First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes
Abstract
1. Introduction
2. Results
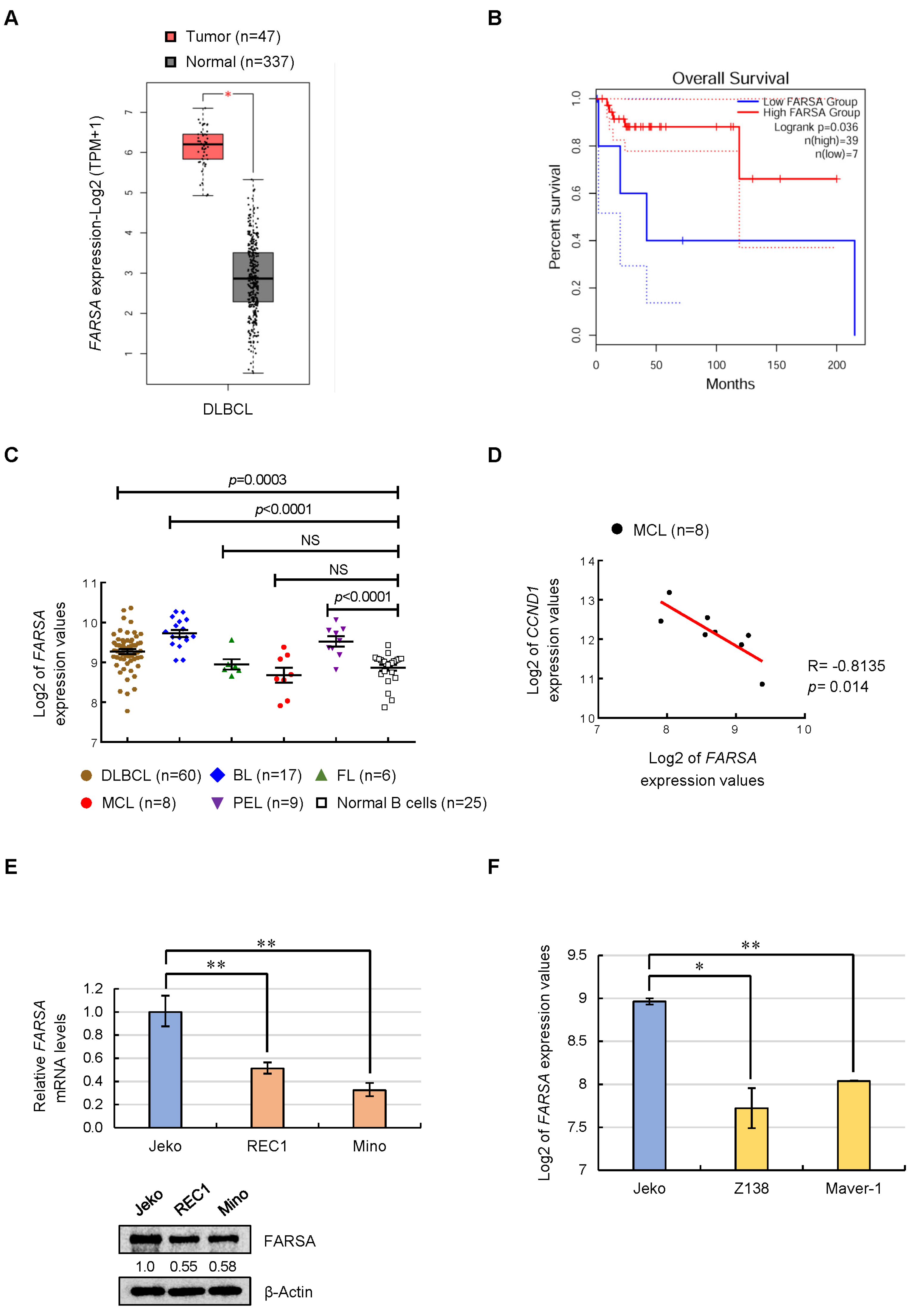
2.1. Expression Feature of FARSA in NHLs and MCL Cell Lines
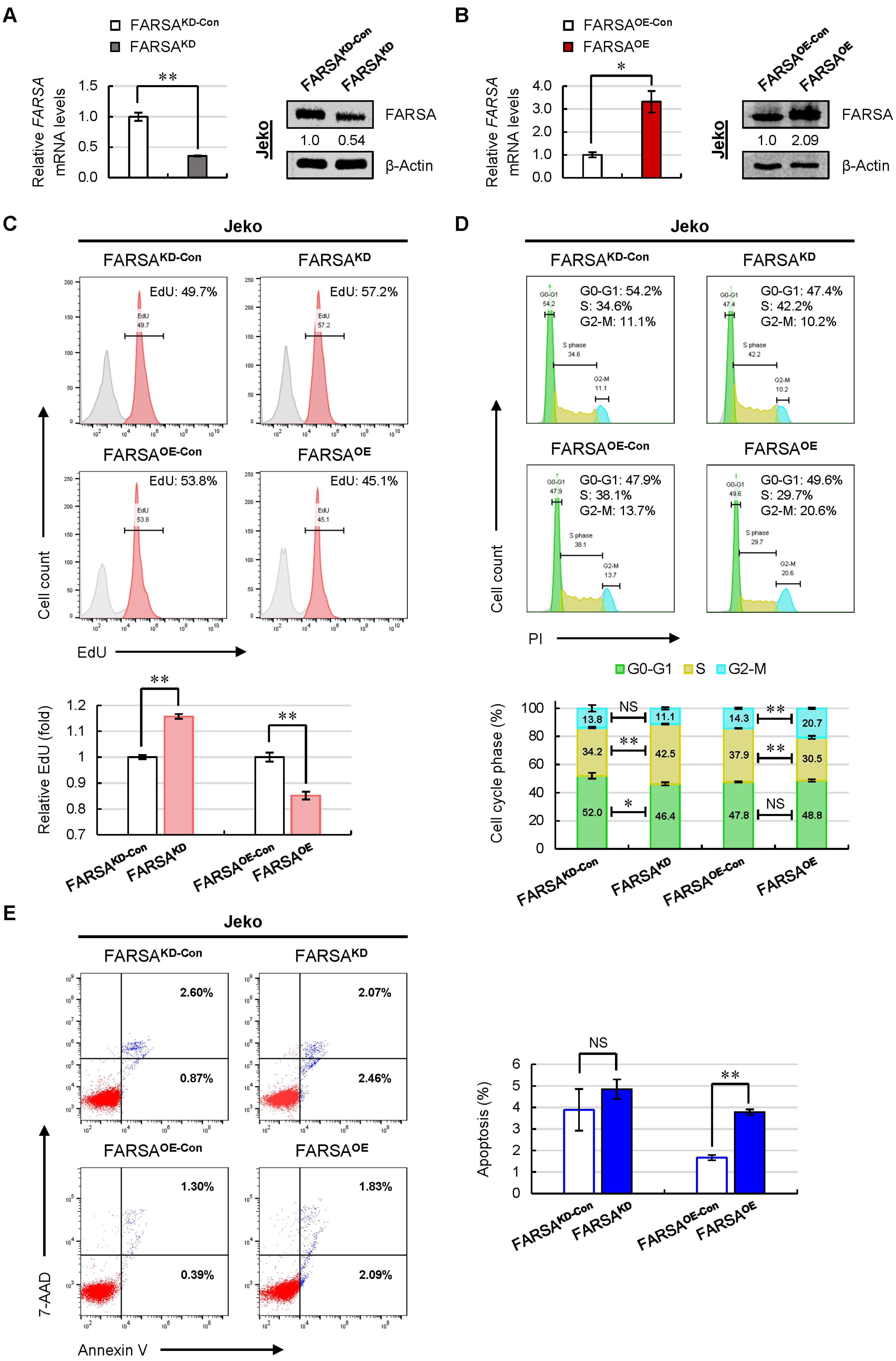
2.2. Knockdown of FARSA Accelerates Cell Proliferation and Cell Cycle in FARSA-Higher MCL Cells
2.3. Overexpression of FARSA Induces Remarkable Cell-Cycle Arrest and Apoptosis in FARSA-Lower MCL Cells
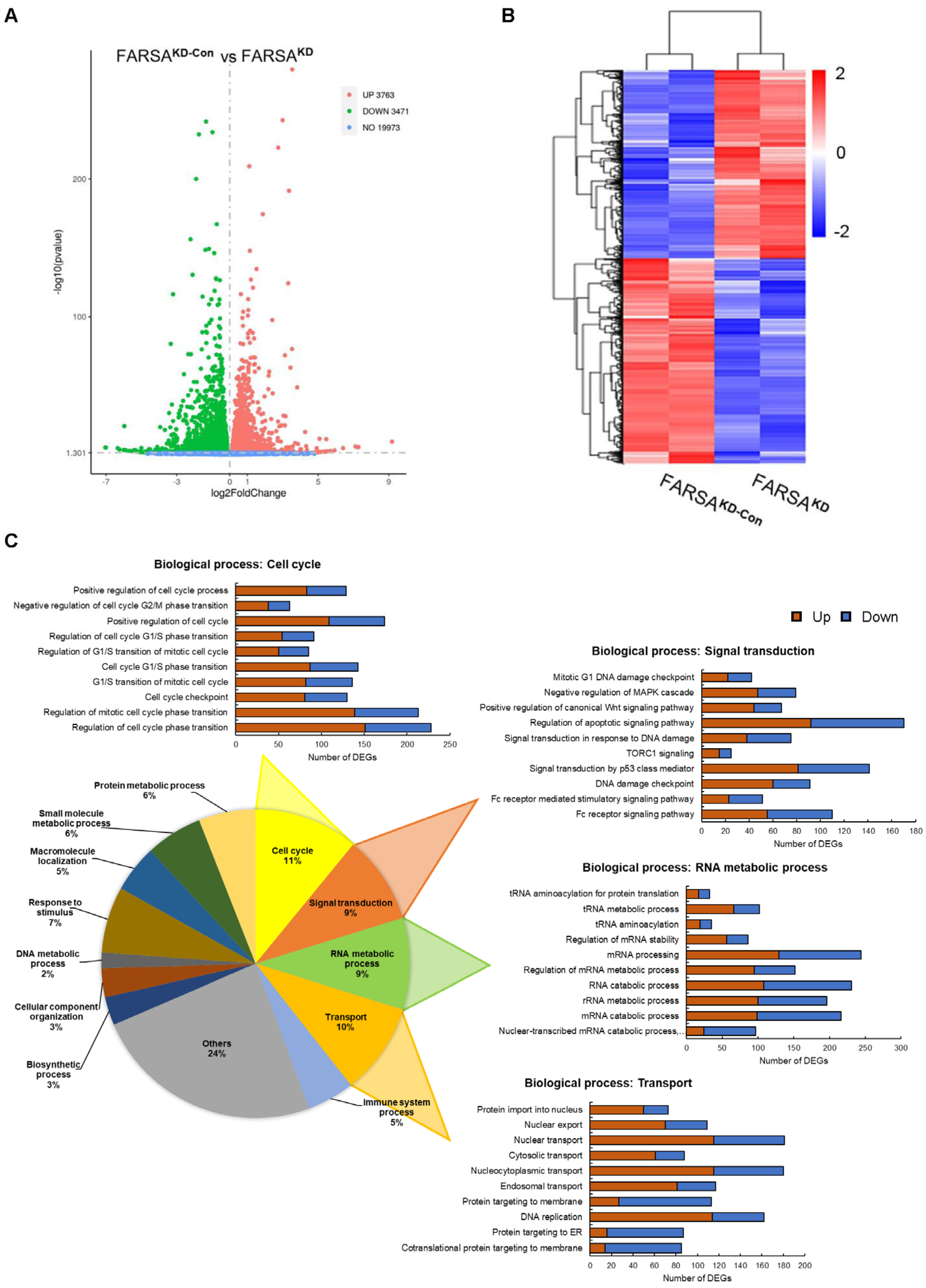
2.4. RNA-Seq Profiles of FARSAKD Jeko Cells Reveal Noncanonical Roles of FARSA in MCL
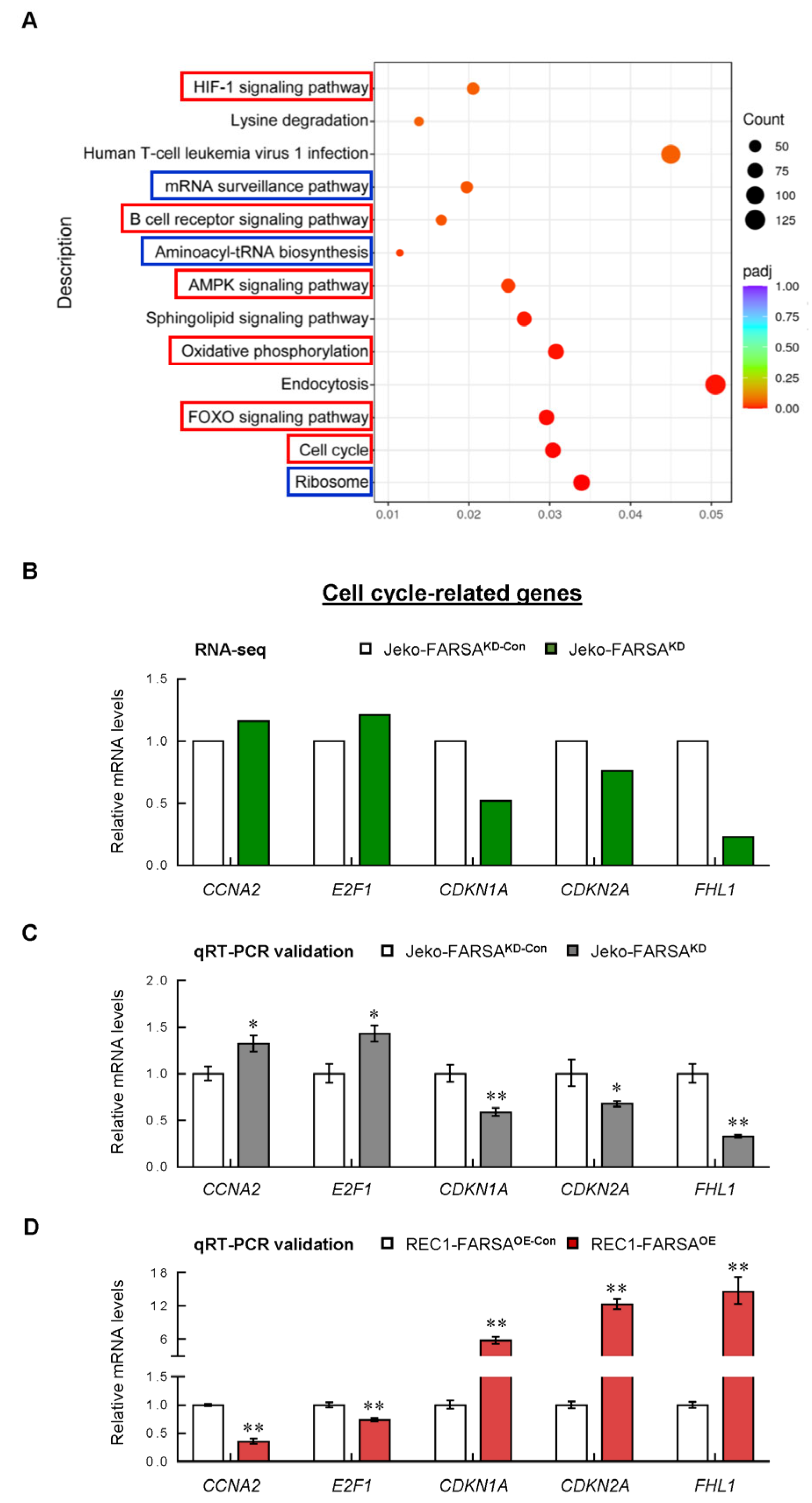
2.5. Cell Cycle- and Cancer-Related Genes Are Significantly Altered in FARSA-Manipulated MCL Cells
2.6. FARSA-Mediated Enhancement of PI3K-AKT Signaling in MCL Cells
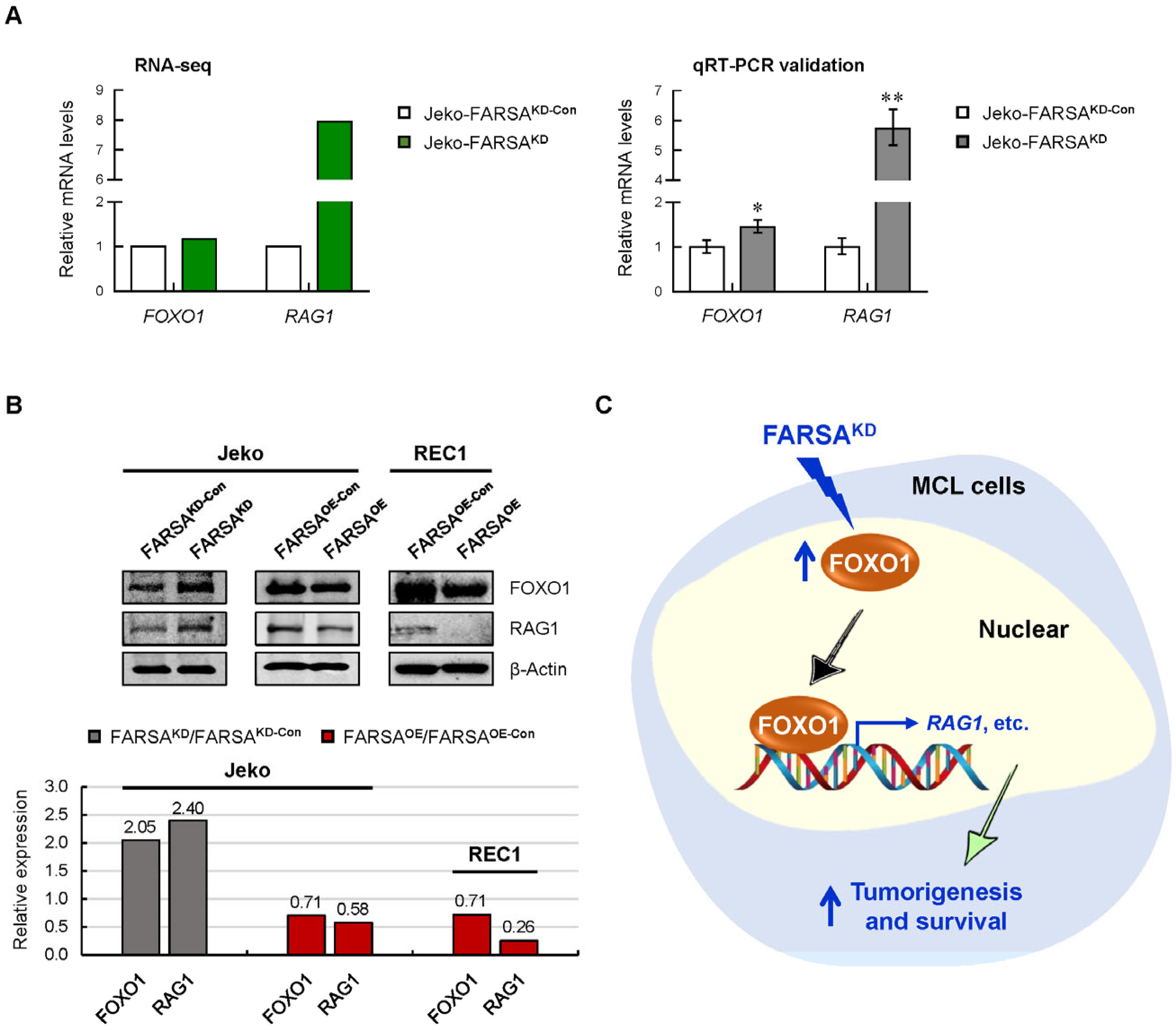
2.7. FARSA-Mediated Activation of the FOXO1-RAG1 Signaling in MCL Cells
3. Discussion
4. Materials and Methods
4.1. Clinical Data Sources
4.2. Cell Lines and Culture
4.3. Lentiviral Infection and Generation of Stable Cell Lines
4.4. RNA Isolation and qRT-PCR
4.5. Immunoblotting and Semi-Quantitative Analysis
4.6. Cell Proliferation Assay
4.7. Cell Cycle and Cell Apoptosis Assays
4.8. RNA-Seq Profiling and Analysis
4.9. GO Terms and Pathway Enrichment Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aaRS(s) | aminoacyl-transfer RNA synthetase(s) |
| ATF5 | activating transcription factor 5 |
| BTZ | Bortezomib |
| CCNA2 | cyclin A2 |
| CCND1 | cyclin D1 |
| CDK4/6 | cyclin dependent kinase 4/6 |
| CDKN1A/2A | cyclin dependent kinase inhibitor 1A/2A |
| cyto | cytoplasmic |
| DEGs | differentially expressed genes |
| E2F1 | E2F transcription factor 1 |
| EIF4G1 | eukaryotic translation initiation factor 4 gamma 1 |
| EPRS1 | glutamyl-prolyl-tRNA synthetase 1 |
| FARS/PheRS | phenylalanyl-tRNA synthetase |
| FARSA | phenylalanyl-tRNA synthetase subunit alpha |
| FARSB | phenylalanyl-tRNA synthetase subunit beta |
| FHL1 | four and a half LIM domains 1 |
| FOXO | forkhead box O |
| GARS1 | glycyl-tRNA synthetase 1 |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| IARS1 | isoleucyl-tRNA synthetase 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KRAS | KRAS proto-oncogene |
| MAP2K1 | MAPK kinase 1 |
| MAPK1 | mitogen-activated protein kinase 1 |
| MCL | mantle cell lymphoma |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MYC | MYC proto-oncogene, bHLH transcription factor |
| NHLs | non-Hodgkin’s lymphomas |
| p-AKT | phosphorylated AKT |
| PIK3CA/B | phosphatidylinositol-4,5-bisphosphaste 3-kinase catalytic subunit alpha/beta |
| qRT-PCR | quantitative real-time PCR |
| RAG1 | recombination activating 1 |
| RNA-seq | RNA sequencing |
| SD | standard deviation |
| SEM | standard error of mean |
| STN | Sotrastaurin |
| TARS1 | threonyl-tRNA synthetase 1 |
| TCGA | Cancer Genome Atlas |
| TERT | telomerase reverse transcriptase |
| tRNA | transfer RNA |
| WARS1 | tryptophanyl-tRNA synthetase 1 |
References
- Kim, S.; You, S.; Hwang, D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat. Rev. Cancer 2011, 11, 708–718. [Google Scholar] [CrossRef]
- Wang, J.; Vallee, I.; Dutta, A.; Wang, Y.; Mo, Z.; Liu, Z.; Cui, H.; Su, A.I.; Yang, X.L. Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer. Genes 2020, 11, 1384. [Google Scholar] [CrossRef]
- Katsyv, I.; Wang, M.; Song, W.M.; Zhou, X.; Zhao, Y.; Park, S.; Zhu, J.; Zhang, B.; Irie, H.Y. EPRS is a critical regulator of cell proliferation and estrogen signaling in ER+ breast cancer. Oncotarget 2016, 7, 69592–69605. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, J.Y.; Ryu, Y.L.; Kim, C.; Kong, J.; Oh, S.; Kang, B.S.; Ahn, H.W.; Ahn, S.G.; Jeong, J.; et al. Stabilization of Cyclin-Dependent Kinase 4 by Methionyl-tRNA Synthetase in p16(INK4a)-Negative Cancer. ACS Pharmacol. Transl. Sci. 2018, 1, 21–31. [Google Scholar] [CrossRef]
- Nam, S.H.; Kim, D.; Lee, D.; Lee, H.M.; Song, D.G.; Jung, J.W.; Kim, J.E.; Kim, H.J.; Kwon, N.H.; Jo, E.K.; et al. Lysyl-tRNA synthetase-expressing colon spheroids induce M2 macrophage polarization to promote metastasis. J. Clin. Invest. 2018, 128, 5034–5055. [Google Scholar] [CrossRef]
- Hyeon, D.Y.; Kim, J.H.; Ahn, T.J.; Cho, Y.; Hwang, D.; Kim, S. Evolution of the multi-tRNA synthetase complex and its role in cancer. J. Biol. Chem. 2019, 294, 5340–5351. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.T.; Lu, J.; Brunssen, D.; Suter, B. A translation-independent function of PheRS activates growth and proliferation in Drosophila. Dis. Model Mech. 2021, 14, dmm048132. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Zhou, H.; Ripmaster, T.; Hittelman, W.N.; Schimmel, P.; White, R.A. Expression of a gene encoding a tRNA synthetase-like protein is enhanced in tumorigenic human myeloid leukemia cells and is cell cycle stage- and differentiation-dependent. Proc. Natl. Acad. Sci. USA 1997, 94, 6164–6169. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Rule, S. Mantle cell lymphoma: Evolving management strategies. Blood 2015, 125, 48–55. [Google Scholar] [CrossRef]
- Maddocks, K.; Blum, K.A. Treatment strategies in mantle cell lymphoma. Cancer Treat. Res. 2015, 165, 251–270. [Google Scholar] [CrossRef]
- Fu, K.; Weisenburger, D.D.; Greiner, T.C.; Dave, S.; Wright, G.; Rosenwald, A.; Chiorazzi, M.; Iqbal, J.; Gesk, S.; Siebert, R.; et al. Cyclin D1-negative mantle cell lymphoma: A clinicopathologic study based on gene expression profiling. Blood 2005, 106, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Rizzatti, E.G.; Perez-Galan, P.; Liu, D.; Wang, Q.; Munson, P.J.; Raghavachari, N.; White, T.; Tweito, M.M.; Dunleavy, K.; et al. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin. Cancer Res. 2011, 17, 5101–5112. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, J.; Sun, M.; Li, G.; Li, B.; Zhang, H. 3-Methyladenine but not antioxidants to overcome BACH2-mediated bortezomib resistance in mantle cell lymphoma. Cancer Cell Int. 2021, 21, 279. [Google Scholar] [CrossRef]
- Blanchard, J.M. Cyclin A2 transcriptional regulation: Modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem. Pharmacol. 2000, 60, 1179–1184. [Google Scholar] [CrossRef]
- Sander, B.; Flygare, J.; Porwit-Macdonald, A.; Smith, C.I.; Emanuelsson, E.; Kimby, E.; Liden, J.; Christensson, B. Mantle cell lymphomas with low levels of cyclin D1 long mRNA transcripts are highly proliferative and can be discriminated by elevated cyclin A2 and cyclin B1. Int. J. Cancer 2005, 117, 418–430. [Google Scholar] [CrossRef]
- Guo, D.; Wang, H.; Sun, L.; Liu, S.; Du, S.; Qiao, W.; Wang, W.; Hou, G.; Zhang, K.; Li, C.; et al. Identification of key gene modules and hub genes of human mantle cell lymphoma by coexpression network analysis. PeerJ 2020, 8, e8843. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Alinari, L.; Prince, C.J.; Edwards, R.B.; Towns, W.H.; Mani, R.; Lehman, A.; Zhang, X.; Jarjoura, D.; Pan, L.; Kinghorn, A.D.; et al. Dual targeting of the cyclin/Rb/E2F and mitochondrial pathways in mantle cell lymphoma with the translation inhibitor silvestrol. Clin. Cancer Res. 2012, 18, 4600–4611. [Google Scholar] [CrossRef]
- Jones, R.J.; Chen, Q.; Voorhees, P.M.; Young, K.H.; Bruey-Sedano, N.; Yang, D.; Orlowski, R.Z. Inhibition of the p53 E3 ligase HDM-2 induces apoptosis and DNA damage—Independent p53 phosphorylation in mantle cell lymphoma. Clin. Cancer Res. 2008, 14, 5416–5425. [Google Scholar] [CrossRef]
- Hutter, G.; Scheubner, M.; Zimmermann, Y.; Kalla, J.; Katzenberger, T.; Hubler, K.; Roth, S.; Hiddemann, W.; Ott, G.; Dreyling, M. Differential effect of epigenetic alterations and genomic deletions of CDK inhibitors [p16(INK4a), p15(INK4b), p14(ARF)] in mantle cell lymphoma. Genes Chromosomes Cancer 2006, 45, 203–210. [Google Scholar] [CrossRef]
- Niu, C.; Liang, C.; Guo, J.; Cheng, L.; Zhang, H.; Qin, X.; Zhang, Q.; Ding, L.; Yuan, B.; Xu, X.; et al. Downregulation and growth inhibitory role of FHL1 in lung cancer. Int. J. Cancer 2012, 130, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Lian, P.; Cheng, L.; Du, P.; Guan, X.; Wang, H.; Ding, L.; Gao, Z.; Huang, X.; Xiao, F.; et al. FHL1 inhibits the growth of tongue squamous cell carcinoma cells via G1/S cell cycle arrest. Mol. Med. Rep. 2015, 12, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Bisikirska, B.; Bansal, M.; Shen, Y.; Teruya-Feldstein, J.; Chaganti, R.; Califano, A. Elucidation and Pharmacological Targeting of Novel Molecular Drivers of Follicular Lymphoma Progression. Cancer Res. 2016, 76, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Herzog, L.O.; Walters, B.; Buono, R.; Lee, J.S.; Mallya, S.; Fung, A.; Chiu, H.; Nguyen, N.; Li, B.; Pinkerton, A.B.; et al. Targeting eIF4F translation initiation complex with SBI-756 sensitises B lymphoma cells to venetoclax. Br. J. Cancer 2021, 124, 1098–1109. [Google Scholar] [CrossRef]
- Panero, J.; Alves-Paiva, R.M.; Roisman, A.; Santana-Lemos, B.A.; Falcao, R.P.; Oliveira, G.; Martins, D.; Stanganelli, C.; Slavutsky, I.; Calado, R.T. Acquired TERT promoter mutations stimulate TERT transcription in mantle cell lymphoma. Am. J. Hematol. 2016, 91, 481–485. [Google Scholar] [CrossRef]
- Amin, R.H.; Schlissel, M.S. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat. Immunol. 2008, 9, 613–622. [Google Scholar] [CrossRef]
- Dengler, H.S.; Baracho, G.V.; Omori, S.A.; Bruckner, S.; Arden, K.C.; Castrillon, D.H.; DePinho, R.A.; Rickert, R.C. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat. Immunol. 2008, 9, 1388–1398. [Google Scholar] [CrossRef]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 2011, 1813, 1938–1945. [Google Scholar] [CrossRef]
- Rudelius, M.; Pittaluga, S.; Nishizuka, S.; Pham, T.H.; Fend, F.; Jaffe, E.S.; Quintanilla-Martinez, L.; Raffeld, M. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood 2006, 108, 1668–1676. [Google Scholar] [CrossRef]
- Perez-Galan, P.; Dreyling, M.; Wiestner, A. Mantle cell lymphoma: Biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef]
- Ochodnicka-Mackovicova, K.; Bahjat, M.; Bloedjes, T.A.; Maas, C.; de Bruin, A.M.; Bende, R.J.; van Noesel, C.J.; Guikema, J.E. NF-kappaB and AKT signaling prevent DNA damage in transformed pre-B cells by suppressing RAG1/2 expression and activity. Blood 2015, 126, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Klemm, L.; Park, E.; Papaemmanuil, E.; Ford, A.; Kweon, S.M.; Trageser, D.; Hasselfeld, B.; Henke, N.; Mooster, J.; et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat. Immunol. 2015, 16, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Demir, S.; Gehringer, F.; Osswald, C.D.; Seyfried, F.; Enzenmuller, S.; Eckhoff, S.M.; Maier, T.; Holzmann, K.; Debatin, K.M.; et al. Tight regulation of FOXO1 is essential for maintenance of B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 2929–2942. [Google Scholar] [CrossRef]
- Kabrani, E.; Chu, V.T.; Tasouri, E.; Sommermann, T.; Bassler, K.; Ulas, T.; Zenz, T.; Bullinger, L.; Schultze, J.L.; Rajewsky, K.; et al. Nuclear FOXO1 promotes lymphomagenesis in germinal center B cells. Blood 2018, 132, 2670–2683. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, W.W.; Chen, J.C.; Zhang, H.L.; Liu, J.; Lin, Y.; Lin, P.C.; Wu, B.X.; An, Y.P.; Huang, L.; et al. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRbeta. Nat. Commun. 2022, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Chen, C.; Sun, X.; Guo, P.; Vessella, R.L.; Wang, R.X.; Chung, L.W.; Zhou, W.; Dong, J.T. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006, 66, 6998–7006. [Google Scholar] [CrossRef]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef]
- Xie, L.; Ushmorov, A.; Leithauser, F.; Guan, H.; Steidl, C.; Farbinger, J.; Pelzer, C.; Vogel, M.J.; Maier, H.J.; Gascoyne, R.D.; et al. FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood 2012, 119, 3503–3511. [Google Scholar] [CrossRef]
- Lee, C.W.; Chang, K.P.; Chen, Y.Y.; Liang, Y.; Hsueh, C.; Yu, J.S.; Chang, Y.S.; Yu, C.J. Overexpressed tryptophanyl-tRNA synthetase, an angiostatic protein, enhances oral cancer cell invasiveness. Oncotarget 2015, 6, 21979–21992. [Google Scholar] [CrossRef]
- Lu, S.; Wang, L.J.; Lombardo, K.; Kwak, Y.; Kim, W.H.; Resnick, M.B. Expression of Indoleamine 2, 3-dioxygenase 1 (IDO1) and Tryptophanyl-tRNA Synthetase (WARS) in Gastric Cancer Molecular Subtypes. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 360–368. [Google Scholar] [CrossRef]
- Yang, P.P.; Yu, X.H.; Zhou, J. Tryptophanyl-tRNA synthetase (WARS) expression in uveal melanoma—Possible contributor during uveal melanoma progression. Biosci. Biotechnol. Biochem. 2020, 84, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Margolin, A.A.; Stolovitzky, G.; Klein, U.; Dalla-Favera, R.; Califano, A. Reverse engineering of regulatory networks in human B cells. Nat. Genet. 2005, 37, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Rahal, R.; Frick, M.; Romero, R.; Korn, J.M.; Kridel, R.; Chan, F.C.; Meissner, B.; Bhang, H.E.; Ruddy, D.; Kauffmann, A.; et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat. Med. 2014, 20, 87–92. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Yang, K.; Wang, J.; Li, G.; Zhang, H. First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes. Int. J. Mol. Sci. 2023, 24, 1608. https://doi.org/10.3390/ijms24021608
Feng M, Yang K, Wang J, Li G, Zhang H. First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes. International Journal of Molecular Sciences. 2023; 24(2):1608. https://doi.org/10.3390/ijms24021608
Chicago/Turabian StyleFeng, Min, Kun Yang, Jia Wang, Guilan Li, and Han Zhang. 2023. "First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes" International Journal of Molecular Sciences 24, no. 2: 1608. https://doi.org/10.3390/ijms24021608
APA StyleFeng, M., Yang, K., Wang, J., Li, G., & Zhang, H. (2023). First Report of FARSA in the Regulation of Cell Cycle and Survival in Mantle Cell Lymphoma Cells via PI3K-AKT and FOXO1-RAG1 Axes. International Journal of Molecular Sciences, 24(2), 1608. https://doi.org/10.3390/ijms24021608





