In Vivo Degradation Behavior of Magnesium Alloy for Bone Implants with Improving Biological Activity, Mechanical Properties, and Corrosion Resistance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of the MAO-Coated ZK60 Plates
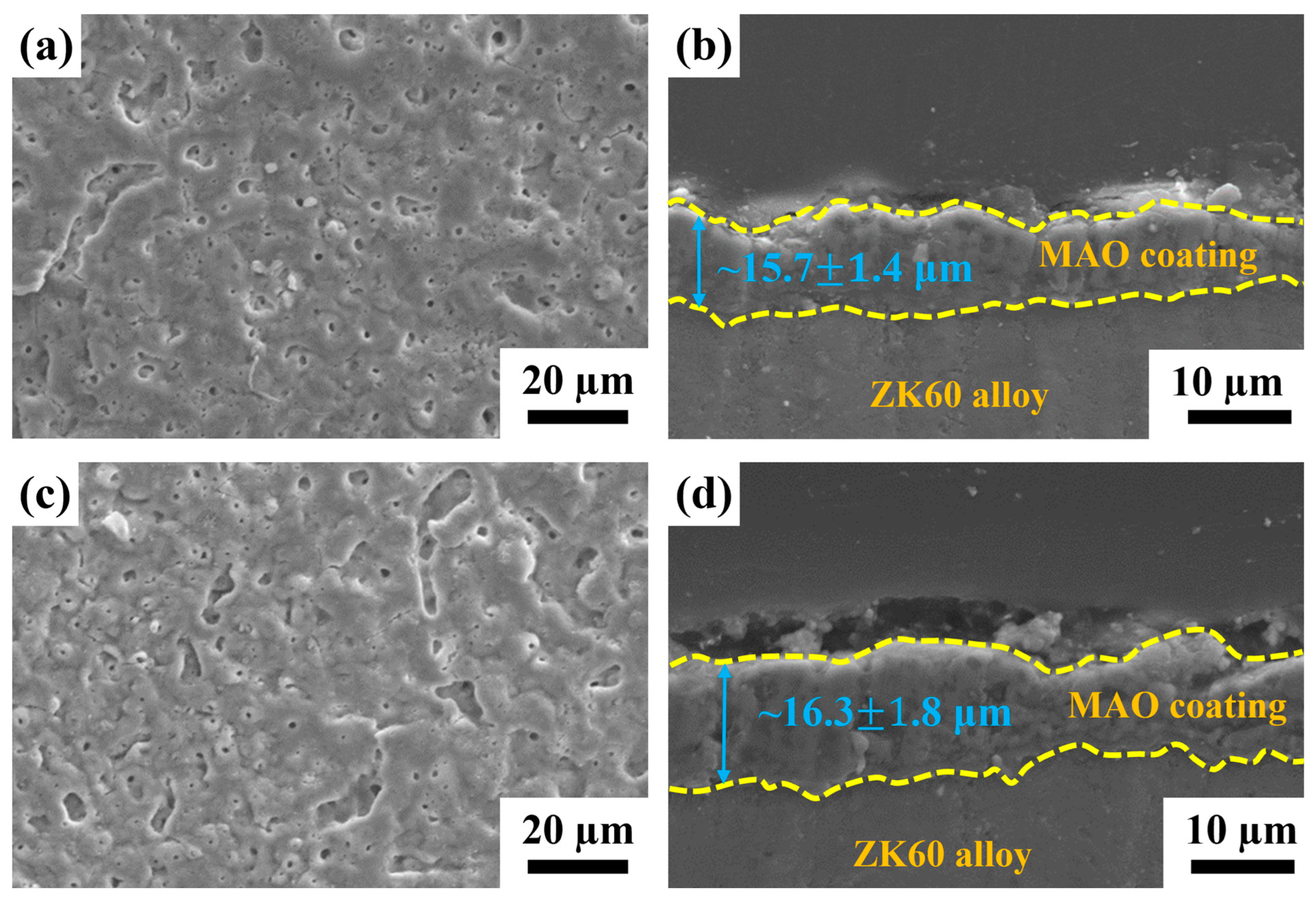
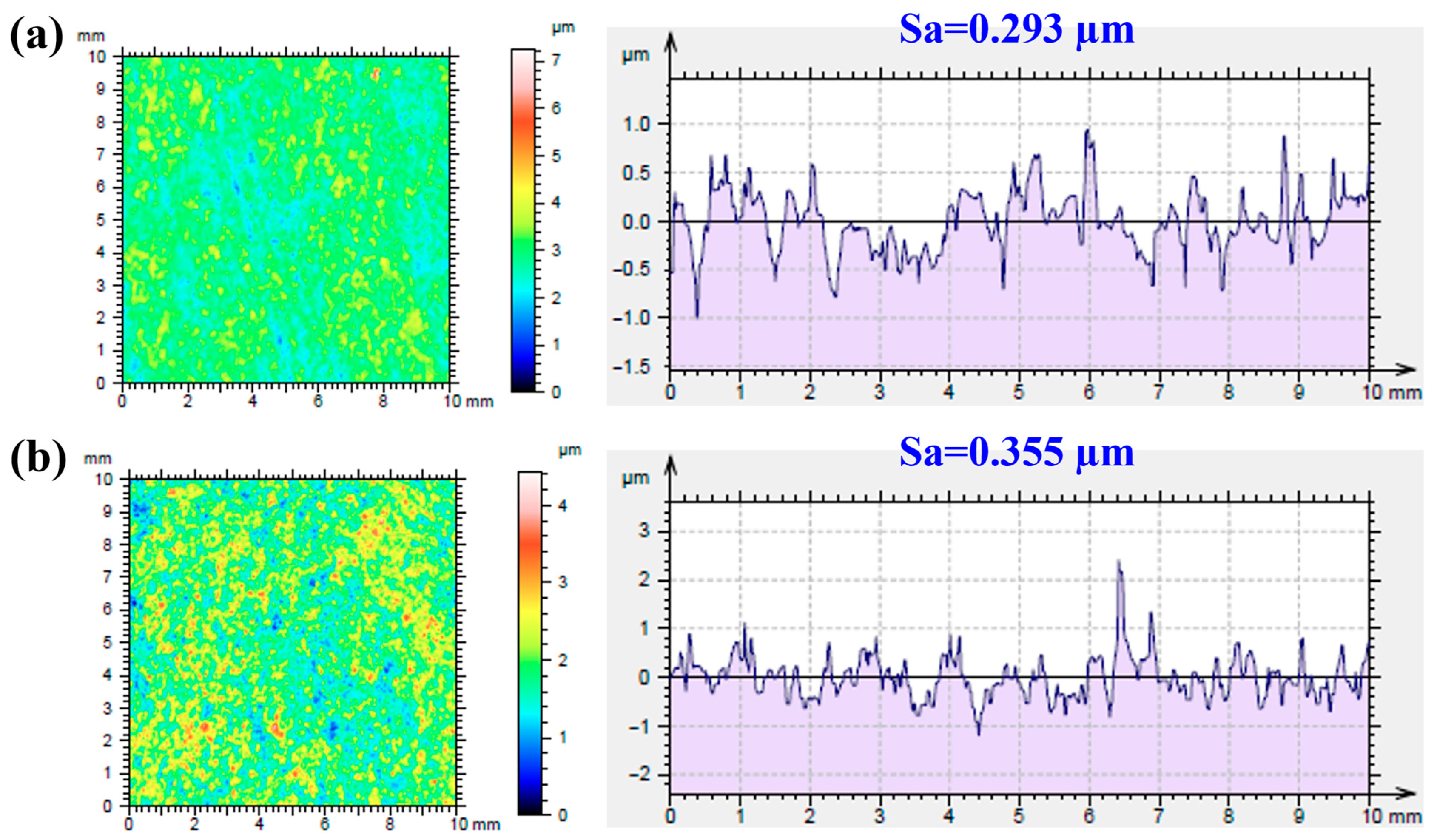
2.1.1. Microstructural Observations
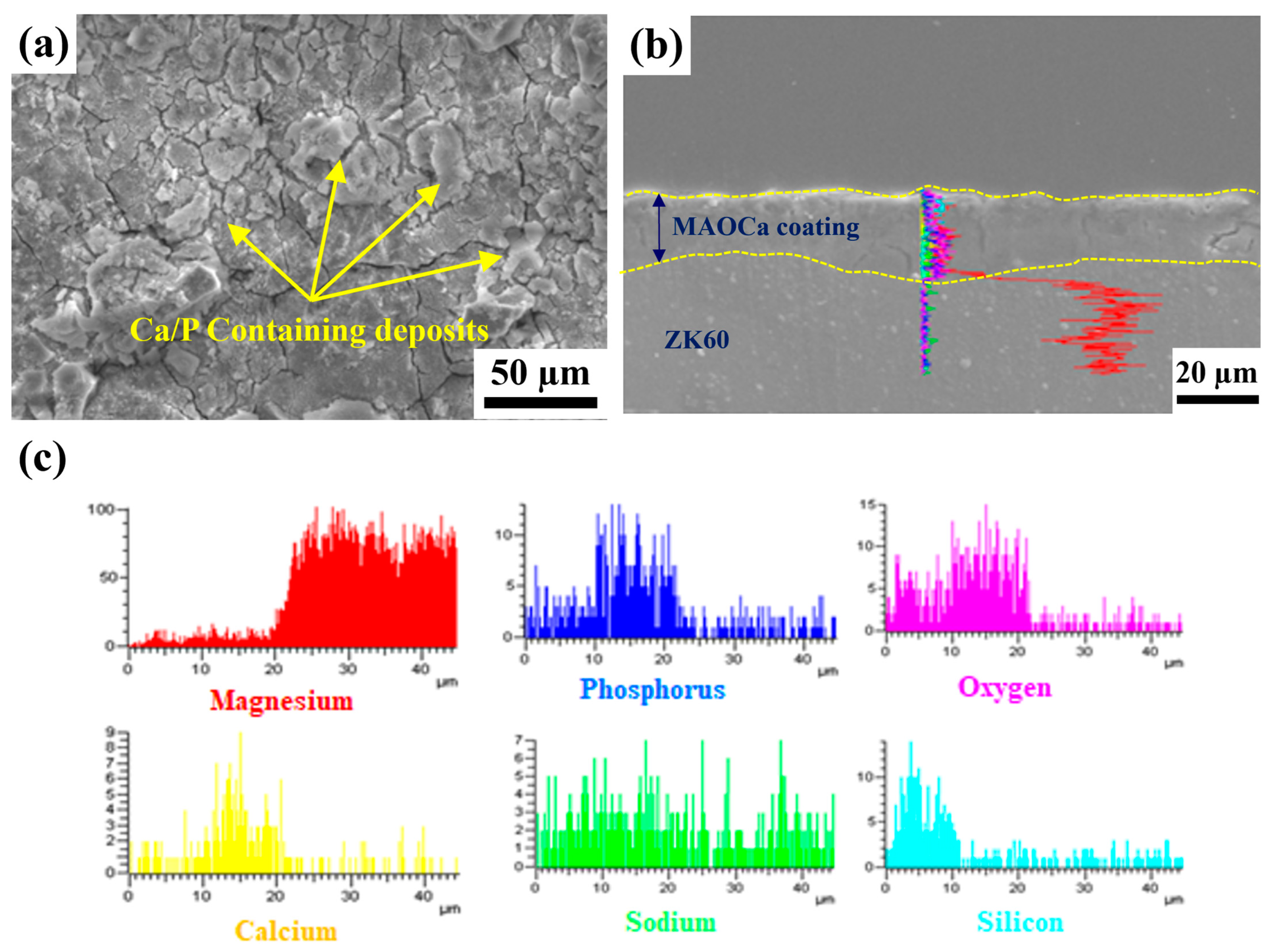
2.1.2. Chemical Composition
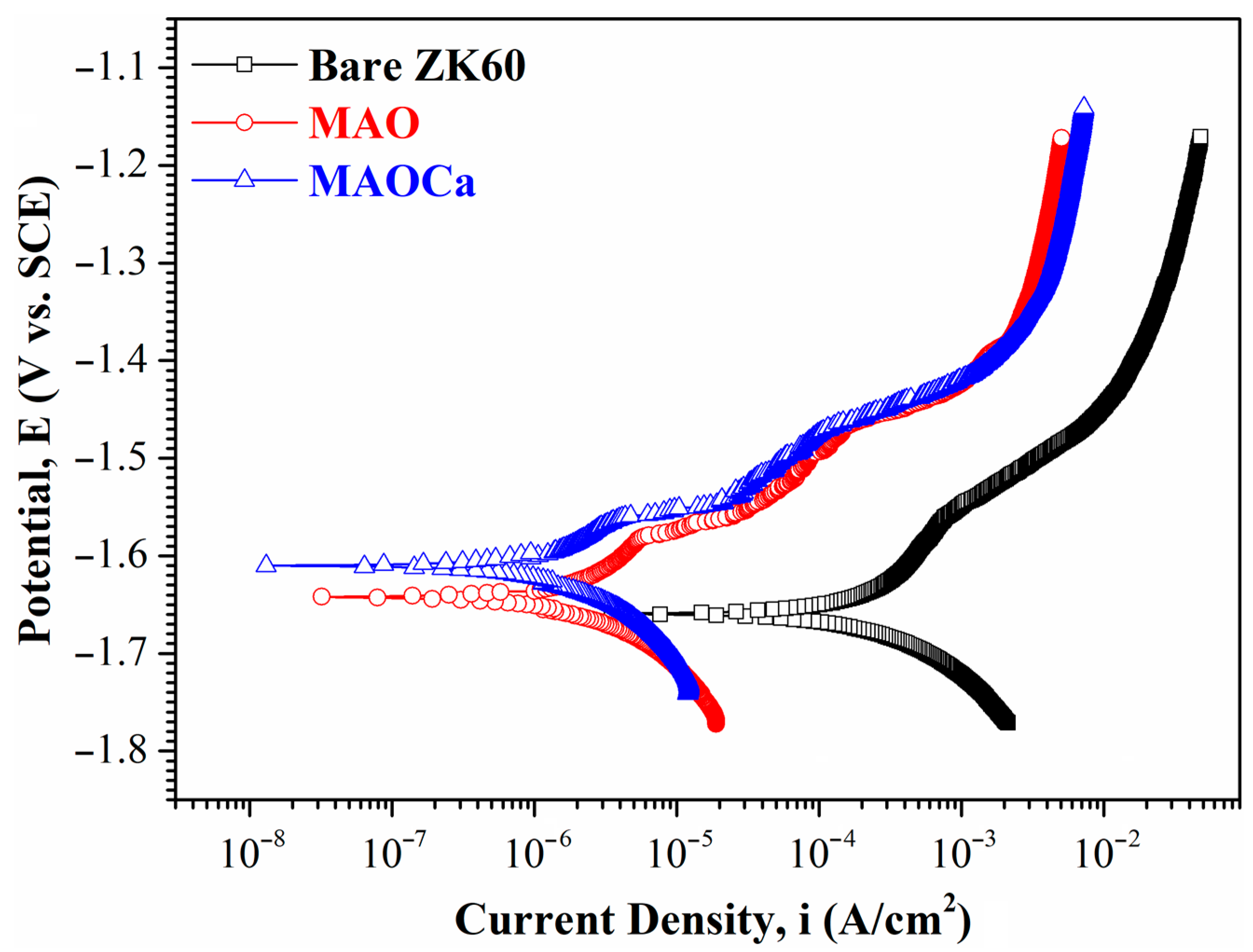
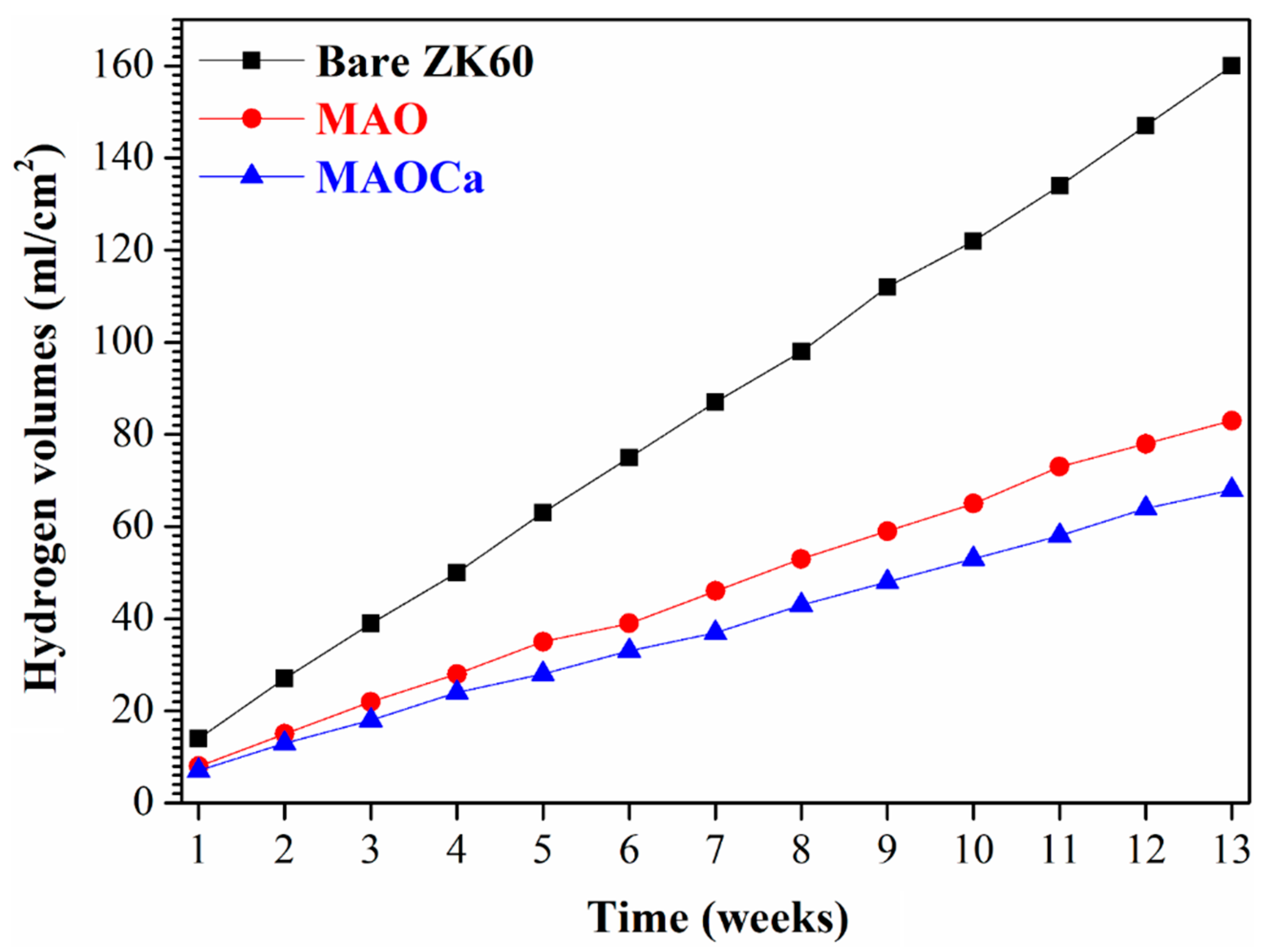
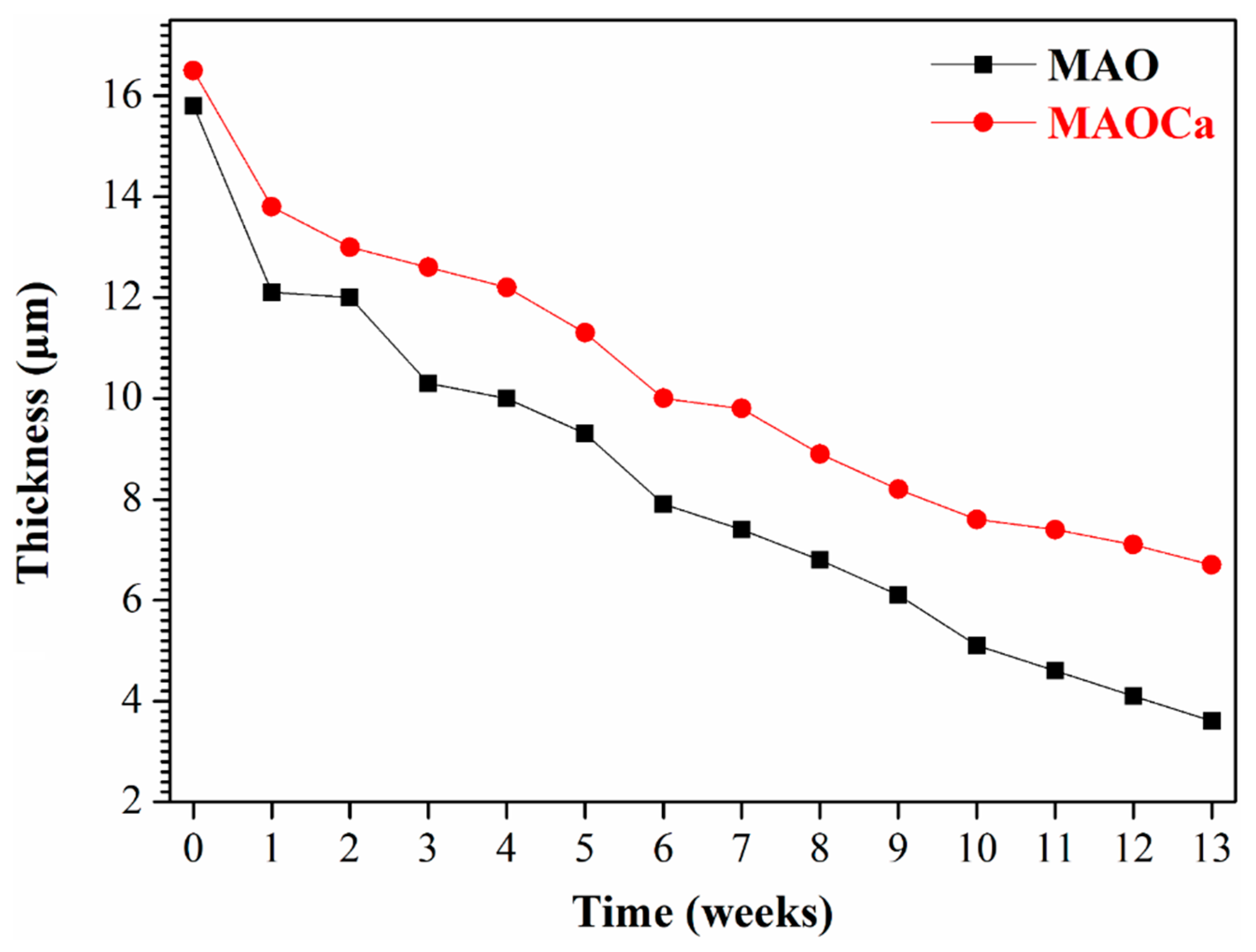
2.1.3. Corrosion Resistance
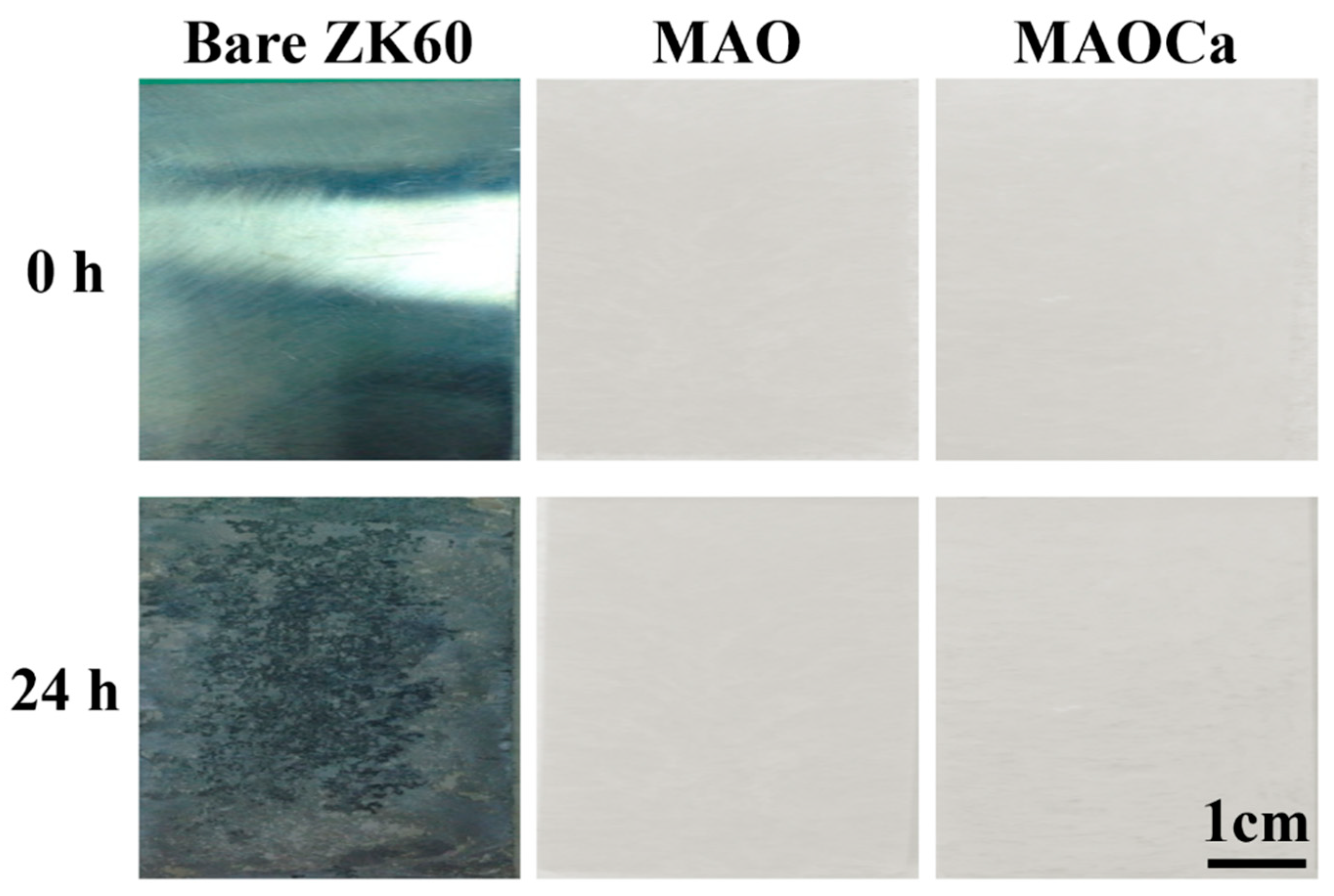
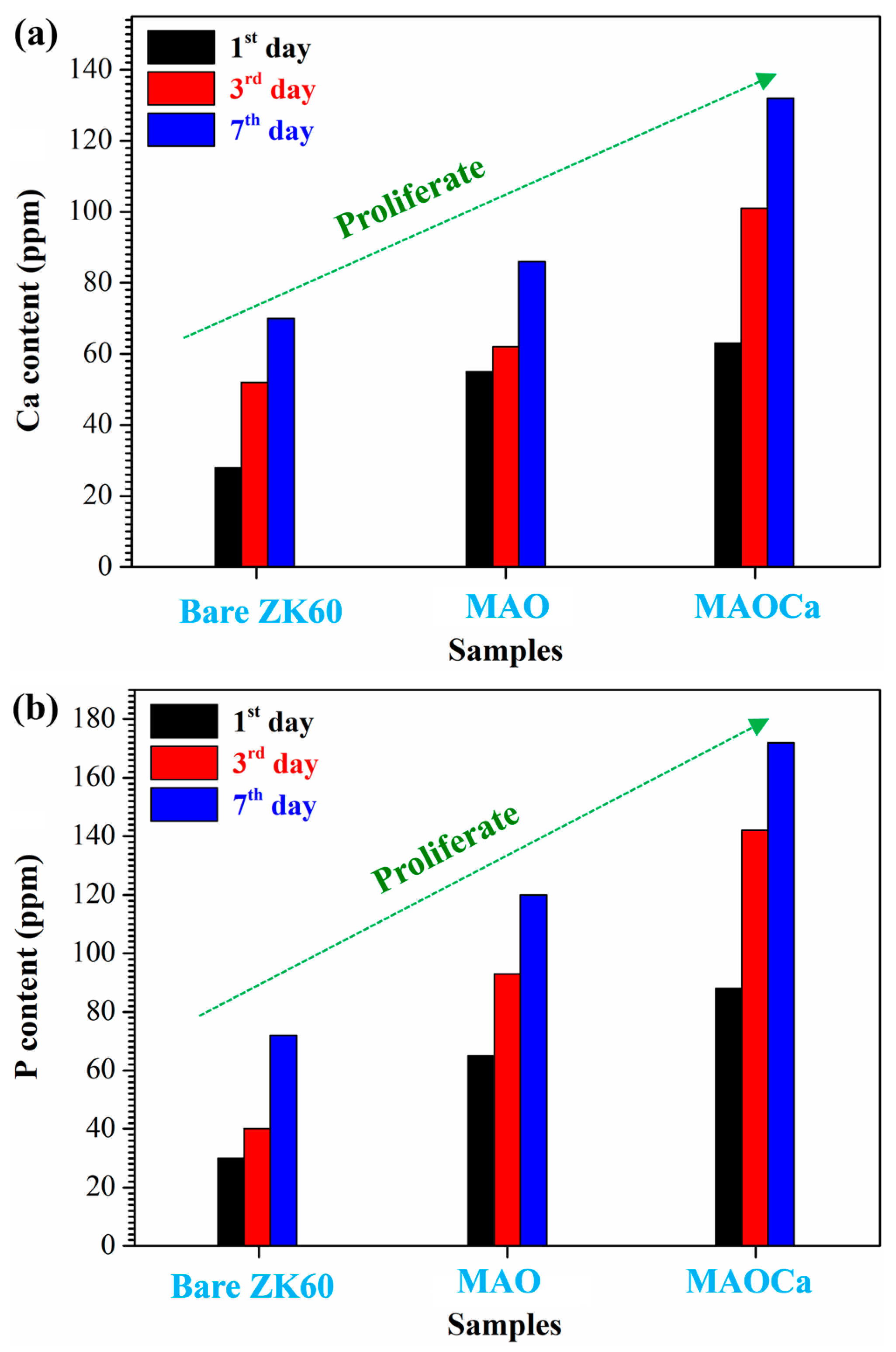
2.1.4. Biological Activity Enhancement
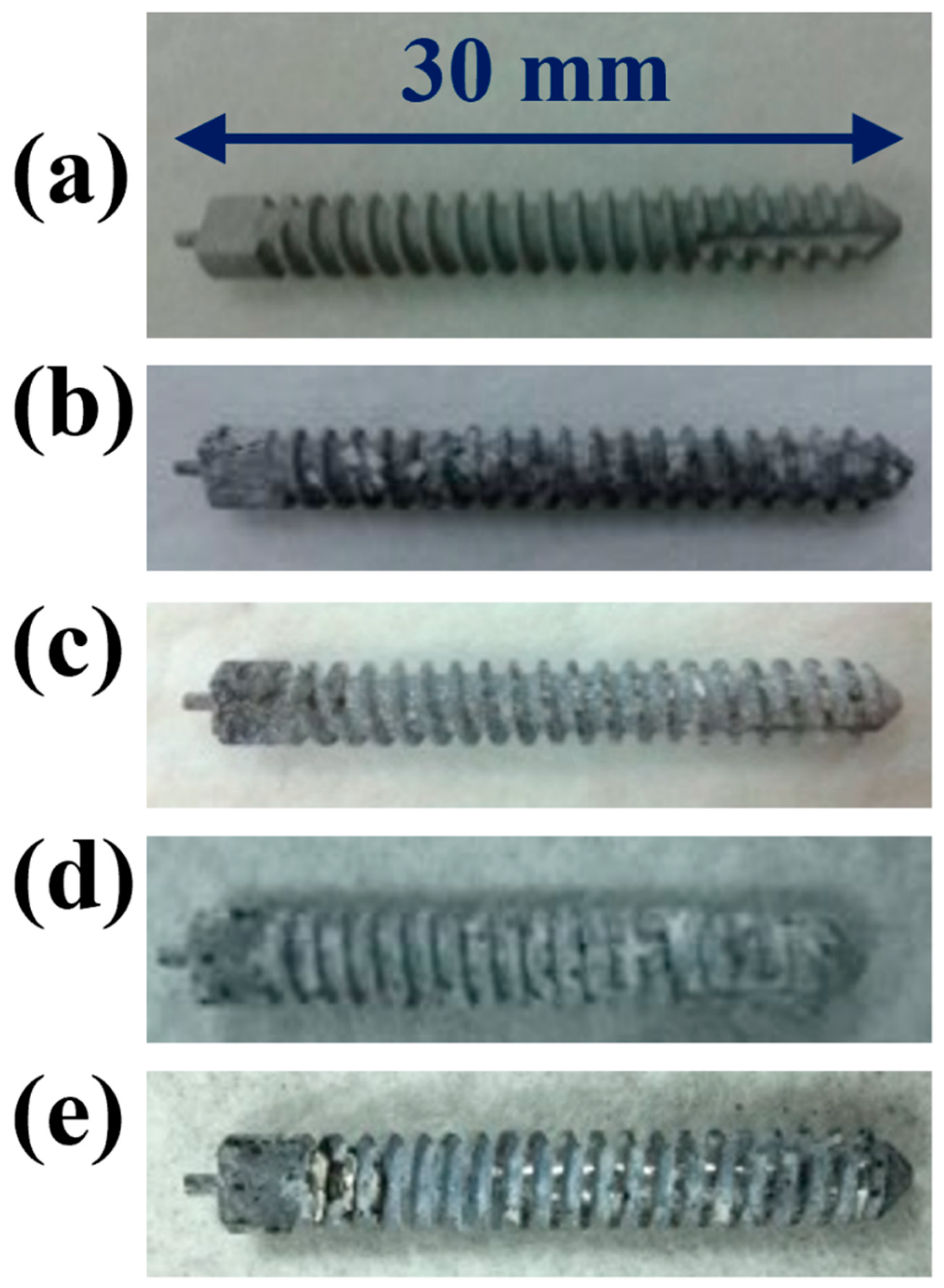
2.2. Mechanical Properties of the MAOCa-Coated ZK60 Bone Screws
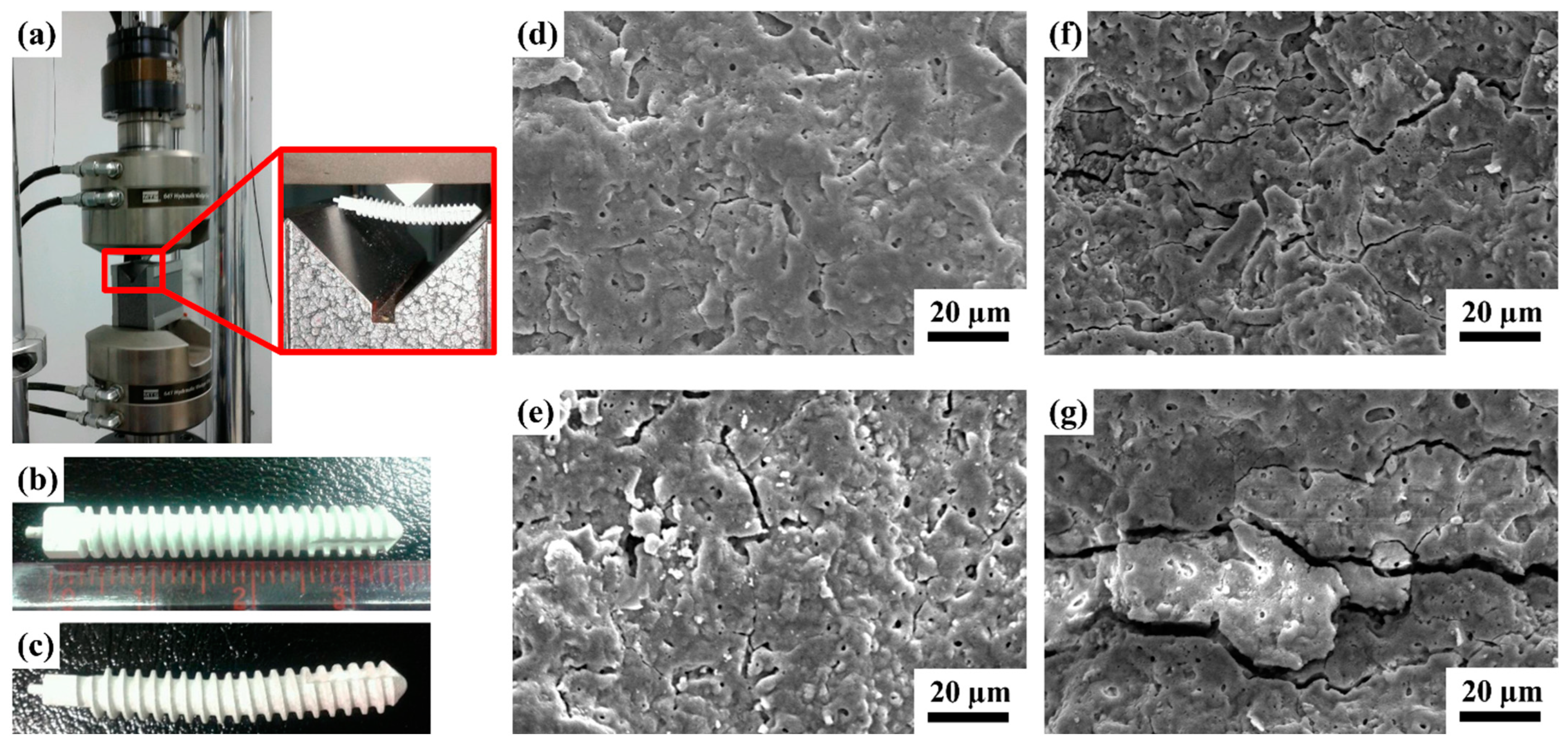
2.2.1. Results of the Three-Point Flexure Test
2.2.2. Adhesion Test Analysis
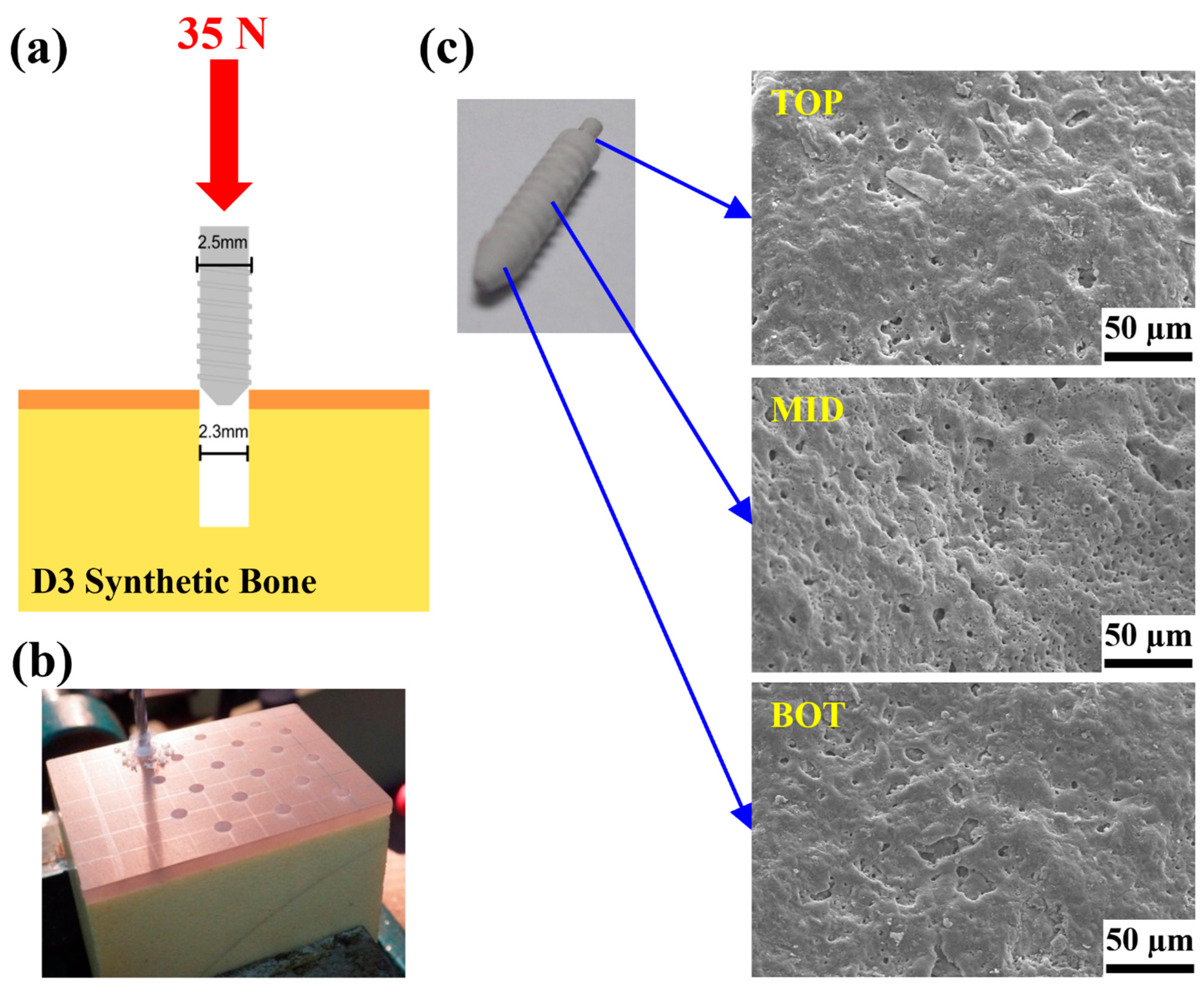
2.2.3. Locking Force Analysis
2.3. Animal Experiments and In Vitro Cell Test
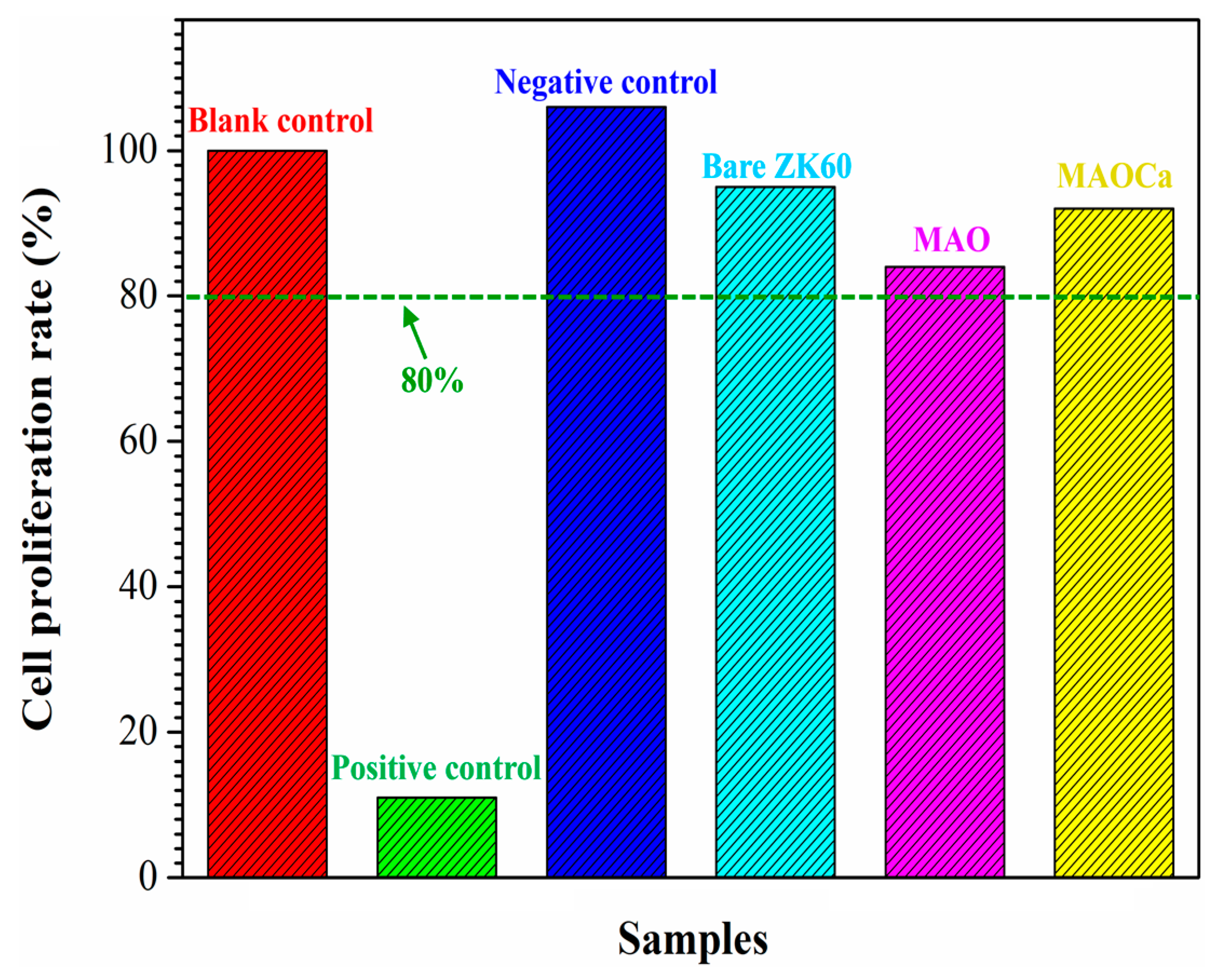
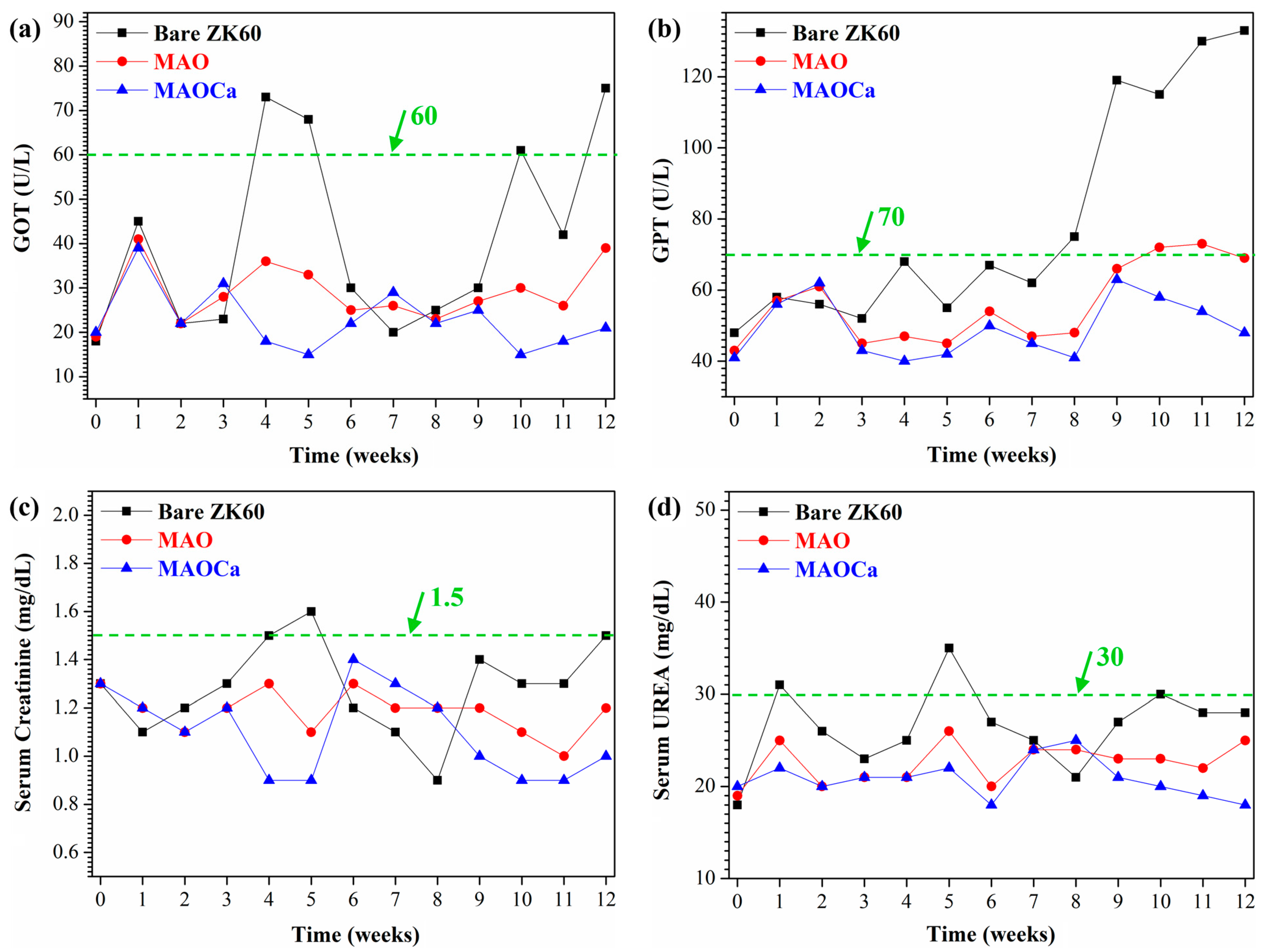
2.3.1. Biocompatibility Analysis
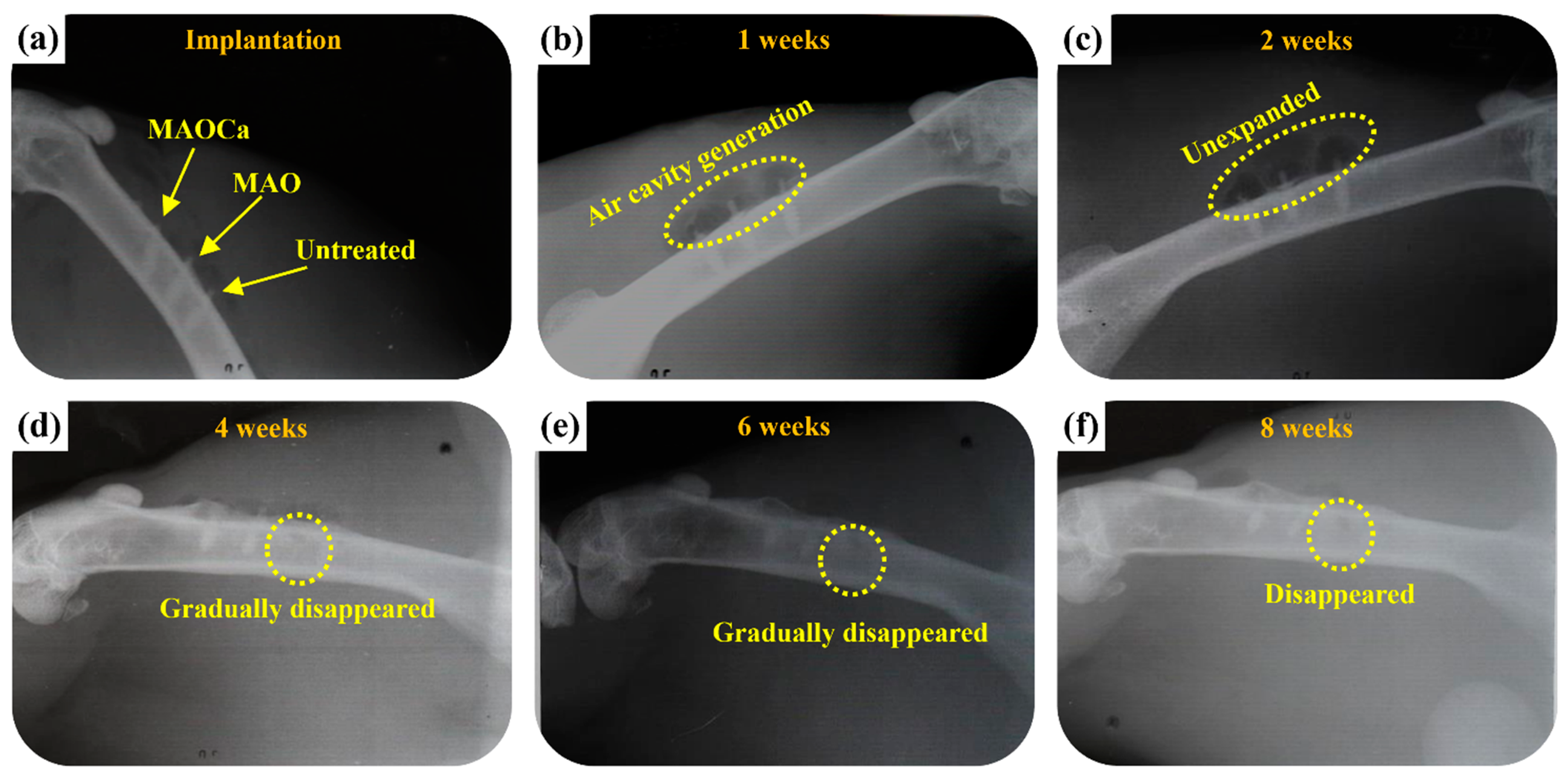
2.3.2. Radiological Examination
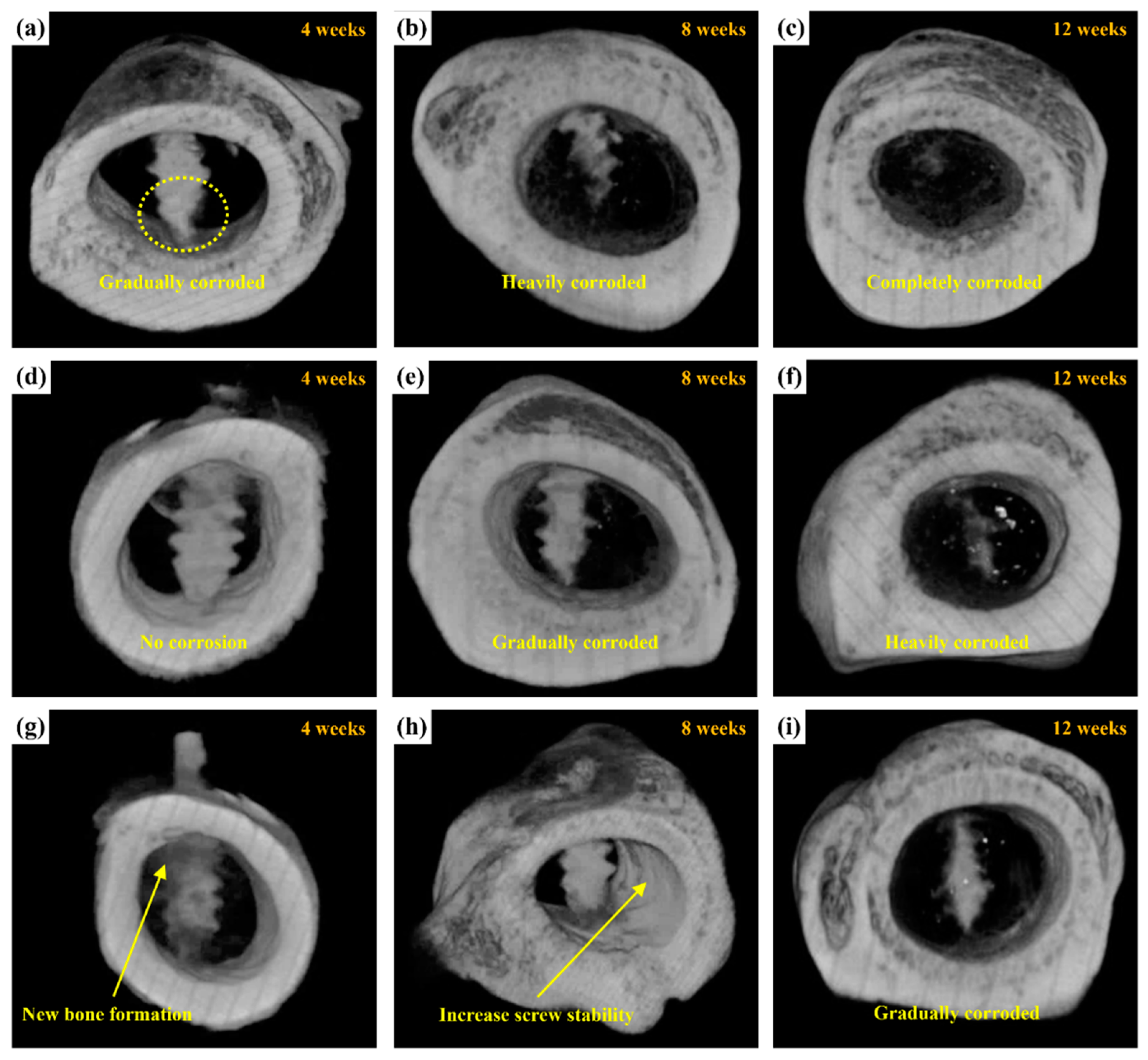
2.3.3. Micro-CT Scanning
3. Experimental Section
3.1. Preparation of Specimens
3.2. Characterization
3.3. Electrochemical Measurements and Corrosion Test
3.4. Animal Surgery and Implant Harvest
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.Y.; Chang, K.L. Effect of cerium ion on the microstructure and properties of permanganate conversion coating on LZ91 magnesium alloy. Appl. Surf. Sci. 2020, 509, 144767. [Google Scholar] [CrossRef]
- Jian, S.Y.; Yang, C.Y.; Chang, J.K. Robust corrosion resistance and self-healing characteristics of a novel Ce / Mn conversion coatings on EV31 magnesium alloys. Appl. Surf. Sci. 2020, 510, 145385. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, Y.; Gao, H. Improving the corrosion resistance and osteogenic differentiation of ZK60 magnesium alloys by hydroxyapatite/graphene/graphene oxide composite coating. Ceram. Int. 2022, 48, 16131–16141. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Rahman, M.; Balu, R.; Dutta, N.K.; Choudhury, N.R. In Vitro Corrosion Resistance of a Layer-by-Layer Engineered Hybrid Coating on ZK60 Magnesium Alloy. Sustainability 2022, 14, 2459. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Yuan, K.; Qiao, Y. Review of the Effect of Surface Coating Modification on Magnesium Alloy Biocompatibility. Materials 2022, 15, 3291. [Google Scholar] [CrossRef]
- Pan, H.; Kang, R.; Li, J.; Xie, H.; Zeng, Z.; Huang, Q.; Yang, C.; Ren, Y.; Qin, G. Mechanistic investigation of a low-alloy Mg–Ca-based extrusion alloy with high strength–ductility synergy. Acta Mater. 2020, 186, 278–290. [Google Scholar] [CrossRef]
- Chen, J.; Lin, W.; Liang, S.; Zou, L.; Wang, C.; Wang, B.; Yan, M.; Cui, X. Effect of alloy cations on corrosion resistance of LDH/MAO coating on magnesium alloy. Appl. Surf. Sci. 2019, 463, 535–544. [Google Scholar] [CrossRef]
- Jian, S.Y.; Tzeng, Y.C.; Ger, M.D.; Chang, K.L.; Shi, G.N.; Huang, W.H.; Chen, C.Y.; Wu, C.C. The study of corrosion behavior of manganese-based conversion coating on LZ91 magnesium alloy: Effect of addition of pyrophosphate and cerium. Mater. Des. 2020, 192, 108707. [Google Scholar] [CrossRef]
- Sarian, M.N.; Iqbal, N.; Sotoudehbagha, P.; Razavi, M.; Ahmed, Q.U.; Sukotjo, C.; Hermawan, H. Potential bioactive coating system for high-performance absorbable magnesium bone implants. Bioact. Mater. 2021, 12, 42–63. [Google Scholar] [CrossRef]
- Rout, P.K.; Roy, S.; Ganguly, S.; Rathore, D.K. A review on properties of magnesium-based alloys for biomedical applications. Biomed. Phys. Eng. Express 2022, 8, 042002. [Google Scholar] [CrossRef]
- Mao, G.; Jin, X.; Sun, J.; Han, X.; Zeng, M.; Qiu, Y.; Bian, W. Microalloying Design of Biodegradable Mg–2Zn–0.05Ca Promises Improved Bone-Implant Applications. ACS Biomater. Sci. Eng. 2021, 7, 2755–2766. [Google Scholar] [CrossRef]
- Jacobsen, N.; Pettersen, A.H. Occupational health problems and adverse patient reactions in orthodontics. Eur. J. Orthod. 1989, 11, 254–264. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Vahey, J.W.; Simonian, P.T.; Conrad, E.U., III. Carcinogenicity and metallic implants. Am. J. Orthop. 1995, 24, 319–324. [Google Scholar]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Le, G.R.Z. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar]
- Van, B.C.A.; Grote, J.J.; Kuijpers, W.; Blok-van, H.C.J.G.; Daems, W.T. Bioreactions at the tissue/hydroxyapatite interface. Biomaterials 1985, 6, 243–251. [Google Scholar]
- Ganeles, J.; Listgarten, M.A.; Evian, C.I. Ultrastructure of durapatite-periodontal tissue interface in human intrabony defects. J. Periodontol. 1986, 57, 133–140. [Google Scholar] [CrossRef]
- Guéhennec, L.L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Jian, S.Y.; Ho, M.L.; Shih, B.C.; Wang, Y.J.; Weng, L.W.; Wang, M.W.; Tseng, C.C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Kim, H.W.; Koh, Y.H.; Li, L.H.; Lee, S.; Kim, H.E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, H.E.; Koh, Y.H.; Kim, H.W.; Jang, J.H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomater. 2009, 5, 2196–2205. [Google Scholar] [CrossRef]
- Shi, J.Z.; Chen, C.Z.; Yu, H.J.; Zhang, S.J. Application of magnetron sputtering for producing bioactive ceramic coatings on implant materials. Bull. Mater. Sci. 2008, 31, 877–884. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Yao, Z.P.; Li, L.L.; Jiang, Z.H. Adjustment of the ratio of Ca/P in the ceramic coating on Mg alloy by plasma electrolytic oxidation. Appl. Surf. Sci. 2009, 255, 6724–6728. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Ding, L.; Cui, X.; Zhao, Y.; Zhang, R. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Laing, P.G.; Ferguson Jr, A.B.; Hodge, E.S. Tissue reaction in rabbit muscle exposed to metallic implants. J. Biomed. Mater. Res. 1967, 1, 135–149. [Google Scholar] [CrossRef]
- Elshahawy, W.M.; Watanabe, I.; Kramer, P. In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent. Mater. 2009, 25, 1551–1555. [Google Scholar] [CrossRef]
- Calin, M.; Gebert, A.; Ghinea, A.C.; Gostin, P.F.; Abdi, S.; Mickel, C.; Eckert, J. Designing biocompatible Ti-based metallic glasses for implant applications. Mater. Sci. Eng. C 2013, 33, 875–883. [Google Scholar] [CrossRef]
- McLachlan, D.R.C.; Bergeron, C.; Smith, J.E.; Boomer, D.; Rifat, S.L. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology 1996, 46, 401–405. [Google Scholar]
- Granchi, D.; Cenni, E.; Ciapetti, G.; Savarino, L.; Stea, S.; Gamberini, S.; Gori, A.; Pizzoferrato, A. Cell death induced by metal ions: Necrosis or apoptosis? J. Mater. Sci. Mater. Med. 1998, 9, 31–37. [Google Scholar] [CrossRef]
- Jian, S.Y.; Aktug, S.L.; Huang, H.T.; Ho, C.J.; Lin, S.Y.; Chen, C.H.; Wang, M.W.; Tseng, C.C. The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics. Int. J. Mol. Sci. 2021, 22, 4706. [Google Scholar] [CrossRef]
- Karthega, M.; Raman, V.; Rajendran, N. Influence of potential on the electrochemical behaviour of β titanium alloys in Hank’s solution. Acta Biomater. 2007, 3, 1019–1023. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Lin, J.; Li, Y.; Ping, D.; Yamabe-Mitarai, Y.; Wen, C. Investigations into Ti–(Nb,Ta)–Fe alloys for biomedical applications. Acta Biomater. 2016, 32, 336–347. [Google Scholar] [CrossRef]
- López, M.F.; Gutiérrez, A.; Jiménez, J.A. In vitro corrosion behaviour of titanium alloys without vanadium. Electrochim. Acta 2002, 47, 1359–1364. [Google Scholar] [CrossRef]
- Kumar, A.M.; Hassan, S.F.; Sorour, A.A.; Paramsothy, M.; Gupta, M. Electrochemical Corrosion and In vitro Biocompatibility Performance of AZ31Mg/Al2O3 Nanocomposite in Simulated Body Fluid. J. Mater. Eng. Perform. 2018, 27, 3419–3428. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Tim, D.S.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B.; Chapman, M.W.; Sharkey, N.A.; Zissimos, S.L.; Bay, B.; Shors, E.G. Bone ingrowth and mechanical properties of coralline hydroxyapatite 1 yr after implantation. Biomaterials 1993, 14, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O.C. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, X.; Tan, L.; Wan, P.; Yu, X.; Li, Q.; Yang, K. Effect of preparation parameters on the properties of hydroxyapatite containing micro-arc oxidation coating on biodegradable ZK60 magnesium alloy. Ceram. Int. 2014, 40, 10043–10051. [Google Scholar] [CrossRef]
- Klompmaker, J.; Jansen, H.W.B.; Veth, R.P.H.; Nielsen, H.K.L.; Degroot, J.H.; Pennings, A.J. Porous polymer implants for repair of full-thickness defects of articular cartilage: An experimental study in rabbit and dog. Biomaterials 1992, 13, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. A 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ramires, P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Alonso, A.; Sanz, M. Long-term results and survival rate of implants treated with guided bone regeneration: A 5-year case series prospective study. Clin. Oral Implant. Res. 2005, 16, 294–301. [Google Scholar] [CrossRef]
- Kamachimudali, U.; Sridhar, T.; Raj, B. Corrosion of bio implants. Sādhanā 2003, 28, 601–637. [Google Scholar] [CrossRef]
- Merritt, K.; Brown, S.A. Effect of proteins and pH on fretting corrosion and metal ion release. J. Biomed. Mater. Res. 1988, 22, 111–120. [Google Scholar] [CrossRef]
- Williams, R.L.; Brown, S.A.; Merritt, K. Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials 1988, 9, 181–186. [Google Scholar] [CrossRef]
- Hench, L.L.; Ethridge, E.C. Biomaterials—The Interfacial Problem. Adv. Biomed. Eng. 1975, 5, 35–150. [Google Scholar]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1936. [Google Scholar] [CrossRef]
- Neil, W.C.; Forsyth, M.; Howlett, P.C.; Hutchinson, C.R.; Hinton, B.R.W. Corrosion of heat-treated magnesium alloy ZE41. Corros. Sci. 2011, 53, 3299–3308. [Google Scholar] [CrossRef]
| Element—Atomic% | |||||||
|---|---|---|---|---|---|---|---|
| O | Mg | Na | Si | P | Ca | Total | |
| MAO | 43.6 | 42.5 | 0.6 | 11.7 | 1.6 | - | 100 |
| MAOCa | 55.8 | 28.3 | 0.2 | 9.2 | 2.4 | 4.1 | 100 |
| Elemental Analysis at 10 nm from the Surface | Before Immersion | After 48 h Immersion | ||||
|---|---|---|---|---|---|---|
| Bare ZK60 | MAO | MAOCa | Bare ZK60 | MAO | MAOCa | |
| Ca | 0% | 0% | 4.01% | 7.45% | 14.6% | 18.8% |
| P | 0% | 1.82% | 2.03% | 6.12% | 8.92% | 11.1% |
| The ZK60 Mg Alloy Bone Screw Depression Distance (mm) | ||||
|---|---|---|---|---|
| 0.5 | 0.75 | 1.0 | 2.0 | |
| The angle of deformation | 3.3° | 5° | 6.6° | 13.1° |
| Load force | 70 N | 90 N | 201 N | 254 N |
| ZK60 Mg Alloy Bone Screw | |
|---|---|
| Locking force of SBF non-immersed screw (A) | 251 N |
| Locking force of screw immersed for 6 weeks (B) | 233 N |
| Residual locking force of screw immersed for 6 weeks (B/A) | 92% |
| Locking force of screw immersed for 10 weeks (C) | 213 N |
| Residual locking force of screw immersed for 10 weeks (C/A) | 84% |
| Name | Na2SiO3 | NaOH | Na3PO4 | Ca3PO4 | EDTA |
|---|---|---|---|---|---|
| MAO | 60 g/L | 70 g/L | 20 g/L | - | - |
| MAOCa | 60 g/L | 70 g/L | 20 g/L | 10.5 g/L | 7.5 g/L |
| Electrolyte information | All electrolytes were from ECHO CHEMICAL CO., LTD. (Miaoli County 35145, Taiwan). | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.-Y.; Lin, C.-F.; Tsai, T.-L.; Wang, P.-H.; Chen, C.-H.; Lin, S.-Y.; Tseng, C.-C. In Vivo Degradation Behavior of Magnesium Alloy for Bone Implants with Improving Biological Activity, Mechanical Properties, and Corrosion Resistance. Int. J. Mol. Sci. 2023, 24, 1602. https://doi.org/10.3390/ijms24021602
Jian S-Y, Lin C-F, Tsai T-L, Wang P-H, Chen C-H, Lin S-Y, Tseng C-C. In Vivo Degradation Behavior of Magnesium Alloy for Bone Implants with Improving Biological Activity, Mechanical Properties, and Corrosion Resistance. International Journal of Molecular Sciences. 2023; 24(2):1602. https://doi.org/10.3390/ijms24021602
Chicago/Turabian StyleJian, Shun-Yi, Chiu-Feng Lin, Tung-Lin Tsai, Pei-Hua Wang, Chung-Hwan Chen, Sung-Yen Lin, and Chun-Chieh Tseng. 2023. "In Vivo Degradation Behavior of Magnesium Alloy for Bone Implants with Improving Biological Activity, Mechanical Properties, and Corrosion Resistance" International Journal of Molecular Sciences 24, no. 2: 1602. https://doi.org/10.3390/ijms24021602
APA StyleJian, S.-Y., Lin, C.-F., Tsai, T.-L., Wang, P.-H., Chen, C.-H., Lin, S.-Y., & Tseng, C.-C. (2023). In Vivo Degradation Behavior of Magnesium Alloy for Bone Implants with Improving Biological Activity, Mechanical Properties, and Corrosion Resistance. International Journal of Molecular Sciences, 24(2), 1602. https://doi.org/10.3390/ijms24021602







