were obtained by treatment with Me3SiBr in dry CH2Cl2 to yield TMS esters, followed by cleavage in a MeOH/CH2Cl2 mixture.
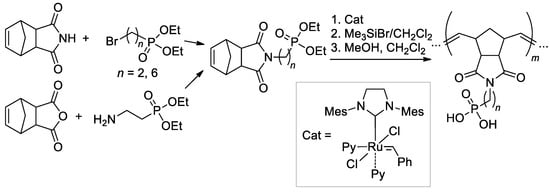
Scheme 38.
Synthesis of norbornene-based phosphonate monomers and ROMP with a formation of –P(O)(OH)2-functionalized polymers [146].
2.5.3. Nucleophilic Polycondensation
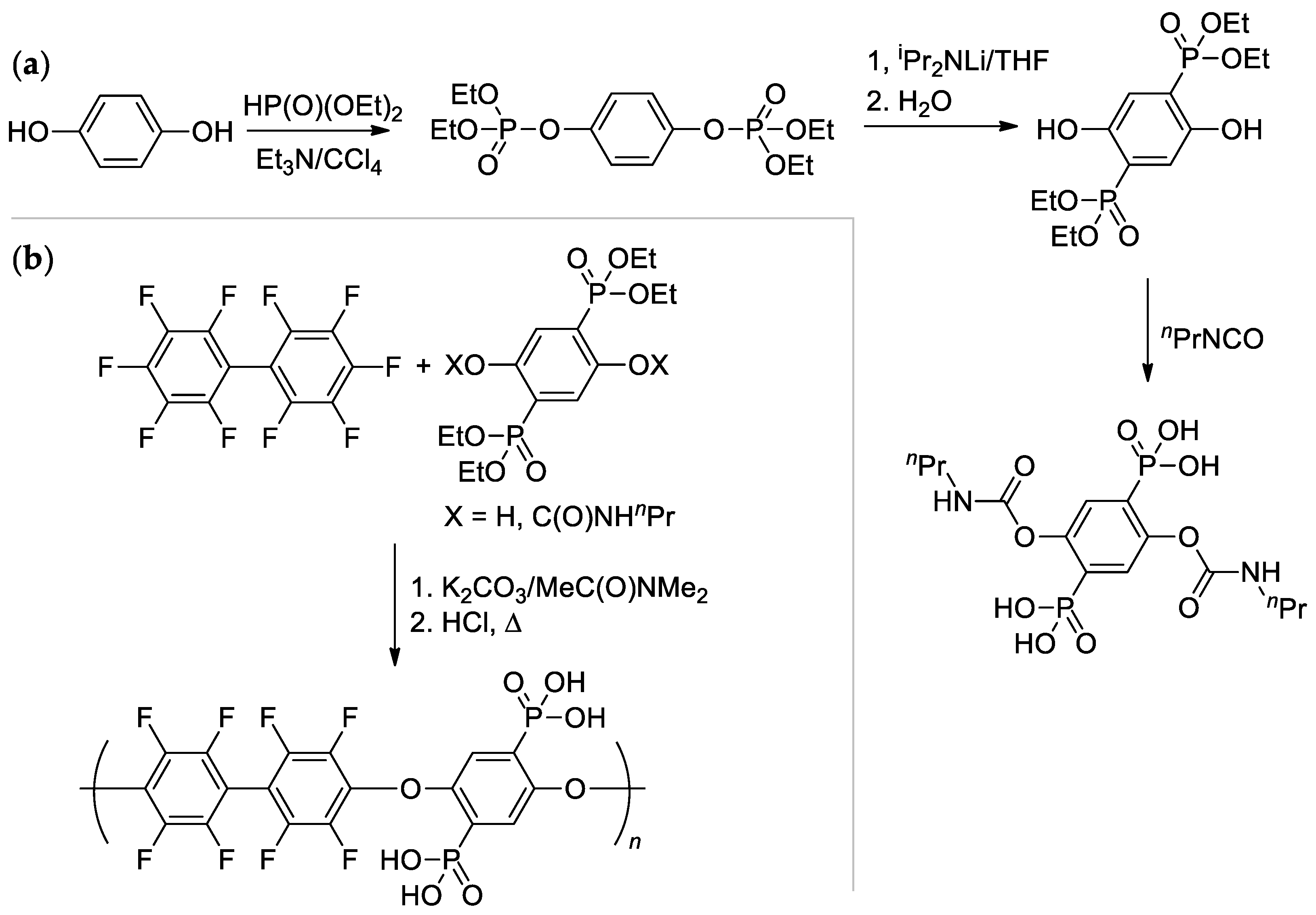
The high reactivity of the C–F bond in the p-position of RC6F5 was also exhibited in the reaction of perfluoro-1,1′-biphenyl with diphosphonated hydroquinone in the presence of K2CO3 [147] (Scheme 39). Copolymer with Mn = 28.4 kDa was obtained; intermediate modification of (2,5-dihydroxy-1,4-phenylene)diphosphonic acid by the reaction with nPrNCO resulted in dicarbamate derivative, more efficient in polycondensation (milder conditions, Mn = 37.9 kDa).
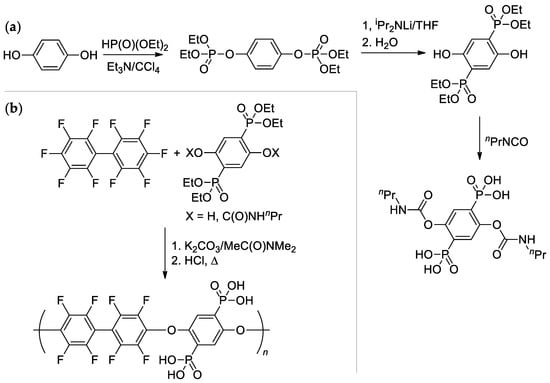
Scheme 39.
(a) Synthesis of diphosphonated hydroquinone and its dicarbamate derivative; (b) Polycondensation with perfluoro-1,1′-biphenyl with a formation of high-MW copolymer [147].
2.5.4. Reductive Coupling
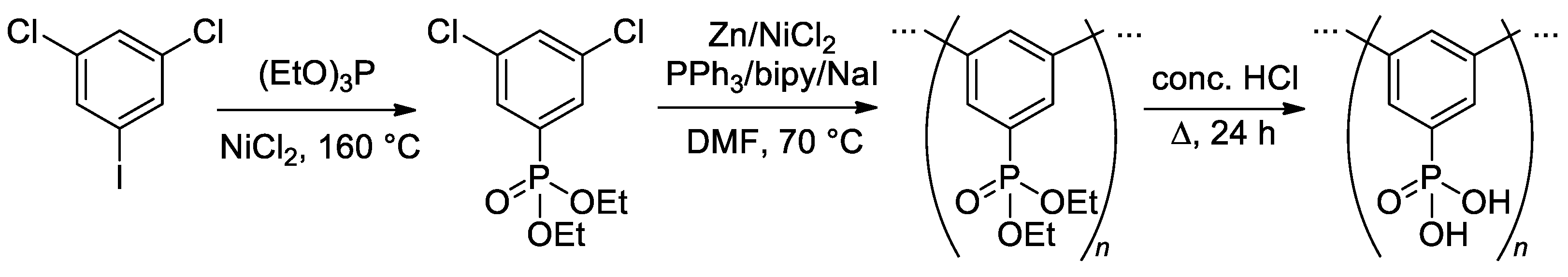
Nickel-catalyzed reductive coupling in the presence of metallic zinc was used in the synthesis of a prospective polymer, poly(1,3-phenylene-5-phosphonic acid) (PmPPA) [148]. During the synthesis of PmPPA (Scheme 40), –P(O)(OEt)2 group was introduced by nickel-catalyzed Arbuzov reaction of 1,3-dichloro-5-iodobenzene with triethyl phosphite, and after reductive coupling phosphonate groups were hydrolized by HCl.
Scheme 40.
Synthesis of poly(1,3-phenylene-5-phosphonic acid) (PmPPA) [148].
3. Properties and Applications of Sidechain PCPAs
3.1. Physico-Chemical Characteristics and Solution Behavior of SideChain PCPAs
3.1.1. Physical State and Mechanical Properties
The physical state and characteristics of sidechain PCPAs can vary widely due to the diversity of the structures of macromolecules. Obviously, no one canceled the common patterns of the influence of the polymer microstructure on physicomechanical characteristics, but non-selective methods of the synthesis of sidechain PCPAs (mostly free-radical polymerization, see Section 2) make irrelevant the issue of polymer tacticity. Discussions about the influence of the polymer microstructure on polymer properties make sense only for well-researched polymers, synthesized by different methods, and PVPA is actually the only polymer in the group under consideration.
Depending on the molecular mass value, the samples of regioregular and atactic PVPA, obtained by free-radical polymerization, represent viscous liquid [77] or amorphous solid [66,149]. The sample morphology drastically changed with increasing water content. For example, for a relatively high-MW polymer (Mw = 62 kDa) up to ~45% relative humidity (RH) (~17% water sorption), the material remained a white powder. Above 45% RH, a transparent film was formed. With further increasing humidity, this film became sticky and turned into a gel at about 100% RH [149]. In the amorphous PVPA, no melting transition was detected, and a glass transition at ~220 °C was masked by dehydration with a formation of phosphonic acid anhydride [75] (on other data, for low-MW amorphous PVPA the glass temperature Tg = 141 °C [150]).
The behavior of cross-linked copolymer of HEMA, MOEP (0–100 mol% relative to HEMA), and BMEP (0.5 wt%) (see Scheme 4, obtained by γ-initiated free-radical polymerization, [108]), when immersed in water was found to be dependent on the fraction of MOEP in the polymer. The polymers with 0–20 mol% MOEP did not fracture during swelling and displayed concentration-dependent water sorption, while the copolymers with greater than 30 mol% MOEP exhibited catastrophic fracturing during swelling, resulting in the destruction of the sample geometry. In this way, with high content of acidic groups, even cross-linking does not ensure the stability of the copolymer shape (and this should be considered in developing formulations of hydrogels and composites).
3.1.2. Solution and Colloidal Behavior
The solution behavior of sidechain PCPAs mainly depends on the nature of the polymer backbone and the relative number of phosphonate (or phosphate) fragments. For example, PVPA is soluble in water and ethanol and can be purified by precipitation of the saturated ethanol solution in ethyl acetate [69]. Highly phosphonated PmPPA is soluble in water and water/alcohol mixtures but poorly soluble in pure methanol [148].
A high similarity of the solution behavior of poly(acrylic acid) and PVPA was demonstrated by Wegner and Coll. [151]. The results of the more recent study of the solution behavior of PVPA and random VPA/CH2=CHP(O)(OMe)2 copolymers by SEC-MALLS measurements [73] are presented in Table 1. The coefficients calculated from MHS plots were between 0.5 and 0.8 meaning that randomly coiled polymers were formed.

Table 1.
Characteristics of VPA/CH2=CHP(O)(OMe)2 copolymers obtained by SEC-MALLS measurements (sample concentration of 1 mg/mL) [73].
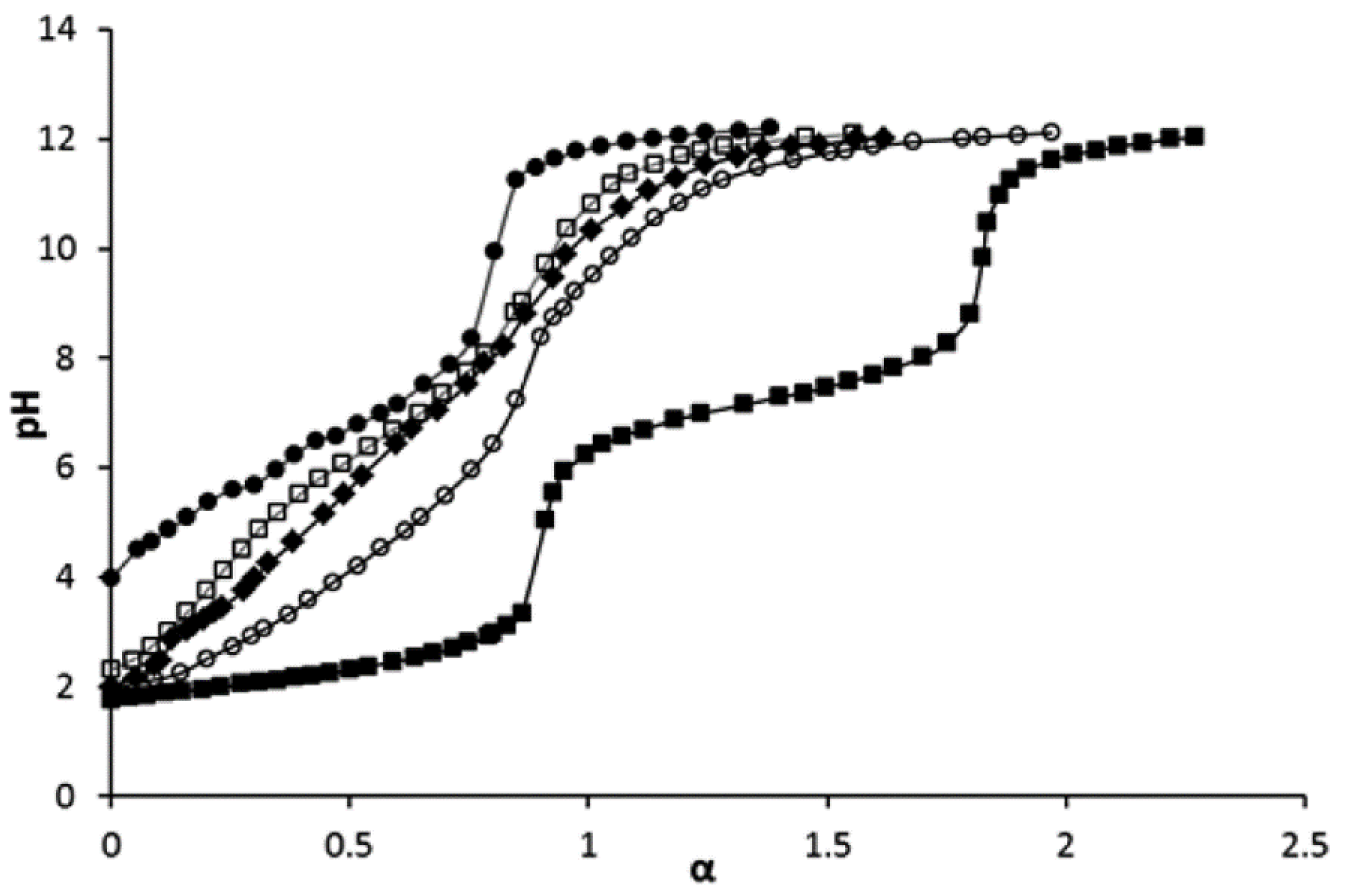
As demonstrated by Wegner et al. [57], while the VPA monomer shows the two dissociation steps expected, PVPA behaves as a monoprotic acid. The results of the study of copolymers of VPA and acrylic acid by potentiometric titration are presented in Figure 8 [95]. The nominal degrees of neutralization α of the poly(acrylic acid) (PVPA-0), copolymers containing 30 and 60 mol% of VPA (PVPA-30 and PVPA-60, respectively), poly(VPA) (PVPA-100) were calculated by taking into account elemental analysis data. The results of this study were in line with [57,65] with respect to PVPA. As opposed to the homopolymer, the titration curves of the copolymers showed two neutralization steps, which may be attributed to the contributions of VPA units with a pKa1 = 2.49 (step 1) and acrylic acid unit with a pKa2 = 7.74 (step 2).
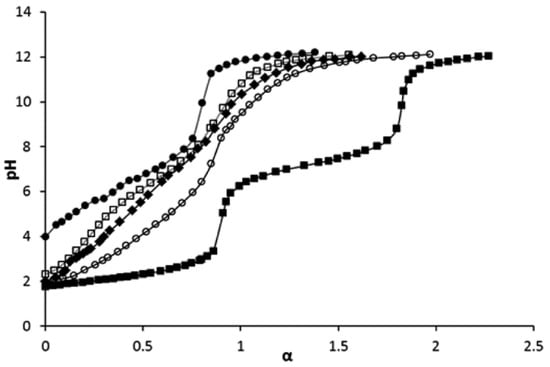
Figure 8.
pH titration curves of VPA (■), PVPA-0 (●), PVPA-30 (□), PVPA-60 (♦), and PVPA-100 (○), (co)polymer concentration was 1 mg∙mL−1 for all cases, α is nominal degree of neutralization. Reprinted with permission from [95]. Copyright (2016) American Chemical Society.
In contrast with PVPA, for PmPPA (see Scheme 40) the pH titration curves showed distinct pH steps for the addition of one and two equivalents of base [148].
The solution behavior of polymers affects the ability to use certain methods of the preparation of polymer films and scaffolds. Among other approaches, ES molding has been successfully used in obtaining fibrous materials for different applications [152,153]. However, this method remains practically unused for sidechain PCPAs, despite their polyelectrolyte nature. Only the works of Lee [154,155] and Akar et al. [103] reported the results of the studies on the subject. In [154], ES fibrous mats with good morphology were prepared from an aqueous solution of the poly(vinyl alcohol) (PVA)/PVPA mixture. To prevent swelling, the samples were subjected to thermal treatment or chemical cross-linking (methanol/glutaraldehyde). In the next work [155], ES fibrous materials with lower content of PVPA were prepared and stabilized by heating at 150 °C for 24 h in vacuo, chemical cross-linking was not used to avoid toxicity. In [103], the preparation of the ES fibrous materials from a copolymer of VPA, acrylonitrile, and methyl acrylate is described, and copolymer solutions in DMF were used.
Amphiphilic diblock copolymers usually demonstrated complex solution behavior. For example, PMEMA-b-poly(MOEP) and PMEMA-b-poly(MMPN) (for their synthesis, see Scheme 23) in basic aqueous media formed core-shell micelles as the PMEMA block formed insoluble micelle core. It is noteworthy that PMEMA-b-poly(MMPN) diblock copolymers interacted strongly with Ca2+ with a formation of reverse micellar structures. [29].
As was shown in [110], complex random copolymer, containing phosphorylcholine and doxorubicin fragments can self-assemble into nanoparticles with doxorubicin as the core, and hydrophilic P(MPC-co-PEGMA-BZ, see Section 3.3.4) as the shell in an aqueous solution.
3.2. Metal Complexation of the Sidechain PCPAs, Polymer-Inorganic Hybrids, and Composites
3.2.1. Complexation of Sidechain PCPAs with Metal Ions
The reaction of PVPA with calcium tetraphosphate (Equation (2)) in 2:3 molar ratio results in the formation of Ca-PVPA and HAp [150].
2PVPA + 3Ca4(PO4)2O → 2Ca-PVPA + Ca10(PO4)6(OH)2
Cross-linked polymers presented in Scheme 28 demonstrated excellent adsorption of Pb2+ and Cu2+ ions. The values of the maximum adsorption capacity Qm were 7.19 and 17.0 mmol∙g−1, respectively [115]. Another cross-linked copolymer was obtained from a terpolymer of MOEP, HEMA and 2-hydroxypropane-1,3-diyl bis(2-methylacrylate) by radical polymerization with acrylamide and N,N′-(propane-1,3-diyl)diacrylamide to mimic aquatic caddisworm silk and natural processes of Ca2+ complexation [106]. The clear homogeneous hydrogel was formed, and in the presence of divalent metal ions, Mg2+, Ca2+, and Zn2+, the hydrogels shrank to about 65% of the initial volume and became translucent. Above a critical phosphate sidechain density, hydrogels equilibrated with Ca2+ or Zn2+ ions displayed increased initial stiffness, strain-rate dependent yield behavior, and required 100 times more work to fracture than hydrogels equilibrated with Mg2+ or Na+ ions. The toughness of the bio-inspired hydrogels exceeded the toughness of cartilage and meniscus, suggesting potential application as prosthetic biomaterials, according to the authors of the study [106]. Mg2+ complexation by MOEP/HEMA copolymer in aqueous media was the subject of a separate study by the same research team [23].
Results of the study of VPA homopolymer and VPA copolymers with acrylamide and vinylsulfonic acid have demonstrated a high potential if the use of VPA copolymers as antiscalants for (Fe, Mg) silicates [156], the best efficiency was detected for a copolymer of VPA with vinylsulfonic acid (1:5 comonomer ratio).
The product of the hydrolytic cross-linking of the CH2=CHP(O)(OMe)2, CH2=CF2, and CH2=CHSi(OEt)3 terpolymer exhibited very high Eu3+ extraction from its aqueous solutions [101]. Hyper-cross-linked homopolymer of (CH2=C(Me)C(O)OCH2CH2O)2P(O)OH demonstrated adsorption capacity for UO22+ up to 800 mg∙g−1 and excellent selectivity for U(VI) adsorption over coexisting ions. In addition, this polymer also displayed high adsorption capacities for Gd, Sm, Ce, Nd, Tb, and Eu [111].
The ability of the –P(O)(OH)2 fragment to chemical binding with the metal ions largely determines the use of phosphates as anticorrosive agents. Copolymers of CF2=CH2 with CH2=C(CF3)C(O)OCH2P(O)(OH)2 (79–96 mol% of CF2=CH2, Mn up to 10 kDa) were synthesized by tert-amyl peroxy-2-ethylhexanoate initiated free-radical copolymerization in dimethyl carbonate [36]. Steel plates, coated with the copolymer, displayed satisfactory anticorrosion properties under a simulated seawater environment. An original and efficient approach to development of anticorrosive agents was proposed by Kousar and Moratti [157], via the use of methacrylate copolymers containing –(CH2)2(CF2)7CH3 and –CH2P(O)(OH)2 substituents. These copolymers formed monolayers at the stainless-steel surface (the contact angle >128°) and were stable after being submerged in water for a week. The best set of characteristics was achieved for copolymers containing phosphonated and fluorinated monomers in a 2:1 ratio.
3.2.2. Effects of the Sidechain PCPAs on Crystal Growth and Morphology
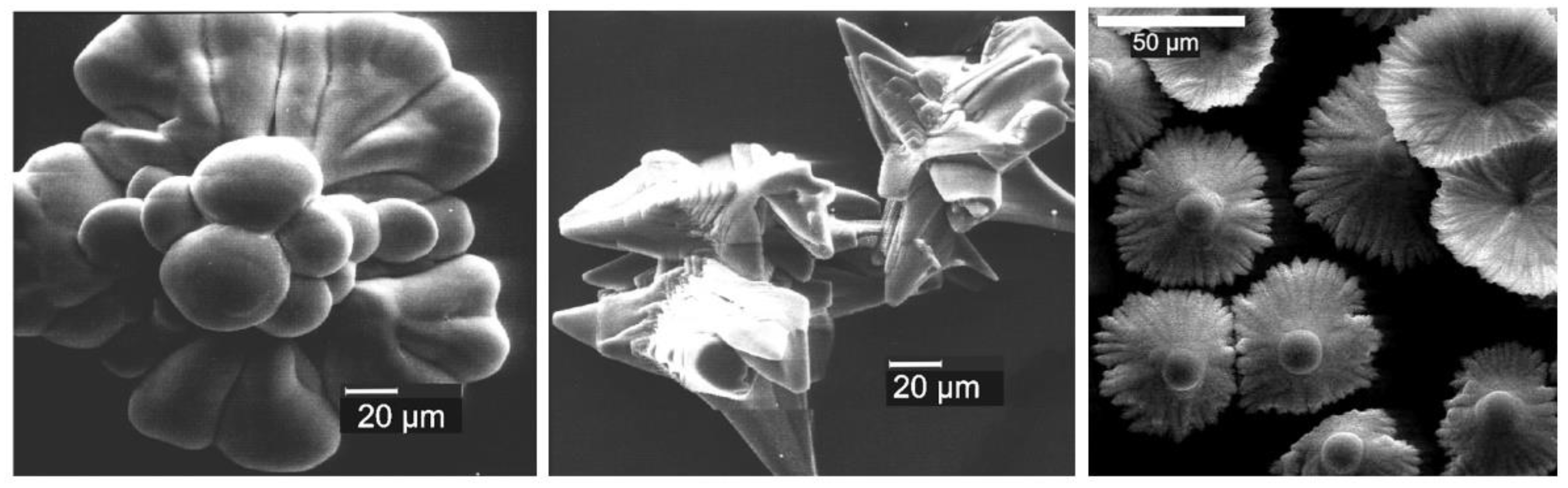
A number of works from the Penczek group were devoted to the study of the synthesis and morphology of CaCO3 particles obtained in the presence of sidechain PCPAs, and an amazing diversity of the crystallite’s forms was observed [119,120,122,158,159] (Figure 9).
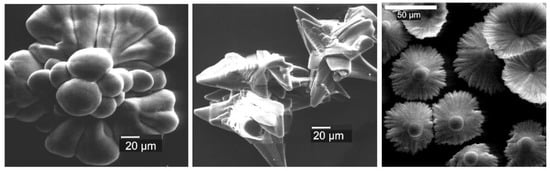
Figure 9.
Examples of CaCO3 microcrystals obtained in the presence of PEG-b-(poly[2-(2-hydroxy ethyl)ethylene] with variable degrees of phosphate substitution. Reprinted with permission from [122]. Copyright (2002) Wiley-VCH GmbH.
Diblock copolymer mPEG-b-poly(EMEPN) (see Scheme 26) [114] demonstrated an interesting concentration effect on the formation of CaCO3 particles (Figure 10). Cölfen and Antonietti [160] have made a certain contribution to this issue; however, a detailed comparative analysis of their studies is complicated by the lack of detailed information on the structure of copolymers used.

Figure 10.
SEM images of CaCO3 obtained on a glass slip by the gas diffusion reaction after 1 week in 3 mL of solution with 10 mmol/L of Ca2+ in the presence of different concentrations of phosphonated copolymer. (a) 10−2 g∙L−1; (b) A typical spherical structure in image (a); (c) The surface structure of particle (b); (d) 10−3 g∙L−1; (e) one modified spherical structure in image (d) and the surface structure (inset); (f) one calcite rhombohedral structure in image (d) with a hole and the porous surface structure in the hole (inset). Reprinted with permission from [114]. Copyright (2005) Wiley-VCH GmbH.
Concentration effects, observed by the Penczek group, can be interpreted in view of the study of Dimova and Coll. [125] of the interaction of double hydrophilic block copolymers (see Scheme 31) with calcite crystals by isothermal titration calorimetry. It was shown that the interaction of copolymers with relatively large CaCO3 crystals is an exothermic process (well fitted by a Langmuir adsorption model). In the absence of large crystals, the observed endothermic process was attributed to polymer interaction with small clusters or aggregates of calcium carbonate of the type (CaCO3)x(H2O)y where 1 < x < 100. The formation of similar aggregates cannot but affect the crystallization process in the presence of PCPAs. Morphological control of BaSO4 microstructure by copolymers PEG-b-PMMA and PEG-b-PMMA-PO3H2(1%) was studied by Cölfen and Coll. [128].
3.2.3. Hybrid Nanoparticle Formation by Sidechain PCPAs
The ability of divalent transition metal ions to induce micellization by mixing aqueous solutions of the partly ionized poly(acrylamide)-b-PVPA copolymers (see Scheme 22) and metal ions was studied as a function of the nature of the metal cation [96]. Based on DLS measurements, the authors concluded that the micellization efficiency followed the sequence: Ni2+, Co2+ < Zn2+ < Mn2+ < Cu2+.
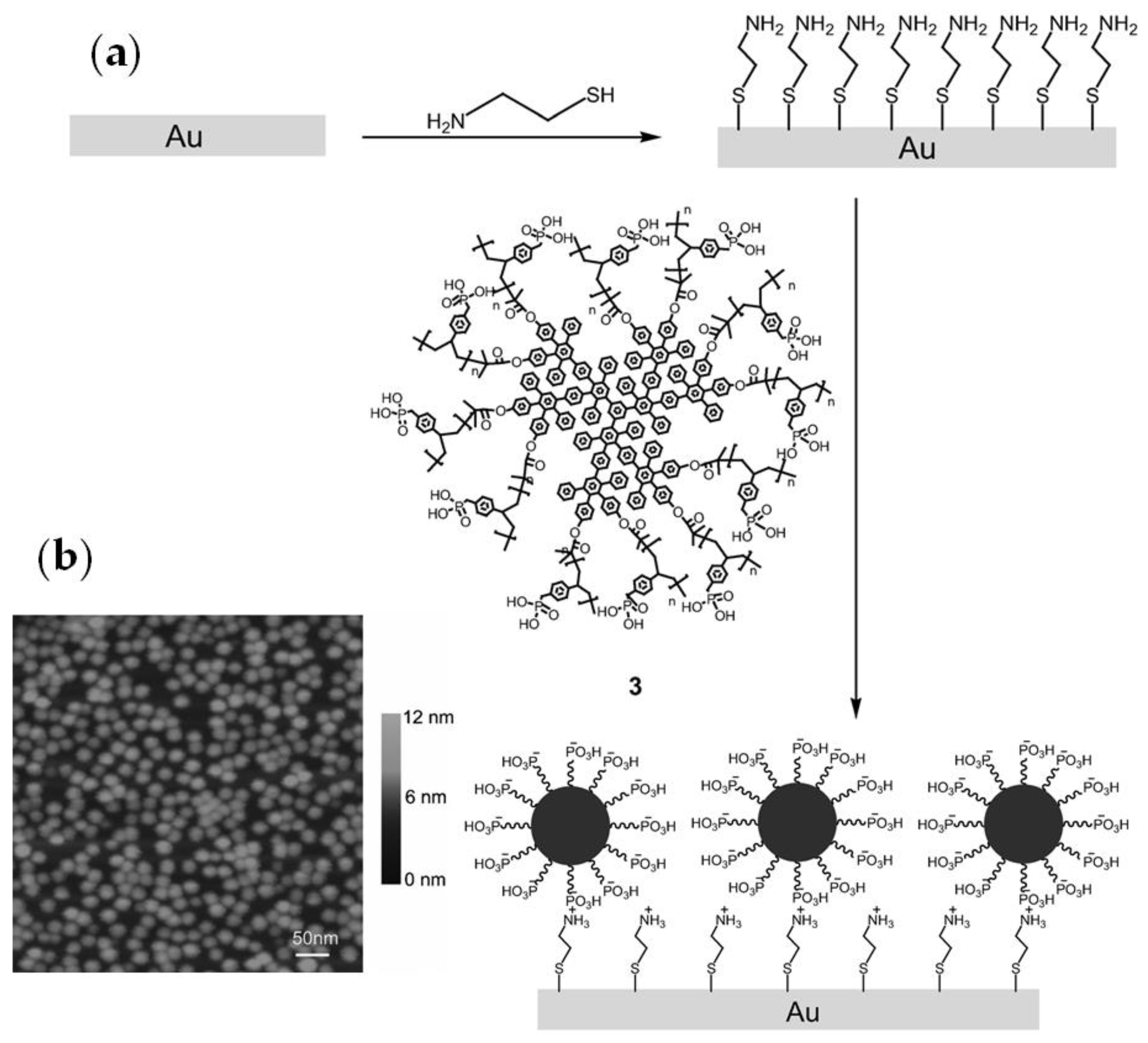
After treatment of the gold surface by HS(CH2)2NH2, the self-assembled film (SAF) of the core-shell copolymer three (Figure 11a) was studied [74]. The self-assembly of a core-shell macromolecule three on gold was characterized by atomic force microscopy (AFM). As can be seen in Figure 11b, separated globular particles appear to be uniformly monodisperse with a diameter of ~20 nm.
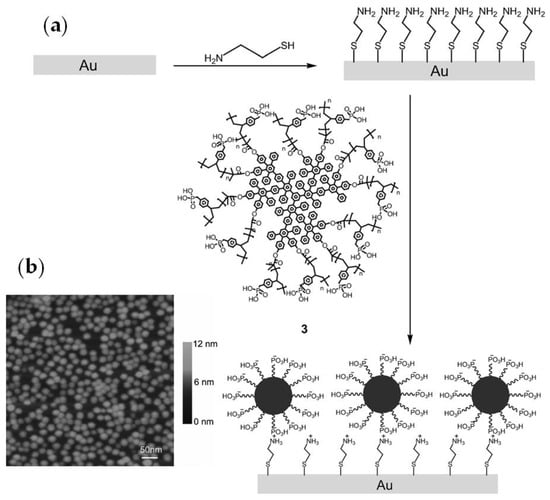
Figure 11.
(a) The deposition of core-shell macromolecule 3 on a modified gold substrate through electrostatic interaction; (b) AFM image of a self-assembled film of 3 on gold. Reprinted with permission from [74]. Copyright (2012) Wiley-VCH GmbH.
When considering and using PVPA as a biomimetic analog of matrix phosphoproteins, Tay and Coll. [60] studied mineralization of cross-linked collagen in the presence of Ca2+, PO43−, OH− ions and poly(acrylic acid). Intrafibrillar and extrafibrillar mineralization via a bottom-up nanoparticle assembly based on the non-classical crystallization pathway were identified. Selected area electron diffraction patterns of highly mineralized collagen fibrils were nearly identical to those of natural bone, with apatite crystallites preferentially aligned along the collagen fibril axes. Conversely, only large mineral spheres with no preferred association with collagen fibrils were observed in the absence of PVPA.
3.2.4. Polymer-Inorganic Composites
Composites of the Ca-PVPA and HAp were prepared by warm-pressing powder mixtures of Ca4(PO4)2O and PVPA at a weight ratio of 3.5:1 at temperatures of up to 300 °C and pressure up to 690 kpsi within 1 h [150]. The highest achieved values for the tensile strength and elastic modulus were 53 MPa and 32 GPa, respectively. The tensile strength values of the composites were lower than those of long bones (121–149 MPa), in the range of those reported for dentin (29.6–105.5 MPa), and higher than that reported for enamel (10.3 MPa). Elastic moduli of the composites were higher than long bones (17.2–18.6 GPa) and dentin (0.26–14.7 GPa) but lower than those of enamel (84.1–130 GPa). No further biomedical studies for these composites were conducted.
Attempt to prepare ferrimagnetic PVPA/BaFe12O19 nanocomposite was not entirely successful due to the adsorption of PVPA anions during the preparation of the nanocomposite that strongly influenced the magnetic properties, resulting in much lower saturation magnetization values [63].
A number of composite samples were prepared from phosphorylated poly(vinyl alcohol) and nano-sized HAp (the mean crystallite size 19 nm, 0–60 wt% loaded in composite formulation) [126]. In composites, obtained by chemical methods (with the use of an aqueous polymer solution), the phosphorylated polymer has demonstrated dispersant properties. With an increase in the content of HAp in composite, the tensile strength goes through the maximum of 26.2 MPa (50 wt% of HAp), Young’s modulus increases from 151 MPa (o% HAp) to 668 MPa (60% HAp), and elongation at break decreases from 39.2 to 4.8%.
3.3. Biomedical Applications of Sidechain PCPAs
3.3.1. Sidechain PCPAs and Cell Viability/Metabolism and Differentiation
For sidechain PCPAs, hydrolytic degradation should lead to the formation of –OH functionalized polymers with unpredictable properties. Research in this area is fragmentary; however, the results of the effect of PCPAs and their metabolites on cell adhesion and proliferation showed that sidechain PCPAs rarely demonstrate toxicity.
In particular, when studying ES mats based on PVPA/PVA, no toxicity was detected in experiments with MG-63 osteoblast-like cells for untreated materials, but cross-linked membranes (MeOH/glutaraldehyde) turned out to be toxic, the cell proliferation was also suppressed significantly [154]. In the follow-up study [155], PVPA/PVA ES mats demonstrated low cytotoxicity with respect to M3TCT3-E1 preosteoblast cells, which increased with the increase in PVPA content in the composite. At the same time, the presence of PVPA facilitated cell proliferation.
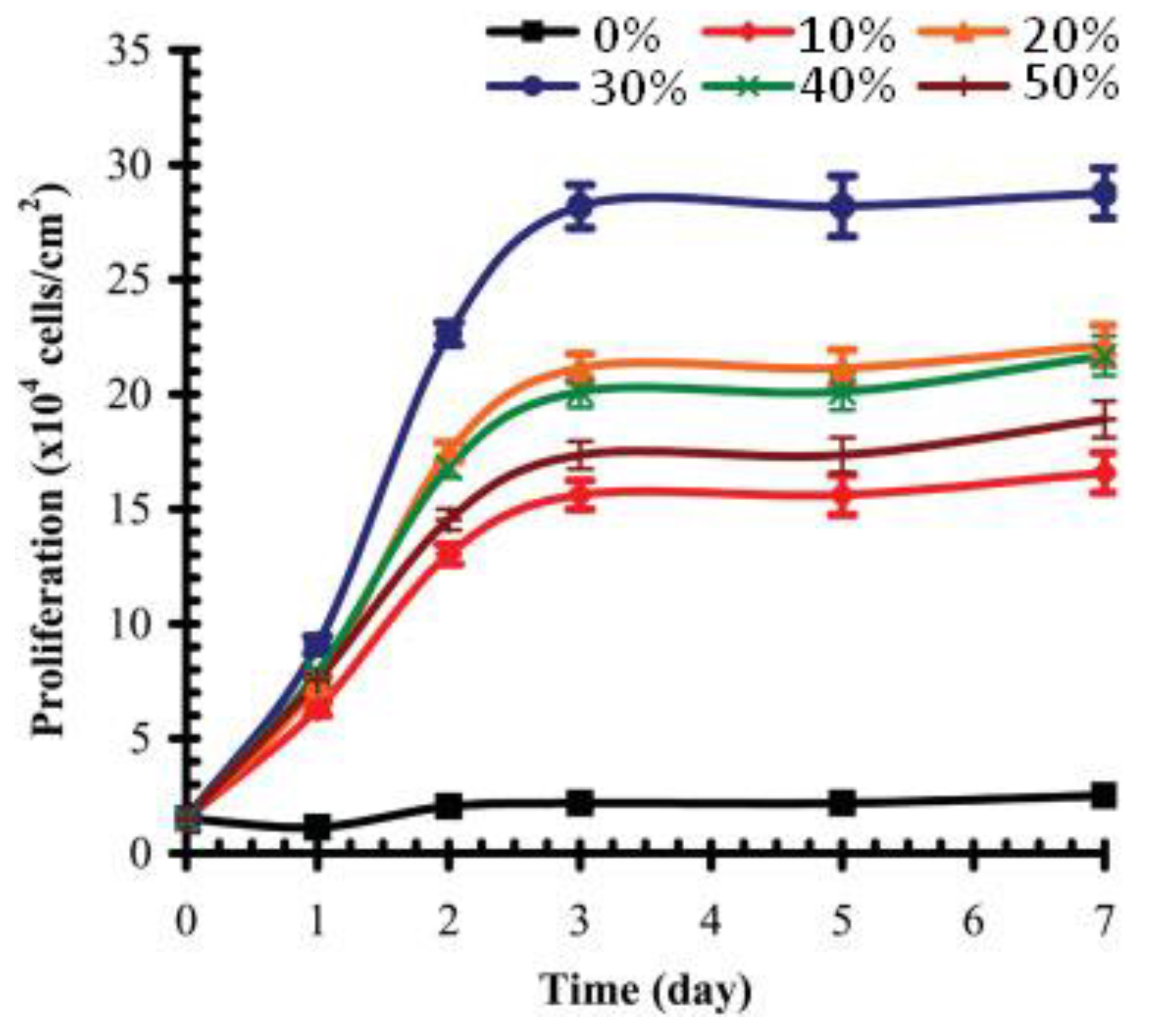
When studying osteogenic MC3T3-E1 subclone 4 cells on the surface of silicon, grafted by VPA—acrylamide copolymers, Gemeinhart and Coll. have demonstrated that cell adhesion and proliferation have a clear maximum that corresponds to 30 mol% content of VPA in the comonomer feed [100] (Figure 12). MC3T3-E1 maturation began at about the 20th day and was defined by matrix calcification and increased alkaline phosphatase activity. The mineralization of PVPA-modified surfaces was potentially due to both cellular differentiation and polymer-based calcification, but experiments in the absence of cells did not show mineralization. In this way, the cell-mediated mineralization, observed for the pVPA30-grafted surface, would benefit from osteointegration, which demonstrates high prospects for the use of VPA copolymers in the further design of bone tissue engineering scaffolds.
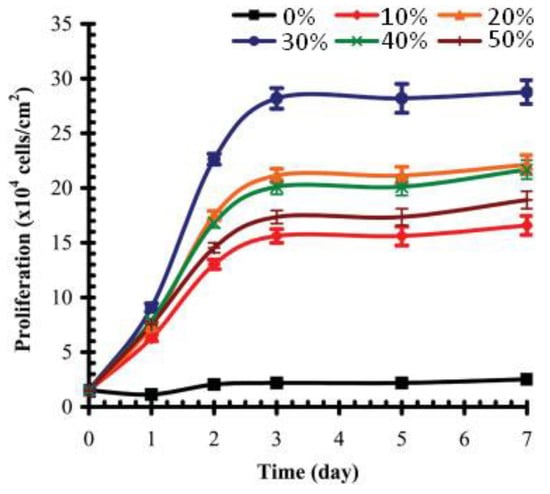
Figure 12.
Number (proliferation) of MC3T3-E1 subclone 4 preosteoblast cells present on substrates modified with graft copolymers created from feed compositions of increasing VPA. Surfaces were modified with polyacrylamide (pVPA0), PVPA (pVPA100), and 10% (pVPA10), 20% (pVPA20), 30% (pVPA30), 40% (pVPA40), and 50% (pVPA50) VPA in the comonomer feed. Reprinted with permission from [100]. Copyright (2006) Wiley-VCH GmbH.
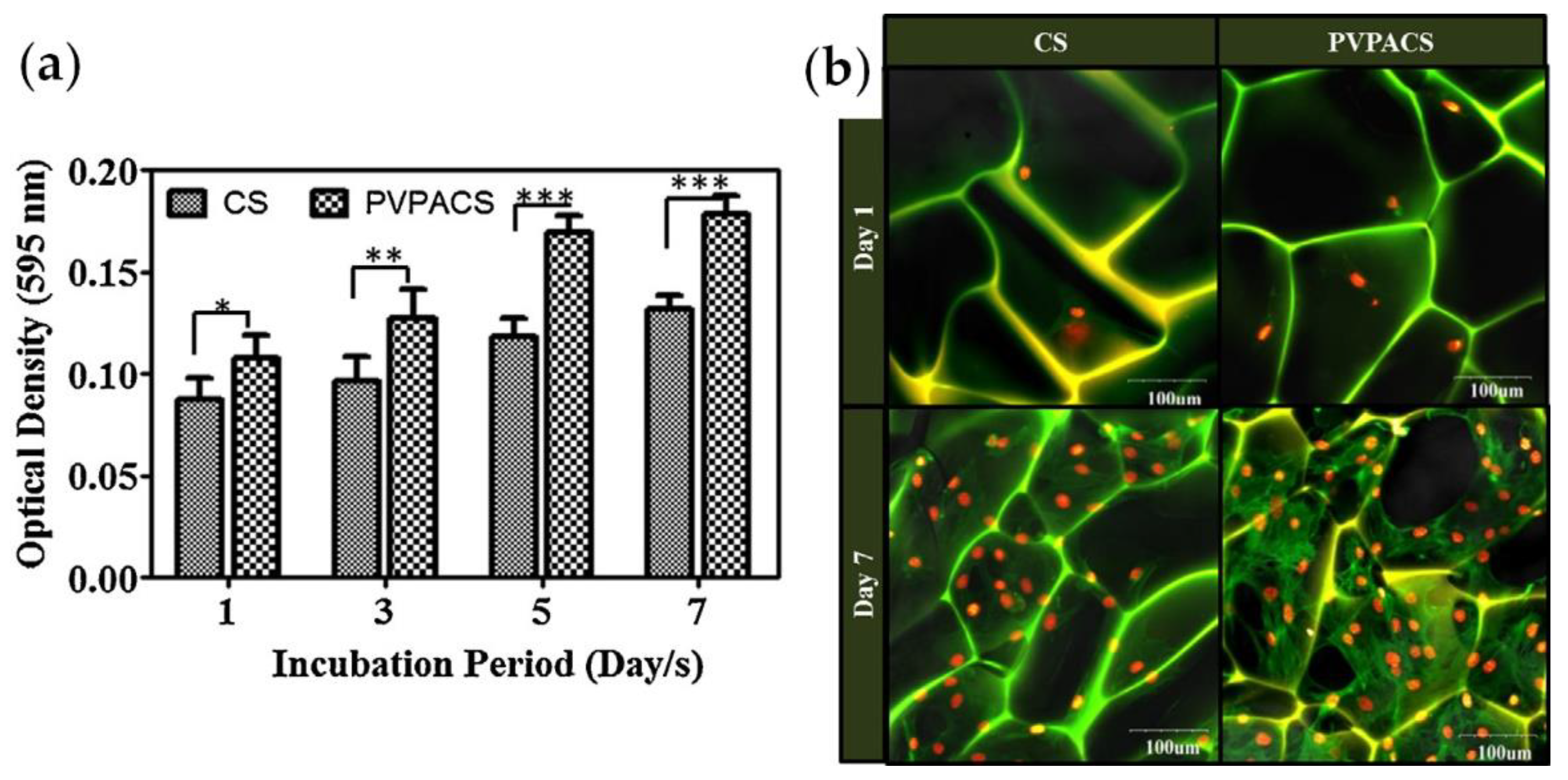
PVPA/chitosan composites were prepared by simultaneous adsorption of PVPA and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide mediated cross-linking; the porous three-dimensional matrices were prepared from the 2% aqueous solutions of chitosan (CS) or composite (PVPACS) containing 1% AcOH by thermally induced phase separation at −80 °C followed by sublimation of ice crystals [161]. MTT results for MC3T3-E1 pre-osteoblast cells have shown a higher degree of proliferation for PVPACS matrices compared with CS matrices (Figure 13).
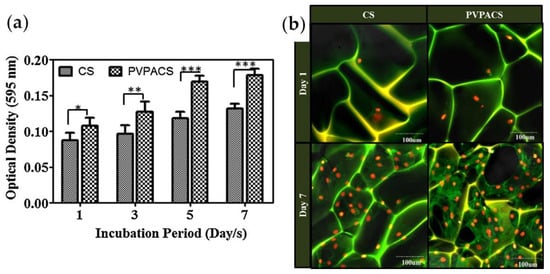
Figure 13.
(a) MTT assay of MC3T3-E1 pre-osteoblast cells proliferation on CS and PVPACS matrices after 1, 3, 5 and 7 days of incubation period, data represent the mean ± SD forfour replicates and significantly different values (*** p < 0.001, ** p < 0.01, * p < 0.05); (b) Confocal images of MC3T3-E1 pre-osteoblast cells cultured on CS and PVPACS matrices after 1 and 7 days of the incubation period. Reprinted with permission from [161]. Copyright (2014) Elsevier B. V.
Copolymers of acrylic acid, VPA, and ethylene glycol diacrylate as a cross-linking agent formed hydrogel. Freeze-drying the hydrogel resulted in obtaining the mesoporous materials. The pore diameter and formation of an interconnected pore network depended on the VPA content [97]. The experiments with human osteosarcoma-derived osteoblast (SaOS-2) cells showed that an increase in the VPA content supports cell adhesion and proliferation. The authors have said that hydrogels with 30 or 50 mol% VPA are ideal for use as bone void fillers, providing high swelling and increased osteoblast-like cell attachment and proliferation.
3.3.2. Biocompatibility of Sidechain PCPAs and Prospects for Use in Bone Surgery
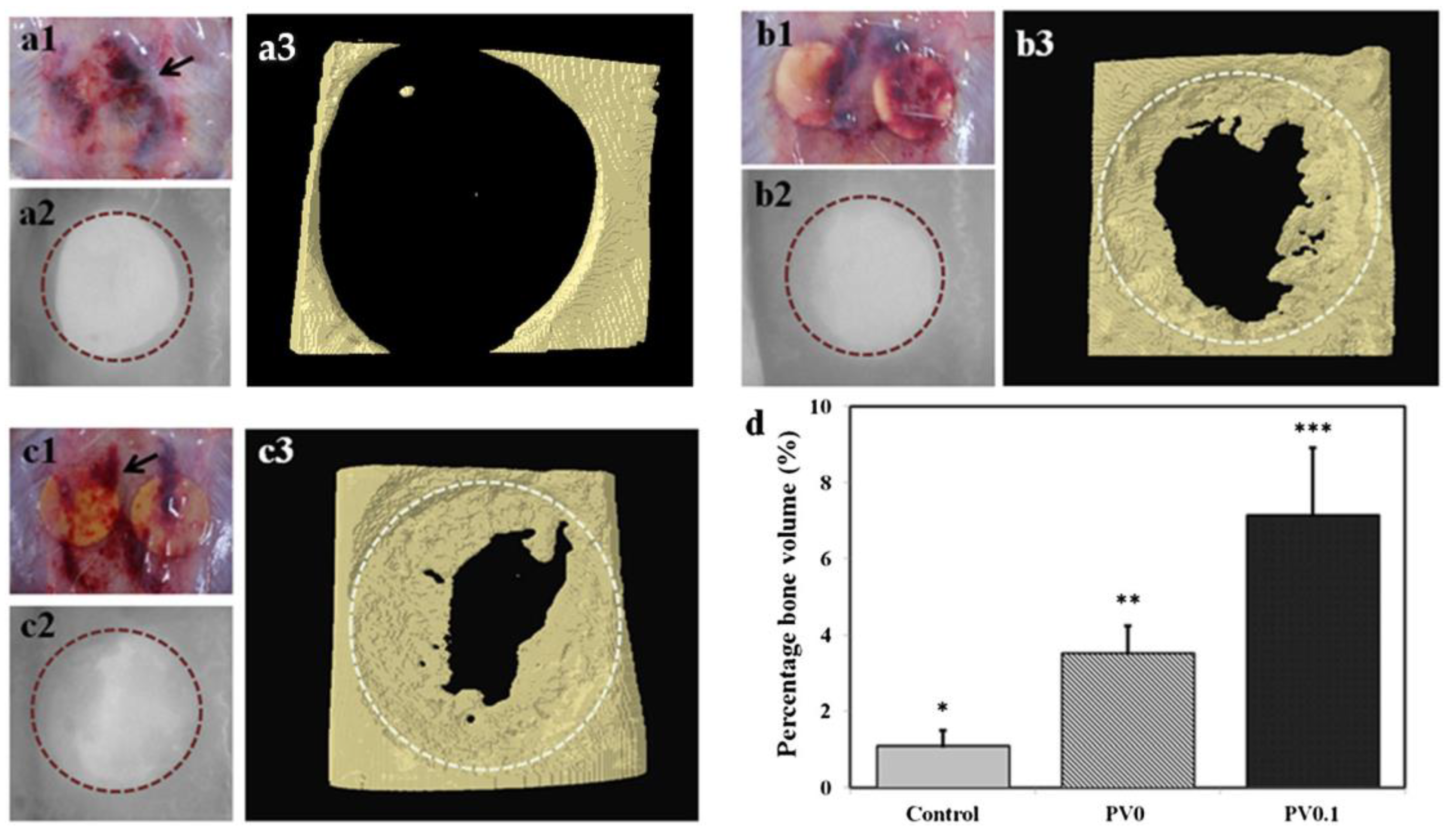
The effect of PVPA in addition to in vivo biocompatibility of PVPA/PVA ES mats was preliminarily investigated by the implantation of the PV0.1 membrane (1 wt% PVPA) in a defect compared with a defect with PV0 implanted (without PVPA) and with a defect without an implant (control) [155] (Figure 14). The results showed that the in vitro cell growth behavior on the PVPA-added membrane resulted in favorable tissue growth in vivo (confirmed by micro-CT results and histological studies). The histological observations derived from the rat skull defect implanted with PV0.1 also showed that the membrane was still intact after 4 weeks of grafting. The authors concluded that the efficiency of tissue growth is due to the phosphate groups, which facilitate the bonding process of the implant to the native host tissue.
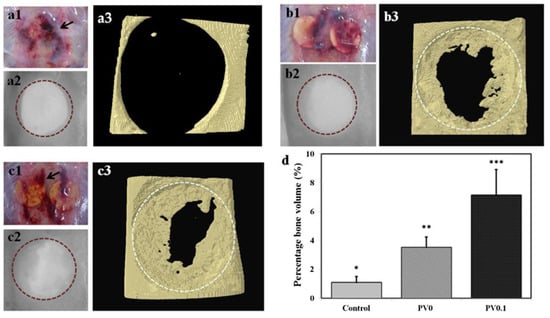
Figure 14.
Comparison of defects without implant (control) (a), with PV0 (b) and PV0.1 (c). Images shown were obtained during extraction after 4 weeks of implantation (1), scanned micrograph from micro-computed tomography (2) and reconstructed three-dimensional structure of the defects (3). Blood clots (black arrows) may have resulted from the trauma of the surgery because the presence in the control (a1). Percentage bone volume was also computed, showing statistically higher values obtained in defects implanted with PV0.1 compared with nonimplanted defects (*** p < 0.001, ** p < 0.01, * p < 0.05) (d). Reprinted with permission from [155]. Copyright (2014) Wiley-VCH GmbH.
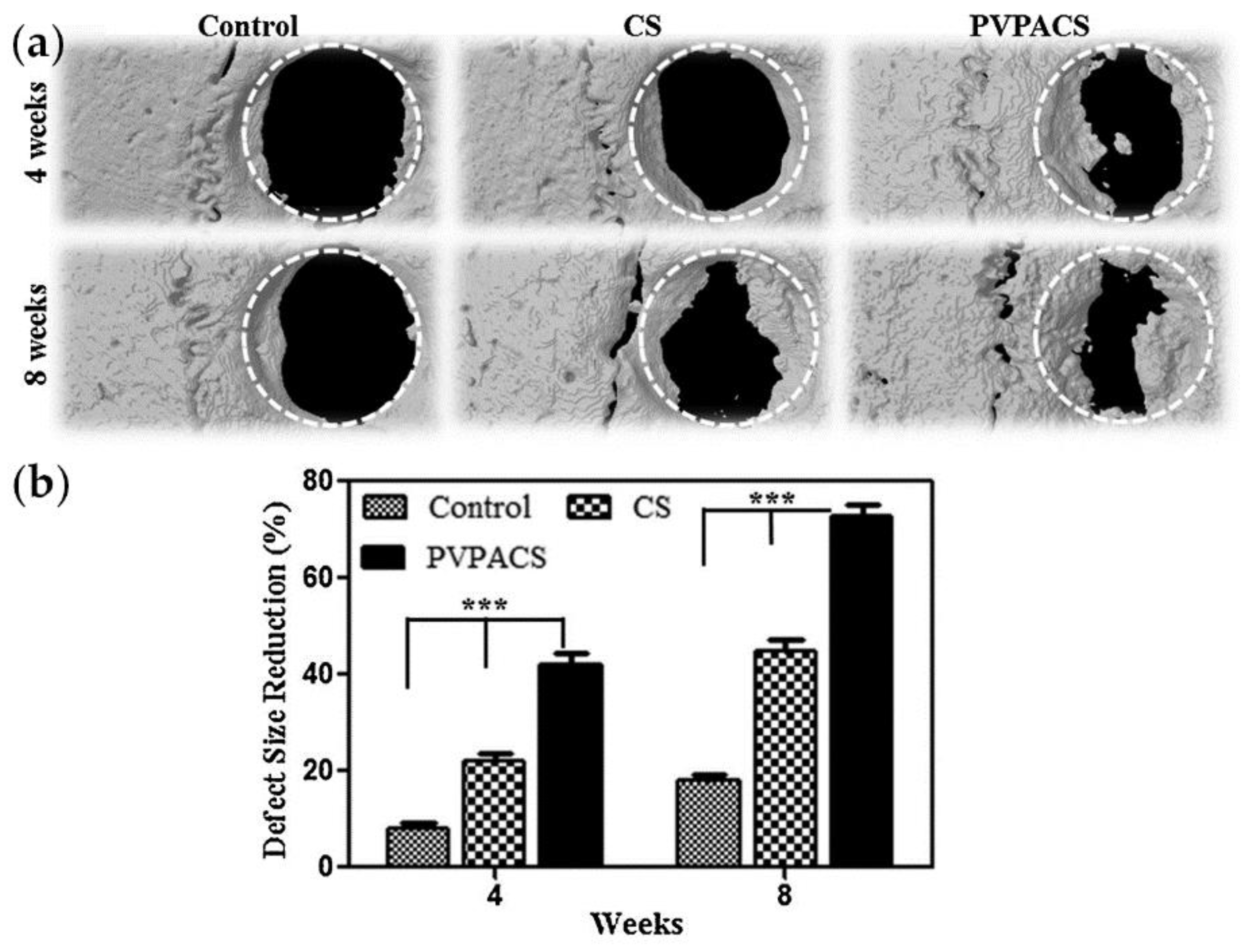
PVPA/chitosan composite porous three-dimensional matrices were used for the implantation of full-thickness calvarial bone defects (diameter of 5 mm) in Sprague Dawley male rats [161]. Bone formation was limited by CS-treated groups, commencing only from the periphery of the host bone. However, with PVPACS treated group, bone formation was markedly higher, and some initiated in the center of the defect after 4 and 8 weeks of implantation (Figure 15). The osteoinductive effect of PVPA was also confirmed by hystological studies.
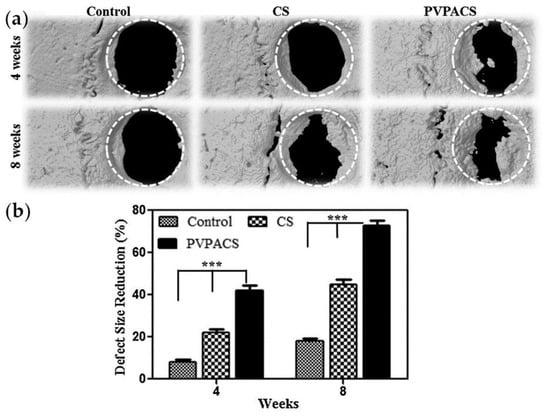
Figure 15.
(a) Reconstructed three-dimensional images of μCT scans taken after 4 and 8 weeks of matrix implantation in rat calvarias; (b) Percentage size reduction quantified within standard ROI placed concentrically over the defect site from each μCT scan and time point. Asterisks define significant differences (*** p < 0.05). Reprinted with permission from [161]. Copyright (2014) Elsevier B. V.
A complex porous composite material, based on poly(lactic-co-glycolic acid) microspheres, the copolymer of fumaric acid and oligo(ethylene glycol), and BMEP (see Scheme 4) as a cross-linker, was studied as a bone substituent after treatment with BMP-2 [162]. It was demonstrated that it is the presence of BMEP that enhance bone formation in the context of BMP-2.
3.3.3. Sidechain PCPAs and Composites for Dental Applications
Dental self-etch adhesives (SEAs) are widely employed to adhere a restorative material to a tooth. Since the functionality of SEAs is provided by polymer formation, these de facto monomeric compounds are also discussed in this section.
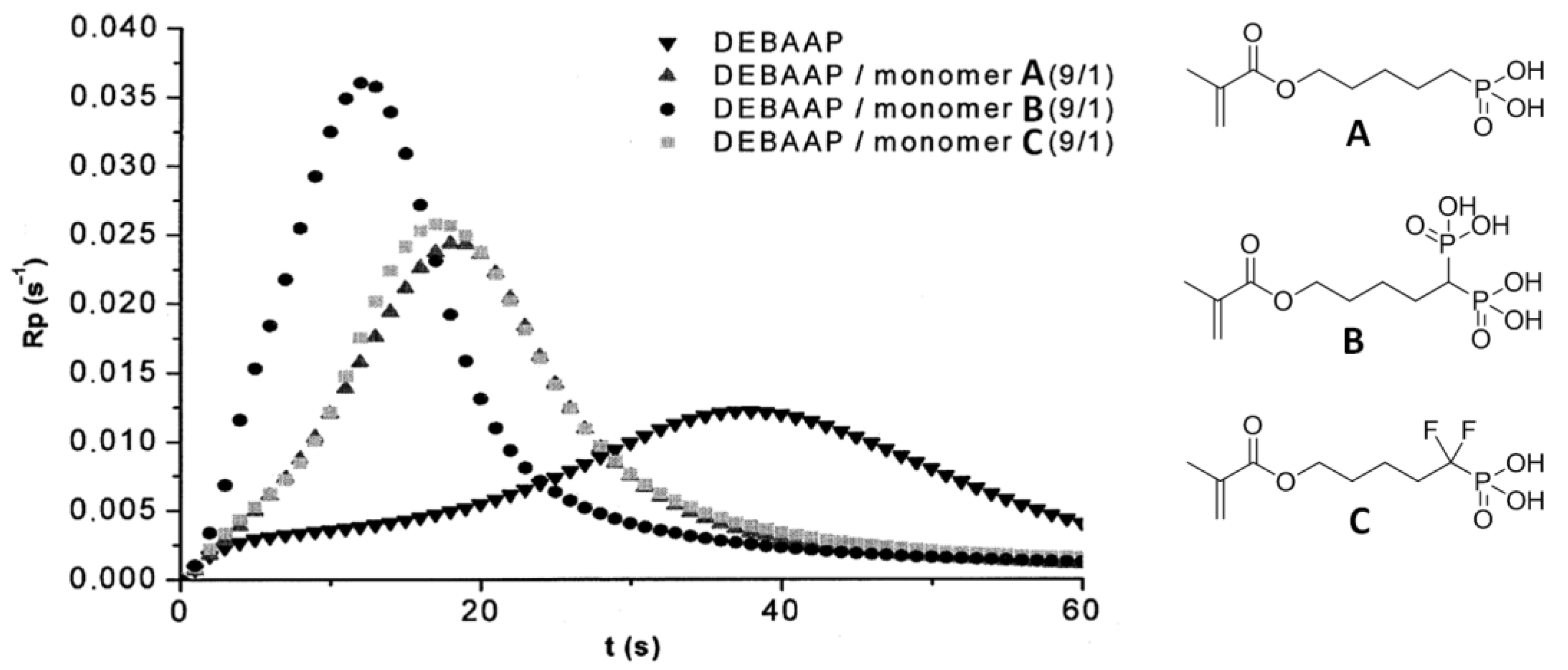
Le Pluart and Coll. [34] studied three different phosphonic, bisphosphonic, and difluoromethylphosphonic acid methacrylate monomers (see Scheme 7 and structures A–C in Figure 16).
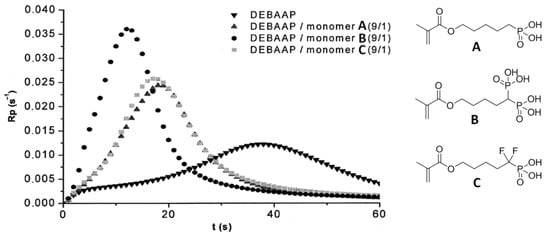
Figure 16.
(Co)polymerization rate (Rp) as a function of time for DEBAAP and monomers A–C. Reprinted with permission from [34]. Copyright (2012) Elsevier B. V.
The main objective of their study was to evaluate the influence of the nature of the acidic group on the reactivity and adhesive properties of the monomers. Photopolymerization of N,N′-diethyl-1,3-bis(acrylamido)propane (DEBAAP) with monomers A–C demonstrated higher reactivity of B, the difference between A and C was minimal. Dentin shear bond strength measurements have shown that primers based on B and C are significantly more efficient than the ones based on A. The dentin shear bond strength (SBS) values were 15.4, 19.7, and 20.6 MPa for A, B, and C, respectively. Therefore, monomers B and C appear to be great candidates for light-cured adhesive formulations.
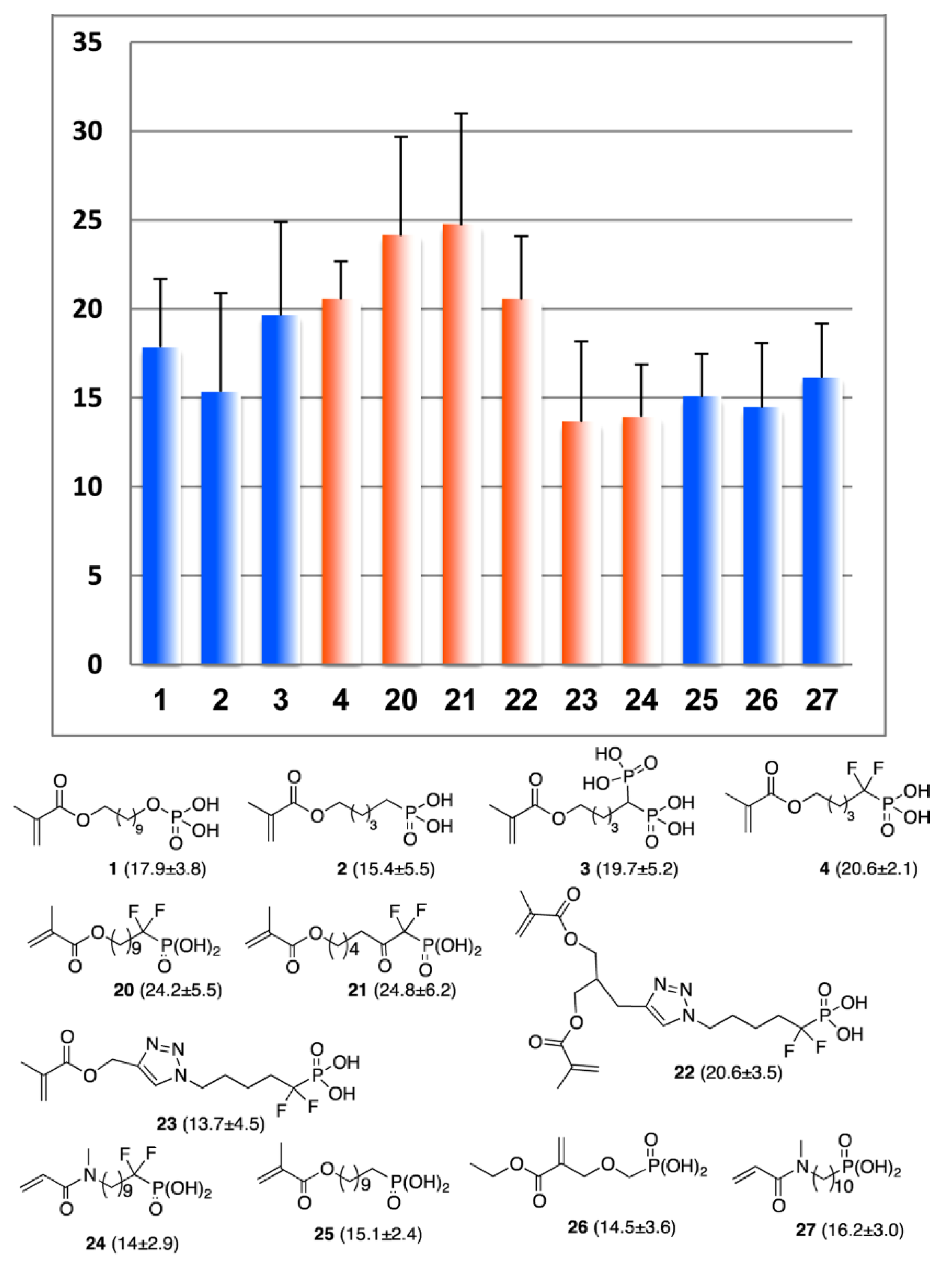
In further studies [35], new monomers with different spacers between methacrylate and –CF2P(O)(OH)2 fragments were synthesized (see Scheme 7) and used for the preparation of the primers with N,N-diethyl-1,3-bis(acrylamido)propane (10 mol%), containing photoinitiator (camphorquinone), coinitiator (ethyl 4-(dimethylamino)benzoate) and stabilizer (2,6-di-tert-butyl-4-methylphenol). Primers, coupled with the AdheSE bonding resin [163], were used to generate a bond between a standard Z100 A3 composite (3M/ESPE) and dentin. The results of the SBS measurements are presented in Figure 17. Dentin SBS measurements showed that –CF2P(O)(OH)2 containing primers are significantly more efficient than the –CH2P(O)(OH)2 containing methacrylates. Of particular note is the influence of the –(CH2)n– spacer length: the use of the SEP containing monomer 20 (n = 10, Figure 17) resulted in an SBS value of 24.2 MPa, whereas widespread monomers 1 and 25 had values of 17.9 and 15.1 MPa, respectively, and monomer 4 (n = 4) was characterized by SBS value of 20.6 MPa.
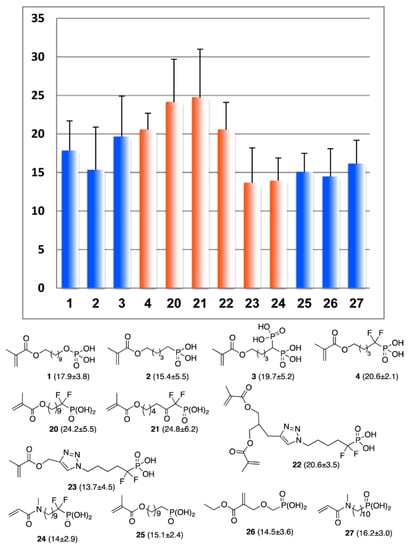
Figure 17.
Mean shear bond strength (SBS) values (MPa) and standard deviation (MPa) for fluorophosphonate acids SEP, SBS values for fluorine-containing methacrylates are highlighted with orange. Reprinted with permission from [35]. Copyright (2014) American Chemical Society.
3.3.4. Drug Delivery and Drug Release with the Use of Sidechain PCPAs
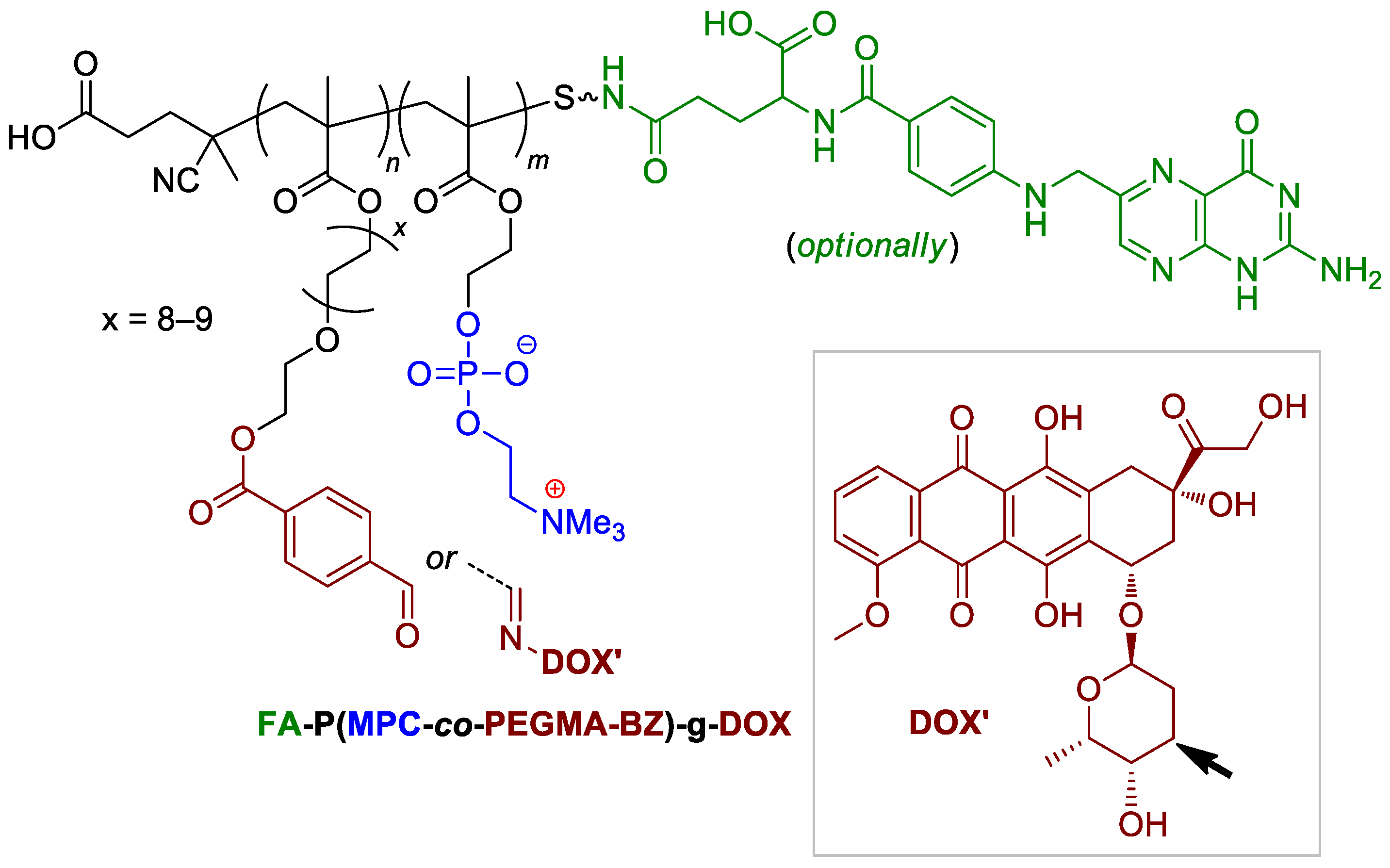
Since zwitterionic polymers have excellent properties of hydration, anti-bacterial adhesion, long-term circulation and suppress nonspecific protein adsorption in vivo, Ni and Coll. described a novel folate-targeted and acid-labile polymeric prodrug under the microenvironment of tumor cells, based on the use MPC (Scheme 4) comonomer [110]. Functionalized copolymers containing the fragment of folic acid were able to chemical binding with doxorubicin (see Scheme 1 and Scheme 41), and the presence of phosphocholine fragments provided the delivery and release of the drug that was bonded with the neighboring fragment. The copolymers have demonstrated marked colloidal behavior with the critical aggregation concentration ~50 mg∙L−1. Nanoparticles were stable at pH 7.4 and tended to aggregate at a pH of 5.0. Colloid stability correlated with the drug release rates (after 98 h, 80 and 15% of DOX released at pH 5.0 and 7.4, respectively). The DOX-free copolymer was found to be non-toxic for normal cells (L929) and cancer cells (HeLa HepG2), whereas DOX-bonded copolymers have demonstrated a marked antitumor efficacy (IC50 of 0.76 mg∙L−1 and 0.62 mg∙L−1 after 48 h of incubation for PMPD and FA-PMPD, respectively), but were inferior to doxorubicin.
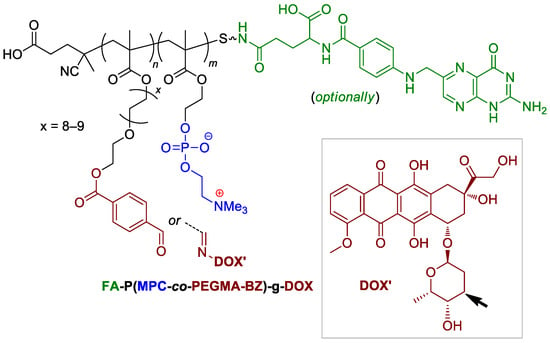
Scheme 41.
Structure of MPC-based copolymer for doxorubicin delivery [110].
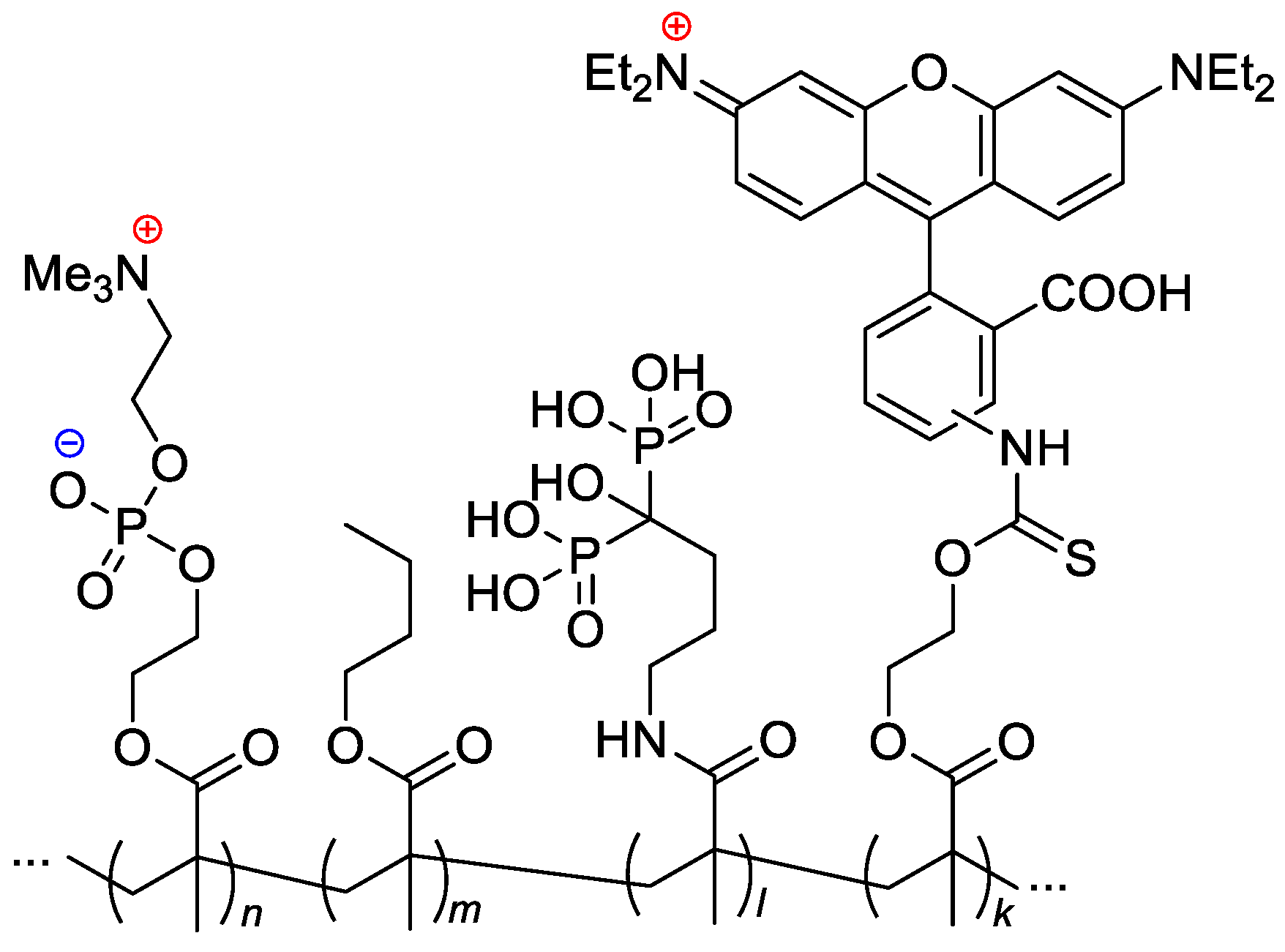
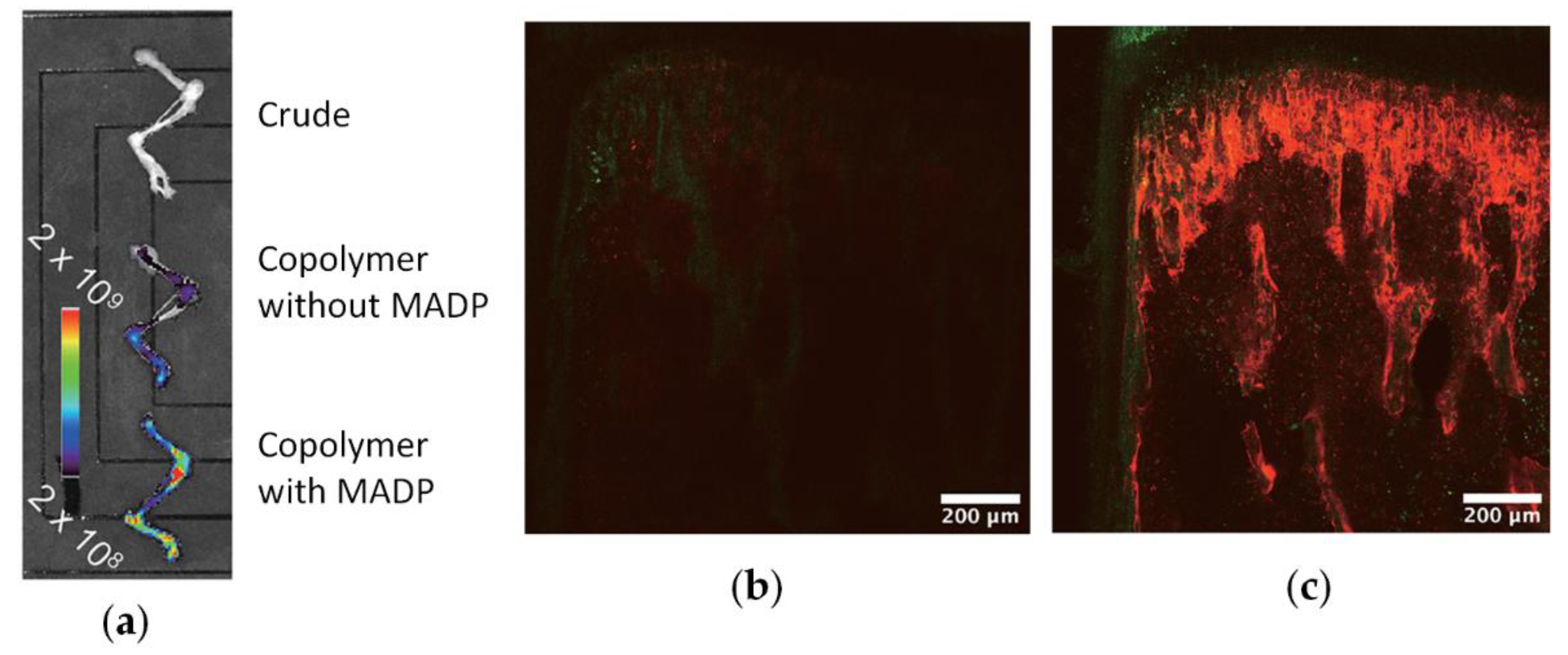
Given that current chemotherapies have limited effectiveness in eliminating bone metastasis, Iwasaki and Coll. [40] made a separate study to provide regional chemotherapy for this metastatic tumor. A bone-targeting drug carrier was designed by introducing the osteotropic bisphosphonate alendronate units into an amphiphilic phospholipid polymer (see Scheme 24). The copolymer formed nanoparticles (d < 30 nm), and diphosphonate units were exposed to the outer layer of the particles. These particles were found to be able to encapsulate a hydrophobic anticancer drug docetaxel. The complex formation did not hamper the pharmacological effect of the drug against several breast cancer cell lines. The fluorescence observations evaluated by an in vivo imaging system and fluorescence microscopy showed that the addition of diphosphonate units to the polymer–drug complex enhanced bone accumulation (Figure 18).
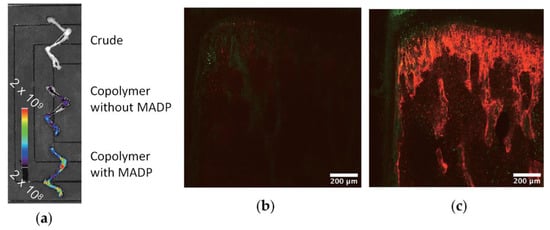
Figure 18.
(a) An ex vivo fluorescence image of leg bone; (b,c) Confocal microscopy of the sagittal section of the proximal end of the tibia, harvested from mice 24 h after intravenous injection of copolymer/docetaxel for copolymer without and with MADP monomer units, rhodamine label was excited at 561 nm. Reprinted with permission from [40]. Copyright (2020) Wiley-VCH GmbH.
3.4. Polyelectrolytes Based on Sidechain PCPAs
Proton conduction in solid-state polyelectrolytes has aroused considerable interest in recent years in connection with the development of fuel cells, batteries, and water electrolyzers [164]. Sidechain PCPAs are regarded as prospective materials due to the presence of acid groups and the stable polymer backbone. Over the past decades, the number of research papers on the issue of sidechain PCPAs-based polyelectrolytes has significantly increased, and in this section we will look at some representative examples, taking into account the molecular structure of the polymers.
For polymer electrolytes, the critical structural variable is the concentration of the ionic functional groups that affect the water uptake and ion conductivity, expressed by the ion exchange capacity (IEC, molar equivalents of ion exchange group per g of dry polymer (mequiv∙g−1 or mmol∙g−1). Because –P(O)(OH)2-functionalized compounds are weaker acids than –SO3H-substituted polymers, phosphonic acids have lower conductivity in the presence of water. As a result, low-temperature electrochemical devices typically use sulfonated polymer electrolytes. However, for electrochemical devices operating at 100 °C and higher temperatures, phosphonic/phosphoric acid systems have an advantage because of their ability to provide proton conductivity in the absence of water. So in addition to proton conductivity, the high thermal stability of the polymers is desirable.
3.4.1. PVPA-Based Polyelectrolytes
In water-free proton-conducting solid polyelectrolytes, proton transfer can be carried out only by hydrogen bond breaking and forming due to the absence of mobile species other than protons. The mechanism of the complex proton motion in solid PVPA was studied by Spiess and Coll. by solid-state NMR [165,166]. They confirmed the mobility of the P–OH protons but found that the formation of anhydride fragments leads to a decrease in proton conductivity through the decrease in the number of charge carriers and blockage of charge transport through immobilization of charge carriers together with a hindered reorientation of the anhydride groups. The 1H chemical shift of the P–OH protons provided evidence for the presence of a hydrogen-bond network (Figure 19), which allows for proton transport via a Grotthus-type mechanism [165]. Combined 2H line shape analysis and ab initio MD calculations [166] characterized the dynamical proton motion via the rearrangement of the H-bonding network, which involves both intra- and inter-chain transfers.
Figure 19.
Molecular structure and hydrogen-bonding network of the model system of PVPA as used in the first-principles molecular dynamics simulations and chemical shift calculations. Hydrogen bonds, which are shown as broken lines, are assumed for hydrogen and oxygen pairs with O…H distances below 2.3 Å and O–H…O angles above 90°. Reprinted with permission from [165]. Copyright (2007) American Chemical Society.
The proton conductivity of PVPA was not as high as expected (~0.8 mS∙cm−1 at 140 °C in dry conditions [167]), and the activation energy was around 60 kJ∙mol−1 [168]. Since poly(ethylene oxide) (PEO) has a low glass transition temperature (210 K) and contains a huge number of oxygen atoms in the backbone which can form hydrogen bonds with phosphonic acid groups, Meyer and Coll. [167] proposed to build a dynamic hydrogen bond network in PVPA/PEO blends. However, even in homogeneous PVPA/PEO blends although the fast mobility of PEO chains, the proton conductivity decreased with increasing PEO content.
In further studies of the same scientific group, the influence of water sorption on the proton conductivity of PVPA was assessed [149]. High-MW almost atactic polymer (Mw = 62 kDa) was selected for experiments upon annealing and drying; it was demonstrated that H2O and self-condensation products coexist in the sample.
To improve the characteristics of PVPA-based membranes, Sen and Coll. have studied blends of PVPA and Nafion (Scheme 42), and polymer membranes were prepared by means of film casting from Nafion/PVPA solutions [169]. Nafion/PVPA electrolyte with S/P molar ratio of 1:1 was thermally stable up to 400 °C, the presence of –SO3H groups blocked the formation of a phosphonic acid anhydride. The proton conductivity of the Nafion/PVPA blend membrane at 130 °C in an anhydrous state was 1.1 × 10−5 S∙cm−1. Under humidified conditions, the conductivities of the blends increased by four orders of magnitude, up to 1.2 × 10−2 S∙cm−1.
Scheme 42.
The structures of reference polymer Nafion 117 and polymers used for the preparation of composites.
The effect of the basic polymeric component in the composite with PVPA was studied by Zhang and Coll. on the example of PVPA-treated SiO2/poly(4-vinylpyridine) (PVP, Scheme 42) nanocomposite [170]. PVP-decorated SiO2 nanoparticles (diameter of the silica core 7–10 nm), containing 94 wt% of PVP, were treated by aq. PVPA solution in different N/P molar ratios, and freeze-dried. The proton conductivity of the membranes obtained was up to 10 mS∙cm−1 under humidified conditions (for 80 wt% PVPA composite) and inferior to PVPA-based membrane.
PVPA/mPBI (Scheme 42) blend membranes were prepared using DMSO polymer solutions and studied by Sharif and Coll. in 2021 [171]. The activation energies of proton transport were calculated in the ranges of 7.1–40.8 kJ∙mol−1 and 37.4–49.7 kJ∙mol−1 at RH = 100 and 0%, respectively. H3PO4-doped PVPA/mPBI (1:1 mol/mol) have demonstrated the highest proton conductivity of 79.6 mS∙cm−1 at 150 °C, which is comparable to Nafion membrane conductivity (mS∙cm−1) under humidified condition at 80 °C. The methanol permeabilities of the blend membranes were about 1000 times lower than that of Nafion.
The complex character of the proton conductivity of PVPA (as well as evident problems arising from the formation of anhydrides at elevated temperatures) prompted the researchers to synthesize and study VPA copolymers. So, for example, free-standing films were obtained from copolymers of VPA and 1-vinyl-1,2,4-triazole (49–67 mol% of VPA) by evaporation of the aqueous solutions and studied [98]. Suddenly, the formation of phosphonic acid anhydride in copolymers was comparable to that in pure PVPA (~30–40% before and ~60% after annealing at 160 °C, 31P magic angle spinning (MAS) NMR data). The proton conductivities of the copolymer with maximum VPA content in an anhydrous state was ~1 mS∙cm−1 at 120 °C. The hydrogen-bonding network of the copolymers was analyzed and the presence of two different types of hydrogen bonding, O–H…O and O–H…N, was proved by 1H double-quantum MAS NMR. However, at high temperatures (140 °C) highly mobile acidic protons were only detected for O–H…O bonding. Copolymers of VPA with 5-(methacrylamido)tetrazole (2:1 comonomer ratio) have demonstrated the maximum proton conductivity of 10 mS∙cm−1 at 150 °C [105]. The copolymer of VPA and styrene was studied in mixing with 1-propylimidazole [172], but the results have been mediocre (good thermal stability but very low conductivity even in the presence of water).
PVPA-grafted poly(vinylidene fluoride) (see Section 2.4.5) membranes were prepared from the DMF solutions [140], and the maximum proton conductivity of 2.3 mS∙cm−1 was detected at 150 °C for copolymer with 20 wt% PVPA content.
The idea of the use of PVPA/inorganic composites as electrolyte membranes was tested in the study of Aslan and Bozkurt [62] who prepared PVPA/TiO2 composites containing 5, 10, 15, or 20 wt% of TiO2. The maximum proton conductivity under water-free conditions was detected for 10% TiO2 composite at 120 °C and found to be 4 mS∙cm−1. For PVPA/BN composites (3, 5, 10, and 15 wt% of the hexagonal BN) high thermal stability was detected (up to 200 °C), and maximum proton conductivity was measured for PVPA-15%HBN as 6.51 mS∙cm−1 at 150 °C in anhydrous conditions [61].
3.4.2. Polyelectrolytes Based on Other Non-Fluorinated Polymers
PmPPA (see Scheme 40) [148] has demonstrated high thermal stability (up to 300 °C) without softening before decomposition. Temperature-dependent conductivity measurements under 1 atm water vapor pressure revealed that the conductivity of PmPPA amounted to 6 mS∙cm−1 at 115 °C and decreased to 2 mS∙cm−1 at a higher temperature. Without humidification, no reproducible results were obtained, which was attributed by the authors to irreversible condensation reactions, so PmPPA can be used as a proton conductor in the presence of water, but not in the dry state.
AIBA-initiated free-radical polymerization of styryl phosphonic acids with –CH2O(CH2)n– (n = 2, 6) spacers between the aromatic ring and P atom (Scheme 8) resulted in the formation of PCPAs with relatively high thermal stability (no anhydride formation up to 140 °C) [49]. The proton conductivities were found to be ~0.26 mS∙cm−1 at 140 °C under nominally anhydrous conditions, and the length of the alkanediyl spacer did not affect the conductivity. Functionalized poly(styrene-ethylene/butylene-styrene) block copolymers of an even more simple structure (–CH2– spacers between the aromatic ring and –P(O)(OH)2, see Section 2.4.3) have demonstrated a lowering of the tensile strength and an increase in proton conductivity. With an increase in the degree of phosphonation [130], the maximum value of protonic conductivity at 140 °C was 5.82 mS∙cm−1.
Although fluorinated polymers as polyelectrolytes are discussed in the next section, we felt it more appropriate to consider –CH2CF2P(O)(OH)2-functionalized polystyrene [50] just after non-fluorinated analogs. The IEC of this polymer was 7.3 mmol∙g−1, and the conductivity of the dried sample at 120 °C reached 0.018 mS∙cm−1.
A similar study with a variation of the length of –(CH2)n– spacers between ‘PE’ backbone and –P(O)(OH)2 groups was conducted by Ueda and Coll. [45]. Phosphonated poly(acrylates) were obtained via free-radical polymerization of CH2=CHC(O)O(CH2)nP(O)(OEt)2 (n = 2, 4, 6) followed by hydrolytic cleavage, polymer membranes were prepared by cross-linking with the use of 5 wt% of benzoyl peroxide. The IEC values were 5.44, 4.76, and 4.09 mequiv∙g−1, respectively. These membranes have demonstrated high proton conductivity under humidification at 80 °C, and the sample based on acrylate with the longest –(CH2)6– spacer was not inferior to Nafion 117 (Scheme 42) in conductivity.
In the development of proton-exchange membranes, star polymers may have some advantages compared to linear analogs owing to the compact and branched architecture of star polymers. An original approach to similar polymers was proposed by Müllen and Coll. [74], core-shell macromolecule containing polyaromatic core and polystyrene with –CH2P(O)(OH)2 groups in the position para (Figure 11a) was synthesized and studied. The polymer showed relatively high thermal stability (up to 180 °C), and no anhydride formation was detected by 31P MAS NMR. The maximum conductivity of 3.9 mS∙cm−1 was detected in a humidified atmosphere at 115 °C.
Phosphonated poly(styrene-b-methylbutylene)s (see Scheme 35) were used in the preparation of membranes, containing ionic liquids (obtained from imidazole (Im), 2-methylimidazole (2MIm) or 2-ethyl-4-methylimidazole (2E4MIm) and bis(trifluoromethane)sulfonimide (TFSI)) [137]. The introduction of alkyl substituents in imidazole resulted in increase of the proton conductivity in the temperature range of 60−150 °C, the maximum value of 2 mS∙cm−1 was reached at 150 °C. The studies of the composites with ionic liquids were continued [138] mostly in view of self-assembled morphologies. It was demonstrated that with minimal changes in the numbers of comonomer units in copolymer, and depending on the type of the ionic liquid and thermal history, well-defined lamellae, gyroid, hexagonal cylinder (HEX), body-centered cubic, and A15 lattice morphologies are formed. The samples having the A15 lattice showed higher morphology factors (0.83–0.96) than those with the HEX phases (0.42–0.69), this structural advantage of the A15 lattice over the HEX for constructing less tortuous ion-conduction pathways resulted in higher proton conductivity (Figure 20).
Figure 20.
(a) Ionic conductivity of [2E4MIm][TFSI]-containing composites of copolymer (11 styrene units with 4 –P(O)(OH)2 groups and 18 methylbutylene units) with different thermal histories; (b) Schematic drawings that illuminate ion-conduction pathways across PSP phases for A15 and HEX structures. Reprinted with permission from [138]. Copyright (2016) Wiley-VCH GmbH.
Block copolymers of diethyl styryl phosphonate or diethyl styryl bis(phosphonate) (SP and SbP, respectively, see Scheme 9) were synthesized by ATRP, –OC(O)CMe2Br-functionalized poly(isobutylene) (pIB) was used as a macroinitiator; homopolymers were also obtained [51]. After hydrolytic cleavage (see Section 2.3.4), poly(phosphonic acid)s were dissolved in THF/MeOH or mixed with Im, 2MIm, or 2E4MIm / TFSI-based ionic liquids in THF/MeOH media, polyelectrolite membranes were obtained by solvent casting and subsequent vacuum drying. Wide-angle X-ray scattering (WAXS) profiles of the membrane samples (pSP, pSbP, pIB66-b-pSP30, pIB66-b-pSbP16, pIB66-b-pSbP28 without and with ionic liquids, Figure 21) and results of the small-angle X-ray scattering (SAXS) studies showed that block copolymers display long-range ordered self-assembled morphologies, in pSbP-based polymers ion aggregation was avoided featuring the confinement of polymer chains to few-nanometer domains.
Figure 21.
WAXS profiles of phosphonated polymers in the presence and absence of ionic liquids: (a) neat homopolymers; (b) neat block copolymers; (c) [2E4MIm][TFSI]-containing homopolymers; (d) [2E4MIm][TFSI]-containing block copolymers. Ion clustering in PSP and PSbP (right panels of (a)). λ values in (c,d) indicate concentrations of ionic liquid. Characteristic peaks are indicated by ↓, ▼, and ▽. Reprinted with permission from [51]. Copyright (2018) American Chemical Society.
In the presence of ionic liquids, virtually homogeneous ionic phases with suppressed ion clustering were detected for pSbP-based block copolymer. pIB66-b-pSbP16-based membranes have demonstrated the highest conductivity despite lower IEC presumably due to a narrow polymer domain with excluded ion aggregation (Figure 22). Given that the Tg of pSbP is more than 20 °C higher than the Tg of pSP, the use of pSbP-based membranes appears a promising way for improving both the conductivity and mechanical strength of solid-state polyelectrolytes.
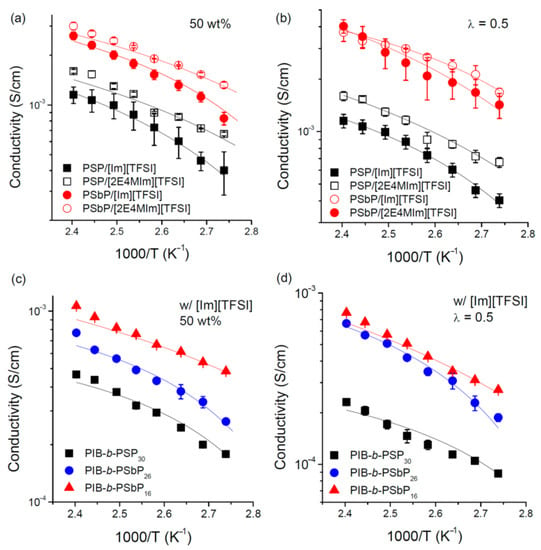
Figure 22.
Temperature-dependent through-plane conductivities of (a,b) ionic liquid-containing phosphonated homopolymers and (c,d) phosphonated block copolymers. The solid lines in (a–d) indicate VTF fits. Reprinted with permission from [51]. Copyright (2018) American Chemical Society.
Polystyrene, containing 1,4-perfluorophenylene linkage between aryl and –P(O)(OH)2 fragments (see Scheme 10), under anhydrous conditions at 120 °C exhibited a conductivity of 9.91 × 10−7 S∙cm−1 [52].
3.4.3. Polyelectrolytes Based on Fluorinated Polymers
For phosphonated copolymers (CF2CFClCH2CH(OCH2CH2X))n (X = Cl, H, P(O)(OH)2) with different degrees of phosphonation, the dependence of the conductivity on ionic exchange capacity (IEC) was investigated at 20 °C and 95% relative humidity [129]. The level of conductivity was in the range of 0.02–20 mS∙cm−1, which is comparable to the Nafion 115 (up to ~50 mS∙cm−1). The conductivity was strongly dependent on the IEC: the conductivities of the copolymer with 36% phosphonate functionalization (IEC of 2.9 mequiv∙g−1) and 93% phosphonate functionalization (IEC of 7.0 mequiv∙g−1) differed by almost 3 orders of magnitude. When decreasing relative humidity from 95 to 25%, the conductivity values decreased tenfold. An increase in the temperature from 90 to 120 °C resulted in an increase of the proton conductivity by twice. This result indicates that water molecules and phosphonic acid hydrogen bonding are involved in the proton conduction mechanism.
Around the same time, one first and promising step toward the development of efficient solid-state polyelectrolytes has been made by Atanasov and Kerres [131]. Poly(4-vinyl-2,3,5,6-tetrafluorophenylphosphonic acid) (PWN2010) (see Scheme 33) with a degree of phosphonation ~90% and IEC = 7 mmol∙g−1 was found to be thermally stable up to 349 °C, and has not Tg. The water uptake of PWN largely exceeded the one of Nafion 117, whereas the numbers of the water molecules per functional group (λ) of PWN2010 and Nafion 117 were similar. The conductivity of PWN2010, measured at 1 atm water vapor pressure (see Figure 23), was found to be by a factor of 4–10 higher than similar phosphonated polymers such as PVPA and PmPPA. It was even higher than the conductivity of Nafion 117 (Scheme 42), being up to two times larger at T = 155 °C. Similar to PmPPA, PWN2010 showed reduced dependence on the conductivity from the water content at higher temperatures (T = 135–155 °C). The absence of conductivity hysteresis indicated the absence of the anhydride formation in stark contrast to PVPA and PmPPA.
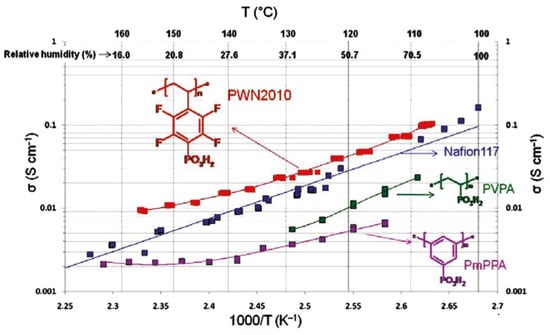
Figure 23.
The conductivity of PWN2010 as a function of temperature at water partial pressure of p(H2O) = 1 atm. Conductivities of Nafion 117, PVPA, and PmPPA, measured at the same conditions and setup, are given as a comparison. Reprinted with permission from [131]. Copyright (2011) American Chemical Society.
In [133], Atanasov and Coll. reported the results of further studies of PWN. They convincingly showed that PWN really does not form anhydride at moderately elevated temperatures. Even at 250 °C 48% dehydration required annealing within 5 h! At 150 °C in dry N2, the proton conductivity was 0.4 mS∙cm−1. Stable polymer films were prepared by blending PWN with poly(benzimidazole) PBIOO (Scheme 42), which after doping with PA reached a peak power density of about 230 mW∙cm−2 at 150 °C. Another important aspect in the potential use of PWN is its poor mechanical properties at high degrees of the phosphonation, and in [132] the synthesis and studies of poly(pentafluorostyrene)s with controlled phosphonation degrees (17–66%) were presented. Ion conductivity was found to increase with both temperature and phosphonation degree reaching 57 mS∙cm−1 at 120 °C (RH ~90%). In the fuel cell, the blend showed the best peak power density of 400 mW∙cm−2 against 350 mW∙cm−2 for Nafion 212. A blend membrane consisting of 82 wt% PWN and 18 wt% benzimidazole copolymer F6PBI (Scheme 42) with IEC ~ 2.1 mmol∙g−1 was produced and showed better thermal and radical resistance, lower water uptake but similar ion-conductivity. With the use of these blends, full-featured prototypes of the fuel cells were developed and tested.
In trying to improve PWN characteristics, triblock copolymers with poly(diaryl sulfone) were synthesized (see Scheme 34) and studied [136]. Copolymer membranes were prepared from N,N-dimethylacetamide solutions. Under hydrated conditions, copolymers have demonstrated high values of proton conductivity (Figure 24).
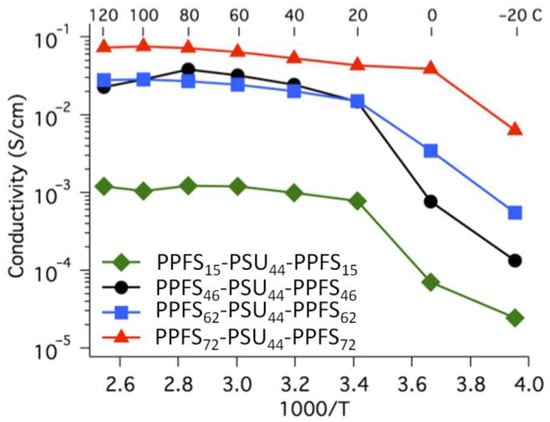
Figure 24.
Proton conductivity of block copolymer membranes as a function of temperature under fully hydrated conditions. Reprinted with permission from [136]. Copyright (2013) Wiley-VCH GmbH.
The conductivity value for copolymer membrane, based on perfluoro-1,1′-biphenyl and diphosphonated hydroquinone (see Scheme 39), reached 92 mS∙cm−1 when fully hydrated at ambient temperature [147] which is comparable with standard Nafion membranes [173] and substantially higher than reported for certain other phosphonated polymers [44,129,139].
In [134], the most promising results of the studies of PWN-based polymer membranes were discussed and summarized, and the advantages (film processing, proton conductivity, acid retention with water) and disadvantages (hydrophobicity, thermal stability) of these polymers in comparison with Nafion were demonstrated.
The high efficiency of the synergy of PWN-type polymer and Nafion was demonstrated in the very recent work of Kin and Coll. [135]. They proposed that the presence of highly acidic –SO3H groups could enhance the anhydrous proton conduction of fuel cell electrodes due to proton transfer to the phosphonic acid groups. The fuel cell, exhibiting a rated power density of 780 mW∙cm−2 at 160 °C, with minimal degradation during 2500 h of operation and 700 thermal cycles from 40 to 160 °C under load, was developed by the results of the study.
3.5. Other Applications of Sidechain PCPAs
3.5.1. Sidechain PCPAs as Flame Retardants
The phosphorus-containing FRs surpass halogen-based FRs in safety and are being intensively researched [12]. In a recent review of Özer and Gaan, devoted to phosphorus-based flame retardant coatings for textiles [174], low-MW compounds have mainly been described.
As with main-chain PCPAs, sidechain polyacids have been little studied as FRs. In 2013 Fang and Coll. reported on a study of the construction of Zn2+- and Cu2+-doped FR coatings on the surface of ramie fabrics [175]. PVPA and branched polyethylenimine/M2+ were used in the layer-by-layer assembly technique. As a result, the thermal and fire stability of the coated fabric was improved, e.g., a significant residue (up to 25.2 wt %) was left (<1% for the control). The results of this study were somewhat expanded and corrected one year later [176].
El Hage and Coll. had chosen not to use metal salts, and studied different methods of VPA grafting [141] (Figure 7). Flame retardant properties of VPA-grafted flax fabrics can be explicitly illustrated by Figure 25, thus confirming the correctness of a chosen way.
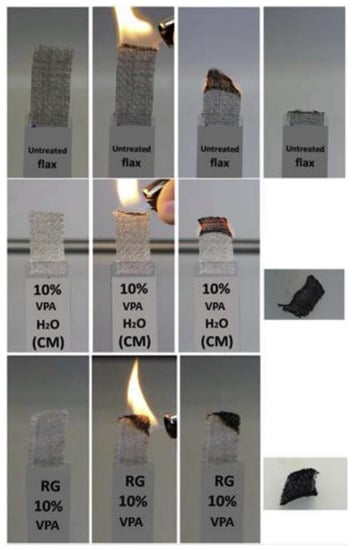
Figure 25.
Preliminary fire test on untreated (top), chemically modified (middle), and irradiated (bottom) flax fabrics. Reprinted with permission from [141]. Copyright (2018) Elsevier B. V.
As was shown in [103], even small amounts of VPA in copolymer with acrylonitrile or acrylonitrile/methyl methacrylate reduces significantly the flammability of the materials. The authors assumed that the presence of –P(O)(OH)2 groups in acrylonitrile-based polymers accelerates intramolecular cyclization of the nitrile units (Scheme 43).
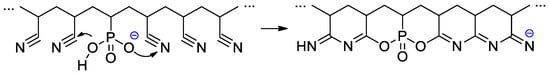
Scheme 43.
The proposed mechanism, and the effect of phosphonate and phosphonic acid on cyclization during heat treatment of acrylonitrile copolymers. [103].
Copolymers of methyl methacrylate and the most synthetically available phosphate-containing acrylate-type monomer MEPN (see Scheme 7) were studied by Marić and Coll. in 2020 [109]. They showed that the char yield content increased from 1.2 to 24.4 wt% for copolymers containing 6 to 28 mol% of MEPN. The limiting oxygen index (LOI) value (the minimum fraction of oxygen in an O2/N2 mixture supporting the burning of a vertical sample) was estimated by Van Krevelen empirical Equation (3) for halogen-free polymers [177]:
where CY is char yield. The LOI of pure poly(methyl methacrylate) and copolymer with 28 mol% MEPN content were 17.8 and 27.3 respectively. Note that the LOI of pure poly(MEPN) was 34.1.
LOI = 17.5 + 0.4 × CY
3.5.2. Sidechain PCPA-Based Polymer Networks for Enzyme Immobilization
In the study of Toppare and Coll. [178], proton conducting polymer blend was prepared by mixing PVPA with poly(1-vinylimidazole) at various ratios (Scheme 44). The polymer network having the most suitable stoichiometric ratio for substantial proton conductivity was used for the immobilization of invertase. Despite the fact that the value of the Michaelis-Menten constant (KM) for immobilized invertase was 3.5 times lower than the KM of the free enzyme (6.8 vs. 24.3 mM), the use of polymer matrix allowed to have the activity of the enzyme kept constant after 25 repetitive usages.
Scheme 44.
Protonation of PVPA and poly(1-vinylimidazole) with a formation of polyelectrolyte network [178].
4. Conclusions
In our review, we tried to show all the diversity of the synthetic approaches to sidechain PCPAs and the great potential of their applications. Sidechain PCPAs are different from other organic polyacids by higher acidity and, more significantly, by the ability to participate in biomineralization processes. The high chemical stability of the backbone of the sidechain PCPAs is a clear advantage in the development of the solid-state polyelectrolytes but is a serious obstacle to their use in tissue engineering and bone surgery.
Both for main-chain and sidechain PCPAs, the potential of the current polymer chemistry and science of catalysis remains largely unexploited. The very idea to combine polyester (or other) biodegradable backbone with –P(O)(OH)2-functionalized substituents has not yet been implemented despite its apparent promise.
Author Contributions
Conceptualization, P.V.I.; methodology, I.E.N. and P.V.I.; writing—original draft preparation, P.V.I.; writing—review and editing, I.E.N. and P.V.I.; visualization, P.V.I.; supervision, I.E.N.; project administration, I.E.N.; funding acquisition, I.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the TIPS RAS State Plan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.N.; Sahin, S.; Virga, E.; de Beer, S.; de Smet, L.C.P.M.; de Vos, W.M. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Appl. Polym. Mater. 2021, 3, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Steininger, E. Chapter 5: Phosphorylation of the polymers. J. Macromol. Chem. C Polym. Rev. 1968, 2, 57–72. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Hiranphinyophat, S.; Iwasaki, Y. Controlled biointerfaces with biomimetic phosphorus-containing polymers. Sci. Technol. Adv. Biomater. 2021, 22, 301–316. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. [Google Scholar] [CrossRef]
- Ogliari, F.A.; da Silva, E.D.; Lima, G.D.; Madruga, F.C.; Henn, S.; Bueno, M.; Ceschi, M.A.; Petzhold, C.L.; Piva, E. Synthesis of phosphate monomers and bonding to dentin: Esterification methods and use of phosphorus pentoxide. J. Dent. 2008, 36, 171–177. [Google Scholar] [CrossRef]
- Wehbi, M.; Mehdi, A.; Negrell, C.; David, G.; Alaaeddine, A.; Améduri, B. Phosphorus-Containing Fluoropolymers: State of the Art and Applications. ACS Appl. Mater. Interfaces 2020, 12, 38–59. [Google Scholar] [CrossRef]
- David, G.; Negrell-Guirao, C.; Iftene, F.; Boutevin, B.; Chougrani, K. Recent progress on phosphonate vinyl monomers and polymers therefore obtained by radical (co)polymerization. Polym. Chem. 2012, 3, 265–274. [Google Scholar] [CrossRef]
- Anbar, M.; Farley, E.P. Potential Use of Organic Polyphosphonates as Adhesives in the Restoration of Teeth. J. Dent. Res. 1974, 53, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y. Vinylphosphonic acid and some of its derivatives. Russ. Chem. Bull. 1959, 8, 2043–2045. [Google Scholar] [CrossRef]
- Rochlitz, F.; Vilcsek, H. β-Chloroethylphosphonic Dichloride: Its Synthesis and Use. Angew. Chem. Int. Ed. 1962, 1, 652–656. [Google Scholar] [CrossRef]
- Kleiner, H.-J.; Dürsch, W. Process for the Preparation of Vinylphosphonic Acid Diesters and Vinylphosphonic Acid. U.S. Patent 4493803, 15 January 1985. Available online: https://patents.google.com/patent/US4493803A/en?oq=US4493803+(A) (accessed on 20 November 2022).
- Kleiner, H.-J.; Roscher, G. Process for Preparing Vinyl-Phosphonic Acids. U.S. Patent 5811575, 22 September 1998. Available online: https://patents.google.com/patent/US5811575A/en?oq=US5811575+(A) (accessed on 20 November 2022).
- Zotov, S.B.; Tuzhikov, M.O.; Tuzhikov, O.I.; Khokhlova, T.V. Microwave synthesis of vinylphosphonic acid and its derivatives. Russ. J. Appl. Chem. 2012, 85, 639–643. [Google Scholar] [CrossRef]
- Svara, J.; Weferling, N.; Hofmann, T. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry, 2nd ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Becker, L.W. Poly (Alkenyl) Phosphonic Acid and Methods of Use Thereof. U.S. Patent 4446046, 1 May 1984. Available online: https://patents.google.com/patent/US4446046A/en (accessed on 20 November 2022).
- Zeuner, F.; Moszner, N.; Völkel, T.; Vogel, K.; Rheinberger, V. Synthesis and Dental Aspects of Acrylic Phosphoric and Phosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144, 133–136. [Google Scholar] [CrossRef]
- Song, I.T.; Stewart, R.J. Complex coacervation of Mg(ii) phospho-polymethacrylate, a synthetic analog of sandcastle worm adhesive phosphoproteins. Soft Matter 2018, 14, 379–386. [Google Scholar] [CrossRef]
- Browne, J.E.; Driver, M.J.; Russell, J.C.; Sammes, P.G. Preparation of phospholipid analogues using the phosphoramidite route. J. Chem. Soc. Perkin Trans. 2000, 653–657. [Google Scholar] [CrossRef]
- Avci, D.; Albayrak, A.Z. Synthesis and copolymerization of new phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2003, 41, 2207–2217. [Google Scholar] [CrossRef]
- Avci, D.; Mathias, L.J. Synthesis and polymerization of phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2002, 40, 3221–3231. [Google Scholar] [CrossRef]
- Moszner, N.; Zeuner, F.; Fischer, U.K.; Rheinberger, V. Monomers for adhesive polymers, 2. Synthesis and radical polymerisation of hydrolytically stable acrylic phosphonic acids. Macromol. Chem. Phys. 1999, 200, 1062–1067. [Google Scholar] [CrossRef]
- Sahin, G.; Avci, D.; Karahan, O.; Moszner, N. Synthesis and photopolymerizations of new phosphonated methacrylates from alkyl α-hydroxymethacrylates and glycidyl methacrylate. J. Appl. Polym. Sci. 2009, 114, 97–106. [Google Scholar] [CrossRef]
- Samav, Y.; Akpinar, B.; Kocak, G.; Bütün, V. Preparation of Responsive Zwitterionic Diblock Copolymers Containing Phosphate and Phosphonate Groups. Macromol. Res. 2020, 28, 1134–1141. [Google Scholar] [CrossRef]
- El Asri, Z.; Chougrani, K.; Negrell-Guirao, C.; David, G.; Boutevin, B.; Loubat, C. An efficient process for synthesizing and hydrolyzing a phosphonated methacrylate: Investigation of the adhesive and anticorrosive properties. J. Polym. Sci. A Polym. Chem. 2008, 46, 4794–4803. [Google Scholar] [CrossRef]
- Bressy-Brondino, C.; Boutevin, B.; Hervaud, Y.; Gaboyard, M. Adhesive and anticorrosive properties of poly(vinylidene fluoride) powders blended with phosphonated copolymers on galvanized steel plates. J. Appl. Polym. Sci. 2002, 83, 2277–2287. [Google Scholar] [CrossRef]
- Catel, Y.; Bock, T.; Moszner, N. Monomers for adhesive polymers. XV. Synthesis, photopolymerization, and adhesive properties of polymerizable β-ketophosphonic acids. J. Polym. Sci. A Polym. Chem. 2014, 52, 3550–3563. [Google Scholar] [CrossRef]
- Suzuki, S.; Whittaker, M.R.; Grøndahl, L.; Monteiro, M.J.; Wentrup-Byrne, E. Synthesis of Soluble Phosphate Polymers by RAFT and Their in Vitro Mineralization. Biomacromolecules 2006, 7, 3178–3187. [Google Scholar] [CrossRef]
- Catel, Y.; Besse, V.; Zulauf, A.; Marchat, D.; Pfund, E.; Pham, T.-N.; Bernache-Assolant, D.; Degrange, M.; Lequeux, T.; Madec, P.-J.; et al. Synthesis and evaluation of new phosphonic, bisphosphonic and difluoromethylphosphonic acid monomers for dental application. Eur. Polym. J. 2012, 48, 318–330. [Google Scholar] [CrossRef]
- Derbanne, M.; Zulauf, A.; Le Goff, S.; Pfund, E.; Sadoun, M.; Pham, T.-N.; Lequeux, T. Fluorophosphonylated Monomers for Dental Applications. Org. Process Res. Dev. 2014, 18, 1010–1019. [Google Scholar] [CrossRef]
- Banerjee, S.; Wehbi, M.; Manseri, A.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Poly(vinylidene fluoride) Containing Phosphonic Acid as Anticorrosion Coating for Steel. ACS Appl. Mater. Interfaces 2017, 9, 6433–6443. [Google Scholar] [CrossRef] [PubMed]
- Catel, Y.; Degrange, M.; Le Pluart, L.; Madec, P.-J.; Pham, T.-N.; Picton, L. Synthesis, photopolymerization and adhesive properties of new hydrolytically stable phosphonic acids for dental applications. J. Polym. Sci. A Polym. Chem. 2008, 46, 7074–7090. [Google Scholar] [CrossRef]
- Salman, S.; Albayrak, A.Z.; Avci, D.; Aviyente, V. Synthesis and modeling of new phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2005, 43, 2574–2583. [Google Scholar] [CrossRef]
- Moszner, N.; Zeuner, F.; Pfeiffer, S.; Schurte, I.; Rheinberger, V.; Drache, M. Monomers for Adhesive Polymers, 3. Synthesis, Radical Polymerization and Adhesive Properties of Hydrolytically Stable Phosphonic Acid Monomers. Macromol. Mater. Eng. 2001, 286, 225–231. [Google Scholar] [CrossRef]
- Otaka, A.; Yamaguchi, T.; Saisho, R.; Hiraga, T.; Iwasaki, Y. Bone-targeting phospholipid polymers to solubilize the lipophilic anticancer drug. J. Biomater. Mater. Res. 2020, 108, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Pavlinec, J.; Lamparth, I.; Zeuner, F.; Angermann, J. Monomers for Adhesive Polymers, 6. Synthesis and Radical Polymerisation of 1,3-Bis(methacrylamido)propane-2-yl Dihydrogen Phosphate. Macromol. Rapid Commun. 2006, 27, 1115–1120. [Google Scholar] [CrossRef]
- Pavlinec, J.; Zeuner, F.; Angermann, J.; Moszner, N. Monomers for Adhesive Polymers, 5. Macromol. Chem. Phys. 2005, 206, 1878–1886. [Google Scholar] [CrossRef]
- Sibold, N.; Madec, P.J.; Masson, S.; Pham, T.N. Synthesis and characterization of (co)polymers containing a phosphonate function for use in dental composites. Polymer 2002, 43, 7257–7267. [Google Scholar] [CrossRef]
- Fukuzaki, N.; Nakabayashi, K.; Nakazawa, S.; Murata, S.; Higashihara, T.; Ueda, M. Highly phosphonated poly(N-phenylacrylamide) for proton exchange membranes. J. Polym. Sci. A Polym. Chem. 2011, 49, 93–100. [Google Scholar] [CrossRef]
- Higashihara, T.; Fukuzaki, N.; Tamura, Y.; Rho, Y.; Nakabayashi, K.; Nakazawa, S.; Murata, S.; Ree, M.; Ueda, M. Polymer electrolyte membrane based on polyacrylate with phosphonic acid via long alkyl side chains. J. Mater. Chem. A 2013, 1, 1457–1464. [Google Scholar] [CrossRef]
- Avci, D.; Mathias, L.J. Synthesis and photopolymerizations of phosphate-containing acrylate/(di)methacrylate monomers from 3-(acryloyloxy)-2-hydroxypropyl methacrylate. Polym. Bull. 2005, 54, 11–19. [Google Scholar] [CrossRef]
- Yeniad, B.; Albayrak, A.Z.; Olcum, N.C.; Avci, D. Synthesis and photopolymerizations of new phosphonated monomers for dental applications. J. Polym. Sci. A Polym. Chem. 2008, 46, 2290–2299. [Google Scholar] [CrossRef]
- Yamabe, M.; Akiyama, K.; Akatsuka, Y.; Kato, M. Novel phosphonated perfluorocarbon polymers. Eur. Polym. J. 2000, 36, 1035–1041. [Google Scholar] [CrossRef]
- Lee, S.-I.; Yoon, K.-H.; Song, M.; Peng, H.; Page, K.A.; Soles, C.L.; Yoon, D.Y. Structure and Properties of Polymer Electrolyte Membranes Containing Phosphonic Acids for Anhydrous Fuel Cells. Chem. Mater. 2012, 24, 115–122. [Google Scholar] [CrossRef]
- Alter, C.; Hoge, B. Synthesis and characterization of a novel difluoromethylene phosphonic acid functionalized polymer. J. Appl. Polym. Sci. 2018, 135, 46765. [Google Scholar] [CrossRef]
- Jang, S.; Kim, S.Y.; Jung, H.Y.; Park, M.J. Phosphonated Polymers with Fine-Tuned Ion Clustering Behavior: Toward Efficient Proton Conductors. Macromolecules 2018, 51, 1120–1128. [Google Scholar] [CrossRef]
- Alter, C.; Neumann, B.; Stammler, H.-G.; Hoge, B. Synthesis and characterization of a novel highly phosphonated water-insoluble polymer. J. Appl. Polym. Sci. 2020, 137, 48235. [Google Scholar] [CrossRef]
- Funk, R.L.; Stallman, J.B.; Wos, J.A. Claisen rearrangements of enol phosphates. J. Am. Chem. Soc. 1993, 115, 8847–8848. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Yu, R.; Shi, L.; Cai, Q.; Lu, M.; Heretsch, P. General Synthetic Approach to Functionalized Dihydrooxepines. Org. Lett. 2013, 15, 1994–1997. [Google Scholar] [CrossRef]
- Jackson, J.A.; Hammond, G.B.; Wiemer, D.F. Synthesis of .alpha.-phosphono lactones and esters through a vinyl phosphate-phosphonate rearrangement. J. Org. Chem. 1989, 54, 4750–4754. [Google Scholar] [CrossRef]
- Lee, K.; Jackson, J.A.; Wiemer, D.F. Stereocontrol in Horner-Wadsworth-Emmons condensations of α-phosphono lactones with aldehydes: A synthesis of integerrinecic acid and senecic acid lactones. J. Org. Chem. 1993, 58, 5967–5971. [Google Scholar] [CrossRef]
- Bingöl, B.; Meyer, W.H.; Wagner, M.; Wegner, G. Synthesis, Microstructure, and Acidity of Poly(vinylphosphonic acid). Macromol. Rapid Commun. 2006, 27, 1719–1724. [Google Scholar] [CrossRef]
- Komber, H.; Steinert, V.; Voit, B. 1H, 13C, and 31P NMR Study on Poly(vinylphosphonic acid) and Its Dimethyl Ester. Macromolecules 2008, 41, 2119–2125. [Google Scholar] [CrossRef]
- Millaruelo, M.; Steinert, V.; Komber, H.; Klopsch, R.; Voit, B. Synthesis of Vinylphosphonic Acid Anhydrides and their Copolymerization with Vinylphosphonic Acid. Macromol. Chem. Phys. 2008, 209, 366–374. [Google Scholar] [CrossRef]
- Kim, Y.K.; Gu, L.; Bryan, T.E.; Kim, J.R.; Chen, L.; Liu, Y.; Yoon, J.C.; Breschi, L.; Pashley, D.H.; Tay, F.R. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010, 31, 6618–6627. [Google Scholar] [CrossRef]
- Tutgun, M.S.; Sinirlioglu, D.; Celik, S.U.; Bozkurt, A. Effect of hexagonal boron nitride on poly(vinyl phosphonic acid) composite polymer electrolytes for fuel cells. Polym. Sci. A 2016, 58, 810–817. [Google Scholar] [CrossRef]
- Asian, A.; Bozkurt, A. Nanocomposite polymer electrolyte membranes based on poly(vinylphosphonic acid)/TiO2 nanoparticles. J. Mater. Res. 2012, 27, 3090–3095. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Sozeri, H.; Toprak, M.S.; Aslan, A.; Baykal, A. Poly(vinyl phosphonic acid) (PVPA)–BaFe12O19 Nanocomposite. J. Supercond. Nov. Magn. 2012, 25, 1185–1193. [Google Scholar] [CrossRef]
- David, G.; Boutevin, B.; Seabrook, S.; Destarac, M.; Woodward, G.; Otter, G. Radical Telomerisation of Vinyl Phosphonic Acid with a Series of Chain Transfer Agents. Macromol. Chem. Phys. 2007, 208, 635–642. [Google Scholar] [CrossRef]
- Taherkhani, Z.; Abdollahi, M.; Sharif, A. Synthesis and microstructural characterization of low to high molecular weight poly(vinylphosphonic acid)s: Effect of molecular weight and temperature on acidity and polyelectrolyte behavior. J. Polym. Res. 2017, 24, 132. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tayouo, R.; Seabrook, S.; Ameduri, B.; Boutevin, B.; Woodward, G.; Destarac, M. A Process for Polymerizing Vinyl Phosphonic Acid with C6F13I Perfluoroalkyl Iodide Chain-Transfer Agent. Macromol. Chem. Phys. 2008, 209, 75–83. [Google Scholar] [CrossRef]
- Blidi, I.; Geagea, R.; Coutelier, O.; Mazières, S.; Violleau, F.; Destarac, M. Aqueous RAFT/MADIX polymerisation of vinylphosphonic acid. Polym. Chem. 2012, 4, 609–612. [Google Scholar] [CrossRef]
- Seiler, L.; Loiseau, J.; Leising, F.; Boustingorry, P.; Harrisson, S.; Destarac, M. Acceleration and improved control of aqueous RAFT/MADIX polymerization of vinylphosphonic acid in the presence of alkali hydroxides. Polym. Chem. 2017, 8, 3825–3832. [Google Scholar] [CrossRef]
- Ellis, J.; Wilson, A.D. Polyphosphonate cements: A new class of dental materials. J. Mater. Sci. Lett. 1990, 9, 1058–1060. [Google Scholar] [CrossRef]
- Braybrook, J.H.; Nicholson, J.W. Incorporation of crosslinking agents into poly(vinyl phosphonic acid) as a route to glass–polyalkenoate cements of improved compressive strength. J. Mater. Chem. 1993, 3, 361–365. [Google Scholar] [CrossRef]
- Jin, S.; Gonsalves, K.E. Synthesis and Characterization of Functionalized Poly(ε-caprolactone) Copolymers by Free-Radical Polymerization. Macromolecules 1998, 31, 1010–1015. [Google Scholar] [CrossRef]
- Bingöl, B.; Hart-Smith, G.; Barner-Kowollik, C.; Wegner, G. Characterization of Oligo(vinyl phosphonate)s by High-Resolution Electrospray Ionization Mass Spectrometry: Implications for the Mechanism of Polymerization. Macromolecules 2008, 41, 1634–1639. [Google Scholar] [CrossRef]
- Macarie, L.; Pekar, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in aqueous solution of homo- and copolymers of vinylphosphonic acid derivatives obtained by UV-curing. Macromol. Res. 2017, 25, 214–221. [Google Scholar] [CrossRef]
- Yin, M.; Kang, N.; Cui, G.; Liu, Z.; Wang, F.; Yang, W.; Klapper, M.; Müllen, K. Synthesis, Electrochemical Properties and Self-Assembly of a Proton-Conducting Core–Shell Macromolecule. Chem. Eur. J. 2012, 18, 2239–2243. [Google Scholar] [CrossRef]
- Wagner, T.; Manhart, A.; Deniz, N.; Kaltbeitzel, A.; Wagner, M.; Brunklaus, G.; Meyer, W.H. Vinylphosphonic Acid Homo- and Block Copolymers. Macromol. Chem. Phys. 2009, 210, 1903–1914. [Google Scholar] [CrossRef]
- Rabinowitz, R. The Reactions of Phosphonic Acid Esters with Acid Chlorides. A Very Mild Hydrolytic Route. J. Org. Chem. 1963, 28, 2975–2978. [Google Scholar] [CrossRef]
- Kawauchi, T.; Ohara, M.; Udo, M.; Kawauchi, M.; Takeichi, T. Preparation of isotactic-rich poly(dimethyl vinylphosphonate) and poly(vinylphosphonic acid) via the anionic polymerization of dimethyl vinylphosphonate. J. Polym. Sci. A Polym. Chem. 2010, 48, 1677–1682. [Google Scholar] [CrossRef]
- Rabe, G.W.; Komber, H.; Häussler, L.; Kreger, K.; Lattermann, G. Polymerization of Diethyl Vinylphosphonate Mediated by Rare-Earth Tris(amide) Compounds. Macromolecules 2010, 43, 1178–1181. [Google Scholar] [CrossRef]
- Seemann, U.B.; Dengler, J.E.; Rieger, B. High-Molecular-Weight Poly(vinylphosphonate)s by Single-Component Living Polymerization Initiated by Rare-Earth-Metal Complexes. Angew. Chem. Int. Ed. 2010, 49, 3489–3491. [Google Scholar] [CrossRef]
- Salzinger, S.; Seemann, U.B.; Plikhta, A.; Rieger, B. Poly(vinylphosphonate)s Synthesized by Trivalent Cyclopentadienyl Lanthanide-Induced Group Transfer Polymerization. Macromolecules 2011, 44, 5920–5927. [Google Scholar] [CrossRef]
- Zhang, N.; Salzinger, S.; Rieger, B. Poly(vinylphosphonate)s with Widely Tunable LCST: A Promising Alternative to Conventional Thermoresponsive Polymers. Macromolecules 2012, 45, 9751–9758. [Google Scholar] [CrossRef]
- Salzinger, S.; Soller, B.S.; Plikhta, A.; Seemann, U.B.; Herdtweck, E.; Rieger, B. Mechanistic Studies on Initiation and Propagation of Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates. J. Am. Chem. Soc. 2013, 135, 13030–13040. [Google Scholar] [CrossRef]
- Soller, B.S.; Salzinger, S.; Jandl, C.; Pöthig, A.; Rieger, B. C–H Bond Activation by σ-Bond Metathesis as a Versatile Route toward Highly Efficient Initiators for the Catalytic Precision Polymerization of Polar Monomers. Organometallics 2015, 34, 2703–2706. [Google Scholar] [CrossRef]
- Soller, B.S.; Sun, Q.; Salzinger, S.; Jandl, C.; Pöthig, A.; Rieger, B. Ligand Induced Steric Crowding in Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates: Does Enthalpy Matter? Macromolecules 2016, 49, 1582–1589. [Google Scholar] [CrossRef]
- Pahl, P.; Schwarzenböck, C.; Herz, F.A.D.; Soller, B.S.; Jandl, C.; Rieger, B. Core-First Synthesis of Three-Armed Star-Shaped Polymers by Rare Earth Metal-Mediated Group Transfer Polymerization. Macromolecules 2017, 50, 6569–6576. [Google Scholar] [CrossRef]
- Weger, M.; Pahl, P.; Schmidt, F.; Soller, B.S.; Altmann, P.J.; Pöthig, A.; Gemmecker, G.; Eisenreich, W.; Rieger, B. Isospecific Group-Transfer Polymerization of Diethyl Vinylphosphonate and Multidimensional NMR Analysis of the Polymer Microstructure. Macromolecules 2019, 52, 7073–7080. [Google Scholar] [CrossRef]
- Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates. Macromol. Rapid Commun. 2012, 33, 1327–1345. [Google Scholar] [CrossRef] [PubMed]
- Soller, B.S.; Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Precision Polymerization of Vinylphosphonates and Conjugated Nitrogen-Containing Vinyl Monomers. Chem. Rev. 2016, 116, 1993–2022. [Google Scholar] [CrossRef]
- Zhang, N.; Salzinger, S.; Deubel, F.; Jordan, R.; Rieger, B. Surface-Initiated Group Transfer Polymerization Mediated by Rare Earth Metal Catalysts. J. Am. Chem. Soc. 2012, 134, 7333–7336. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, Y.; Salzinger, S.; Zhang, N.; Dong, D.; Rieger, B. Poly(vinylphosphonate)s functionalized polymer microspheres via rare earth metal-mediated group transfer polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 2919–2925. [Google Scholar] [CrossRef]
- Altenbuchner, P.T.; Soller, B.S.; Kissling, S.; Bachmann, T.; Kronast, A.; Vagin, S.I.; Rieger, B. Versatile 2-Methoxyethylaminobis(phenolate)yttrium Catalysts: Catalytic Precision Polymerization of Polar Monomers via Rare Earth Metal-Mediated Group Transfer Polymerization. Macromolecules 2014, 47, 7742–7749. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.; Ling, J.; Shen, Z. Syntheses and properties of poly(diethyl vinylphosphonate) initiated by lanthanide tris(borohydride) complexes: Polymerization controllability and mechanism. J. Polym. Sci. A Polym. Chem. 2013, 51, 2409–2415. [Google Scholar] [CrossRef]
- Li, C.; Saga, Y.; Onozawa, S.; Kobayashi, S.; Sato, K.; Fukaya, N.; Han, L.-B. Wet and Dry Processes for the Selective Transformation of Phosphonates to Phosphonic Acids Catalyzed by Brønsted Acids. J. Org. Chem. 2020, 85, 14411–14419. [Google Scholar] [CrossRef]
- Harsági, N.; Keglevich, G. The Hydrolysis of Phosphinates and Phosphonates: A Review. Molecules 2021, 26, 2840. [Google Scholar] [CrossRef]
- Dey, R.E.; Zhong, X.; Youle, P.J.; Wang, Q.G.; Wimpenny, I.; Downes, S.; Hoyland, J.A.; Watts, D.C.; Gough, J.E.; Budd, P.M. Synthesis and Characterization of Poly(vinylphosphonic acid-co-acrylic acid) Copolymers for Application in Bone Tissue Scaffolds. Macromolecules 2016, 49, 2656–2662. [Google Scholar] [CrossRef]
- Layrac, G.; Gérardin, C.; Tichit, D.; Harrisson, S.; Destarac, M. Hybrid polyion complex micelles from poly(vinylphosphonic acid)-based double hydrophilic block copolymers and divalent transition metal ions. Polymer 2015, 72, 292–300. [Google Scholar] [CrossRef]
- Dey, R.E.; Wimpenny, I.; Gough, J.E.; Watts, D.C.; Budd, P.M. Poly(vinylphosphonic acid-co-acrylic acid) hydrogels: The effect of copolymer composition on osteoblast adhesion and proliferation. J. Biomed. Mater. Res. 2018, 106, 255–264. [Google Scholar] [CrossRef]
- Çelik, S.Ü.; Akbey, Ü.; Graf, R.; Bozkurt, A.; Spiess, H.W. Anhydrous proton-conducting properties of triazole–phosphonic acidcopolymers: A combined study with MAS NMR. Phys. Chem. Chem. Phys. 2008, 10, 6058–6066. [Google Scholar] [CrossRef]
- Najafi, V.; Kabiri, K.; Ziaee, F.; Omidian, H.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Farhadnejad, H. Synthesis and characterization of alcogels based on ethylene glycol methyl ether methacrylate-vinyl phosphonic acid copolymers. J. Polym. Res. 2012, 19, 9866. [Google Scholar] [CrossRef]
- Gemeinhart, R.A.; Bare, C.M.; Haasch, R.T.; Gemeinhart, E.J. Osteoblast-like cell attachment to and calcification of novel phosphonate-containing polymeric substrates. J. Biomed. Mater. Res. A 2006, 78, 433–440. [Google Scholar] [CrossRef]
- Wehbi, M.; Mehdi, A.; Alaaeddine, A.; Jaber, N.; Ameduri, B. Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes. Polymers 2020, 12, 1955. [Google Scholar] [CrossRef]
- Nazarova, O.; Chesnokova, E.; Nekrasova, T.; Zolotova, Y.; Dobrodumov, A.; Vlasova, E.; Fischer, A.; Bezrukova, M.; Panarin, E. New Copolymers of Vinylphosphonic Acid with Hydrophilic Monomers and Their Eu3+ Complexes. Polymers 2022, 14, 590. [Google Scholar] [CrossRef]
- Yılmaz, M.; Akar, A.; Köken, N.; Kızılcan, N. Polymers of vinylphosphonic acid, acrylonitrile, and methyl acrylate and their nanofibers. J. Appl. Polym. Sci. 2020, 137, 49023. [Google Scholar] [CrossRef]
- Kavlak, S.; Güner, A.; Rzayev, Z.M.O. Functional terpolymers containing vinylphosphonic acid: The synthesis and characterization of poly(vinylphosphonic acid-co-styrene-co-maleic anhydride). J. Appl. Polym. Sci. 2012, 125, 3617–3629. [Google Scholar] [CrossRef]
- Sinirlioglu, D.; Celik, S.U.; Muftuoglu, A.E.; Bozkurt, A. Proton Conducting Copolymer Electrolytes Based on Vinyl Phosphonic Acid and 5-(Methacrylamido)tetrazole. Macromol. Chem. Phys. 2014, 215, 269–279. [Google Scholar] [CrossRef]
- Lane, D.D.; Kaur, S.; Weerasakare, G.M.; Stewart, R.J. Toughened hydrogels inspired by aquatic caddisworm silk. Soft Matter 2015, 11, 6981–6990. [Google Scholar] [CrossRef] [PubMed]
- Uğur, M.H.; Kayaman-Apohan, N.; Avci, D.; Güngör, A. Phosphoric acid functional UV-cured proton conducting polymer membranes for fuel cells. Ionics 2015, 21, 3097–3107. [Google Scholar] [CrossRef]
- George, K.A.; Wentrup-Byrne, E.; Hill, D.J.T.; Whittaker, A.K. Investigation into the Diffusion of Water into HEMA-co-MOEP Hydrogels. Biomacromolecules 2004, 5, 1194–1199. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Marić, M. Thermal characteristics and flame retardance behavior of phosphoric acid-containing poly(methacrylates) synthesized by RAFT polymerization. Mater. Today Commun. 2020, 25, 101618. [Google Scholar] [CrossRef]
- Li, L.; Song, Y.; He, J.; Zhang, M.; Liu, J.; Ni, P. Zwitterionic shielded polymeric prodrug with folate-targeting and pH responsiveness for drug delivery. J. Mater. Chem. B 2019, 7, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Tan, D.; Yu, Q.; Lin, J.; Zhu, T.; Li, T.; Li, L. Ultra-High Performance of Hyper-Crosslinked Phosphate-Based Polymer for Uranium and Rare Earth Element Adsorption in Aqueous Solution. Langmuir 2019, 35, 13860–13871. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamaguchi, E. Synthesis of well-defined thermoresponsive polyphosphoester macroinitiators using organocatalysts. Macromolecules 2010, 43, 2664–2666. [Google Scholar] [CrossRef]
- Zhou, F.; Huck, W.T.S. Three-stage switching of surface wetting using phosphate-bearing polymer brushes. Chem. Commun. 2005, 5999–6001. [Google Scholar] [CrossRef]
- Wang, T.; Rother, G.; Cölfen, H. A New Method to Purify Highly Phosphonated Block Copolymers and Their Effect on Calcium Carbonate Mineralization. Macromol. Chem. Phys. 2005, 206, 1619–1629. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Ali, S.A. Removal of heavy metal ions using a novel cross-linked polyzwitterionic phosphonate. Sep. Purif. Technol. 2012, 98, 94–101. [Google Scholar] [CrossRef]
- Clayton, J.O.; Jensen, W.L. Reaction of Paraffin Hydrocarbons with Phosphorus Trichloride and Oxygen to Produce Alkanephosphonyl Chlorides. J. Am. Chem. Soc. 1948, 70, 3880–3882. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Sopchak, W.P. The reaction of phosphorus trichloride and oxygen with polymers. J. Polym. Sci. 1960, 47, 417–433. [Google Scholar] [CrossRef]
- Allan, J.M.; Dooley, R.L.; Shalaby, S.W. Surface phosphonylation of low-density polyethylene. J. Appl. Polym. Sci. 2000, 76, 1870–1875. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lapienis, G.; Basko, M.; Bartczak, Z.; Dworak, A.; Penczek, S. Dihydrophilic block copolymers with ionic and nonionic blocks. I. Poly(ethylene oxide)-b-polyglycidol with OP(O)(OH)2, COOH, or SO3H functions: Synthesis and influence for CaCO3 crystallization. J. Polym. Sci. A Polym. Chem. 2001, 39, 955–963. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Penczek, S. Poly(ethylene glycol)-b-phosphorylated polyglycidols as CaCO3 crystal growth modifiers. II. Macromolecular architecture versus the crystal size and shape and crystallization inhibition. J. Polym. Sci. A Polym. Chem. 2007, 45, 90–98. [Google Scholar] [CrossRef]
- Dworak, A.; Baran, G.; Trzebicka, B.; Wałach, W. Polyglycidol-block-poly(ethylene oxide)-block-polyglycidol: Synthesis and swelling properties. React. Funct. Polym. 1999, 42, 31–36. [Google Scholar] [CrossRef]
- Rudloff, J.; Antonietti, M.; Cölfen, H.; Pretula, J.; Kaluzynski, K.; Penczek, S. Double-Hydrophilic Block Copolymers with Monophosphate Ester Moieties as Crystal Growth Modifiers of CaCO3. Macromol. Chem. Phys. 2002, 203, 627–635. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Simultaneous introduction of phosphonic and carboxylic acid functions to hydroxylated macromolecules. J. Polym. Sci. A Polym. Chem. 2004, 42, 432–443. [Google Scholar] [CrossRef]
- Förster, S.; Krämer, E. Synthesis of PB−PEO and PI−PEO Block Copolymers with Alkyllithium Initiators and the Phosphazene Base t-BuP4. Macromolecules 1999, 32, 2783–2785. [Google Scholar] [CrossRef]
- Dimova, R.; Lipowsky, R.; Mastai, Y.; Antonietti, M. Binding of Polymers to Calcite Crystals in Water: Characterization by Isothermal Titration Calorimetry. Langmuir 2003, 19, 6097–6103. [Google Scholar] [CrossRef]
- Pramanik, N.; Biswas, S.K.; Pramanik, P. Synthesis and Characterization of Hydroxyapatite/Poly(Vinyl Alcohol Phosphate) Nanocomposite Biomaterials. Int. J. Appl. Ceram. Technol. 2008, 5, 20–28. [Google Scholar] [CrossRef]
- Kieczykowski, G.R.; Jobson, R.B.; Melillo, D.G.; Reinhold, D.F.; Grenda, V.J.; Shinkai, I. Preparation of (4-Amino-1-Hydroxybutylidene)bisphosphonic Acid Sodium Salt, MK-217 (Alendronate Sodium). An Improved Procedure for the Preparation of 1-Hydroxy-1,1-bisphosphonic Acids. J. Org. Chem. 1995, 60, 8310–8312. [Google Scholar] [CrossRef]
- Li, M.; Cölfen, H.; Mann, S. Morphological control of BaSO4 microstructures by double hydrophilic block copolymer mixtures. J. Mater. Chem. 2004, 14, 2269–2276. [Google Scholar] [CrossRef]
- Tayouo, R.; David, G.; Améduri, B.; Rozière, J.; Roualdès, S. New Fluorinated Polymers Bearing Pendant Phosphonic Acid Groups. Proton Conducting Membranes for Fuel Cell. Macromolecules 2010, 43, 5269–5276. [Google Scholar] [CrossRef]
- Elumalai, V.; Annapooranan, R.; Ganapathikrishnan, M.; Sangeetha, D. A synthesis study of phosphonated PSEBS for high temperature proton exchange membrane fuel cells. J. Appl. Polym. Sci. 2018, 135, 45954. [Google Scholar] [CrossRef]
- Atanasov, V.; Kerres, J. Highly Phosphonated Polypentafluorostyrene. Macromolecules 2011, 44, 6416–6423. [Google Scholar] [CrossRef]
- Atanasov, V.; Oleynikov, A.; Xia, J.; Lyonnard, S.; Kerres, J. Phosphonic acid functionalized poly(pentafluorostyrene) as polyelectrolyte membrane for fuel cell application. J. Power Souces 2017, 343, 364–372. [Google Scholar] [CrossRef]
- Atanasov, V.; Gudat, D.; Ruffmann, B.; Kerres, J. Highly phosphonated polypentafluorostyrene: Characterization and blends with polybenzimidazole. Eur. Polym. J. 2013, 49, 3977–3985. [Google Scholar] [CrossRef]
- Atanasov, V.; Lee, A.S.; Park, E.J.; Maurya, S.; Baca, E.D.; Fujimoto, C.; Hibbs, M.; Matanovic, I.; Kerres, J.; Kim, Y.S. Synergistically integrated phosphonated poly(pentafluorostyrene) for fuel cells. Nat. Mater. 2021, 20, 370–377. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, A.S.; Atanasov, V.; Kerres, J.; Park, E.J.; Adhikari, S.; Maurya, S.; Manriquez, L.D.; Jung, J.; Fujimoto, C.; et al. Protonated phosphonic acid electrodes for high power heavy-duty vehicle fuel cells. Nat. Energy 2022, 7, 248–259. [Google Scholar] [CrossRef]
- Shao, Z.; Sannigrahi, A.; Jannasch, P. Poly(tetrafluorostyrenephosphonic acid)–Polysulfone Block Copolymers and Membranes. J. Polym. Sci. A Polym. Chem. 2013, 51, 4657–4666. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, S.Y.; Kim, O.; Park, M.J. Effect of the Protogenic Group on the Phase Behavior and Ion Transport Properties of Acid-Bearing Block Copolymers. Macromolecules 2015, 48, 6142–6152. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, O.; Park, M.J. Ion Transport in Nanostructured Phosphonated Block Copolymers Containing Ionic Liquids. Macromol. Rapid Commun. 2016, 37, 1116–1123. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. Polysulfone ionomers functionalized with benzoyl(difluoromethylenephosphonic acid) side chains for proton-conducting fuel-cell membranes. J. Polym. Sci. A Polym. Chem. 2007, 45, 269–283. [Google Scholar] [CrossRef]
- Sinirlioglu, D.; Muftuoglu, A.E.; Bozkurt, A. Investigation of perfluorinated proton exchange membranes prepared via a facile strategy of chemically combining poly(vinylphosphonic acid) with PVDF by means of poly(glycidyl methacrylate) grafts. J. Polym. Res. 2015, 22, 154. [Google Scholar] [CrossRef]
- Hajj, R.; El Hage, R.; Sonnier, R.; Otazaghine, B.; Gallard, B.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.-M. Grafting of phosphorus flame retardants on flax fabrics: Comparison between two routes. Polym. Degrad. Stab. 2018, 147, 25–34. [Google Scholar] [CrossRef]
- Farrokhi, M.; Abdollahi, M.; Alizadeh, A. An efficient method for straightforward phosphorylation of ethylene/vinyl alcohol copolymers using trialkyl phosphite/iodine. Polymer 2019, 169, 215–224. [Google Scholar] [CrossRef]
- Fiss, B.G.; Hatherly, L.; Stein, R.S.; Friščić, T.; Moores, A. Mechanochemical Phosphorylation of Polymers and Synthesis of Flame-Retardant Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 7951–7959. [Google Scholar] [CrossRef]
- Opper, K.L.; Fassbender, B.; Brunklaus, G.; Spiess, H.W.; Wagener, K.B. Polyethylene Functionalized with Precisely Spaced Phosphonic Acid Groups. Macromolecules 2009, 42, 4407–4409. [Google Scholar] [CrossRef]
- Opper, K.L.; Markova, D.; Klapper, M.; Müllen, K.; Wagener, K.B. Precision Phosphonic Acid Functionalized Polyolefin Architectures. Macromolecules 2010, 43, 3690–3698. [Google Scholar] [CrossRef]
- Bingöl, B.; Kroeger, A.; Jannasch, P. Well-defined phosphonated homo- and copolymers via direct ring opening metathesis polymerization. Polymer 2013, 54, 6676–6688. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Ghassemi, H.; Shockravi, A.; Zawodzinski, T.; Schiraldi, D. Phosphonated poly(arylene ether)s as potential high temperature proton conducting materials. Polymer 2011, 52, 4709–4717. [Google Scholar] [CrossRef]
- Rager, T.; Schuster, M.; Steininger, H.; Kreuer, K.-D. Poly(1,3-phenylene-5-phosphonic Acid), a Fully Aromatic Polyelectrolyte with High Ion Exchange Capacity. Adv. Mater. 2007, 19, 3317–3321. [Google Scholar] [CrossRef]
- Kaltbeitzel, A.; Schauff, S.; Steininger, H.; Bingöl, B.; Brunklaus, G.; Meyer, W.H.; Spiess, H.W. Water sorption of poly(vinylphosphonic acid) and its influence on proton conductivity. Solid State Ion. 2007, 178, 469–474. [Google Scholar] [CrossRef]
- Greish, Y.E.; Brown, P.W. Formation and Properties of Hydroxyapatite–Calcium Poly(vinyl phosphonate) Composites. J. Am. Cheram. Soc. 2002, 85, 1738–1744. [Google Scholar] [CrossRef]
- Strandberg, C.; Rosenauer, C.; Wegner, G. Poly(vinyl phosphonic acid): Hydrodynamic Properties and SEC-Calibration in Aqueous Solution. Macromol. Rapid Commun. 2010, 31, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the prism of time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Franco, R.A.; Min, Y.-K.; Yang, H.-M.; Lee, B.-T. On Stabilization of PVPA/PVA Electrospun Nanofiber Membrane and Its Effect on Material Properties and Biocompatibility. J. Nanimater. 2012, 2012, 393042. [Google Scholar] [CrossRef]
- Franco, R.A.; Sadiasa, A.; Lee, B.-T. Utilization of PVPA and its effect on the material properties and biocompatibility of PVA electrospun membrane. Polym. Adv. Technol. 2014, 25, 55–65. [Google Scholar] [CrossRef]
- Topçu, G.; Çelik, A.; Baba, A.; Demir, M.M. Design of Polymeric Antiscalants Based on Functional Vinyl Monomers for (Fe, Mg) Silicates. Energy Fuels 2017, 31, 8489–8496. [Google Scholar] [CrossRef]
- Kousar, F.; Moratti, S.C. Synthesis of fluorinated phosphorus-containing copolymers and their immobilization and properties on stainless steel. RSC Adv. 2021, 11, 38189–38201. [Google Scholar] [CrossRef] [PubMed]
- Penczek, S.; Kaluzynski, K.; Pretula, J. Hybrids of dihydrophylic ionic-nonionic block copolymers and CaCO3. Determination of the number of CaCO3 molecules attached to the ionic groups. Polym. Sci. Ser. A 2009, 51, 1282. [Google Scholar] [CrossRef]
- Penczek, S.; Kaluzynski, K.; Pretula, J. Determination of copolymer localization in polymer–CaCO3 hybrids formed in mediated crystallization. J. Polym. Sci. A Polym. Chem. 2009, 47, 4464–4467. [Google Scholar] [CrossRef]
- Cölfen, H.; Antonietti, M. Crystal Design of Calcium Carbonate Microparticles Using Double-Hydrophilic Block Copolymers. Langmuir 1998, 14, 582–589. [Google Scholar] [CrossRef]
- Abueva, C.D.G.; Lee, B.-T. Poly(vinylphosphonic acid) immobilized on chitosan: A glycosaminoglycan-inspired matrix for bone regeneration. Int. J. Biol. Macromol. 2014, 64, 294–301. [Google Scholar] [CrossRef]
- Olthof, M.G.L.; Tryfonidou, M.A.; Liu, X.; Pouran, B.; Meij, B.P.; Dhert, W.J.A.; Yaszemski, M.J.; Lu, L.; Alblas, J.; Kempen, D.H.R. Phosphate Functional Groups Improve Oligo[(Polyethylene Glycol) Fumarate] Osteoconduction and BMP-2 Osteoinductive Efficacy. Tissue Eng. Part A 2018, 24, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Antonson, S.A.; Antonson, D.E.; Bush, P. Effect of moist/dry/desiccated dentin on shear-bond strength of universal adhesives. Dent. Mater. 2014, 30, e81–e82. [Google Scholar] [CrossRef]
- Kim, Y.S. Polymer Electrolytes with High Ionic Concentration for Fuel Cells and Electrolyzers. ACS Appl. Polym. Mater. 2021, 3, 1250–1270. [Google Scholar] [CrossRef]
- Lee, Y.J.; Bingöl, B.; Murakhtina, T.; Sebastiani, D.; Meyer, W.H.; Wegner, G.; Spiess, H.W. High-Resolution Solid-State NMR Studies of Poly(vinyl phosphonic acid) Proton-Conducting Polymer: Molecular Structure and Proton Dynamics. J. Phys. Chem. B 2007, 111, 9711–9721. [Google Scholar] [CrossRef]
- Lee, Y.J.; Murakhtina, T.; Sebastiani, D.; Spiess, H.W. 2H Solid-State NMR of Mobile Protons: It Is Not Always the Simple Way. J. Am. Chem. Soc. 2007, 129, 12406–12407. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, H.; Graf, R.; Meyer, W.H.; Spiess, H.W.; Wegner, G. Phase Behavior and Proton Conduction in Poly(vinylphosphonic acid)/Poly(ethylene oxide) Blends. Macromolecules 2010, 43, 3876–3881. [Google Scholar] [CrossRef]
- Bingöl, B. Synthesis and Characterization of Poly(vinylphosphonic acid) for Proton Exchange Membranes in Fuel Cells. Ph.D. Dissertation, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 7 February 2007. [Google Scholar]
- Sen, U.; Acar, O.; Celik, S.U.; Bozkurt, A.; Ata, A.; Tokumasu, T.; Miyamoto, A. Proton-conducting blend membranes of Nafion/poly(vinylphosphonic acid) for proton exchange membrane fuel cells. J. Polym. Res. 2013, 20, 217. [Google Scholar] [CrossRef]
- Jiang, F.; Kaltbeitzel, A.; Zhang, J.; Meyer, W.H. Nano-spheres stabilized poly(vinyl phosphonic acid) as proton conducting membranes for PEMFCs. Int. J. Hydrogen Energy 2014, 39, 11157–11164. [Google Scholar] [CrossRef]
- Taherkhani, Z.; Abdollahi, M.; Sharif, A.; Barati, S. Poly(benzimidazole)/poly(vinylphosphonic acid) blend membranes with enhanced performance for high temperature polymer electrolyte membrane fuel cells. Solid State Ion. 2021, 364, 115635. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nohara, T.; Tabata, K.; Yamakado, R.; Shimada, R.; Nakazaki, H.; Saito, T.; Makino, T.; Arita, T.; Masuhara, A. Proton-conductive polymeric ionic liquids block copolymer of poly(vinylphosphonic acid)/1-propylimidazole-b-polystyrene for polymer electrolyte membrane fuel cells. Jpn. J. Appl. Phys. 2022, 61, SD1034. [Google Scholar] [CrossRef]
- Slade, S.; Campbell, S.A.; Ralph, T.R.; Walsh, F.C. Ionic Conductivity of an Extruded Nafion 1100 EW Series of Membranes. J. Electrochem. Soc. 2002, 149, A1556–A1564. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yan, H.; Peng, M.; Fang, Z. Modification of ramie fabric with a metal-ion-doped flame-retardant coating. J. Appl. Polym. Sci. 2013, 129, 2986–2997. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yan, H.; Peng, M.; Fang, Z.; Li, Y.; Hao, W. Flame-retardant coating by alternate assembly of poly(vinylphosphonic acid) and polyethylenimine for ramie fabrics. Chin. J. Polym. Sci. 2014, 32, 305–314. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Isikli, S.; Tuncagil, S.; Bozkurt, A.; Toppare, L. Immobilization of Invertase in a Novel Proton Conducting Poly(vinylphosphonic acid)–poly(1-vinylimidazole) Network. J. Macromol. Sci. A 2010, 47, 639–646. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).