The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health?
Abstract
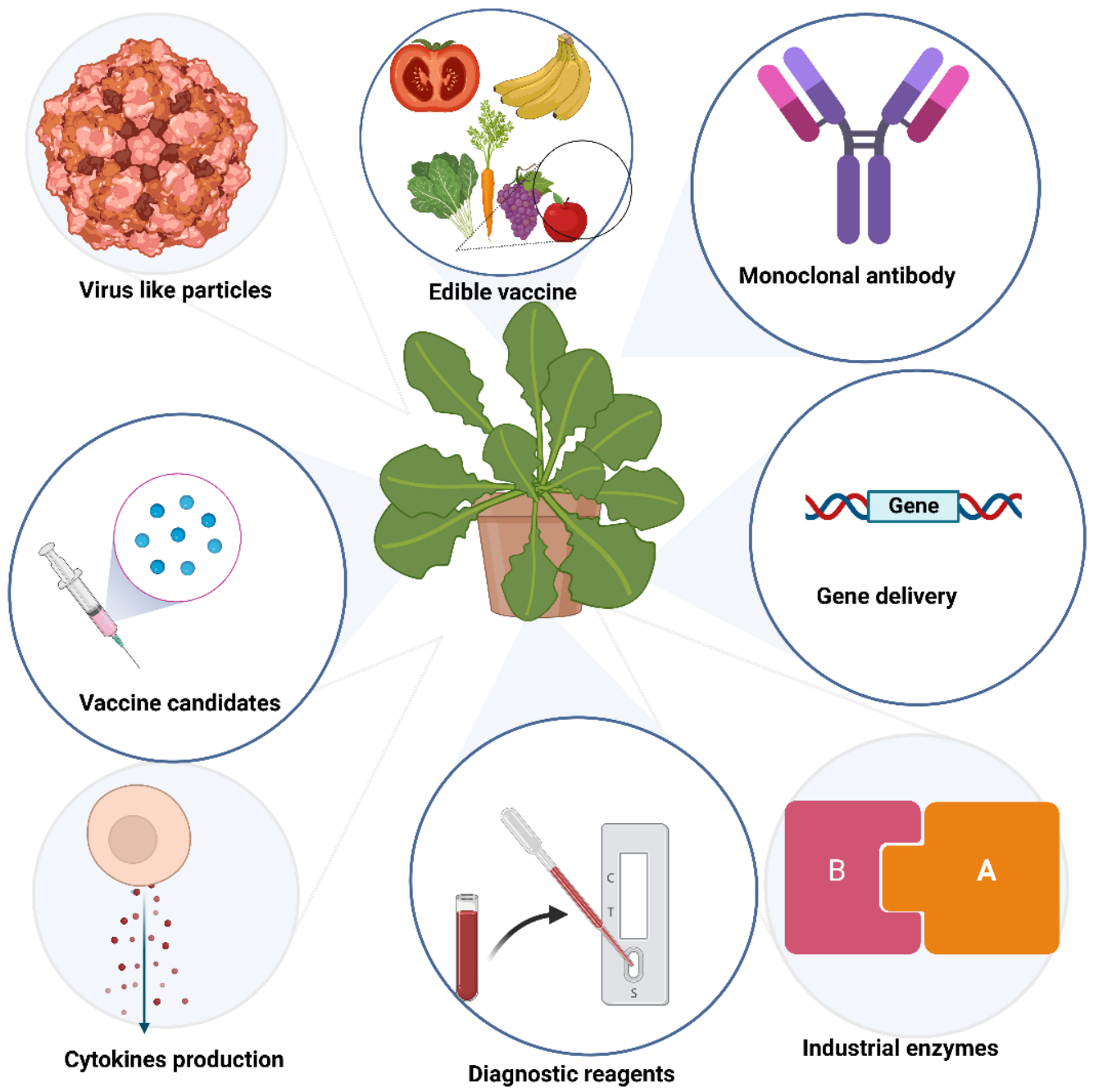
1. Introduction
Historical Review of Plant Molecular Farming
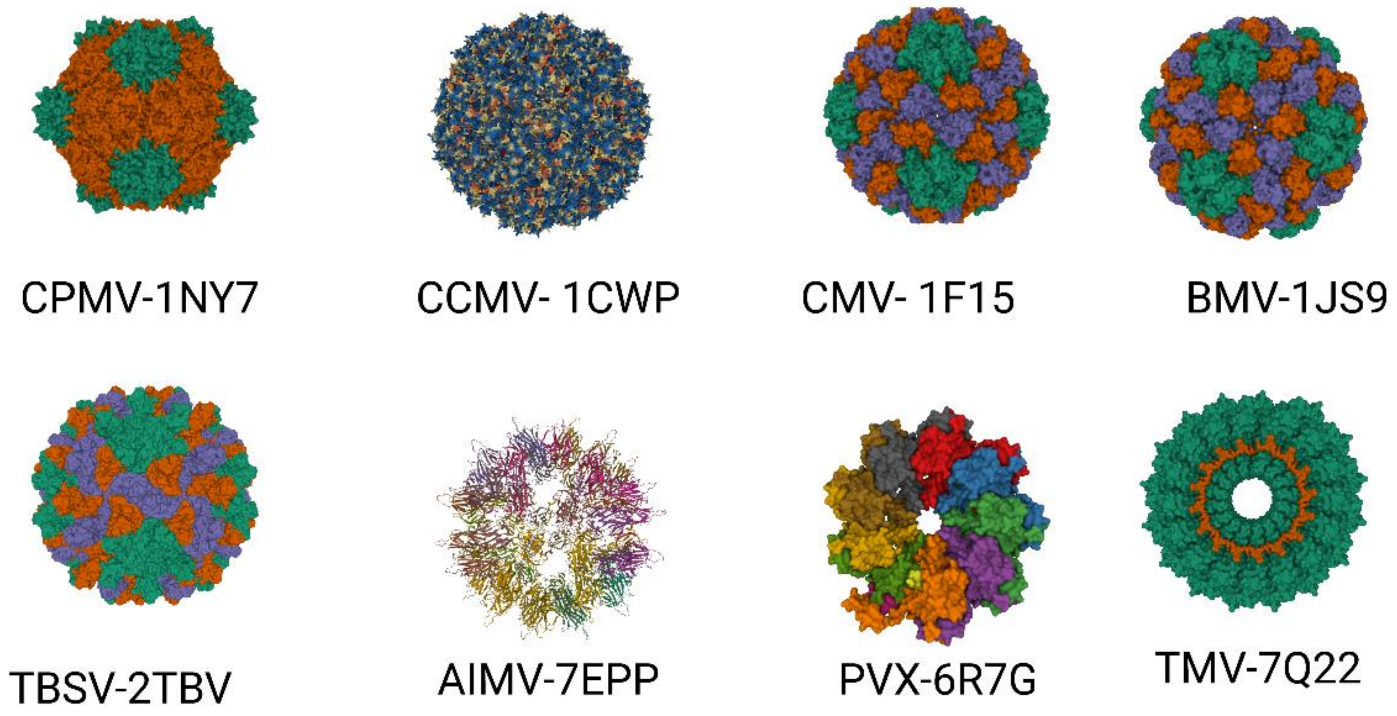
2. Important Plant Viruses for Plant Biotechnology
2.1. Tobacco Mosaic Virus (TMV)
2.2. Cowpea Mosaic Virus (CPMV)
2.3. Cowpea Chlorotic Mottle Virus (CCMV)
2.4. Brome Mosaic Virus (BMV)
3. Plant Viruses as a Source of Vectors for Transient Expression
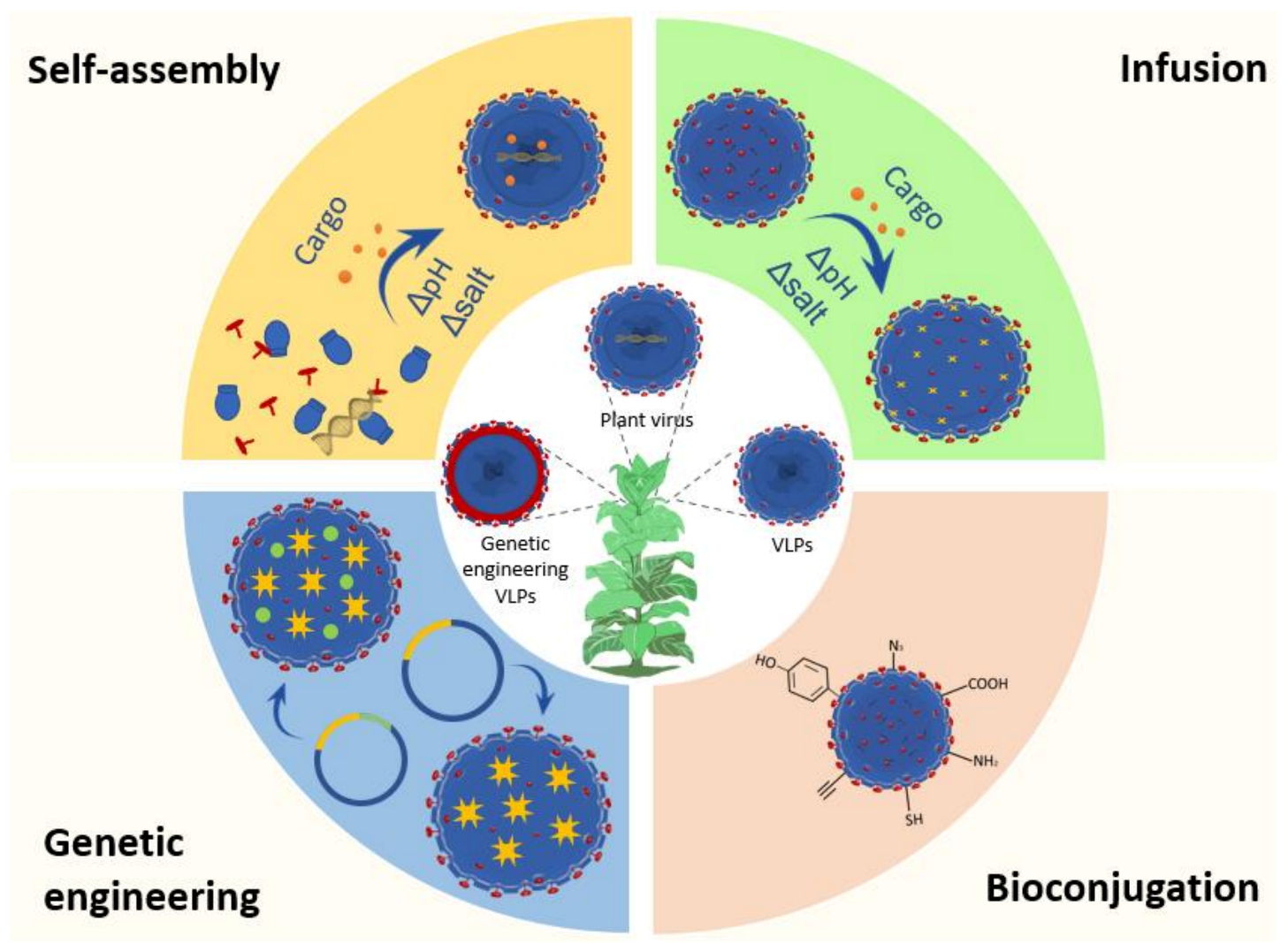
4. Functionalization Methods of VNPs
5. Application of Plant VNPs for Delivering Bioactive Cargos
5.1. Anticancer Drugs
5.2. Imaging Agents
5.3. Nucleic Acid
5.4. Non-Biological Synthetic Nanoparticles
5.5. Plant-Derived VNPs Loading Enzymes
5.6. Plant-Derived VNPs Decorated with Antibodies and Nanobodies
5.7. Plant Viruses and VNPs Used for Cancer Immunotherapy
5.8. Plant Viruses VLPs-Based Display Platform for Immunogenic Peptides
6. Methods of Application of Vaccines Produced in Plants
Can Plant-Based Oral Vaccines Help the One Health Approach?
7. Products, Market Size with Some Examples
8. Challenges in Developing Biopharmaceuticals in Plants
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steinmetz, N.F.; Lin, T.; Lomonossoff, G.P.; Johnson, J.E. Structure-Based Engineering of an Icosahedral Virus for Nanomedicine and Nanotechnology. In Viruses and Nanotech; Manchester, M., Steinmetz, N.F., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 23–58. ISBN 978-3-540-69379-6. [Google Scholar]
- Aljabali, A.A.A.; Shukla, S.; Lomonossoff, G.P.; Steinmetz, N.F.; Evans, D.J. CPMV-DOX Delivers. Mol. Pharm. 2013, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wen, H.; Wen, Q.; Chen, X.; Wang, Y.; Xuan, W.; Liang, J.; Wan, S. Cucumber Mosaic Virus as Drug Delivery Vehicle for Doxorubicin. Biomaterials 2013, 34, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Odon, V.; Kormelink, R. Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses. Front. Plant Sci. 2019, 10, 803. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Sanders, P.R.; Flick, J.S.; Adams, S.P.; Bittner, M.L.; Brand, L.A.; Fink, C.L.; Fry, J.S.; et al. Expression of Bacterial Genes in Plant Cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Witcher, D.R.; Hood, E.E.; Peterson, D.; Bailey, M.; Bond, D.; Kusnadi, A.; Evangelista, R.; Nikolov, Z.; Wooge, C.; Mehigh, R.; et al. Commercial Production of β-Glucuronidase (GUS): A Model System for the Production of Proteins in Plants. Mol. Breed. 1998, 4, 301–312. [Google Scholar] [CrossRef]
- Hood, E.E.; Witcher, D.R.; Maddock, S.; Meyer, T.; Baszczynski, C.; Bailey, M.; Flynn, P.; Register, J.; Marshall, L.; Bond, D.; et al. Commercial Production of Avidin from Transgenic Maize: Characterization of Transformant, Production, Processing, Extraction and Purification. Mol. Breed. 1997, 3, 291–306. [Google Scholar] [CrossRef]
- Woodard, S.L.; Mayor, J.M.; Bailey, M.R.; Barker, D.K.; Love, R.T.; Lane, J.R.; Delaney, D.E.; McComas-Wagner, J.M.; Mallubhotla, H.D.; Hood, E.E.; et al. Maize (Zea Mays)-Derived Bovine Trypsin: Characterization of the First Large-Scale, Commercial Protein Product from Transgenic Plants. Biotechnol. Appl. Biochem. 2003, 38, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J.M. The Expression of a Nopaline Synthase—Human Growth Hormone Chimaeric Gene in Transformed Tobacco and Sunflower Callus Tissue. Plant Mol Biol 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- During, K. Wound-Inducible Expression and Secretion of T4 Lysozyme and Monoclonal Antibodies in Nicotiana Tabacum. Ph.D. Thesis, Mathematisch-Naturwissenschaftlichen Fakultat der Universität zu Köln, Köln, Germany, 1988. [Google Scholar]
- Arntzen, C. Plant-made Pharmaceuticals: From ‘Edible Vaccines’ to Ebola Therapeutics. Plant Biotechnol. J. 2015, 13, 1013–1016. [Google Scholar] [CrossRef]
- Loza-Rubio, E.; Rojas, E.; Gómez, L.; Olivera, M.T.J.; Gómez-Lim, M.A. Development of an Edible Rabies Vaccine in Maize Using the Vnukovo Strain. Dev. Biol. 2008, 131, 477–482. [Google Scholar]
- Zahmanova, G.; Falzarano, D.; Naimov, S.; Kostova, M.; Boncheva, R.; Dukiandjiev, S.; Minkov, I.; Andonov, A. Oral immunization with truncated hepatitis B virus nucleocapsid expressed in transgenic potatoes. Comptes Rendus L’acade’mie Bulg. Des Sci. 2008, 61, 1293–1300. [Google Scholar]
- Arntzen, C.; Plotkin, S.; Dodet, B. Plant-Derived Vaccines and Antibodies: Potential and Limitations. Vaccine 2005, 23, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Noncompliance History. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/compliance-and-inspections/CT_Compliance_history (accessed on 8 December 2022).
- Rybicki, E.P. Plant-Made Vaccines for Humans and Animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-Produced Vaccines: Promise and Reality. Drug Discov. Today 2009, 14, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Mazalovska, M.; Toneva, V.; Minkov, I.; Lomonossoff, G. Production of Chimeric Virus-like Particles Bearing M2e Influenza Epitope in Nicotiana Benthamiana Plants. J. Biotechnol. 2015, 208, S109. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Kotlyarov, R.Y.; Stuchinskaya, M.D.; Nikolaeva, L.I.; Zahmanova, G.; Ravin, N.V. High-Yield Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope and Receptor Binding Domain of SARS-CoV-2 in Plants Using Viral Vectors. Int. J. Mol. Sci. 2022, 23, 15684. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 Spike Protein and Its Receptor Binding Domain in Plant Cell Suspension Cultures. Front. Plant Sci. 2022, 13, 995429. [Google Scholar] [CrossRef]
- Jung, J.-W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-Based Expression and Characterization of SARS-CoV-2 Virus-like Particles Presenting a Native Spike Protein. Plant Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Kopertekh, L.; Schiemann, J. Transient Production of Recombinant Pharmaceutical Proteins in Plants: Evolution and Perspectives. Curr. Med. Chem. 2019, 26, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Vermij, P.; Waltz, E. USDA Approves the First Plant-Based Vaccine. Nat. Biotechnol. 2006, 24, 233–234. [Google Scholar]
- Maxmen, A. Drug-Making Plant Blooms. Nature 2012, 485, 160. [Google Scholar] [CrossRef] [PubMed]
- Medicago Inc Medicago and GSK Announce the Approval by Health Canada of COVIFENZ®, an Adjuvanted Plant-Based COVID-19 Vaccine. Available online: https://medicago.com/en/press-release/covifenz/ (accessed on 26 February 2022).
- Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, Immunogenicity, and Safety of a Plant-Derived, Quadrivalent, Virus-like Particle Influenza Vaccine in Adults (18–64 Years) and Older Adults (≥65 Years): Two Multicentre, Randomised Phase 3 Trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Roossinck, M.J. Plant Virus Ecology. PLoS Pathog. 2013, 9, e1003304. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Balke, I.; Zeltins, A. Use of Plant Viruses and Virus-like Particles for the Creation of Novel Vaccines. Adv. Drug Deliv. Rev. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The Use of Tobacco Mosaic Virus and Cowpea Mosaic Virus for the Production of Novel Metal Nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular Pharming—VLPs Made in Plants. Curr Opin Biotechnol 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Evans, D.J. The Bionanoscience of Plant Viruses: Templates and Synthons for New Materials. J. Mater. Chem. 2008, 18, 3746–3754. [Google Scholar] [CrossRef]
- Sainsbury, F.; Lomonossoff, G.P. Transient Expressions of Synthetic Biology in Plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.K.; Orlova, E.V. 4.6Å Cryo-EM Reconstruction of Tobacco Mosaic Virus from Images Recorded at 300keV on a 4k×4k CCD Camera. J. Struct. Biol. 2010, 171, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Schramm, G.; Schumacher, G.; Zillig, W. An Infectious Nucleoprotein from Tobacco Mosaic Virus. Nature 1955, 175, 549–550. [Google Scholar] [CrossRef]
- Harrison, B.D.; Wilson, T.M.A.; Butler, P.J.G. Self–Assembly of Tobacco Mosaic Virus: The Role of an Intermediate Aggregate in Generating Both Specificity and Speed. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1999, 354, 537–550. [Google Scholar] [CrossRef]
- Okada, Y. Molecular Assembly of Tobacco Mosaic Virus in Vitro. Adv. Biophys. 1986, 22, 95–149. [Google Scholar] [CrossRef] [PubMed]
- Shire, S.J.; Steckert, J.J.; Adams, M.L.; Schuster, T.M. Kinetics and Mechanism of Tobacco Mosaic Virus Assembly: Direct Measurement of Relative Rates of Incorporation of 4S and 20S Protein. Proc. Natl. Acad. Sci. USA 1979, 76, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Kegel, W.K.; van der Schoot, P. Physical Regulation of the Self-Assembly of Tobacco Mosaic Virus Coat Protein. Biophys. J. 2006, 91, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Pitek, A.S.; Hu, H.; Shukla, S.; Steinmetz, N.F. Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 39468–39477. [Google Scholar] [CrossRef]
- Bruckman, M.A.; Steinmetz, N.F. Chemical Modification of the Inner and Outer Surfaces of Tobacco Mosaic Virus (TMV). Methods Mol. Biol. 2014, 1108, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Hern, S.; Jiang, K.; Flask, C.A.; Yu, X.; Steinmetz, N.F. Tobacco Mosaic Virus Rods and Spheres as Supramolecular High-Relaxivity MRI Contrast Agents. J. Mater. Chem. B 2013, 1, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Czapar, A.E.; VanMeter, A.; Randolph, L.N.; Steinmetz, N.F. Tobacco Mosaic Virus-Based Protein Nanoparticles and Nanorods for Chemotherapy Delivery Targeting Breast Cancer. J. Control. Release 2016, 231, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kernan, D.L.; Wen, A.M.; Pitek, A.S.; Steinmetz, N.F. Featured Article: Delivery of Chemotherapeutic VcMMAE Using Tobacco Mosaic Virus Nanoparticles. Exp. Biol. Med. 2017, 242, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Carpenter, B.L.; Wen, A.M.; Ghiladi, R.A.; Steinmetz, N.F. High Aspect Ratio Nanotubes Formed by Tobacco Mosaic Virus for Delivery of Photodynamic Agents Targeting Melanoma. ACS Biomater. Sci. Eng. 2016, 2, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, N.; Ishikawa, M.; Meshi, T.; Okada, Y. Expression of Bacterial Chloramphenicol Acetyltransferase Gene in Tobacco Plants Mediated by TMV-RNA. EMBO J. 1987, 6, 307–311. [Google Scholar] [CrossRef]
- Lomonossoff, G.P. Cowpea Mosaic Virus. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 569–574. ISBN 978-0-12-374410-4. [Google Scholar]
- Lomonossoff, G.P.; Johnson, J.E. The Synthesis and Structure of Comovirus Capsids. Prog. Biophys. Mol. Biol. 1991, 55, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, Z.; Usha, R.; Stauffacher, C.V.; Dai, J.B.; Schmidt, T.; Johnson, J.E. The Refined Crystal Structure of Cowpea Mosaic Virus at 2.8 A Resolution. Virology 1999, 265, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Cañizares, M.C.; Lomonossoff, G.P. Cowpea Mosaic Virus: The Plant Virus–Based Biotechnology Workhorse. Annu. Rev. Phytopathol. 2010, 48, 437–455. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Barclay, J.E.; Steinmetz, N.F.; Lomonossoff, G.P.; Evans, D.J. Controlled Immobilisation of Active Enzymes on the Cowpea Mosaic Virus Capsid. Nanoscale 2012, 4, 5640–5645. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Barclay, J.E.; Lomonossoff, G.P.; Evans, D.J. Virus Templated Metallic Nanoparticles. Nanoscale 2010, 2, 2596–2600. [Google Scholar] [CrossRef]
- Evans, D.J. Bionanoscience at the Plant Virus–Inorganic Chemistry Interface. Inorg. Chim. Acta 2010, 363, 1070–1076. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Evans, D.J. Utilisation of Plant Viruses in Bionanotechnology. Org. Biomol. Chem. 2007, 5, 2891–2902. [Google Scholar] [CrossRef]
- Porta, C.; Spall, V.E.; Loveland, J.; Johnson, J.E.; Barker, P.J.; Lomonossoff, G.P. Development of Cowpea Mosaic Virus as a High-Yielding System for the Presentation of Foreign Peptides. Virology 1994, 202, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, K.; Uttenthal, Å.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.O.; Langeveld, J.P.M.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant–Derived Vaccine Protects Target Animals against a Viral Disease. Nat. Biotechnol. 1997, 15, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Montague, N.P.; Thuenemann, E.C.; Saxena, P.; Saunders, K.; Lenzi, P.; Lomonossoff, G.P. Recent Advances of Cowpea Mosaic Virus-Based Particle Technology. Hum. Vaccines 2011, 7, 383–390. [Google Scholar] [CrossRef]
- Saunders, K.; Sainsbury, F.; Lomonossoff, G.P. Efficient Generation of Cowpea Mosaicvirus Empty Virus-like Particles by the Proteolytic Processing of Precursors in Insect Cells and Plants. Virology 2009, 393, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, E.L.; Meshcheriakova, Y.; Thompson, R.F.; Lomonossoff, G.P.; Ranson, N.A. The Structures of a Naturally Empty Cowpea Mosaic Virus Particle and Its Genome-Containing Counterpart by Cryo-Electron Microscopy. Sci. Rep. 2017, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Hesketh, E.L.; Saxena, P.; Meshcheriakova, Y.; Ku, Y.-C.; Hoang, L.T.; Johnson, J.E.; Ranson, N.A.; Lomonossoff, G.P.; Reddy, V.S. Crystal Structure and Proteomics Analysis of Empty Virus-like Particles of Cowpea Mosaic Virus. Structure 2016, 24, 567–575. [Google Scholar] [CrossRef]
- Wang, Q.; Kaltgrad, E.; Lin, T.; Johnson, J.E.; Finn, M.G. Natural Supramolecular Building Blocks. Wild-Type Cowpea Mosaic Virus. Chem. Biol. 2002, 9, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Tiu, B.D.B.; Advincula, R.C.; Steinmetz, N.F. Nanomanufacture of Free-Standing, Porous, Janus-Type Films of Polymer-Plant Virus Nanoparticle Arrays. Methods Mol. Biol. 2018, 1776, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kruse, I.; Peyret, H.; Saxena, P.; Lomonossoff, G.P. Encapsidation of Viral RNA in Picornavirales: Studies on Cowpea Mosaic Virus Demonstrate Dependence on Viral Replication. J. Virol. 2019, 93, e01520-18. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. When Plant Virology Met Agrobacterium: The Rise of the Deconstructed Clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Caspar, D.L.; Klug, A. Physical Principles in the Construction of Regular Viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24. [Google Scholar] [CrossRef]
- Douglas, T.; Young, M. Host–Guest Encapsulation of Materials by Assembled Virus Protein Cages. Nature 1998, 393, 152–155. [Google Scholar] [CrossRef]
- Wilts, B.D.; Schaap, I.A.T.; Schmidt, C.F. Swelling and Softening of the Cowpea Chlorotic Mottle Virus in Response to PH Shifts. Biophys. J. 2015, 108, 2541–2549. [Google Scholar] [CrossRef]
- Hema, M.; Vishnu Vardhan, G.P.; Savithri, H.S.; Murthy, M.R.N. Chapter 6—Emerging Trends in the Development of Plant Virus-Based Nanoparticles and Their Biomedical Applications. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 61–82. ISBN 978-0-12-816328-3. [Google Scholar]
- Minten, I.J.; Ma, Y.; Hempenius, M.A.; Vancso, G.J.; Nolte, R.J.M.; Cornelissen, J.J.L.M. CCMV Capsid Formation Induced by a Functional Negatively Charged Polymer. Org. Biomol. Chem. 2009, 7, 4685–4688. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; Evans, D.J. Applications of Plant Viruses in Bionanotechnology. In Plant Viral Vectors; Palmer, K., Gleba, Y., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 61–87. ISBN 978-3-642-40829-8. [Google Scholar]
- Young, M.; Debbie, W.; Uchida, M.; Douglas, T. Plant Viruses as Biotemplates for Materials and Their Use in Nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384. [Google Scholar] [CrossRef]
- Wen, A.M.; Lee, K.L.; Cao, P.; Pangilinan, K.; Carpenter, B.L.; Lam, P.; Veliz, F.A.; Ghiladi, R.A.; Advincula, R.C.; Steinmetz, N.F. Utilizing Viral Nanoparticle/Dendron Hybrid Conjugates in Photodynamic Therapy for Dual Delivery to Macrophages and Cancer Cells. Bioconjug. Chem. 2016, 27, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Han, S.S. Icosahedral Plant Viral Nanoparticles—Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Colloid Interface Sci. 2017, 248, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H.V.; Fauquet, C.M.; Bishop, D.H.L.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Classification and Nomenclature of Viruses. In Virus Taxonomy Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Lucas, R.W.; Larson, S.B.; McPherson, A. The Crystallographic Structure of Brome Mosaic Virus, Edited by I. A. Wilson. J. Mol. Biol. 2002, 317, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Cuillel, M.; Zulauf, M.; Jacrot, B. Self-Assembly of Brome Mosaic Virus Protein into Capsids: Initial and Final States of Aggregation. J. Mol. Biol. 1983, 164, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Incardona, N.L.; Kaesberg, P. A PH-Induced Structural Change in Bromegrass Mosaic Virus. Biophys. J. 1964, 4, 11–21. [Google Scholar] [CrossRef]
- Porta, C.; Lomonossoff, G.P. Viruses as Vectors for the Expression of Foreign Sequences in Plants. Biotechnol. Genet. Eng. Rev. 2002, 19, 245–292. [Google Scholar] [CrossRef]
- Howell, S.H.; Walker, L.L.; Dudley, R.K. Cloned Cauliflower Mosaic Virus DNA Infects Turnips (Brassica Rapa). Science 1980, 208, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Lico, C.; Chen, Q.; Santi, L. Viral Vectors for Production of Recombinant Proteins in Plants. J. Cell. Physiol. 2008, 216, 366–377. [Google Scholar] [CrossRef]
- Grimsley, N.; Hohn, B.; Hohn, T.; Walden, R. “Agroinfection,” an Alternative Route for Viral Infection of Plants by Using the Ti Plasmid. Proc. Natl. Acad. Sci. USA 1986, 83, 3282–3286. [Google Scholar] [CrossRef]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A New Platform for Expressing Recombinant Vaccines in Plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel PEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef]
- Sainsbury, F.; Lavoie, P.-O.; D’Aoust, M.-A.; Vézina, L.-P.; Lomonossoff, G.P. Expression of Multiple Proteins Using Full-Length and Deleted Versions of Cowpea Mosaic Virus RNA-2. Plant Biotechnol. J. 2008, 6, 82–92. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, Y.; Liu, G.; Wei, Y.; Hu, W.; Shi, H. Agrobacterium-Mediated Gene Transient Overexpression and Tobacco Rattle Virus (TRV)-Based Gene Silencing in Cassava. Int. J. Mol. Sci. 2019, 20, 3976. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Hammond-Kosack, K.E.; Kanyuka, K. Barley Stripe Mosaic Virus-Mediated Tools for Investigating Gene Function in Cereal Plants and Their Pathogens: Virus-Induced Gene Silencing, Host-Mediated Gene Silencing, and Virus-Mediated Overexpression of Heterologous Protein. Plant Physiol. 2012, 160, 582–590. [Google Scholar] [CrossRef]
- Marillonnet, S.; Giritch, A.; Gils, M.; Kandzia, R.; Klimyuk, V.; Gleba, Y. In Planta Engineering of Viral RNA Replicons: Efficient Assembly by Recombination of DNA Modules Delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Lin, S.-S.; Hung, T.-H.; Li, T.-K.; Lin, N.-C.; Shen, T.-L. Multiple Domains of the Tobacco Mosaic Virus P126 Protein Can Independently Suppress Local and Systemic RNA Silencing. Mol. Plant Microbe Interact. 2012, 25, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kearney, C.M. An Efficient Foxtail Mosaic Virus Vector System with Reduced Environmental Risk. BMC Biotechnol. 2010, 10, 88. [Google Scholar] [CrossRef]
- Dugdale, B.; Mortimer, C.L.; Kato, M.; James, T.A.; Harding, R.M.; Dale, J.L. In Plant Activation: An Inducible, Hyperexpression Platform for Recombinant Protein Production in Plants. Plant Cell 2013, 25, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, J.; Phoolcharoen, W.; Mason, H.S. Geminiviral Vectors Based on Bean Yellow Dwarf Virus for Production of Vaccine Antigens and Monoclonal Antibodies in Plants. Hum. Vaccines 2011, 7, 331–338. [Google Scholar] [CrossRef]
- Regnard, G.L.; Halley-Stott, R.P.; Tanzer, F.L.; Hitzeroth, I.I.; Rybicki, E.P. High Level Protein Expression in Plants through the Use of a Novel Autonomously Replicating Geminivirus Shuttle Vector. Plant Biotechnol. J. 2010, 8, 38–46. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, S.A.; Popovich, A.H. Efficient Expression of Foreign Proteins in Roots from Tobravirus Vectors. Virology 2000, 267, 29–35. [Google Scholar] [CrossRef]
- Cañizares, M.C.; Liu, L.; Perrin, Y.; Tsakiris, E.; Lomonossoff, G.P. A Bipartite System for the Constitutive and Inducible Expression of High Levels of Foreign Proteins in Plants. Plant Biotechnol. J. 2006, 4, 183–193. [Google Scholar] [CrossRef]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. PEAQ: Versatile Expression Vectors for Easy and Quick Transient Expression of Heterologous Proteins in Plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K. Plant Virus Expression Vectors: A Powerhouse for Global Health. Biomedicines 2017, 5, 44. [Google Scholar] [CrossRef]
- Chapman, S.; Kavanagh, T.; Baulcombe, D. Potato Virus X as a Vector for Gene Expression in Plants. Plant J. 1992, 2, 549–557. [Google Scholar] [CrossRef]
- Harrison, B.D.; Wilson, T.M.A.; Turpen, T.H. Tobacco Mosaic Virus and the Virescence of Biotechnology. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1999, 354, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Lin, T.; Lomonossoff, G. PRESENTATION OF HETEROLOGOUS PEPTIDES ON PLANT VIRUSES: Genetics, Structure, and Function. Annu. Rev. Phytopathol. 1997, 35, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Lomonossoff, G.P. Scope for Using Plant Viruses to Present Epitopes from Animal Pathogens. Rev. Med. Virol. 1998, 8, 25–41. [Google Scholar] [CrossRef]
- Gleba, Y.; Marillonnet, S.; Klimyuk, V. Engineering Viral Expression Vectors for Plants: The “full Virus” and the “Deconstructed Virus” Strategies. Curr. Opin. Plant Biol. 2004, 7, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, K.; Dent, M.; Hurtado, J.; Stahnke, J.; Lai, H.; Zhou, X.; Chen, Q. Efficient Agroinfiltration of Plants for High-Level Transient Expression of Recombinant Proteins. J. Vis. Exp. 2013, 23, 50521. [Google Scholar] [CrossRef] [PubMed]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of Recombinant Antigens and Antibodies in Nicotiana Benthamiana Using ‘Magnifection’ Technology: GMP-Compliant Facilities for Small-and Large-Scale Manufacturing. In Plant Viral Vectors; Palmer, K., Gleba, Y., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–154. ISBN 978-3-642-40829-8. [Google Scholar]
- Mardanova, E.S.; Takova, K.H.; Toneva, V.T.; Zahmanova, G.G.; Tsybalova, L.M.; Ravin, N.V. A Plant-Based Transient Expression System for the Rapid Production of Highly Immunogenic Hepatitis E Virus-like Particles. Biotechnol. Lett. 2020, 42, 2441–2446. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Ravin, N.V. Chapter Ten—Transient Expression of Recombinant Proteins in Plants Using Potato Virus X Based Vectors. In Recombinant Protein Expression: Eukaryotic Hosts; O’Dell, W.B., Kelman, Z., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 660, pp. 205–222. [Google Scholar]
- Takova, K.; Koynarski, T.; Minkov, G.; Toneva, V.; Mardanova, E.; Ravin, N.; Lukov, G.L.; Zahmanova, G. Development and Optimization of an Enzyme Immunoassay to Detect Serum Antibodies against the Hepatitis E Virus in Pigs, Using Plant-Derived ORF2 Recombinant Protein. Vaccines 2021, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana Benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Lenzi, P.; Love, A.J.; Taliansky, M.; Bécares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.G.; Minkov, I.N.; Matić, S.; et al. The Use of Transient Expression Systems for the Rapid Production of Virus-like Particles in Plants. Curr. Pharm. Des. 2013, 19, 5564–5573. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Mazalovska, M.; Takova, K.; Toneva, V.; Minkov, I.; Peyret, H.; Lomonossoff, G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana Benthamiana. Life 2021, 11, 64. [Google Scholar] [CrossRef]
- Zahmanova, G.; Naimov, S.; Mazalovska, M.; Valkova, R.; Minkov, I. Transient Expression of Modified Hepatitis B Capsid Protein in Nicotiana Benthamiana Plants for Viral Nanoparticles Production. J. BioSci. Biotechnol. 2014, 3, SE/ONLINE: 11–16. [Google Scholar]
- Lindbo, J.A. TRBO: A High-Efficiency Tobacco Mosaic Virus RNA-Based Overexpression Vector. Plant Physiol. 2007, 145, 1232–1240. [Google Scholar] [CrossRef]
- Minten, I.J.; Hendriks, L.J.A.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Controlled Encapsulation of Multiple Proteins in Virus Capsids. J. Am. Chem. Soc. 2009, 131, 17771–17773. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meshcheriakova, Y.; Lomonossoff, G.P. Synthetic Plant Virology for Nanobiotechnology and Nanomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1447. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, K.; Wang, Q. Tailoring the Self-Assembly Behaviors of Recombinant Tobacco Mosaic Virus by Rationally Introducing Covalent Bonding at the Protein–Protein Interface. Small 2016, 12, 4955–4959. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, L.; Michel, J.-P.; Gingery, M. The Disassembly, Reassembly and Stability of CCMV Protein Capsids. J. Virol. Methods 2007, 146, 311–316. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.; Zhang, W.; Secundo, F.; Li, C.; Zhang, Z.-P.; Zhang, X.-E.; Li, F. Cargo-Compatible Encapsulation in Virus-Based Nanoparticles. Nano Lett. 2019, 19, 2700–2706. [Google Scholar] [CrossRef]
- Miao, Y.; Johnson, J.E.; Ortoleva, P.J. All-Atom Multiscale Simulation of Cowpea Chlorotic Mottle Virus Capsid Swelling. J. Phys. Chem. B 2010, 114, 11181–11195. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.; Guenther, R.H.; Lommel, S.A.; Franzen, S. Infusion of Dye Molecules into Red Clover Necrotic Mosaic Virus. Chem. Commun. 2008, 1, 88–90. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Alzoubi, L.; Hamzat, Y.; Alqudah, A.; Obeid, M.A.; Al Zoubi, M.S.; Ennab, R.M.; Alshaer, W.; Albatayneh, K.; Al-Trad, B.; et al. A Potential MRI Agent and an Anticancer Drug Encapsulated within CPMV Virus-Like Particles. Comb. Chem. High Throughput Screen. 2021, 24, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Hassan, S.S.; Pabari, R.M.; Shahcheraghi, S.H.; Mishra, V.; Charbe, N.B.; Chellappan, D.K.; Dureja, H.; Gupta, G.; Almutary, A.G.; et al. The Viral Capsid as Novel Nanomaterials for Drug Delivery. Future Sci. OA 2021, 7, FSO744. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Guenther, R.H.; Sit, T.L.; Opperman, C.H.; Lommel, S.A.; Willoughby, J.A. Loading and Release Mechanism of Red Clover Necrotic Mosaic Virus Derived Plant Viral Nanoparticles for Drug Delivery of Doxorubicin. Small 2014, 10, 5126–5136. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.B.; Guenther, R.H.; Tama, F.; Sit, T.L.; Brooks, C.L.; Mikhailov, A.M.; Orlova, E.V.; Baker, T.S.; Lommel, S.A. Removal of Divalent Cations Induces Structural Transitions in Red Clover Necrotic Mosaic Virus, Revealing a Potential Mechanism for RNA Release. J. Virol. 2006, 80, 10395–10406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Zhou, J.; Li, X.; Wang, F. Application of Plant Viruses as a Biotemplate for Nanomaterial Fabrication. Molecules 2018, 23, 2311. [Google Scholar] [CrossRef]
- Arcangeli, C.; Circelli, P.; Donini, M.; Aljabali, A.A.A.; Benvenuto, E.; Lomonossoff, G.P.; Marusic, C. Structure-Based Design and Experimental Engineering of a Plant Virus Nanoparticle for the Presentation of Immunogenic Epitopes and as a Drug Carrier. J. Biomol. Struct. Dyn. 2014, 32, 630–647. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Izadpanah, K.; Ahmadi, F. Generation of Recombinant Protein Shells of Johnson Grass Chlorotic Stripe Mosaic Virus in Tobacco Plants and Their Use as Drug Carrier. J. Virol. Methods 2017, 248, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Manchester, M. Viral Nanoparticles: Tools for Materials Science & Biomedicine; Jenny Stanford Publishing: New York, NY, USA, 2019; ISBN 978-0-429-06745-7. [Google Scholar]
- Esfandiari, N.; Arzanani, M.K.; Soleimani, M.; Kohi-Habibi, M.; Svendsen, W.E. A New Application of Plant Virus Nanoparticles as Drug Delivery in Breast Cancer. Tumour Biol. 2016, 37, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Masarapu, H.; Patel, B.K.; Chariou, P.L.; Hu, H.; Gulati, N.M.; Carpenter, B.L.; Ghiladi, R.A.; Shukla, S.; Steinmetz, N.F. Physalis Mottle Virus-Like Particles as Nanocarriers for Imaging Reagents and Drugs. Biomacromolecules 2017, 18, 4141–4153. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Perez, S.A.; Papamichail, M. Combinatorial Treatments Including Vaccines, Chemotherapy and Monoclonal Antibodies for Cancer Therapy. Cancer Immunol. Immunother. 2009, 58, 317–324. [Google Scholar] [CrossRef]
- Lockney, D.M.; Guenther, R.N.; Loo, L.; Overton, W.; Antonelli, R.; Clark, J.; Hu, M.; Luft, C.; Lommel, S.A.; Franzen, S. The Red Clover Necrotic Mosaic Virus Capsid as a Multifunctional Cell Targeting Plant Viral Nanoparticle. Bioconjug. Chem. 2011, 22, 67–73. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Shi, H.; Gao, S.; Xie, G.; Zhu, M.; Wu, M.; Chen, J.; Niu, Z. Integration of Cell-Penetrating Peptides with Rod-like Bionanoparticles: Virus-Inspired Gene-Silencing Technology. Nano Lett. 2018, 18, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, F.; Tian, Y.; Wu, M.; Zhou, Q.; Jiang, S.; Niu, Z. Size Dependent Cellular Uptake of Rod-like Bionanoparticles with Different Aspect Ratios. Sci. Rep. 2016, 6, 24567. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.D.; Steinmetz, N.F. Tobacco Mosaic Virus Delivery of Mitoxantrone for Cancer Therapy. Nanoscale 2018, 10, 16307–16313. [Google Scholar] [CrossRef]
- Le, D.H.T.; Commandeur, U.; Steinmetz, N.F. Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Rudin, M.; Weissleder, R. Molecular Imaging in Drug Discovery and Development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef]
- Jung, B.; Rao, A.L.N.; Anvari, B. Optical Nano-Constructs Composed of Genome-Depleted Brome Mosaic Virus Doped with a near Infrared Chromophore for Potential Biomedical Applications. ACS Nano 2011, 5, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Röder, J.; Dickmeis, C.; Fischer, R.; Commandeur, U. Systemic Infection of Nicotiana Benthamiana with Potato Virus X Nanoparticles Presenting a Fluorescent ILOV Polypeptide Fused Directly to the Coat Protein. BioMed Res. Int. 2018, 2018, 9328671. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Dickmeis, C.; Nagarajan, A.S.; Fischer, R.; Commandeur, U.; Steinmetz, N.F. Molecular Farming of Fluorescent Virus-Based Nanoparticles for Optical Imaging in Plants, Human Cells and Mouse Models. Biomater. Sci. 2014, 2, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Jiang, K.; Simpson, E.J.; Randolph, L.N.; Luyt, L.G.; Yu, X.; Steinmetz, N.F. Dual-Modal Magnetic Resonance and Fluorescence Imaging of Atherosclerotic Plaques in Vivo Using VCAM-1 Targeted Tobacco Mosaic Virus. Nano Lett. 2014, 14, 1551–1558. [Google Scholar] [CrossRef]
- Lewis, J.D.; Destito, G.; Zijlstra, A.; Gonzalez, M.J.; Quigley, J.P.; Manchester, M.; Stuhlmann, H. Viral Nanoparticles as Tools for Intravital Vascular Imaging. Nat. Med. 2006, 12, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Stein, B.D.; Cheng, H.; Malyutin, A.; Tsvetkova, I.B.; Baxter, D.V.; Remmes, N.B.; Verchot, J.; Kao, C.; Bronstein, L.M.; et al. Magnetic Virus-like Nanoparticles in N. Benthamiana Plants: A New Paradigm for Environmental and Agronomic Biotechnological Research. ACS Nano 2011, 5, 4037–4045. [Google Scholar] [CrossRef]
- Davidson, B.L.; McCray, P.B. Current Prospects for RNA Interference-Based Therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Luly, K.M.; Tzeng, S.Y.; Green, J.J. Nanoparticle Designs for Delivery of Nucleic Acid Therapeutics as Brain Cancer Therapies. Adv. Drug Deliv. Rev. 2021, 179, 113999. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Small Interfering RNA (SiRNA) Based Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, X.-Y.; Lu, A.; Wang, X.-Y.; Jiang, L.-X.; Wang, J.-C. Non-Viral Vectors for RNA Delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional Oncogenesis in 4 Patients after Retrovirus-Mediated Gene Therapy of SCID-X1. J. Clin. Invest. 2008, 118, 3132–3142. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Hunt, K.K. Adenoviral Gene Therapy. Oncologist 2002, 7, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The Influence of Innate and Pre-Existing Immunity on Adenovirus Therapy. J. Cell. Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, N.F. Viral Nanoparticles as Platforms for Next-Generation Therapeutics and Imaging Devices. Nanomedicine 2010, 6, 634–641. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Dehshahri, A.; Izadpanah, K.; Ahmadi, F. Plant Virus Nanoparticles: Novel and Robust Nanocarriers for Drug Delivery and Imaging. Colloids Surf. B Biointerfaces 2018, 167, 20–27. [Google Scholar] [CrossRef]
- Cai, H.; Shukla, S.; Steinmetz, N.F. The Antitumor Efficacy of CpG Oligonucleotides Is Improved by Encapsulation in Plant Virus-Like Particles. Adv. Funct. Mater. 2020, 30, 1908743. [Google Scholar] [CrossRef]
- Peyret, H.; Groppelli, E.; Clark, D.; Eckersley, N.; Planche, T.; Ma, J.; Lomonossoff, G.P. Production and Use of Encapsidated RNA Mimics as Positive Control Reagents for SARS-CoV-2 RT-QPCR Diagnostics. J. Virol. Methods 2022, 300, 114372. [Google Scholar] [CrossRef] [PubMed]
- Azizgolshani, O.; Garmann, R.F.; Cadena-Nava, R.; Knobler, C.M.; Gelbart, W.M. Reconstituted Plant Viral Capsids Can Release Genes to Mammalian Cells. Virology 2013, 441, 12–17. [Google Scholar] [CrossRef]
- Villagrana-Escareño, M.V.; Reynaga-Hernández, E.; Galicia-Cruz, O.G.; Durán-Meza, A.L.; De la Cruz-González, V.; Hernández-Carballo, C.Y.; Ruíz-García, J. VLPs Derived from the CCMV Plant Virus Can Directly Transfect and Deliver Heterologous Genes for Translation into Mammalian Cells. BioMed Res. Int. 2019, 2019, e4630891. [Google Scholar] [CrossRef]
- Lam, P.; Steinmetz, N.F. Delivery of SiRNA Therapeutics Using Cowpea Chlorotic Mottle Virus-like Particles. Biomater. Sci. 2019, 7, 3138–3142. [Google Scholar] [CrossRef]
- Biddlecome, A.; Habte, H.H.; McGrath, K.M.; Sambanthamoorthy, S.; Wurm, M.; Sykora, M.M.; Knobler, C.M.; Lorenz, I.C.; Lasaro, M.; Elbers, K.; et al. Delivery of Self-Amplifying RNA Vaccines in in Vitro Reconstituted Virus-like Particles. PLoS ONE 2019, 14, e0215031. [Google Scholar] [CrossRef]
- Lam, P.; Gulati, N.M.; Stewart, P.L.; Keri, R.A.; Steinmetz, N.F. Bioengineering of Tobacco Mosaic Virus to Create a Non-Infectious Positive Control for Ebola Diagnostic Assays. Sci. Rep. 2016, 6, 23803. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Xiao, R.; Zhu, G.; Li, Y.; Liu, C.; Yang, R.; Tang, Z.; Li, J.; Huang, W.; et al. The Invasion of Tobacco Mosaic Virus RNA Induces Endoplasmic Reticulum Stress-Related Autophagy in HeLa Cells. Biosci. Rep. 2012, 32, 171–184. [Google Scholar] [CrossRef]
- Loo, L.; Guenther, R.H.; Lommel, S.A.; Franzen, S. Encapsidation of Nanoparticles by Red Clover Necrotic Mosaic Virus. J. Am. Chem. Soc. 2007, 129, 11111–11117. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, H.; Shin, H.-J. Encapsulation and Crystallization of Prussian Blue Nanoparticles by Cowpea Chlorotic Mottle Virus Capsids. Biotechnol. Lett. 2014, 36, 515–521. [Google Scholar] [CrossRef]
- Basu, G.; Allen, M.; Willits, D.; Young, M.; Douglas, T. Metal Binding to Cowpea Chlorotic Mottle Virus Using Terbium(III) Fluorescence. J. Biol. Inorg. Chem. 2003, 8, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bulte, J.W.M.; Liepold, L.; Basu, G.; Zywicke, H.A.; Frank, J.A.; Young, M.; Douglas, T. Paramagnetic Viral Nanoparticles as Potential High-Relaxivity Magnetic Resonance Contrast Agents. Magn. Reason. Med. 2005, 54, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Balci, S.; Bittner, A.M.; Hahn, K.; Scheu, C.; Knez, M.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Copper Nanowires within the Central Channel of Tobacco Mosaic Virus Particles. Electrochim. Acta 2006, 51, 6251–6257. [Google Scholar] [CrossRef]
- Dujardin, E.; Peet, C.; Stubbs, G.; Culver, J.N.; Mann, S. Organization of Metallic Nanoparticles Using Tobacco Mosaic Virus Templates. Nano Lett. 2003, 3, 413–417. [Google Scholar] [CrossRef]
- Knez, M.; Bittner, A.M.; Boes, F.; Wege, C.; Jeske, H.; Maiß, E.; Kern, K. Biotemplate Synthesis of 3-Nm Nickel and Cobalt Nanowires. Nano Lett. 2003, 3, 1079–1082. [Google Scholar] [CrossRef]
- Kobayashi, M.; Seki, M.; Tabata, H.; Watanabe, Y.; Yamashita, I. Fabrication of Aligned Magnetic Nanoparticles Using Tobamoviruses. Nano Lett. 2010, 10, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Lomonossoff, G.P.; Evans, D.J. CPMV-Polyelectrolyte-Templated Gold Nanoparticles. Biomacromolecules 2011, 12, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.N.; Brown, A.D.; Zang, F.; Gnerlich, M.; Gerasopoulos, K.; Ghodssi, R. Plant Virus Directed Fabrication of Nanoscale Materials and Devices. Virology 2015, 479–480, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Meldon, J.H.; Lee, B.; Yi, H. Investigation on the Catalytic Reduction Kinetics of Hexavalent Chromium by Viral-Templated Palladium Nanocatalysts. Catal. Today 2014, 233, 108–116. [Google Scholar] [CrossRef]
- Shahgolzari, M.; Pazhouhandeh, M.; Milani, M.; Yari Khosroushahi, A.; Fiering, S. Plant Viral Nanoparticles for Packaging and in Vivo Delivery of Bioactive Cargos. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1629. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, M.; Koch, C.; Eiben, S.; Geiger, F.; Eber, F.; Gliemann, H.; Poghossian, A.; Wege, C.; Schöning, M.J. A New Class of Biosensors Based on Tobacco Mosaic Virus and Coat Proteins as Enzyme Nanocarrier. Procedia Eng. 2016, 168, 618–621. [Google Scholar] [CrossRef]
- Koch, C.; Wabbel, K.; Eber, F.J.; Krolla-Sidenstein, P.; Azucena, C.; Gliemann, H.; Eiben, S.; Geiger, F.; Wege, C. Modified TMV Particles as Beneficial Scaffolds to Present Sensor Enzymes. Front. Plant Sci. 2015, 6, 1137. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Poghossian, A.; Schöning, M.J.; Wege, C. Penicillin Detection by Tobacco Mosaic Virus-Assisted Colorimetric Biosensors. Nanotheranostics 2018, 2, 184–196. [Google Scholar] [CrossRef]
- Cuenca, S.; Mansilla, C.; Aguado, M.; Yuste-Calvo, C.; Sánchez, F.; Sánchez-Montero, J.M.; Ponz, F. Nanonets Derived from Turnip Mosaic Virus as Scaffolds for Increased Enzymatic Activity of Immobilized Candida Antarctica Lipase B. Front. Plant Sci. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, L.; Cadena-Nava, R.D.; Palomares, L.A.; Ruiz-Garcia, J.; Koay, M.S.T.; Cornelissen, J.J.M.T.; Vazquez-Duhalt, R. Chemotherapy Pro-Drug Activation by Biocatalytic Virus-like Nanoparticles Containing Cytochrome P450. Enzym. Microb. Technol. 2014, 60, 24–31. [Google Scholar] [CrossRef]
- Grilo, A.L.; Mantalaris, A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol. 2019, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Monoclonal Antibodies Market to Hit USD 425 Billion by 2028, Says Global Market Insights Inc. 2022. Available online: Bloomberg.com (accessed on 17 May 2022).
- Ma, J.K.-C.; Hiatt, A.; Hein, M.; Vine, N.D.; Wang, F.; Stabila, P.; van Dolleweerd, C.; Mostov, K.; Lehner, T. Generation and Assembly of Secretory Antibodies in Plants. Science 1995, 268, 716–719. [Google Scholar] [CrossRef]
- Diamos, A.G.; Hunter, J.G.L.; Pardhe, M.D.; Rosenthal, S.H.; Sun, H.; Foster, B.C.; DiPalma, M.P.; Chen, Q.; Mason, H.S. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Front. Bioeng. Biotechnol. 2019, 7, 474. [Google Scholar] [CrossRef]
- Malaquias, A.D.M.; Marques, L.E.C.; Pereira, S.S.; de Freitas Fernandes, C.; Maranhão, A.Q.; Stabeli, R.G.; Florean, E.O.P.T.; Guedes, M.I.F.; Fernandes, C.F.C. A Review of Plant-Based Expression Systems as a Platform for Single-Domain Recombinant Antibody Production. Int. J. Biol. Macromol. 2021, 193, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Edgue, G.; Twyman, R.M.; Beiss, V.; Fischer, R.; Sack, M. Antibodies from Plants for Bionanomaterials. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1462. [Google Scholar] [CrossRef] [PubMed]
- Smolenska, L.; Roberts, I.M.; Learmonth, D.; Porter, A.J.; Harris, W.J.; Wilson, T.M.A.; Cruz, S.S. Production of a Functional Single Chain Antibody Attached to the Surface of a Plant Virus. FEBS Lett. 1998, 441, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Marillonnet, S.; Hause, G.; Klimyuk, V.; Gleba, Y. Immunoabsorbent Nanoparticles Based on a Tobamovirus Displaying Protein A. Proc. Natl. Acad. Sci. USA 2006, 103, 17678–17683. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.S.; Colwell, L.J. Comparative Analysis of Nanobody Sequence and Structure Data. Proteins Struct. Funct. Bioinform. 2018, 86, 697–706. [Google Scholar] [CrossRef]
- Martí, M.; Merwaiss, F.; Butković, A.; Daròs, J.-A. Production of Potyvirus-Derived Nanoparticles Decorated with a Nanobody in Biofactory Plants. Front. Bioeng. Biotechnol. 2022, 10, 877363. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.N.; Li, K. Toll-like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Beiss, V.; Fields, J.; Steinmetz, N.F.; Fiering, S. Cowpea Mosaic Virus Stimulates Antitumor Immunity through Recognition by Multiple MYD88-Dependent Toll-like Receptors. Biomaterials 2021, 275, 120914. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.; Suneja, G.; Ambinder, R.F.; Ard, K.; Baiocchi, R.; Barta, S.K.; Carchman, E.; Cohen, A.; Gupta, N.; Johung, K.L.; et al. Cancer in People Living With HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 986–1017. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.T.; Restifo, N.P. Natural Selection of Tumor Variants in the Generation of “Tumor Escape” Phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Roe, A.J.; Liu, R.; Veliz, F.A.; Commandeur, U.; Wald, D.N.; Steinmetz, N.F. Affinity of Plant Viral Nanoparticle Potato Virus X (PVX) towards Malignant B Cells Enables Cancer Drug Delivery. Biomater. Sci. 2020, 8, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Jobsri, J.; Allen, A.; Rajagopal, D.; Shipton, M.; Kanyuka, K.; Lomonossoff, G.P.; Ottensmeier, C.; Diebold, S.S.; Stevenson, F.K.; Savelyeva, N. Plant Virus Particles Carrying Tumour Antigen Activate TLR7 and Induce High Levels of Protective Antibody. PLoS ONE 2015, 10, e0118096. [Google Scholar] [CrossRef]
- Tyulkina, L.G.; Skurat, E.V.; Frolova, O.Y.; Komarova, T.V.; Karger, E.M.; Atabekov, I.G. New Viral Vector for Superproduction of Epitopes of Vaccine Proteins in Plants. Acta Nat. 2011, 3, 73–82. [Google Scholar] [CrossRef]
- Esfandiari, N. Targeting Breast Cancer With Bio-Inspired Virus Nanoparticles. Arch Breast Cancer 2018, 5, 90–95. [Google Scholar] [CrossRef]
- Esfandiari, N.; Arzanani, M.K.; Koohi-Habibi, M. The Study of Toxicity and Pathogenicity Risk of Potato Virus X/Herceptin Nanoparticles as Agents for Cancer Therapy. Cancer Nanotechnol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Cho, C.-F.; Ablack, A.; Lewis, J.D.; Manchester, M. Cowpea Mosaic Virus Nanoparticles Target Surface Vimentin on Cancer Cells. Nanomedicine 2011, 6, 351–364. [Google Scholar] [CrossRef]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Shukla, S.; Wang, C.; Masarapu, H.; Steinmetz, N.F. Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine. J. Am. Chem. Soc. 2019, 141, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Ablack, A.L.; Wen, A.M.; Lee, K.L.; Lewis, J.D.; Steinmetz, N.F. Increased Tumor Homing and Tissue Penetration of the Filamentous Plant Viral Nanoparticle Potato Virus X. Mol. Pharm. 2013, 10, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Nguyen, H.G.; Chowdhury, S.; Bentley, P.; Bruckman, M.A.; Miermont, A.; Gildersleeve, J.C.; Wang, Q.; Huang, X. Tobacco Mosaic Virus as a New Carrier for Tumor Associated Carbohydrate Antigens. Bioconjug. Chem. 2012, 23, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Steinmetz, N.F. CD47 Blockade and Cowpea Mosaic Virus Nanoparticle In Situ Vaccination Triggers Phagocytosis and Tumor Killing. Adv Health Mater 2019, 8, e1801288. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, B.L.; Brennan, F.R.; Martinez-Torrecuadrada, J.L.; Casal, J.I.; Hamilton, W.D.; Wakelin, D. Characterization of the Immune Response to Canine Parvovirus Induced by Vaccination with Chimaeric Plant Viruses. Vaccine 2002, 20, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Rabindran, S.; Commandeur, U.; Twyman, R.M.; Fischer, R. The Potential of Plant Virus Vectors for Vaccine Production. Drugs R D 2006, 7, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Staczek, J.; Bendahmane, M.; Gilleland, L.B.; Beachy, R.N.; Gilleland, H.E. Immunization with a Chimeric Tobacco Mosaic Virus Containing an Epitope of Outer Membrane Protein F of Pseudomonas Aeruginosa Provides Protection against Challenge with P. Aeruginosa. Vaccine 2000, 18, 2266–2274. [Google Scholar] [CrossRef]
- Bendahmane, M.; Koo, M.; Karrer, E.; Beachy, R.N. Display of Epitopes on the Surface of Tobacco Mosaic Virus: Impact of Charge and Isoelectric Point of the Epitope on Virus-Host Interactions. J. Mol. Biol. 1999, 290, 9–20. [Google Scholar] [CrossRef]
- Porta, C.; Spall, V.E.; Findlay, K.C.; Gergerich, R.C.; Farrance, C.E.; Lomonossoff, G.P. Cowpea Mosaic Virus-Based Chimaeras. Effects of Inserted Peptides on the Phenotype, Host Range, and Transmissibility of the Modified Viruses. Virology 2003, 310, 50–63. [Google Scholar] [CrossRef]
- Uhde-Holzem, K.; McBurney, M.; Tiu, B.D.B.; Advincula, R.C.; Fischer, R.; Commandeur, U.; Steinmetz, N.F. Production of Immunoabsorbent Nanoparticles by Displaying Single-Domain Protein A on Potato Virus X. Macromol. Biosci. 2016, 16, 231–241. [Google Scholar] [CrossRef]
- Carignan, D.; Thérien, A.; Rioux, G.; Paquet, G.; Gagné, M.-È.L.; Bolduc, M.; Savard, P.; Leclerc, D. Engineering of the PapMV Vaccine Platform with a Shortened M2e Peptide Leads to an Effective One Dose Influenza Vaccine. Vaccine 2015, 33, 7245–7253. [Google Scholar] [CrossRef]
- Balke, I.; Zeltins, A. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Lomonossoff, G.P.; Hamilton, W.D.O. Cowpea Mosaic Virus-Based Vaccines. In Plant Biotechnology: New Products and Applications; Hammond, J., McGarvey, P., Yusibov, V., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 177–189. ISBN 978-3-642-60234-4. [Google Scholar]
- Haynes, J.R.; Cunningham, J.; von Seefried, A.; Lennick, M.; Garvin, R.T.; Shen, S.-H. Development of a Genetically-Engineered, Candidate Polio Vaccine Employing the Self-Assembling Properties of the Tobacco Mosaic Virus Coat Protein. Biotechnology 1986, 4, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Usha, R.; Rohll, J.B.; Spall, V.E.; Shanks, M.; Maule, A.J.; Johnson, J.E.; Lomonossoff, G.P. Expression of an Animal Virus Antigenic Site on the Surface of a Plant Virus Particle. Virology 1993, 197, 366–374. [Google Scholar] [CrossRef]
- Turpen, T.H.; Reinl, S.J.; Charoenvit, Y.; Hoffman, S.L.; Fallarme, V.; Grill, L.K. Malaria Epitopes Expressed on the Surface of Recombinant Tobacco Mosaic Virus. Nat. Biotechnol. 1995, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Hamamoto, H.; Takemoto, S.; Watanabe, Y.; Okada, Y. Systemic Production of Foreign Peptides on the Particle Surface of Tobacco Mosaic Virus. FEBS Lett. 1995, 359, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Lomonossoff, G.P.; Johnson, J.E. Use of Macromolecular Assemblies as Expression Systems for Peptides and Synthetic Vaccines. Curr. Opin. Struct. Biol. 1996, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.; Forlenza, M. Oral Vaccination of Fish: Lessons from Humans and Veterinary Species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Expression and Purification of Pharmaceutical Proteins in Plants. Biol. Eng. Trans. 2008, 1, 291–321. [Google Scholar] [CrossRef]
- Lai, H.; Chen, Q. Bioprocessing of Plant-Derived Virus-like Particles of Norwalk Virus Capsid Protein under Current Good Manufacture Practice Regulations. Plant Cell Rep. 2012, 31, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, H. Plant-Derived Virus-like Particles as Vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.; Gerdts, V. Veterinary Vaccines: Alternatives to Antibiotics? Anim. Health Res. Rev. 2008, 9, 187–199. [Google Scholar] [CrossRef]
- Chen, T.-H.; Hu, C.-C.; Liao, J.-T.; Lee, Y.-L.; Huang, Y.-W.; Lin, N.-S.; Lin, Y.-L.; Hsu, Y.-H. Production of Japanese Encephalitis Virus Antigens in Plants Using Bamboo Mosaic Virus-Based Vector. Front. Microbiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Kolotilin, I.; Topp, E.; Cox, E.; Devriendt, B.; Conrad, U.; Joensuu, J.; Stöger, E.; Warzecha, H.; McAllister, T.; Potter, A.; et al. Plant-Based Solutions for Veterinary Immunotherapeutics and Prophylactics. Vet. Res. 2014, 45, 117. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Takova, K.; Valkova, R.; Toneva, V.; Minkov, I.; Andonov, A.; Lukov, G.L. Plant-Derived Recombinant Vaccines against Zoonotic Viruses. Life 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Cherian, S.; Sumithra, T.G.; Raina, O.K.; Sankar, M. Edible Vaccines against Veterinary Parasitic Diseases—Current Status and Future Prospects. Vaccine 2013, 31, 1879–1885. [Google Scholar] [CrossRef]
- Su, H.; Yakovlev, I.A.; van Eerde, A.; Su, J.; Clarke, J.L. Plant-Produced Vaccines: Future Applications in Aquaculture. Front. Plant Sci. 2021, 12, 718775. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, J.J.; Niklander-Teeri, V.; Brandle, J.E. Transgenic Plants for Animal Health: Plant-Made Vaccine Antigens for Animal Infectious Disease Control. Phytochem. Rev. 2008, 7, 553–577. [Google Scholar] [CrossRef]
- Howard, J.A. Commercialization of Plant-Based Vaccines from Research and Development to Manufacturing. Anim. Health Res. Rev. 2004, 5, 243–245. [Google Scholar] [CrossRef]
- Sahoo, A.; Mandal, A.K.; Dwivedi, K.; Kumar, V. A Cross Talk between the Immunization and Edible Vaccine: Current Challenges and Future Prospects. Life Sci. 2020, 261, 118343. [Google Scholar] [CrossRef]
- Mičúchová, A.; Piačková, V.; Frébort, I.; Korytář, T. Molecular Farming: Expanding the Field of Edible Vaccines for Sustainable Fish Aquaculture. Rev. Aquac. 2022, 14, 1978–2001. [Google Scholar] [CrossRef]
- Specht, E.; Mayfield, S. Algae-Based Oral Recombinant Vaccines. Front. Microbiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Kim, M.-N.; Park, J.-Y.; Cha, J.-H.; Chung, H.-J. Edible vaccine for aquacultured fish: Present and prospect. J. Plant Biotechnol. 2010, 37, 269–274. [Google Scholar] [CrossRef]
- Ma, K.; Bao, Q.; Wu, Y.; Chen, S.; Zhao, S.; Wu, H.; Fan, J. Evaluation of Microalgae as Immunostimulants and Recombinant Vaccines for Diseases Prevention and Control in Aquaculture. Front. Bioeng. Biotechnol. 2020, 8, 590431. [Google Scholar] [CrossRef]
- Rybicki, E. History and Promise of Plant-Made Vaccines for Animals. In Prospects of Plant-Based Vaccines in Veterinary Medicine; MacDonald, J., Ed.; Springer International Publishing: Cham, Germany, 2018; pp. 1–22. ISBN 978-3-319-90137-4. [Google Scholar]
- Zhang, X.; Buehner, N.A.; Hutson, A.M.; Estes, M.K.; Mason, H.S. Tomato Is a Highly Effective Vehicle for Expression and Oral Immunization with Norwalk Virus Capsid Protein. Plant Biotechnol. J. 2006, 4, 419–432. [Google Scholar] [CrossRef]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human Immune Responses to a Novel Norwalk Virus Vaccine Delivered in Transgenic Potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef]
- Yusibov, V.; Hooper, D.C.; Spitsin, S.V.; Fleysh, N.; Kean, R.B.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J.; et al. Expression in Plants and Immunogenicity of Plant Virus-Based Experimental Rabies Vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.-S.; Jeon, I.-S.; Jung, Y.-J.; Kim, J.-B.; Park, J.-S.; Ha, S.-H.; Kim, K.-H.; Kim, H.-M.; Yang, J.-S.; Kim, Y.-H. Expression of Hemagglutinin-Neuraminidase Protein of Newcastle Disease Virus in Transgenic Tobacco. Plant Biotechnol. Rep. 2007, 1, 85–92. [Google Scholar] [CrossRef]
- Guerrero-Andrade, O.; Loza-Rubio, E.; Olivera-Flores, T.; Fehérvári-Bone, T.; Gómez-Lim, M.A. Expression of the Newcastle Disease Virus Fusion Protein in Transgenic Maize and Immunological Studies. Transgenic Res. 2006, 15, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Samiullah, T.R.; Shakoor, S.; Latif, A.; Yasmeen, A.; Azam, S.; Shahid, A.A.; Husnain, T.; Rao, A.Q. Early Stage Development of a Newcastle Disease Vaccine Candidate in Corn. Front. Vet. Sci. 2020, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.J.; Ebrahimi, M.M.; Shahsavandi, S.; Amani, J.; Kazemi, R.; Jafari, M.; Salmanian, A.-H. The Immunogenicity of a Novel Chimeric Hemagglutinin-Neuraminidase-Fusion Antigen from Newcastle Disease Virus by Oral Delivery of Transgenic Canola Seeds to Chickens. Mol. Biotechnol. 2020, 62, 344–354. [Google Scholar] [CrossRef]
- Berinstein, A.; Vazquez-Rovere, C.; Asurmendi, S.; Gómez, E.; Zanetti, F.; Zabal, O.; Tozzini, A.; Conte Grand, D.; Taboga, O.; Calamante, G.; et al. Mucosal and Systemic Immunization Elicited by Newcastle Disease Virus (NDV) Transgenic Plants as Antigens. Vaccine 2005, 23, 5583–5589. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, I.A.; Charpin-El Hamri, G.; Fussenegger, M. Heat-Stable Oral Alga-Based Vaccine Protects Mice from Staphylococcus Aureus Infection. J. Biotechnol. 2010, 145, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wigdorovitz, A.; Mozgovoj, M.; Santos, M.J.D.; Parreño, V.; Gómez, C.; Pérez-Filgueira, D.M.; Trono, K.G.; Ríos, R.D.; Franzone, P.M.; Fernández, F.; et al. Protective Lactogenic Immunity Conferred by an Edible Peptide Vaccine to Bovine Rotavirus Produced in Transgenic Plants. J. Gen. Virol. 2004, 85, 1825–1832. [Google Scholar] [CrossRef]
- Pogrebnyak, N.; Golovkin, M.; Andrianov, V.; Spitsin, S.; Smirnov, Y.; Egolf, R.; Koprowski, H. Severe Acute Respiratory Syndrome (SARS) S Protein Production in Plants: Development of Recombinant Vaccine. Proc. Natl. Acad. Sci. USA 2005, 102, 9062–9067. [Google Scholar] [CrossRef]
- Legocki, A.B.; Miedzinska, K.; Czaplińska, M.; Płucieniczak, A.; Wędrychowicz, H. Immunoprotective Properties of Transgenic Plants Expressing E2 Glycoprotein from CSFV and Cysteine Protease from Fasciola Hepatica. Vaccine 2005, 23, 1844–1846. [Google Scholar] [CrossRef]
- Plant-Based Biologics Market Size 2026 | Revised in a New Report. Available online: https://www.researchdive.com/150/plant-based-biologics-market (accessed on 5 December 2022).
- Peyret, H.; Brown, J.K.M.; Lomonossoff, G.P. Improving Plant Transient Expression through the Rational Design of Synthetic 5′ and 3′ Untranslated Regions. Plant Methods 2019, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Montero-Morales, L.; Steinkellner, H. Advanced Plant-Based Glycan Engineering. Front. Bioeng. Biotechnol. 2018, 6, 81. [Google Scholar] [CrossRef]
- Drossard, J. Downstream Processing of Plant-Derived Recombinant Therapeutic Proteins. In Molecular Farming; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 217–231. ISBN 978-3-527-60363-3. [Google Scholar]
- Streatfield, S.J. Approaches to Achieve High-Level Heterologous Protein Production in Plants. Plant Biotechnol. J. 2007, 5, 2–15. [Google Scholar] [CrossRef]
- Jutras, P.V.; D’Aoust, M.-A.; Couture, M.M.-J.; Vézina, L.-P.; Goulet, M.-C.; Michaud, D.; Sainsbury, F. Modulating Secretory Pathway PH by Proton Channel Co-Expression Can Increase Recombinant Protein Stability in Plants. Biotechnol. J. 2015, 10, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Jutras, P.V.; Dodds, I.; van der Hoorn, R.A. Proteases of Nicotiana Benthamiana: An Emerging Battle for Molecular Farming. Curr. Opin. Biotechnol. 2020, 61, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Elelyso® for Gaucher Disease. Available online: https://protalix.com/about/elelyso/ (accessed on 5 December 2022).
- Tran, E.E.H.; Nelson, E.A.; Bonagiri, P.; Simmons, J.A.; Shoemaker, C.J.; Schmaljohn, C.S.; Kobinger, G.P.; Zeitlin, L.; Subramaniam, S.; White, J.M. Mapping of Ebolavirus Neutralization by Monoclonal Antibodies in the ZMapp Cocktail Using Cryo-Electron Tomography and Studies of Cellular Entry. J. Virol. 2016, 90, 7618–7627. [Google Scholar] [CrossRef]
- Reuters Staff Mapp Biopharma’s Ebola Drug Gets FDA Fast Track Status. Reuters 2015. Available online: https://www.reuters.com/article/mapp-biopharmaceutical-fda-idUSL4N11N4K520150917 (accessed on 17 November 2022).
- IBio Inc. IBio Reports Successful Preclinical Immunization Studies with Next-Gen Nucleocapsid COVID-19 Vaccine Candidate. Available online: https://ibioinc.com/ibio-reports-successful-preclinical-immunization-studies-with-next-gen-nucleocapsid-covid-19-vaccine-candidate/ (accessed on 20 November 2021).
- British American Tobacco—BAT Makes Progress on COVID-19 Vaccine Provides Community Support. Available online: https://www.bat.com/group/sites/UK__9D9KCY.nsf/vwPagesWebLive/DOBPMBZC# (accessed on 20 November 2021).
- Baiya Phytopharm Co Ltd.: Baiya SARS-CoV-2 Vax 1 Vaccine—COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/vaccines/130/ (accessed on 20 November 2021).
- Hardy, A. IBIO-201 Demonstrates Ability to Elicit Anti-SARS-CoV-2 Immune Response in Preclinical Studies. Available online: https://biotuesdays.com/2020/08/10/ibio-updates-ibio-201-covid-19-vaccine-candidate/ (accessed on 20 November 2021).
- Hahn-Löbmann, S.; Stephan, A.; Schulz, S.; Schneider, T.; Shaverskyi, A.; Tusé, D.; Giritch, A.; Gleba, Y. Colicins and Salmocins —New Classes of Plant-Made Non-Antibiotic Food Antibacterials. Front. Plant Sci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Starkevič, U.; Bortesi, L.; Virgailis, M.; Ružauskas, M.; Giritch, A.; Ražanskienė, A. High-Yield Production of a Functional Bacteriophage Lysin with Antipneumococcal Activity Using a Plant Virus-Based Expression System. J. Biotechnol. 2015, 200, 10–16. [Google Scholar] [CrossRef]
- Gomord, V.; Fitchette, A.-C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-Specific Glycosylation Patterns in the Context of Therapeutic Protein Production. Plant Biotechnol. J. 2010, 8, 564–587. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Salazar-González, J.A.; Decker, E.L.; Reski, R. Implications of Plant Glycans in the Development of Innovative Vaccines. Expert Rev. Vaccines 2016, 15, 915–925. [Google Scholar] [CrossRef] [PubMed]

| Virus Classification/Genome Organization | Virus | Vector | Achieved High Yield | Recombinant Proteins | Reference |
|---|---|---|---|---|---|
| Tobamovirus + ssRNA | tobacco mosaic virus (TMV) | magnICON | 5 mg/g FWT | GFP | [88] |
| TRBO | 5.5 mg/g FWT | GFP | [89] | ||
| Potexvirus + ssRNA | foxtail mosaic virus (FoMV) | FECT/40 vector | 1.7 mg/g FWT | GFP | [90] |
| potato virus X (PVX) | pEff | 1 mg/g FWT | GFP | [84] | |
| Geminivirusss circular DNA | tobacco yellow dwarf mastrevirus (TYDV) | INPACT | 0.1 mg/g FWT | human Vitronectin | [91] |
| bean yellow dwarf virus (BeYDV) | pBYRp19 | 0.5 mg/g FWT | mAb 6D8 against Ebola virus | [92] | |
| pRIC | 0.55 mg/g FWT | HPV CP L1 | [93] | ||
| Tobravirus + ssRNA | tobacco rattle virus (TRV) | TRV- based vector | 0.01 mg/g of fresh weight root tissue | GNA lectin protein | [94] |
| Comovirus + ss RNA | cowpea mosaic virus (CPMV) | delRNA-2 | - | GFP | [95] |
| pEAQ-HT | 1.5 mg/g | GFP | [96] |
| Disease/Infectious Agents | Antigen | Species | Yield | Immunogenicity | Reference |
|---|---|---|---|---|---|
| Norwalk virus | Capsid protein (NVCP) | Tomato | up to 8% of TSP | Freeze-dried tomato (40 µg VLPs) induced NV-specific serum IgG and mucosal IgA in ≥80% of mice. | [240] |
| Potato | - | 19/20 human volunteers developed an immune response after oral immunization with VLPs. | [241] | ||
| Rabies virus | G and N proteins fused to AlMV CP | Tobacco and spinach | 0.4 ± 0.07 mg/g of fresh leaf tissue | Immunized mice were protected against challenge infection. Human volunteers previously non-immunized demonstrated significant antibody responses after fed. | [242] |
| Newcastle Disease Virus (NDV) | Hemagglutinin-neuraminidase protein (HN) | Tobacco | 0.069% of TSP | Specific immune response after oral administration of chicken was induced. | [243] |
| Fusion (F) | Maize | 0.9–3% TSP | [244] | ||
| Fusion (F) and hemagglutinin-neuraminidase (HN) proteins | Maize | 0.5–0.8% of total seed protein | [245] | ||
| Canola | up to 0.18% and 0.11% TSP in transgenic seeds and leaves | [246] | |||
| Potato | 0.3–0.6 mg/g of total leaf protein | [247] | |||
| S. aureus and Cholera | D2 fibronectin-binding domain with cholera toxin B subunit | C. reinhardtii | Up to 0.7% TSP | Mice fed with whole algae showed mucosal IgA and systemic IgG responses to CTB and D2. A total of 80% survived after lethal challenge. | [248] |
| Bovine rotavirus (BVR) | eBRV4 fused to βGUS | Alfalfa | 0.4–0.9 mg (g TPS)−1 | An effective anti-rotavirus antibody response was induced in mice after oral administration. | [249] |
| Coronavirus | S protein (S1) | Tomato | - | Orally immunized mice showed significantly increased levels of SARS-CoV-specific IgA. | [250] |
| Hog pest virus/F. hepatica | Glycoprotein E2/cysteine proteases | Lettuce | 0.16 mg/g dry mass | Oral immunization of mice induced specific antibodies. | [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahmanova, G.; Aljabali, A.A.; Takova, K.; Toneva, V.; Tambuwala, M.M.; Andonov, A.P.; Lukov, G.L.; Minkov, I. The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? Int. J. Mol. Sci. 2023, 24, 1533. https://doi.org/10.3390/ijms24021533
Zahmanova G, Aljabali AA, Takova K, Toneva V, Tambuwala MM, Andonov AP, Lukov GL, Minkov I. The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? International Journal of Molecular Sciences. 2023; 24(2):1533. https://doi.org/10.3390/ijms24021533
Chicago/Turabian StyleZahmanova, Gergana, Alaa A. Aljabali, Katerina Takova, Valentina Toneva, Murtaza M. Tambuwala, Anton P. Andonov, Georgi L. Lukov, and Ivan Minkov. 2023. "The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health?" International Journal of Molecular Sciences 24, no. 2: 1533. https://doi.org/10.3390/ijms24021533
APA StyleZahmanova, G., Aljabali, A. A., Takova, K., Toneva, V., Tambuwala, M. M., Andonov, A. P., Lukov, G. L., & Minkov, I. (2023). The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? International Journal of Molecular Sciences, 24(2), 1533. https://doi.org/10.3390/ijms24021533









