Targeting DNA Methylation in Leukemia, Myelodysplastic Syndrome, and Lymphoma: A Potential Diagnostic, Prognostic, and Therapeutic Tool
Abstract
1. Introduction
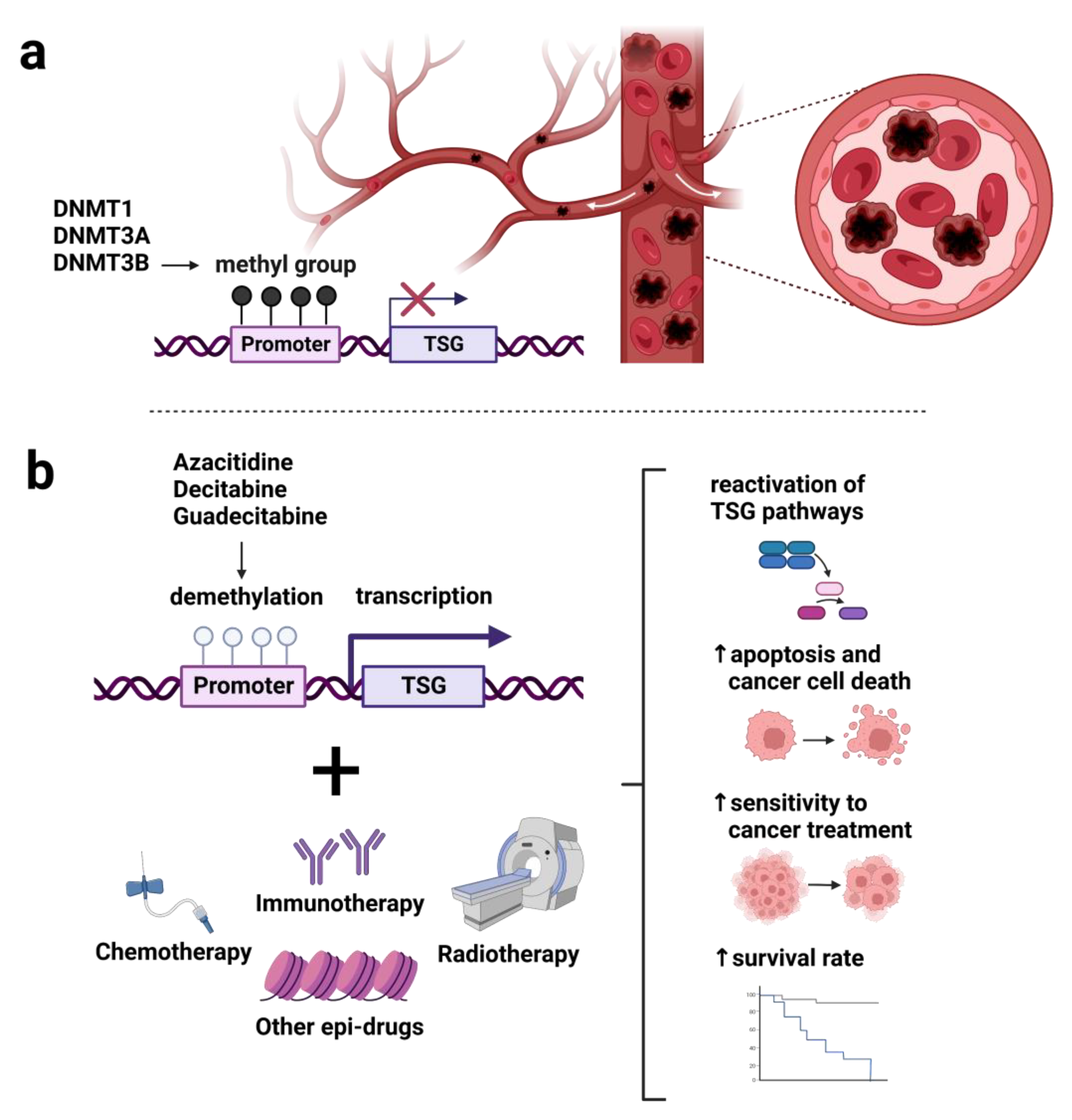
2. Enzymes and Regulators of DNA Methylation in Hematologic Malignancies
2.1. DNA Methyltransferases
2.2. Modifiers and Regulators of DNA Methylation
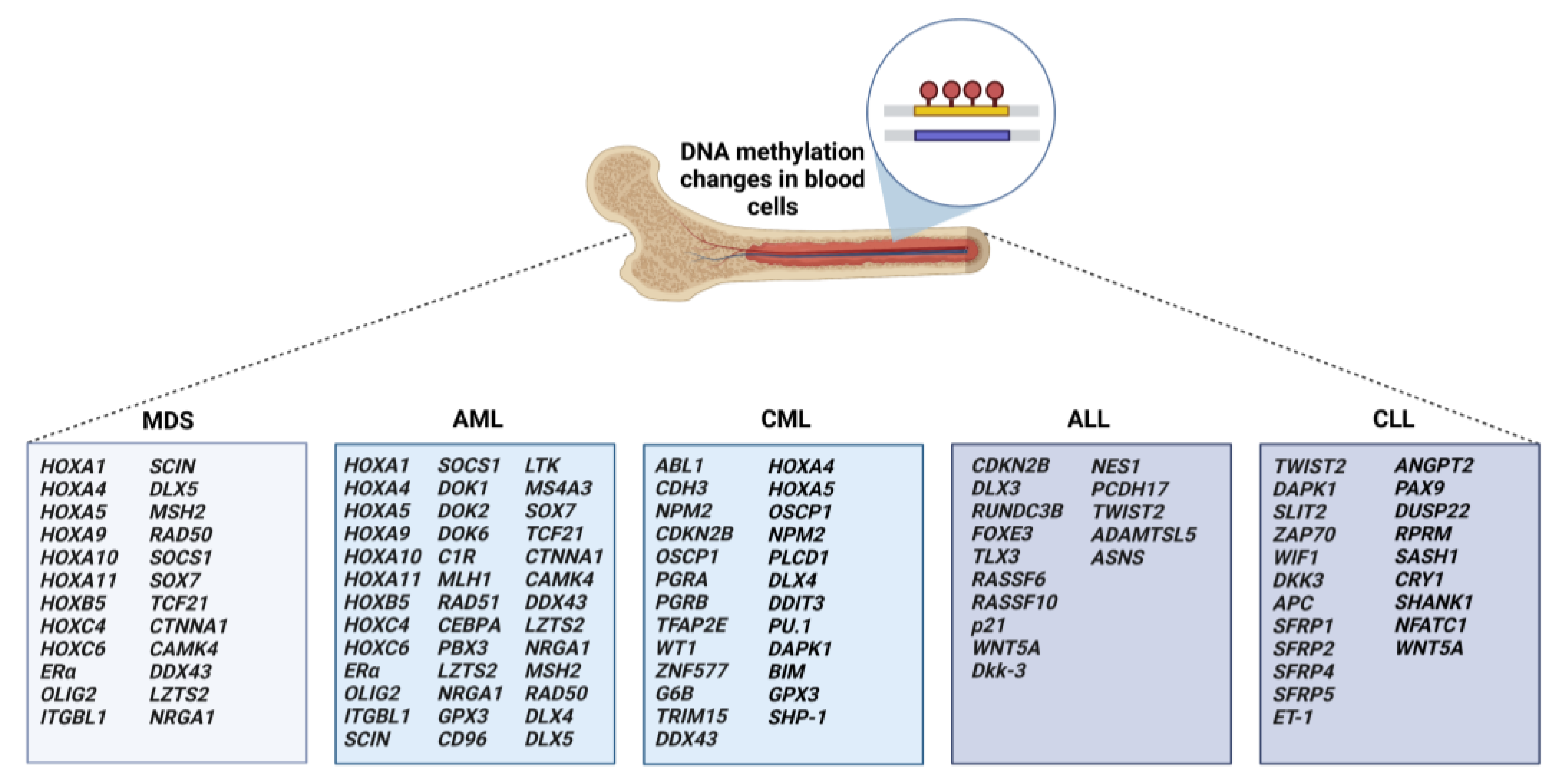
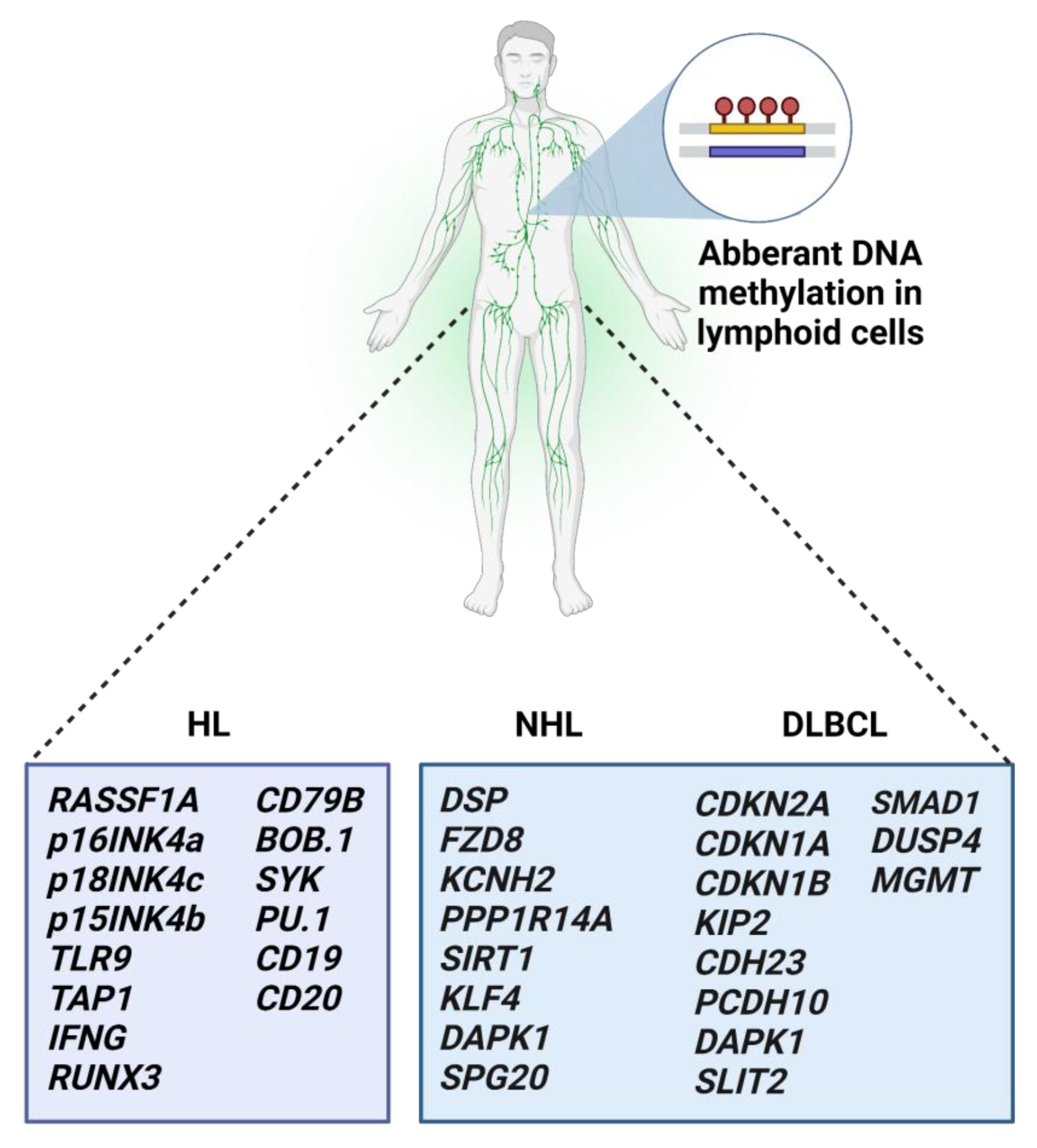
3. DNA Methylation of Target Genes in Leukemias, Myelodysplastic Syndromes, and Lymphomas
3.1. Aberrant Methylation in MDS and AML
3.2. Aberrant Methylation in CML
3.3. Aberrant Methylation in ALL
3.4. Aberrant Methylation in CLL
3.5. Aberrant Methylation in Malignant Lymphomas
4. Therapeutic Implication of DNA Methylation-Based Approach
4.1. HMAs Monotherapy
4.2. Combined HMAs Therapy with Other Treatments
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A landscape takes shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, T. DNA Methylation Reprogramming during Mammalian Development. Genes 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Weisenberger, D.J.; Lakshminarasimhan, R.; Liang, G. The Role of DNA Methylation and DNA Methyltransferases in Cancer. Adv. Exp. Med. Biol. 2022, 1389, 317–348. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Chen, T. Genetic Studies on Mammalian DNA Methyltransferases. Adv. Exp. Med. Biol. 2022, 1389, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Cypris, O.; Frobel, J.; Rai, S.; Franzen, J.; Sontag, S.; Goetzke, R.; de Toledo, M.A.S.; Zenke, M.; Wagner, W. Tracking of epigenetic changes during hematopoietic differentiation of induced pluripotent stem cells. Clin. Epigenet. 2019, 11, 19. [Google Scholar] [CrossRef]
- Farlik, M.; Halbritter, F.; Muller, F.; Choudry, F.A.; Ebert, P.; Klughammer, J.; Farrow, S.; Santoro, A.; Ciaurro, V.; Mathur, A.; et al. DNA Methylation Dynamics of Human Hematopoietic Stem Cell Differentiation. Cell Stem Cell 2016, 19, 808–822. [Google Scholar] [CrossRef]
- Guillamot, M.; Cimmino, L.; Aifantis, I. The Impact of DNA Methylation in Hematopoietic Malignancies. Trends Cancer 2016, 2, 70–83. [Google Scholar] [CrossRef]
- Hoang, N.M.; Rui, L. DNA methyltransferases in hematological malignancies. J. Genet. Genom. 2020, 47, 361–372. [Google Scholar] [CrossRef]
- Blecua, P.; Martinez-Verbo, L.; Esteller, M. The DNA methylation landscape of hematological malignancies: An update. Mol. Oncol. 2020, 14, 1616–1639. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Snow, J.W.; Kim, J.; Orkin, S.H. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell 2009, 5, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Chijiwa, T.; Okamura, T.; Akashi, K.; Fukumaki, Y.; Niho, Y.; Sasaki, H. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood 2001, 97, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.J.; Zheng, W.; Lodh, A.; Yevtodiyenko, A.; Liefwalker, D.; Li, H.; Felsher, D.W.; van Riggelen, J. DNMT3B overexpression contributes to aberrant DNA methylation and MYC-driven tumor maintenance in T-ALL and Burkitt’s lymphoma. Oncotarget 2017, 8, 76898–76920. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Hlady, R.A.; Opavska, J.; Klinkebiel, D.; Novakova, S.; Smith, L.M.; Lewis, R.E.; Karpf, A.R.; Simpson, M.A.; Wu, L.; et al. Essential role for Dnmt1 in the prevention and maintenance of MYC-induced T-cell lymphomas. Mol. Cell. Biol. 2013, 33, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Amara, K.; Ziadi, S.; Hachana, M.; Soltani, N.; Korbi, S.; Trimeche, M. DNA methyltransferase DNMT3b protein overexpression as a prognostic factor in patients with diffuse large B-cell lymphomas. Cancer Sci. 2010, 101, 1722–1730. [Google Scholar] [CrossRef]
- Kamachi, K.; Ureshino, H.; Watanabe, T.; Yoshida, N.; Yamamoto, Y.; Kurahashi, Y.; Fukuda-Kurahashi, Y.; Hayashi, Y.; Hirai, H.; Yamashita, S.; et al. Targeting DNMT1 by demethylating agent OR-2100 increases tyrosine kinase inhibitors-sensitivity and depletes leukemic stem cells in chronic myeloid leukemia. Cancer Lett. 2022, 526, 273–283. [Google Scholar] [CrossRef]
- Lopusna, K.; Nowialis, P.; Opavska, J.; Abraham, A.; Riva, A.; Haney, S.L.; Opavsky, R. Decreases in different Dnmt3b activities drive distinct development of hematologic malignancies in mice. J. Biol. Chem. 2021, 296, 100285. [Google Scholar] [CrossRef]
- Niederwieser, C.; Kohlschmidt, J.; Volinia, S.; Whitman, S.P.; Metzeler, K.H.; Eisfeld, A.K.; Maharry, K.; Yan, P.; Frankhouser, D.; Becker, H.; et al. Prognostic and biologic significance of DNMT3B expression in older patients with cytogenetically normal primary acute myeloid leukemia. Leukemia 2015, 29, 567–575. [Google Scholar] [CrossRef]
- Zhang, H.; Ying, H.; Wang, X. Methyltransferase DNMT3B in leukemia. Leuk. Lymphoma 2020, 61, 263–273. [Google Scholar] [CrossRef]
- Challen, G.A.; Sun, D.; Jeong, M.; Luo, M.; Jelinek, J.; Berg, J.S.; Bock, C.; Vasanthakumar, A.; Gu, H.; Xi, Y.; et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 2011, 44, 23–31. [Google Scholar] [CrossRef]
- Yan, X.J.; Xu, J.; Gu, Z.H.; Pan, C.M.; Lu, G.; Shen, Y.; Shi, J.Y.; Zhu, Y.M.; Tang, L.; Zhang, X.W.; et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat. Genet. 2011, 43, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Kwon, A.; Cho, B.S.; Kim, H.J.; Hwang, K.A.; Kim, M.; Kim, Y. Characteristics of DNMT3A mutations in acute myeloid leukemia. Blood Res. 2020, 55, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Lu, R.; Wang, P.; Yu, Y.; Chen, D.; Gao, L.; Liu, S.; Ji, D.; Rothbart, S.B.; Wang, Y.; et al. Structural basis for DNMT3A-mediated de novo DNA methylation. Nature 2018, 554, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Anteneh, H.; Fang, J.; Song, J. Structural basis for impairment of DNA methylation by the DNMT3A R882H mutation. Nat. Commun. 2020, 11, 2294. [Google Scholar] [CrossRef]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.; et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar] [CrossRef]
- Aref, S.; El Menshawy, N.; El-Ghonemy, M.S.; Zeid, T.A.; El-Baiomy, M.A. Clinicopathologic Effect of DNMT3A Mutation in Adult T-Cell Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, 43–48. [Google Scholar] [CrossRef]
- Walter, M.J.; Ding, L.; Shen, D.; Shao, J.; Grillot, M.; McLellan, M.; Fulton, R.; Schmidt, H.; Kalicki-Veizer, J.; O’Laughlin, M.; et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia 2011, 25, 1153–1158. [Google Scholar] [CrossRef]
- Spencer, D.H.; Russler-Germain, D.A.; Ketkar, S.; Helton, N.M.; Lamprecht, T.L.; Fulton, R.S.; Fronick, C.C.; O’Laughlin, M.; Heath, S.E.; Shinawi, M.; et al. CpG Island Hypermethylation Mediated by DNMT3A Is a Consequence of AML Progression. Cell 2017, 168, 801–816.e13. [Google Scholar] [CrossRef]
- Wang, R.Q.; Chen, C.J.; Jing, Y.; Qin, J.Y.; Li, Y.; Chen, G.F.; Zhou, W.; Li, Y.H.; Wang, J.; Li, D.W.; et al. Characteristics and prognostic significance of genetic mutations in acute myeloid leukemia based on a targeted next-generation sequencing technique. Cancer Med. 2020, 9, 8457–8467. [Google Scholar] [CrossRef]
- Patel, J.P.; Gonen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef]
- Kubuki, Y.; Yamaji, T.; Hidaka, T.; Kameda, T.; Shide, K.; Sekine, M.; Kamiunten, A.; Akizuki, K.; Shimoda, H.; Tahira, Y.; et al. TET2 mutation in diffuse large B-cell lymphoma. J. Clin. Exp. Hematop. 2017, 56, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Zhou, J.D.; Yang, D.Q.; Wang, Y.X.; Wen, X.M.; Guo, H.; Yang, L.; Lian, X.Y.; Lin, J.; Qian, J. TET2 expression is a potential prognostic and predictive biomarker in cytogenetically normal acute myeloid leukemia. J. Cell. Physiol. 2018, 233, 5838–5846. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, L.; Dawlaty, M.M.; Ndiaye-Lobry, D.; Yap, Y.S.; Bakogianni, S.; Yu, Y.; Bhattacharyya, S.; Shaknovich, R.; Geng, H.; Lobry, C.; et al. TET1 is a tumor suppressor of hematopoietic malignancy. Nat. Immunol. 2015, 16, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, F.; Ma, Z.; Yu, M.; Guo, Q.; Huang, J.; Yu, W.; Wang, Y.; Jin, J. High Expression of TET1 Predicts Poor Survival in Cytogenetically Normal Acute Myeloid Leukemia From Two Cohorts. eBioMedicine 2018, 28, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Schmidt, A.; Bontoux, C.; Dourthe, M.E.; Lengline, E.; Andrieu, G.P.; Lhermitte, L.; Graux, C.; Grardel, N.; Cayuela, J.M.; et al. Oncogenetic landscape and clinical impact of IDH1 and IDH2 mutations in T-ALL. J. Hematol. Oncol. 2021, 14, 74. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, W.; Song, R.H.; Liu, S.; Wang, S.; Chen, Y.; Gao, C.; He, C.; Xiao, J.; Zhang, L.; et al. Tumor suppressor CEBPA interacts with and inhibits DNMT3A activity. Sci. Adv. 2022, 8, eabl5220. [Google Scholar] [CrossRef]
- Gao, H.; He, X.; Li, Q.; Wang, Y.; Tian, Y.; Chen, X.; Wang, J.; Guo, Y.; Wang, W.; Li, X. Genome-wide DNA methylome analysis reveals methylation subtypes with different clinical outcomes for acute myeloid leukemia patients. Cancer Med. 2020, 9, 6296–6305. [Google Scholar] [CrossRef]
- Heller, G.; Topakian, T.; Altenberger, C.; Cerny-Reiterer, S.; Herndlhofer, S.; Ziegler, B.; Datlinger, P.; Byrgazov, K.; Bock, C.; Mannhalter, C.; et al. Next-generation sequencing identifies major DNA methylation changes during progression of Ph+ chronic myeloid leukemia. Leukemia 2016, 30, 1861–1868. [Google Scholar] [CrossRef]
- Pei, L.; Choi, J.H.; Liu, J.; Lee, E.J.; McCarthy, B.; Wilson, J.M.; Speir, E.; Awan, F.; Tae, H.; Arthur, G.; et al. Genome-wide DNA methylation analysis reveals novel epigenetic changes in chronic lymphocytic leukemia. Epigenetics 2012, 7, 567–578. [Google Scholar] [CrossRef][Green Version]
- Hetzel, S.; Mattei, A.L.; Kretzmer, H.; Qu, C.; Chen, X.; Fan, Y.; Wu, G.; Roberts, K.G.; Luger, S.; Litzow, M.; et al. Acute lymphoblastic leukemia displays a distinct highly methylated genome. Nat. Cancer 2022, 3, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Haider, Z.; Landfors, M.; Golovleva, I.; Erlanson, M.; Schmiegelow, K.; Flaegstad, T.; Kanerva, J.; Noren-Nystrom, U.; Hultdin, M.; Degerman, S. DNA methylation and copy number variation profiling of T-cell lymphoblastic leukemia and lymphoma. Blood Cancer J. 2020, 10, 45. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, W.; Zhao, J.; Zhang, H. Integrated analysis of genome-wide gene expression and DNA methylation microarray of diffuse large B-cell lymphoma with TET mutations. Mol. Med. Rep. 2017, 16, 3777–3782. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.; Picou, F.; Debeissat, C.; Koubi, M.; Gallay, N.; Hirsch, P.; Ravalet, N.; Bene, M.C.; Maigre, M.; Hunault, M.; et al. Aberrant DNA methylation impacts HOX genes expression in bone marrow mesenchymal stromal cells of myelodysplastic syndromes and de novo acute myeloid leukemia. Cancer Gene Ther. 2022, 29, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Misra, P.; Yadav, S.K.; Sharma, S.; Somasundaram, V. A study on DNA methylation status in promoter region of p15 gene in patients of acute myeloid leukemia and myelodysplastic syndrome. Med. J. Armed Forces India 2021, 77, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Y.; Wu, D.S.; Zhou, H.R.; Shen, J.Z. CpG island methylator phenotype and its relationship with prognosis in adult acute leukemia patients. Hematology 2014, 19, 329–337. [Google Scholar] [CrossRef]
- Kurtovic, N.K.; Krajnovic, M.; Bogdanovic, A.; Suvajdzic, N.; Jovanovic, J.; Dimitrijevic, B.; Colovic, M.; Krtolica, K. Concomitant aberrant methylation of p15 and MGMT genes in acute myeloid leukemia: Association with a particular immunophenotype of blast cells. Med. Oncol. 2012, 29, 3547–3556. [Google Scholar] [CrossRef]
- Hiller, J.K.; Schmoor, C.; Gaidzik, V.I.; Schmidt-Salzmann, C.; Yalcin, A.; Abdelkarim, M.; Blagitko-Dorfs, N.; Dohner, K.; Bullinger, L.; Duyster, J.; et al. Evaluating the impact of genetic and epigenetic aberrations on survival and response in acute myeloid leukemia patients receiving epigenetic therapy. Ann. Hematol. 2017, 96, 559–565. [Google Scholar] [CrossRef]
- Lian, X.Y.; Ma, J.C.; Zhou, J.D.; Zhang, T.J.; Wu, D.H.; Deng, Z.Q.; Zhang, Z.H.; Li, X.X.; He, P.F.; Yan, Y.; et al. Hypermethylation of ITGBL1 is associated with poor prognosis in acute myeloid leukemia. J. Cell. Physiol. 2019, 234, 9438–9446. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhang, W.; Zhou, J.D.; Zhang, T.J.; Ma, J.C.; Xu, Z.J.; Lian, X.Y.; Wu, D.H.; Wen, X.M.; Deng, Z.Q.; et al. Decreased SCIN expression, associated with promoter methylation, is a valuable predictor for prognosis in acute myeloid leukemia. Mol. Carcinog. 2018, 57, 735–744. [Google Scholar] [CrossRef]
- Zhang, T.J.; Xu, Z.J.; Gu, Y.; Wen, X.M.; Ma, J.C.; Zhang, W.; Deng, Z.Q.; Leng, J.Y.; Qian, J.; Lin, J.; et al. Identification and validation of prognosis-related DLX5 methylation as an epigenetic driver in myeloid neoplasms. Clin. Transl. Med. 2020, 10, e29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; So, M.K.; Huh, J. Methylation of DNA Repair Genes as a Prognostic Biomarker in AML of a TCGA-LAML Cohort. Clin. Lab. 2022, 68, 7. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, R.; Sazawal, S.; Mahapatra, M.; Chhikara, S.; Saxena, R. Prognostic relevance of aberrant SOCS-1 gene promoter methylation in myelodysplastic syndromes patients. Int. J. Lab. Hematol. 2015, 37, 265–271. [Google Scholar] [CrossRef] [PubMed]
- He, P.F.; Xu, Z.J.; Zhou, J.D.; Li, X.X.; Zhang, W.; Wu, D.H.; Zhang, Z.H.; Lian, X.Y.; Yao, X.Y.; Deng, Z.Q.; et al. Methylation-associated DOK1 and DOK2 down-regulation: Potential biomarkers for predicting adverse prognosis in acute myeloid leukemia. J. Cell. Physiol. 2018, 233, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.K.; Tang, L.J.; Zhou, J.D.; Xu, Z.J.; Yang, L.; Yuan, Q.; Ma, J.C.; Liu, X.H.; Lin, J.; Qian, J.; et al. DOK6 promoter methylation serves as a potential biomarker affecting prognosis in de novo acute myeloid leukemia. Cancer Med. 2019, 8, 6393–6402. [Google Scholar] [CrossRef]
- Bozic, T.; Lin, Q.; Frobel, J.; Wilop, S.; Hoffmann, M.; Muller-Tidow, C.; Brummendorf, T.H.; Jost, E.; Wagner, W. DNA-methylation in C1R is a prognostic biomarker for acute myeloid leukemia. Clin. Epigenet. 2015, 7, 116. [Google Scholar] [CrossRef]
- Sestakova, S.; Cerovska, E.; Salek, C.; Kundrat, D.; Jeziskova, I.; Folta, A.; Mayer, J.; Racil, Z.; Cetkovsky, P.; Remesova, H. A validation study of potential prognostic DNA methylation biomarkers in patients with acute myeloid leukemia using a custom DNA methylation sequencing panel. Clin. Epigenet. 2022, 14, 22. [Google Scholar] [CrossRef]
- Zhou, J.D.; Yao, D.M.; Zhang, Y.Y.; Ma, J.C.; Wen, X.M.; Yang, J.; Guo, H.; Chen, Q.; Lin, J.; Qian, J. GPX3 hypermethylation serves as an independent prognostic biomarker in non-M3 acute myeloid leukemia. Am. J. Cancer Res. 2015, 5, 1786–1794. [Google Scholar]
- Zhou, J.D.; Zhang, T.J.; Wang, Y.X.; Yang, D.Q.; Yang, L.; Ma, J.C.; Wen, X.M.; Yang, J.; Lin, J.; Qian, J. DLX4 hypermethylation is a prognostically adverse indicator in de novo acute myeloid leukemia. Tumour Biol. 2016, 37, 8951–8960. [Google Scholar] [CrossRef]
- Li, J.; Jin, W.; Tan, Y.; Wang, B.; Wang, X.; Zhao, M.; Wang, K. Distinct gene expression pattern of RUNX1 mutations coordinated by target repression and promoter hypermethylation in acute myeloid leukemia. Front. Med. 2022, 16, 627–636. [Google Scholar] [CrossRef]
- Man, C.H.; Fung, T.K.; Wan, H.; Cher, C.Y.; Fan, A.; Ng, N.; Ho, C.; Wan, T.S.; Tanaka, T.; So, C.W.; et al. Suppression of SOX7 by DNA methylation and its tumor suppressor function in acute myeloid leukemia. Blood 2015, 125, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhou, J.D.; Xu, Z.J.; Zhang, T.J.; Wen, X.M.; Ma, J.C.; Ji, R.B.; Yuan, Q.; Zhang, W.; Chen, Q.; et al. Promoter methylation of the candidate tumor suppressor gene TCF21 in myelodysplastic syndrome and acute myeloid leukemia. Am. J. Transl. Res. 2019, 11, 3450–3460. [Google Scholar] [PubMed]
- Chen, X.X.; Lin, J.; Qian, J.; Qian, W.; Yang, J.; Ma, J.C.; Deng, Z.Q.; An, C.; Tang, C.Y.; Qian, Z.; et al. Methylation of CTNNA1 promoter: Frequent but not an adverse prognostic factor in acute myeloid leukemia. Leuk. Res. 2014, 38, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Liu, D.B.; Xiao, H.X.; Zhou, L.J.; Qu, J. Identification of differentially expressed genes induced by aberrant methylation in acute myeloid leukemia using integrated bioinformatics analyses. Oncol. Lett. 2022, 24, 383. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Q.; Yang, J.; Qian, J.; Deng, Z.Q.; Qian, W.; Chen, X.X.; Ma, J.C.; Xiong, D.S.; Ma, Y.J.; et al. DDX43 promoter is frequently hypomethylated and may predict a favorable outcome in acute myeloid leukemia. Leuk. Res. 2014, 38, 601–607. [Google Scholar] [CrossRef]
- Qu, X.; Othus, M.; Davison, J.; Wu, Y.; Yan, L.; Meshinchi, S.; Ostronoff, F.; Estey, E.H.; Radich, J.P.; Erba, H.P.; et al. Prognostic methylation markers for overall survival in cytogenetically normal patients with acute myeloid leukemia treated on SWOG trials. Cancer 2017, 123, 2472–2481. [Google Scholar] [CrossRef][Green Version]
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future. Cells 2021, 10, 117. [Google Scholar] [CrossRef]
- Jelinek, J.; Gharibyan, V.; Estecio, M.R.; Kondo, K.; He, R.; Chung, W.; Lu, Y.; Zhang, N.; Liang, S.; Kantarjian, H.M.; et al. Aberrant DNA methylation is associated with disease progression, resistance to imatinib and shortened survival in chronic myelogenous leukemia. PLoS ONE 2011, 6, e22110. [Google Scholar] [CrossRef]
- Maupetit-Mehouas, S.; Court, F.; Bourgne, C.; Guerci-Bresler, A.; Cony-Makhoul, P.; Johnson, H.; Etienne, G.; Rousselot, P.; Guyotat, D.; Janel, A.; et al. DNA methylation profiling reveals a pathological signature that contributes to transcriptional defects of CD34+ CD15− cells in early chronic-phase chronic myeloid leukemia. Mol. Oncol. 2018, 12, 814–829. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, J.; Qian, J.; Deng, Z.Q.; Qian, W.; Yang, J.; Li, Y.; Chen, X.X.; Ma, Y.J.; Ma, J.C.; et al. The methylation status of the DDX43 promoter in Chinese patients with chronic myeloid leukemia. Genet. Test. Mol. Biomarkers 2013, 17, 508–511. [Google Scholar] [CrossRef]
- Elias, M.H.; Azlan, H.; Sulong, S.; Baba, A.A.; Ankathil, R. Aberrant DNA methylation at HOXA4 and HOXA5 genes are associated with resistance to imatinib mesylate among chronic myeloid leukemia patients. Cancer Rep. 2018, 1, e1111. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Liu, Q.; Li, Y.; Yang, Z.S.; Yang, L.; Xiang, T.X.; Ren, G.S.; Chen, J.B. Epigenetic inactivation of PLCD1 in chronic myeloid leukemia. Int. J. Mol. Med. 2012, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.D.; Wang, Y.X.; Zhang, T.J.; Yang, D.Q.; Yao, D.M.; Guo, H.; Yang, L.; Ma, J.C.; Wen, X.M.; Yang, J.; et al. Epigenetic inactivation of DLX4 is associated with disease progression in chronic myeloid leukemia. Biochem. Biophys. Res. Commun. 2015, 463, 1250–1256. [Google Scholar] [CrossRef]
- Wang, Y.L.; Qian, J.; Lin, J.; Yao, D.M.; Qian, Z.; Zhu, Z.H.; Li, J.Y. Methylation status of DDIT3 gene in chronic myeloid leukemia. J. Exp. Clin. Cancer Res. 2010, 29, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liang, H.; Yan, J.S.; Tao, R.; Hao, S.G.; Ma, L.Y. Down-regulation of hematopoiesis master regulator PU.1 via aberrant methylation in chronic myeloid leukemia. Int. J. Hematol. 2012, 96, 65–73. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.L.; Lin, J.; Yao, D.M.; Xu, W.R.; Wu, C.Y. Aberrant methylation of the death-associated protein kinase 1 (DAPK1) CpG island in chronic myeloid leukemia. Eur. J. Haematol. 2009, 82, 119–123. [Google Scholar] [CrossRef]
- San Jose-Eneriz, E.; Agirre, X.; Jimenez-Velasco, A.; Cordeu, L.; Martin, V.; Arqueros, V.; Garate, L.; Fresquet, V.; Cervantes, F.; Martinez-Climent, J.A.; et al. Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur. J. Cancer 2009, 45, 1877–1889. [Google Scholar] [CrossRef]
- Yao, D.M.; Zhou, J.D.; Zhang, Y.Y.; Yang, L.; Wen, X.M.; Yang, J.; Guo, H.; Chen, Q.; Lin, J.; Qian, J. GPX3 promoter is methylated in chronic myeloid leukemia. Int. J. Clin. Exp. Pathol. 2015, 8, 6450–6457. [Google Scholar]
- Li, Y.; Yang, L.; Pan, Y.; Yang, J.; Shang, Y.; Luo, J. Methylation and decreased expression of SHP-1 are related to disease progression in chronic myelogenous leukemia. Oncol. Rep. 2014, 31, 2438–2446. [Google Scholar] [CrossRef]
- Wenzinger, C.; Williams, E.; Gru, A.A. Updates in the Pathology of Precursor Lymphoid Neoplasms in the Revised Fourth Edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. Curr. Hematol. Malig. Rep. 2018, 13, 275–288. [Google Scholar] [CrossRef]
- Touzart, A.; Boissel, N.; Belhocine, M.; Smith, C.; Graux, C.; Latiri, M.; Lhermitte, L.; Mathieu, E.L.; Huguet, F.; Lamant, L.; et al. Low level CpG island promoter methylation predicts a poor outcome in adult T-cell acute lymphoblastic leukemia. Haematologica 2020, 105, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Park, J.; Kwon, A.; Choi, H.; Kim, J.; Lee, G.D.; Han, E.; Jekarl, D.W.; Chae, H.; Han, K.; et al. CDKN2B downregulation and other genetic characteristics in T-acute lymphoblastic leukemia. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orto, M.C.; Banelli, B.; Giarin, E.; Accordi, B.; Trentin, L.; Romani, M.; Te Kronnie, G.; Basso, G. Down-regulation of DLX3 expression in MLL-AF4 childhood lymphoblastic leukemias is mediated by promoter region hypermethylation. Oncol. Rep. 2007, 18, 417–423. [Google Scholar]
- Burmeister, D.W.; Smith, E.H.; Cristel, R.T.; McKay, S.D.; Shi, H.; Arthur, G.L.; Davis, J.W.; Taylor, K.H. The expression of RUNDC3B is associated with promoter methylation in lymphoid malignancies. Hematol. Oncol. 2017, 35, 25–33. [Google Scholar] [CrossRef]
- Chatterton, Z.; Burke, D.; Emslie, K.R.; Craig, J.M.; Ng, J.; Ashley, D.M.; Mechinaud, F.; Saffery, R.; Wong, N.C. Validation of DNA methylation biomarkers for diagnosis of acute lymphoblastic leukemia. Clin. Chem. 2014, 60, 995–1003. [Google Scholar] [CrossRef]
- Younesian, S.; Shahkarami, S.; Ghaffari, P.; Alizadeh, S.; Mehrasa, R.; Ghavamzadeh, A.; Ghaffari, S.H. DNA hypermethylation of tumor suppressor genes RASSF6 and RASSF10 as independent prognostic factors in adult acute lymphoblastic leukemia. Leuk. Res. 2017, 61, 33–38. [Google Scholar] [CrossRef]
- Younesian, S.; Shahkarami, S.; Ghaffari, P.; Alizadeh, S.; Mehrasa, R.; Ghaffari, S.H. Residual methylation of tumor suppressor gene promoters, RASSF6 and RASSF10, as novel biomarkers for minimal residual disease detection in adult acute lymphoblastic leukemia. Ann. Hematol. 2019, 98, 2719–2727. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Castillejo, J.A.; Jimenez, A.; Gonzalez, M.G.; Moreno, F.; Rodriguez, M.C.; Barrios, M.; Maldonado, J.; Torres, A. 5’ CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood 2002, 99, 2291–2296. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Cordeu, L.; Vilas-Zornoza, A.; San Jose-Eneriz, E.; Garate, L.; Castillejo, J.A.; Martin, V.; Prosper, F.; Heiniger, A.; et al. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur. J. Cancer 2007, 43, 2736–2746. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Navarro, G.; Barrios, M.; Andreu, E.J.; Prosper, F.; Heiniger, A.; Torres, A. Transcriptional silencing of the Dickkopfs-3 (Dkk-3) gene by CpG hypermethylation in acute lymphoblastic leukaemia. Br. J. Cancer 2004, 91, 707–713. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Barrios, M.; Andreu, E.J.; Prosper, F.; Heiniger, A.; Torres, A. The normal epithelial cell-specific 1 (NES1) gene, a candidate tumor suppressor gene on chromosome 19q13.3-4, is downregulated by hypermethylation in acute lymphoblastic leukemia. Leukemia 2004, 18, 362–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narayan, G.; Freddy, A.J.; Xie, D.; Liyanage, H.; Clark, L.; Kisselev, S.; Un Kang, J.; Nandula, S.V.; McGuinn, C.; Subramaniyam, S.; et al. Promoter methylation-mediated inactivation of PCDH10 in acute lymphoblastic leukemia contributes to chemotherapy resistance. Genes Chromosomes Cancer 2011, 50, 1043–1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uyen, T.N.; Sakashita, K.; Al-Kzayer, L.F.; Nakazawa, Y.; Kurata, T.; Koike, K. Aberrant methylation of protocadherin 17 and its prognostic value in pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2017, 64, e26259. [Google Scholar] [CrossRef] [PubMed]
- Thathia, S.H.; Ferguson, S.; Gautrey, H.E.; van Otterdijk, S.D.; Hili, M.; Rand, V.; Moorman, A.V.; Meyer, S.; Brown, R.; Strathdee, G. Epigenetic inactivation of TWIST2 in acute lymphoblastic leukemia modulates proliferation, cell survival and chemosensitivity. Haematologica 2012, 97, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Choo, C.W.; Alias, H.; Rahman, E.J.A.; Ibrahim, H.M.; Jamal, R.; Hussin, N.H. ADAMTSL5 and CDH11: Putative epigenetic markers for therapeutic resistance in acute lymphoblastic leukemia. Hematology 2017, 22, 386–391. [Google Scholar] [CrossRef]
- Akahane, K.; Kimura, S.; Miyake, K.; Watanabe, A.; Kagami, K.; Yoshimura, K.; Shinohara, T.; Harama, D.; Kasai, S.; Goi, K.; et al. Association of allele-specific methylation of the ASNS gene with asparaginase sensitivity and prognosis in T-ALL. Blood Adv. 2022, 6, 212–224. [Google Scholar] [CrossRef]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Cahill, N.; Rosenquist, R. Uncovering the DNA methylome in chronic lymphocytic leukemia. Epigenetics 2013, 8, 138–148. [Google Scholar] [CrossRef]
- Raval, A.; Lucas, D.M.; Matkovic, J.J.; Bennett, K.L.; Liyanarachchi, S.; Young, D.C.; Rassenti, L.; Kipps, T.J.; Grever, M.R.; Byrd, J.C.; et al. TWIST2 demonstrates differential methylation in immunoglobulin variable heavy chain mutated and unmutated chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 3877–3885. [Google Scholar] [CrossRef]
- Raval, A.; Tanner, S.M.; Byrd, J.C.; Angerman, E.B.; Perko, J.D.; Chen, S.S.; Hackanson, B.; Grever, M.R.; Lucas, D.M.; Matkovic, J.J.; et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell 2007, 129, 879–890. [Google Scholar] [CrossRef]
- Dunwell, T.L.; Dickinson, R.E.; Stankovic, T.; Dallol, A.; Weston, V.; Austen, B.; Catchpoole, D.; Maher, E.R.; Latif, F. Frequent epigenetic inactivation of the SLIT2 gene in chronic and acute lymphocytic leukemia. Epigenetics 2009, 4, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Lucas, D.M.; Stilgenbauer, S.; Ruppert, A.S.; Yu, L.; Zucknick, M.; Mertens, D.; Buhler, A.; Oakes, C.C.; Larson, R.A.; et al. Quantitative DNA methylation analysis identifies a single CpG dinucleotide important for ZAP-70 expression and predictive of prognosis in chronic lymphocytic leukemia. J. Clin. Oncol. 2012, 30, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Pang, R.; Liang, R. Epigenetic dysregulation of the Wnt signalling pathway in chronic lymphocytic leukaemia. J. Clin. Pathol. 2008, 61, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.B.; Taylor, K.H.; Arthur, G.L.; Rahmatpanah, F.B.; Hooshmand, S.I.; Caldwell, C.W. Epigenetic regulation of WNT signaling in chronic lymphocytic leukemia. Epigenomics 2010, 2, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Maffei, R.; Fiorcari, S.; Quadrelli, C.; Zucchini, P.; Benatti, S.; Potenza, L.; Luppi, M.; Marasca, R. The expression of endothelin-1 in chronic lymphocytic leukemia is controlled by epigenetic mechanisms and extracellular stimuli. Leuk. Res. 2017, 54, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Kanduri, M.; Maffei, R.; Fiorcari, S.; Bulgarelli, J.; Marasca, R.; Rosenquist, R. ANGPT2 promoter methylation is strongly associated with gene expression and prognosis in chronic lymphocytic leukemia. Epigenetics 2013, 8, 720–729. [Google Scholar] [CrossRef]
- Rani, L.; Mathur, N.; Gupta, R.; Gogia, A.; Kaur, G.; Dhanjal, J.K.; Sundar, D.; Kumar, L.; Sharma, A. Genome-wide DNA methylation profiling integrated with gene expression profiling identifies PAX9 as a novel prognostic marker in chronic lymphocytic leukemia. Clin. Epigenet. 2017, 9, 57. [Google Scholar] [CrossRef]
- Pan, H.; Renaud, L.; Chaligne, R.; Bloehdorn, J.; Tausch, E.; Mertens, D.; Fink, A.M.; Fischer, K.; Zhang, C.; Betel, D.; et al. Discovery of Candidate DNA Methylation Cancer Driver Genes. Cancer Discov. 2021, 11, 2266–2281. [Google Scholar] [CrossRef]
- Hanoun, M.; Eisele, L.; Suzuki, M.; Greally, J.M.; Huttmann, A.; Aydin, S.; Scholtysik, R.; Klein-Hitpass, L.; Duhrsen, U.; Durig, J. Epigenetic silencing of the circadian clock gene CRY1 is associated with an indolent clinical course in chronic lymphocytic leukemia. PLoS ONE 2012, 7, e34347. [Google Scholar] [CrossRef][Green Version]
- Loi, E.; Moi, L.; Fadda, A.; Satta, G.; Zucca, M.; Sanna, S.; Amini Nia, S.; Cabras, G.; Padoan, M.; Magnani, C.; et al. Methylation alteration of SHANK1 as a predictive, diagnostic and prognostic biomarker for chronic lymphocytic leukemia. Oncotarget 2019, 10, 4987–5002. [Google Scholar] [CrossRef]
- Wolf, C.; Garding, A.; Filarsky, K.; Bahlo, J.; Robrecht, S.; Becker, N.; Zucknick, M.; Rouhi, A.; Weigel, A.; Claus, R.; et al. NFATC1 activation by DNA hypomethylation in chronic lymphocytic leukemia correlates with clinical staging and can be inhibited by ibrutinib. Int. J. Cancer 2018, 142, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Dalla-Favera, R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat. Rev. Cancer 2016, 16, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, M.; Cahill, N.; Goransson, H.; Enstrom, C.; Ryan, F.; Isaksson, A.; Rosenquist, R. Differential genome-wide array-based methylation profiles in prognostic subsets of chronic lymphocytic leukemia. Blood 2010, 115, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Poppova, L.; Pavlova, S.; Gonzalez, B.; Kotaskova, J.; Plevova, K.; Dumbovic, G.; Janovska, P.; Bystry, V.; Panovska, A.; Bezdekova, L.; et al. Memory B-cell like chronic lymphocytic leukaemia is associated with specific methylation profile of WNT5A promoter and undetectable expression of WNT5A gene. Epigenetics 2022, 17, 1628–1635. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, D.; Bordoni, A.; Zucca, E. Epidemiology of hematological malignancies. Ann. Oncol. 2007, 18 (Suppl. S1), i3–i8. [Google Scholar] [CrossRef]
- Murray, P.G.; Qiu, G.H.; Fu, L.; Waites, E.R.; Srivastava, G.; Heys, D.; Agathanggelou, A.; Latif, F.; Grundy, R.G.; Mann, J.R.; et al. Frequent epigenetic inactivation of the RASSF1A tumor suppressor gene in Hodgkin’s lymphoma. Oncogene 2004, 23, 1326–1331. [Google Scholar] [CrossRef]
- Garcia, M.J.; Martinez-Delgado, B.; Cebrian, A.; Martinez, A.; Benitez, J.; Rivas, C. Different incidence and pattern of p15INK4b and p16INK4a promoter region hypermethylation in Hodgkin’s and CD30-Positive non-Hodgkin’s lymphomas. Am. J. Pathol. 2002, 161, 1007–1013. [Google Scholar] [CrossRef]
- Ushmorov, A.; Leithauser, F.; Sakk, O.; Weinhausel, A.; Popov, S.W.; Moller, P.; Wirth, T. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood 2006, 107, 2493–2500. [Google Scholar] [CrossRef]
- Xia, C.; Olsen, T.K.; Zirakzadeh, A.A.; Almamoun, R.; Sjoholm, L.K.; Dahlstrom, J.; Sjoberg, J.; Claesson, H.E.; Johnsen, J.I.; Winqvist, O.; et al. Hodgkin Lymphoma Monozygotic Triplets Reveal Divergences in DNA Methylation Signatures. Front. Oncol. 2020, 10, 598872. [Google Scholar] [CrossRef]
- Bethge, N.; Honne, H.; Hilden, V.; Troen, G.; Eknaes, M.; Liestol, K.; Holte, H.; Delabie, J.; Smeland, E.B.; Lind, G.E. Identification of highly methylated genes across various types of B-cell non-hodgkin lymphoma. PLoS ONE 2013, 8, e79602. [Google Scholar] [CrossRef][Green Version]
- Bethge, N.; Honne, H.; Andresen, K.; Hilden, V.; Troen, G.; Liestol, K.; Holte, H.; Delabie, J.; Lind, G.E.; Smeland, E.B. A gene panel, including LRP12, is frequently hypermethylated in major types of B-cell lymphoma. PLoS ONE 2014, 9, e104249. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R.; Zanetti, E.; Pistoni, M.; Tamagnini, I.; Valli, R.; Braglia, L.; Merli, F. Methylation changes of SIRT1, KLF4, DAPK1 and SPG20 in B-lymphocytes derived from follicular and diffuse large B-cell lymphoma. Leuk. Res. 2017, 57, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R.; Cusenza, V.Y.; Pistoni, M.; Canovi, L.; Cascione, L.; Bertoni, F.; Merli, F. KLF4, DAPK1 and SPG20 promoter methylation is not affected by DNMT1 silencing and hypomethylating drugs in lymphoma cells. Oncol. Rep. 2022, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- Chambwe, N.; Kormaksson, M.; Geng, H.; De, S.; Michor, F.; Johnson, N.A.; Morin, R.D.; Scott, D.W.; Godley, L.A.; Gascoyne, R.D.; et al. Variability in DNA methylation defines novel epigenetic subgroups of DLBCL associated with different clinical outcomes. Blood 2014, 123, 1699–1708. [Google Scholar] [CrossRef]
- Zainuddin, N.; Kanduri, M.; Berglund, M.; Lindell, M.; Amini, R.M.; Roos, G.; Sundstrom, C.; Enblad, G.; Rosenquist, R. Quantitative evaluation of p16(INK4a) promoter methylation using pyrosequencing in de novo diffuse large B-cell lymphoma. Leuk. Res. 2011, 35, 438–443. [Google Scholar] [CrossRef]
- Hagiwara, K.; Li, Y.; Kinoshita, T.; Kunishma, S.; Ohashi, H.; Hotta, T.; Nagai, H. Aberrant DNA methylation of the p57KIP2 gene is a sensitive biomarker for detecting minimal residual disease in diffuse large B cell lymphoma. Leuk. Res. 2010, 34, 50–54. [Google Scholar] [CrossRef]
- Shawky, S.A.; El-Borai, M.H.; Khaled, H.M.; Guda, I.; Mohanad, M.; Abdellateif, M.S.; Zekri, A.N.; Bahanasy, A.A. The prognostic impact of hypermethylation for a panel of tumor suppressor genes and cell of origin subtype on diffuse large B-cell lymphoma. Mol. Biol. Rep. 2019, 46, 4063–4076. [Google Scholar] [CrossRef]
- Cao, B.; Guo, X.; Huang, L.; Wang, B.; Wang, W.; Han, D.; Zhang, W.; Zhong, K. Methylation silencing CDH23 is a poor prognostic marker in diffuse large B-cell lymphoma. Aging 2021, 13, 17768–17788. [Google Scholar] [CrossRef]
- Huang, W.; Xue, X.; Shan, L.; Qiu, T.; Guo, L.; Ying, J.; Lu, N. Clinical significance of PCDH10 promoter methylation in diffuse large B-cell lymphoma. BMC Cancer 2017, 17, 815. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Asmar, F.; Dimopoulos, K.; Nygaard, M.K.; Aslan, D.; Hansen, J.W.; Ralfkiaer, E.; Gronbaek, K. Hypermethylation of DAPK1 is an independent prognostic factor predicting survival in diffuse large B-cell lymphoma. Oncotarget 2014, 5, 9798–9810. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Zhou, L.Y.; Guan, Z.B.; Zeng, W.B.; Zhou, L.L.; Liu, Y.N.; Pan, X.Y. Prognostic significance of DAPK promoter methylation in lymphoma: A meta-analysis. PLoS ONE 2019, 14, e0210943. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Hansen, J.W.; Kristensen, S.S.; Tholstrup, D.; Harslof, L.B.; Pedersen, O.B.; De Nully Brown, P.; Gronbaek, K. Aberrant methylation of cell-free circulating DNA in plasma predicts poor outcome in diffuse large B cell lymphoma. Clin. Epigenet. 2016, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.; Talima, S.; Li, L.; Wei, W.; Rudzki, Z.; Allam, R.M.; Simmons, W.; Tao, Q.; Murray, P.G. Low Expression and Promoter Hypermethylation of the Tumour Suppressor SLIT2, are Associated with Adverse Patient Outcomes in Diffuse Large B Cell Lymphoma. Pathol. Oncol. Res. 2019, 25, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.A.; Robinson, M.D.; Scheifinger, N.A.; Muller, S.; Cogliatti, S.; Tzankov, A.; Muller, A. DUSP4 deficiency caused by promoter hypermethylation drives JNK signaling and tumor cell survival in diffuse large B cell lymphoma. J. Exp. Med. 2015, 212, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, E.J.; Ko, Y.H.; Lee, S.H.; Maeng, L.; Kim, K.M. Prognostic significance of O6-methylguanine DNA methyltransferase and p57 methylation in patients with diffuse large B-cell lymphomas. APMIS 2009, 117, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Clozel, T.; Yang, S.; Elstrom, R.L.; Tam, W.; Martin, P.; Kormaksson, M.; Banerjee, S.; Vasanthakumar, A.; Culjkovic, B.; Scott, D.W.; et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B-cell lymphoma. Cancer Discov. 2013, 3, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hu, G.; Luo, C.; Liang, Z. DNA methyltransferase inhibitors: An updated patent review (2012-2015). Expert Opin. Ther. Pat. 2016, 26, 1017–1030. [Google Scholar] [CrossRef]
- Pappalardi, M.B.; Keenan, K.; Cockerill, M.; Kellner, W.A.; Stowell, A.; Sherk, C.; Wong, K.; Pathuri, S.; Briand, J.; Steidel, M.; et al. Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia. Nat. Cancer 2021, 2, 1002–1017. [Google Scholar] [CrossRef]
- Nieto, M.; Demolis, P.; Behanzin, E.; Moreau, A.; Hudson, I.; Flores, B.; Stemplewski, H.; Salmonson, T.; Gisselbrecht, C.; Bowen, D.; et al. The European Medicines Agency Review of Decitabine (Dacogen) for the Treatment of Adult Patients With Acute Myeloid Leukemia: Summary of the Scientific Assessment of the Committee for Medicinal Products for Human Use. Oncologist 2016, 21, 692–700. [Google Scholar] [CrossRef][Green Version]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Wei, A.H.; Dohner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Roboz, G.J.; Wei, A.H.; Dohner, H.; Pocock, C.; Selleslag, D.; Montesinos, P.; Sayar, H.; Musso, M.; Figuera-Alvarez, A.; et al. Management of adverse events in patients with acute myeloid leukemia in remission receiving oral azacitidine: Experience from the phase 3 randomized QUAZAR AML-001 trial. J. Hematol. Oncol. 2021, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Savona, M.R.; Odenike, O.; Amrein, P.C.; Steensma, D.P.; DeZern, A.E.; Michaelis, L.C.; Faderl, S.; Harb, W.; Kantarjian, H.; Lowder, J.; et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: A multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019, 6, e194–e203. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Kantarjian, H.M.; Yee, K.W.L.; Kropf, P.L.; O’Connell, C.L.; Griffiths, E.A.; Stock, W.; Daver, N.G.; Jabbour, E.; Ritchie, E.K.; et al. Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia. Cancer 2018, 124, 325–334. [Google Scholar] [CrossRef]
- Chung, W.; Kelly, A.D.; Kropf, P.; Fung, H.; Jelinek, J.; Su, X.Y.; Roboz, G.J.; Kantarjian, H.M.; Azab, M.; Issa, J.J. Genomic and epigenomic predictors of response to guadecitabine in relapsed/refractory acute myelogenous leukemia. Clin. Epigenet. 2019, 11, 106. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Roboz, G.; Walsh, K.; Kantarjian, H.; Ritchie, E.; Kropf, P.; O’Connell, C.; Tibes, R.; Lunin, S.; Rosenblat, T.; et al. Guadecitabine (SGI-110) in patients with intermediate or high-risk myelodysplastic syndromes: Phase 2 results from a multicentre, open-label, randomised, phase 1/2 trial. Lancet Haematol. 2019, 6, e317–e327. [Google Scholar] [CrossRef]
- Cahill, K.E.; Karimi, Y.H.; Karrison, T.G.; Jain, N.; Green, M.; Weiner, H.; Fulton, N.; Kadri, S.; Godley, L.A.; Artz, A.S.; et al. A phase 1 study of azacitidine with high-dose cytarabine and mitoxantrone in high-risk acute myeloid leukemia. Blood Adv. 2020, 4, 599–606. [Google Scholar] [CrossRef]
- Pommert, L.; Schafer, E.S.; Malvar, J.; Gossai, N.; Florendo, E.; Pulakanti, K.; Heimbruch, K.; Stelloh, C.; Chi, Y.Y.; Sposto, R.; et al. Decitabine and vorinostat with FLAG chemotherapy in pediatric relapsed/refractory AML: Report from the therapeutic advances in childhood leukemia and lymphoma (TACL) consortium. Am. J. Hematol. 2022, 97, 613–622. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Cherry, E.M.; Abbott, D.; Amaya, M.; McMahon, C.; Schwartz, M.; Rosser, J.; Sato, A.; Schowinsky, J.; Inguva, A.; Minhajuddin, M.; et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv. 2021, 5, 5565–5573. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Srivastava, P.; Matsuzaki, J.; Brumberger, Z.; Wang, E.S.; Kocent, J.; Miller, A.; Roloff, G.W.; Wong, H.Y.; Paluch, B.E.; et al. NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin. Cancer Res. 2018, 24, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Holmberg-Thyden, S.; Dufva, I.H.; Gang, A.O.; Breinholt, M.F.; Schejbel, L.; Andersen, M.K.; Kadivar, M.; Svane, I.M.; Gronbaek, K.; Hadrup, S.R.; et al. Epigenetic therapy in combination with a multi-epitope cancer vaccine targeting shared tumor antigens for high-risk myelodysplastic syndrome—A phase I clinical trial. Cancer Immunol. Immunother. 2022, 71, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Herbrich, S.M.; Pemmaraju, N.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Pierce, S.A.; Jabbour, E.; Wang, S.A.; Bueso-Ramos, C.; et al. A phase 1b/2 study of azacitidine with PD-L1 antibody avelumab in relapsed/refractory acute myeloid leukemia. Cancer 2021, 127, 3761–3771. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Ogasawara, K.; Cavenagh, J.; Silverman, L.R.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for higher-risk myelodysplastic syndromes. Blood Adv. 2022, 6, 2207–2218. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef]
- Nieto, Y.; Valdez, B.C.; Thall, P.F.; Jones, R.B.; Wei, W.; Myers, A.; Hosing, C.; Ahmed, S.; Popat, U.; Shpall, E.J.; et al. Double epigenetic modulation of high-dose chemotherapy with azacitidine and vorinostat for patients with refractory or poor-risk relapsed lymphoma. Cancer 2016, 122, 2680–2688. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Chen, F.; Ding, M.; Dong, M.; Yang, W.; Yin, M.; Wu, J.; Zhang, L.; Fu, X.; et al. Combination of Decitabine and a Modified Regimen of Cisplatin, Cytarabine and Dexamethasone: A Potential Salvage Regimen for Relapsed or Refractory Diffuse Large B-Cell Lymphoma After Second-Line Treatment Failure. Front. Oncol. 2021, 11, 687374. [Google Scholar] [CrossRef]
- Qu, C.; Zou, R.; Wang, P.; Zhu, Q.; Kang, L.; Ping, N.; Xia, F.; Liu, H.; Kong, D.; Yu, L.; et al. Decitabine-primed tandem CD19/CD22 CAR-T therapy in relapsed/refractory diffuse large B-cell lymphoma patients. Front. Immunol. 2022, 13, 969660. [Google Scholar] [CrossRef]
| Study | Design | Disease | Purpose | Patients | Intervention | Status |
|---|---|---|---|---|---|---|
| NCT02935361 | An interventional open-label study single group assignment | CMML MDS AML | To assess the potential risk of treatment-related adverse events and to evaluate ORR and OS | 33 adults | Guadecitabine and atezolizumab as a humanized monoclonal antibody every 28 days up to 2 years in the absence of disease toxicity | Active, not recruiting |
| NCT04167917 | An interventional open-label study single group assignment | AML MDS CML | To confirm the tolerability and safety of the oral HMAs and to evaluate the CBR, OS, PFS, and ORR after epi-drug therapy | 20 adults | DNMT1 inhibitor known as NTX-301 with previously shown anti-leukemic efficacy | Recruiting |
| NCT05042531 | An interventional prospective randomized study parallel assignment | AML | To determine the incidence of adverse events developed during the first and second cycles of azacitidine treatment | 30 adults | Low-dose dasatinib with azacitidine as a maintenance therapy option in chemotherapy-treated patients | Recruiting |
| NCT02878785 | An interventional randomized open-label study factorial assignment | AML | To test the best dose and assess the treatment-related adverse events | 25 adults | A combination of decitabine (5 days every month) with talazoparib (daily oral administration) | Active, not recruiting |
| NCT03164057 | An interventional randomized open-label study parallel assignment | AML MDS | To analyze OS, EFS, and non-hematologic toxic events in patients receiving DNMTi before the chemotherapy | 206 adults/ children | Intravenously administered azacitidine or decitabine before chemotherapy regimens | Active, not recruiting |
| NCT04722952 | An interventional open-label study single-group assignment | AML | To define the efficacy of combined therapies in refractory/relapsed patients and evaluate CR, ORR, OS, EFS, and PFS | 30 adults | PD-1 monoclonal antibody visilizumab plus azacitidine, and a homoharringtonine regimen | Recruiting |
| NCT01806116 | An interventional open-label study single group assignment | MDS AML | To investigate the impact on posttransplant outcomes and evaluate the incidence of GvHD | 30 adults/ children | Intravenous administration of decitabine followed by allogeneic HSCT | Unknown |
| NCT02684162 | An interventional open-label study single-group assignment | AML CML MDS | To confirm CRR, RFS, safety, and toxicity of treatment with/without donor lymphocyte infusion | 55 adults | Guadecitabine and donor lymphocyte infusion in case of the absence of disease progression or toxicity | Active, not recruiting |
| NCT03080766 | An interventional open-label study single-group assignment | AML | To identify CR, DFS, and OS in patients with chromosomal abnormalities associated with bad prognosis | 20 adults | Decitabine treatment with the examination of bone marrow after each therapy course Afterward, patients will receive HSCT plus decitabine. | Recruiting |
| NCT05426798 | An interventional open-label study single-group assignment | AML MDS | To test the tolerability and anti-tumor efficacy of combined treatment in patients with recurrent/refractory disease | 73 adults | Injection of monoclonal antibody TQB2618 with azacytidine/decitabine | Recruiting |
| NCT03298321 | An observational prospective study | AML | To study the potential effect of epi-drug on arrhythmia and left atrium fibrosis | 14 adults | Azacitidine administration | Unknown |
| NCT03066648 | An interventional non-randomized study parallel assignment | AML | To characterize doses for monoclonal antibodies in combination with HMAs and to evaluate dose-limiting toxicities, ORR, PFS, and TTP | 243 adults | Decitabine in combination with MBG453 and/or PDR001 as monoclonal antibodies, or azacitidine together with MBG453 | Active, not recruiting |
| NCT03238248 | An interventional open-label study single-group assignment | MDS | To reveal the efficacy of combined treatment in patients who failed primary therapy with DNMTi and to determine CR and PFS | 71 adults | Azacitidine infusion and antineoplastic pevonedistat as an inhibitor of the Nedd8 activating enzyme | Active, not recruiting |
| NCT02131597 | An interventional open-label study single-group assignment | MDS | To determine CRR, OS, EFS adverse events, and mortality rate in study participants | 107 adults | Administration of guadecitabine up to 24 courses in the absence of progression and toxicity | Active, not recruiting |
| NCT05148234 | An interventional non-randomized open-label study sequential assignment | MDS | To evaluate the efficacy, safety, and tolerability of treatment and determine ORR, CRR, and therapy-associated adverse events | 200 adults | BMS-986253 plus decitabine/cedazuridine | Recruiting |
| NCT04187703 | An interventional open-label study single group assignment | MDS | To analyze ORR, CRR, improvement, and specify treatment-associated adverse events | 20 adults | Low doses of azacitidine and decitabine | Recruiting |
| Study | Study Design | Disease | Purpose | Patients | Intervention | Study Status |
|---|---|---|---|---|---|---|
| NCT04510610 | An interventional open-label study single-group assignment | HL | To define the response duration and PFS in patients with relapsed/refractory disease treated with camrelizumab plus decitabine | 100 adults/ children | Decitabine plus anti-PD-1 monoclonal antibody camrelizumab | Recruiting |
| NCT04233294 | An interventional open-label study single-group assignment | HL | To document the adverse effects of combined therapy and ORR in cancer patients | 100 adults/ children | HDAC inhibitor chidamide in combination with monoclonal antibody camrelizumab plus decitabine | Recruiting |
| NCT03250962 | An interventional randomized open-label study parallel assignment | HL | To test the safety of decitabine with SHR-1210 therapy compared to SHR-1210 monotherapy and investigate treatment-related adverse events | 280 adults/ children | SHR-1210 as an anti-PD-1 monoclonal antibody with/without the decitabine in relapsed or refractory patients | Recruiting |
| NCT04337606 | An interventional randomized open-label study | NHL | To assess the safety, treatment efficacy, and therapy-induced adverse events | 100 adults | Decitabine plus HDAC inhibitor chidamide or monoclonal antibody camrelizumab. | Recruiting |
| NCT04514081 | An interventional randomized open-label study parallel assignment | HL | To compare ORR and adverse events between immunotherapy-resistant patients with chidamide+decitabine+camrelizumab treatment vs. decitabine+camrelizumab | 200 adults/ children | Decitabine with monoclonal antibody camrelizumab or as a combination with camrelizumab+HDAC inhibitor chidamide. | Recruiting |
| NCT03494296 | An observational case-control prospective study | DLBCL | To determine the efficacy and safety of low-dose decitabine with a chemotherapy regiment in relapsed and refractory patients | 150 adults | A combination of chemotherapeutics cyclophosphamide+vindesine+bonisone with decitabine | Recruiting |
| NCT03346642 | An interventional open-label study single-group assignment | DLBCL | To document potential treatment-related adverse events, ORR, CR, PFS, and OS in cancer patients | 30 adults | Combined therapies with gemcitabine, vinorelbine, doxorubicin, and monoclonal antibody SHR-1210 with/without decitabine | Unknown |
| NCT05320640 | An interventional open-label study single-group assignment | NHL solid tumors | To evaluate predictive biomarkers of response, adverse events, ORR, the safety, and efficacy of combined therapy in participants with relapsed/refractory NHL and advanced solid tumors | 100 adults/ children | Chemotherapy-free regimen with HDAC inhibitor chidamide, low-dose hypomethylating agent decitabine, and anti-PD-1/PD-L1/CTLA-4 antibodies | Recruiting |
| NCT03445858 | An interventional open-label study single-group assignment | Lymphoma solid tumors | To detect feasibility, toxicities, tolerability, and anti-tumor effect of combined therapy | 21 adults/ children | Pembrolizumab with decitabine and fixed-dose hypofractionated radiotherapy | Active, not recruiting |
| NCT04553393 | An interventional randomized open-label study parallel assignment | NHL | To assess the effect of combined therapies in relapsed/refractory participants and reveal adverse events, PFS, and OS after the intervention | 80 adults/ children | A combination of decitabine-primed Tandem CAR19/20 engineered T cells with/without chidamide, decitabine | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinkova, L.; Sevcikova, A.; Stevurkova, V.; Fridrichova, I.; Ciernikova, S. Targeting DNA Methylation in Leukemia, Myelodysplastic Syndrome, and Lymphoma: A Potential Diagnostic, Prognostic, and Therapeutic Tool. Int. J. Mol. Sci. 2023, 24, 633. https://doi.org/10.3390/ijms24010633
Kalinkova L, Sevcikova A, Stevurkova V, Fridrichova I, Ciernikova S. Targeting DNA Methylation in Leukemia, Myelodysplastic Syndrome, and Lymphoma: A Potential Diagnostic, Prognostic, and Therapeutic Tool. International Journal of Molecular Sciences. 2023; 24(1):633. https://doi.org/10.3390/ijms24010633
Chicago/Turabian StyleKalinkova, Lenka, Aneta Sevcikova, Viola Stevurkova, Ivana Fridrichova, and Sona Ciernikova. 2023. "Targeting DNA Methylation in Leukemia, Myelodysplastic Syndrome, and Lymphoma: A Potential Diagnostic, Prognostic, and Therapeutic Tool" International Journal of Molecular Sciences 24, no. 1: 633. https://doi.org/10.3390/ijms24010633
APA StyleKalinkova, L., Sevcikova, A., Stevurkova, V., Fridrichova, I., & Ciernikova, S. (2023). Targeting DNA Methylation in Leukemia, Myelodysplastic Syndrome, and Lymphoma: A Potential Diagnostic, Prognostic, and Therapeutic Tool. International Journal of Molecular Sciences, 24(1), 633. https://doi.org/10.3390/ijms24010633






