Molecular Mechanism for Hepatic Glycerolipid Partitioning of n-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels
Abstract
1. Introduction
2. Results
2.1. Incorporation Rate of 14C-Labeling Lipid and 14CO2 Emission Rate into Glycerolipid
2.2. Ememission of 14CO2 and Accumulation rate of 14C-Labeling Lipid in Tissue
2.3. The Ratio of Cholesteryl Ester Metabolized by the Liver
2.4. The Contribution Level of Glycerolipid within the Metabolic Turning Point
3. Discussion
4. Materials and Methods
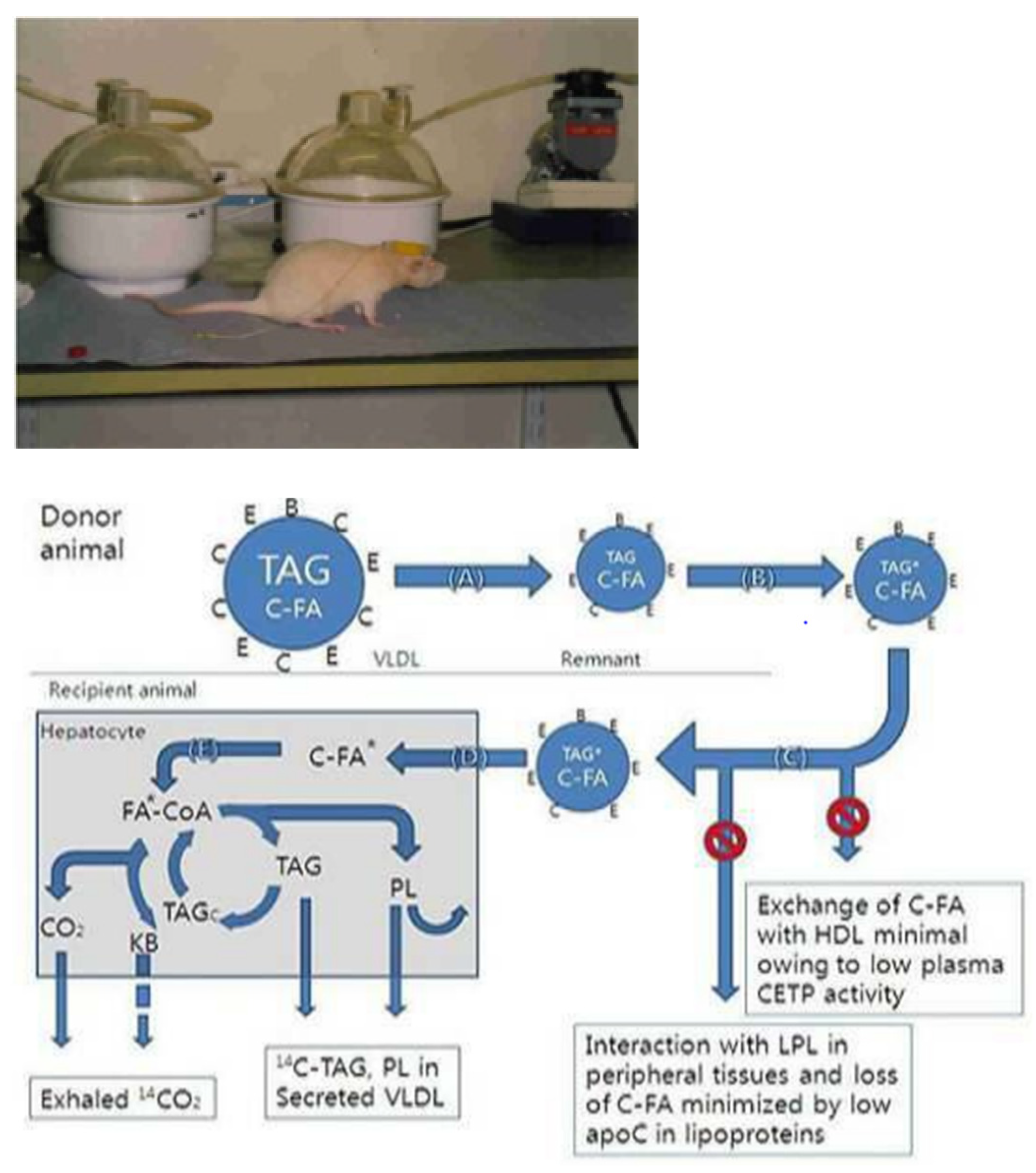
4.1. Animal and Experimental Design
4.2. Experimental Diets and Feeding Management
4.3. Jugular-Vein Cannulation
4.4. Preparation of Lipopolysaccharides
4.5. In Vivo Injection and Respiratory Metabolism Trials
4.6. Thin-Layer Chromatography
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, K.D.; Farooqi, S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Friedman, M.I. Increasing adiposity: Consequence or cause of overeating? JAMA 2014, 311, 2167–2168. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Hall, K.D. Carbohydrates, insulin, and obesity. Science 2021, 372, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of dietary n–3 and n–6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965–6990. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Review; Omega-3 PUFA responders and non-responders and the prevention of lipid dysmetabolism and related diseases. Nutrients 2020, 12, 1363–1381. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Verma, N.; Trivedi, R.K.; Bhardwaj, S.; Shukla, N. Significance of ratio of omega-3 and omega-6 in human health with special reference to flaxseed oil. Int. J. Biol. Chem. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Gómez-Candela, C.; López, L.M.B.; Loria-Kohen, V. Importance of a balanced omega-6/omega-3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrient 2016, 8, 128–135. [Google Scholar] [CrossRef]
- Lee, S.; Um, K.H.; Park, B.S. Effect of n-6/n-3 fatty acid ratio on metabolic partitioning in hyperlipidemic rat. J. Oil Appl. Sci. 2018, 35, 643–646. [Google Scholar]
- Park, S.O.; Zammit, V.A. In vivo monitoring of hepatic glycolipid distribution of n-6/n-3 in jugular-vein-cannulated rats as a nutritional research model for monogastric animal. Arch. Anim. Breed. 2019, 5, 437–446. [Google Scholar] [CrossRef]
- Shin, J.S.; Um, K.H.; Park, B.S. Effects of n-6/n-3 fatty acid ratio on lipid metabolism in obesity model rats. J. Oil Appl. Sci. 2018, 35, 654–666. [Google Scholar]
- Lee, S.G.; Knag, H. Anti-obesity and lipid metabolism effects of Ulmus davidiana var. japonica in mice fed a high-fat diet. J. Microbiol. Biotechnol. 2021, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.; Lee, J.; Yuan, M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int. J. Obes. 2003, 27, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Rennie, S.M.; Park, B.S.; Zammit, V.A. A switch in the direction of the effect of insulin on the partitioning of hepatic fatty acids for the formation of secreted triglycerol occurs in vivo, as predicted from studies with perfused livers. FEBS J. 2000, 267, 935–941. [Google Scholar] [CrossRef]
- Shi, Y.; Cheng, D. Beyond triglyceride synthesis: The dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Umpleby, A.M. Tracing lipid metabolism: The value of stable isotopes. J. Endocrinol. 2015, 226, G1–G10. [Google Scholar] [CrossRef]
- Chen, Z.; Gropler, M.C.; Norris, J.; Lawrence, J.C., Jr.; Harris, T.E.; Finck, B.N. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1738–1744. [Google Scholar] [CrossRef]
- Huynh, F.K.; Green, M.F.; Koves, T.R.; Hirschey, M.D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Meth. Enzymol. 2014, 542, 391–405. [Google Scholar]
- Hodson, L.; Frayn, K.N. Hepatic fatty acid partitioning. Curr. Opin. Lipidol. 2011, 22, 216–224. [Google Scholar] [CrossRef]
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA metabolism and partitioning. Ann. Rev. Nutr. 2014, 34, 1–30. [Google Scholar] [CrossRef]
- Constantin, R.P.; Nascimento, G.S.D.; Salgueiro, C.L.; Bracht, A.; Ishii-Iwamoto, E.L.; Yamamoto, N.S.; Constantin, J. Citrus flavanones affect hepatic fatty acid oxidation in rats by acting as prooxidant agents. Biomed. Res. Int. 2013, 2013, 342973–342985. [Google Scholar] [CrossRef] [PubMed]
- Li, L.O.; Elis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acylCoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, F.; Wang, H.; Haas, J.T. Triacylglycerol synthesisenzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, K.; Tachibe, M.; Kaneko, N.; Kishida, T. Hydroxypropylation of high amylose maize starch changes digestion and fermentation-dependent parameters in rats. J. Nutr. Sci. 2013, 3, e17. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Madiraju, S.R.M. Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 2008, 29, 647–676. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhang, B.; Wang, J.F.; Xue, C.J.; Yanagita, T. The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis. 2010, 9, 4–9. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.J.; Lee, J.; Lee, J.K.; Byun, J.; Kim, I.; Ha, J.H. Lowering n-6/n-3 ratio as an important dietary intervention to prevent LPS-inducible dyslipidemia and hepatic abnormalities in ob/ob mice. Int. J. Mol. Sci. 2022, 23, 6384. [Google Scholar] [CrossRef]
- Angela, M.; Gajda, M.S. High fat diets for diet-induced obesity models. In A Brief Review of the Scientific Literature; Research Diets, Inc.: New Brunswick, NJ, USA, 2008. [Google Scholar]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Thrivikraman, K.V.; Huot, R.L.; Plotsky, P.M. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res. Protoc. 2002, 10, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Cid-Hernandez, M.; Dellamary-Toral, F.A.L.; Gonzalez-Ortiz, M.J.; Sanchez-Pena, M.J.; Pacheco-Moises, F.P. Two dimensional thin layer chromatography-bioautography designed to separate and locate metabolites with antioxidant activity contained on Spirulina platensis. Int. J. Anal. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT® 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
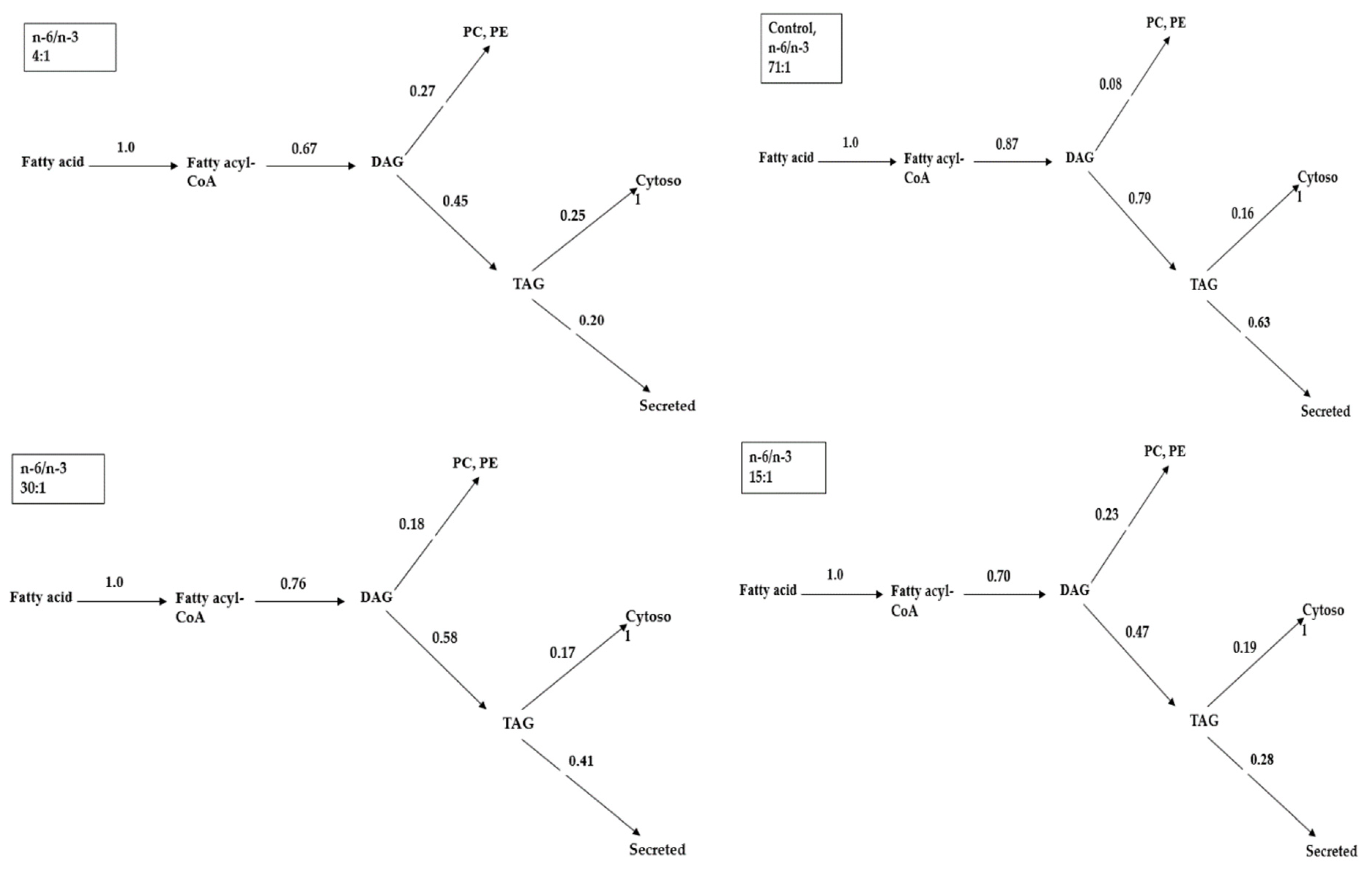
| n-6/n-3 | Incorporation of [3H], [14C] Label (% of Injected Dose) | 14CO2 Emission | |||
|---|---|---|---|---|---|
| Plasma | Liver | ||||
| [3H] | [14C] | [3H] | [14C] | ||
| Control (71:1) | 1.10 ± 0.02 | 23.70 ± 0.95 1,a | 95.35 ± 3.63 | 40.01 ± 1.22 a | 9.78 ± 0.33 d |
| 4:1 | 1.13 ± 0.01 | 17.20 ± 0.68 c | 94.75 ± 3.98 | 28.87 ± 1.08 d | 25.25 ± 0.77 a |
| 15:1 | 1.12 ± 0.01 | 17.89 ± 0.72 c | 95.60 ± 5.02 | 31.86 ± 1.02 c | 24.77 ± 0.63 b |
| 30:1 | 1.10 ± 0.01 | 20.62 ± 0.78 b | 94.52 ± 3.78 | 37.03 ± 1.3 b | 13.58 ± 0.55 c |
| n-6/n-3 | Absorption of [14C] Lipid (%) | 14CO2 Emission (% of Absorbed Dose) | Tissue [14C] Lipid Accumulation (% of Absorbed Dose/g of Tissue) | ||
|---|---|---|---|---|---|
| Adipose Tissue | Liver | Muscle (Hind Leg) | |||
| Control (71:1) | 70.15 ± 2.31 1,a | 27.71 ± 1.10 d | 2.01 ± 0.06 a | 1.87 ± 0.06 a | 0.81 ± 0.07 a |
| 4:1 | 57.43 ± 2.02 d | 45.08 ± 1.77 a | 0.58 ± 0.14 b | 0.33 ± 0.01 c | 0.22 ± 0.02 c |
| 15:1 | 60.59 ± 2.48 c | 43.77 ± 1.56 b | 0.62 ± 0.20 b | 0.87 ± 0.09 b | 0.47 ± 0.03 b |
| 30:1 | 68.72 ± 2.61 b | 30.35 ± 1.07 c | 1.89 ± 0.07 a | 0.92 ± 0.10 b | 0.75 ± 0.10 a |
| n-6/n-3 | Total Glycerolipid (% of Cholesterol [14C]-oleate metabolized in Liver) | % Secreted | Phospholipid/Total Glycerolipid (%) | 14CO2/[14C]-Total Glycerolipid (%) | |
|---|---|---|---|---|---|
| Phospholipid (% of Total Glycerolipid Labelled) | TAG (% of Total Triacylglycerol Labelled) | ||||
| Control (71:1) | 78.51 ± 3.01 1,a | 8.67 ± 0.33 c | 64.58 ± 2.27 a | 11.04 ± 0.38 c | 39.50 ± 1.41 d |
| 4:1 | 70.22 ± 2.22 c | 17.66 ± 0.61 a | 51.26 ± 1.82 d | 25.15 ± 0.96 a | 78.50 ± 2.61 a |
| 15:1 | 75.07 ± 2.01 b | 14.17 ± 0.52 b | 57.01 ± 1.88 c | 18.87 ± 0.66 b | 72.23 ± 2.75 b |
| 30:1 | 76.66 ± 2.17 b | 13.91 ± 0.45 b | 59.87 ± 2.01 b | 18.15 ± 0.53 b | 44.16 ± 1.55 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammit, V.A.; Park, S.-O. Molecular Mechanism for Hepatic Glycerolipid Partitioning of n-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels. Int. J. Mol. Sci. 2023, 24, 1576. https://doi.org/10.3390/ijms24021576
Zammit VA, Park S-O. Molecular Mechanism for Hepatic Glycerolipid Partitioning of n-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels. International Journal of Molecular Sciences. 2023; 24(2):1576. https://doi.org/10.3390/ijms24021576
Chicago/Turabian StyleZammit, Victor A, and Sang-O Park. 2023. "Molecular Mechanism for Hepatic Glycerolipid Partitioning of n-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels" International Journal of Molecular Sciences 24, no. 2: 1576. https://doi.org/10.3390/ijms24021576
APA StyleZammit, V. A., & Park, S.-O. (2023). Molecular Mechanism for Hepatic Glycerolipid Partitioning of n-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels. International Journal of Molecular Sciences, 24(2), 1576. https://doi.org/10.3390/ijms24021576





