Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Anthropometric and Echocardiographic Evaluations and CRTd Implant
4.3. Laboratory Analysis
4.4. RNA Serum Extraction and miR-130a-5p Analysis in CRTd Patients
4.5. Echo Doppler Measurements of the Brachial Artery and Assessment of Endothelial Function
4.6. Study Endpoints
4.7. Statistical Analyses
5. Conclusions
6. Clinical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Santini, L.; Capria, A.; Di Molfetta, A.; Mahfouz, K.; Panattoni, G.; Minni, V.; Sergi, D.; Forleo, G.B.; Romeo, F. Endothelial dysfunction is a marker of systemic response to the cardiac resynchronization therapy in heart failure. J. Card. Fail. 2013, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Warriner, D.R.; Lawford, P.; Sheridan, P.J. Measures of endothelial dysfunction predict response to cardiac resynchronisation therapy. Open Heart 2016, 3, e000391. [Google Scholar] [CrossRef]
- Giannessi, D.; Del Ry, S.; Vitale, R.L. The role of endothelins and their receptors in heart failure. Pharmacol. Res. 2001, 43, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Aprocitentan: New Insights. Front. Cardiovasc Med. 2022, 9, 1093406. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Clozel, M. Mechanisms of ET-1-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 2007, 50, 621–628. [Google Scholar] [CrossRef]
- Sikkeland, L.I.; Dahl, C.P.; Ueland, T.; Andreassen, A.K.; Gude, E.; Edvardsen, T.; Holm, T.; Yndestad, A.; Gullestad, L.; Kongerud, J.; et al. Increased levels of inflammatory cytokines and endothelin-1 in alveolar macrophages from patients with chronic heart failure. PLoS ONE 2012, 7, e36815. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Du, J.Y.; Wang, D.D.; Yang, X. miRNA-130a improves cardiac function by down-regulating TNF-alpha expression in a rat model of heart failure. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8454–8461. [Google Scholar]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. Group ESCSD: 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Filippatos, G.S.; von Haehling, S.; Papassotiriou, J.; Morgenthaler, N.G.; Cicoira, M.; Schefold, J.C.; Rozentryt, P.; Ponikowska, B.; Doehner, W.; et al. Identification of chronic heart failure patients with a high 12-month mortality risk using biomarkers including plasma C-terminal pro-endothelin. PLoS ONE 2011, 6, e14506. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Santamaria, M.; de Lucia, C.; Ruocco, A.; Mauro, C.; Paolisso, G.; Rizzo, M.R.; Barbieri, M.; et al. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. conventional hypoglycemic drugs: Arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc. Diabetol. 2018, 17, 137. [Google Scholar]
- Bertero, T.; Cottrill, K.; Krauszman, A.; Lu, Y.; Annis, S.; Hale, A.; Bhat, B.; Waxman, A.B.; Chau, B.N.; Kuebler, W.M.; et al. The microRNA-130/301 family controls vasoconstriction in pul-monary hypertension. J. Biol. Chem. 2015, 290, 2069–2085. [Google Scholar] [CrossRef]
- Sardu, C.; Marfella, R.; Santulli, G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J. Cardiovasc. Transl. Res. 2014, 7, 362–368. [Google Scholar] [CrossRef]
- Sardu, C.; Barbieri, M.; Santamaria, M.; Giordano, V.; Sacra, C.; Paolisso, P.; Spirito, A.; Marfella, R.; Paolisso, G.; Rizzo, M.R. Multipolar pacing by cardiac resynchronization therapy with a defibrillators treatment in type 2 diabetes mellitus failing heart patients: Impact on responders rate, and clinical outcomes. Cardiovasc. Diabetol. 2017, 16, 75. [Google Scholar] [CrossRef]
- Sardu, C.; Marfella, R.; Santamaria, M.; Papini, S.; Parisi, Q.; Sacra, C.; Colaprete, D.; Paolisso, G.; Rizzo, M.R.; Barbieri, M. Stretch, Injury and Inflammation Markers Evaluation to Predict Clinical Outcomes After Implantable Cardioverter Defibrillator Therapy in Heart Failure Patients With Metabolic Syndrome. Front. Physiol. 2018, 9, 758. [Google Scholar] [CrossRef]
- Jakob, P.; Doerries, C.; Briand, S.; Mocharla, P.; Krankel, N.; Besler, C.; Mueller, M.; Manes, C.; Templin, C.; Baltes, C.; et al. Loss of angiomiR-126 and 130a in angiogenic early outgrowth cells from patients with chronic heart failure: Role for impaired in vivo neovascularization and cardiac repair capacity. Circulation 2012, 126, 2962–2975. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail 2013, 15, 1277–1288. [Google Scholar] [CrossRef]
- Lopez-Sanchez, C.; Franco, D.; Bonet, F.; Garcia-Lopez, V.; Aranega, A.; Garcia-Martinez, V. Negative Fgf8-Bmp2 feed-back is regulated by miR-130a-5p during early cardiac specification. Dev. Biol. 2015, 406, 63–73. [Google Scholar] [CrossRef]
- Akar, J.G.; Al-Chekakie, M.O.; Fugate, T.; Moran, L.; Froloshki, B.; Varma, N.; Santucci, P.; Wilber, D.J.; Matsumura, M.E. Endothelial dysfunction in heart failure identifies responders to cardiac resynchronization therapy. Heart Rhythm 2008, 5, 1229–1235. [Google Scholar] [CrossRef]
- Sardu, C.; Massetti, M.; Scisciola, L.; Trotta, M.C.; Santamaria, M.; Volpicelli, M.; Ducceschi, V.; Signoriello, G.; D’Onofrio, N.; Marfella, L.; et al. Angiotensin receptor/Neprilysin inhibitor effects in CRTd non-responders: From epigenetic to clinical beside. Pharmacol. Res. 2022, 182, 106303. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Jankauskas, S.S.; D’Ascia, S.L.; Sardu, C.; Matarese, A.; Minicucci, F.; Mone, P.; Santulli, G. Refining success of Glycation of ryanodine receptor in circulating lymphocytes predicts the response to cardiac resynchronization therapy. J. Heart Lung Transplant. 2022, 41, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Barbieri, M.; Rizzo, M.R.; Paolisso, P.; Paolisso, G.; Marfella, R. Cardiac Resynchronization Therapy Outcomes in Type 2 Diabetic Patients: Role of MicroRNA Changes. J. Diabetes Res. 2016, 2016, 7292564. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Lund, L.H.; Melin, M.; Benson, L.; Uijl, A.; Dahlström, U.; Braunschweig, F.; Linde, C.; Savarese, G. Cardiac resynchronization therapy with or without defibrillator in patients with heart failure. Europace 2022, 24, 48–57. [Google Scholar] [CrossRef]
- Liu, H.L.; Bao, H.G.; Zheng, C.L.; Teng, C.; Bai, M.H. MiR-130a regulating the biological function of colon cancer by targeting inhibition of PTEN. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1786–1793. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic. Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
| BASELINE | 1-YEAR FOLLOW-UP | |||||
|---|---|---|---|---|---|---|
| PARAMETERS | ED-CRTd (FMD ≤ 7.1, n 590) | NED-CRTd (FMD ≥ 7.1, n 277) | p Value | ED-CRTd (FMD ≤ 7.1, n 326) | NED-CRTd (FMD ≥ 7.1, n 541) | p Value |
| Age, years | 70.7 ± 6.2 | 71.1 ± 5.8 | 0.568 | 71.7 ± 6.6 | 71.8 ± 6.3 | 0.749 |
| Male, n (%) | 429 (72.7) | 199 (71.8) | 0.060 | 233 (71.5) | 393 (72.6) | 0.754 |
| Smokers, n (%) | 295 (49.5) | 128 (46.2) | 0.102 | 179 (54.9) | 283 (52.3) | 0.483 |
| Hypertension, n (%) | 417 (70.7) | 187 (67.5) | 0.344 | 232 (71.2) | 369 (68.2) | 0.363 |
| Dyslipidemia, n (%) | 257 (43.6) | 110 (39.7) | 0.302 | 115 (35.3) | 184 (34.0) | 0.713 |
| Diabetes mellitus, n (%) | 249 (42.2) | 109 (39.3) | 0.376 | 158 (48.5) | 229 (42.3) | 0.090 |
| BMI > 30 kg/m2 (%) | 45 (7.6) | 19 (6.9) | 0.781 | 32 (9.8) | 34 (6.3) | 0.064 |
| Ischemic heart failure (%) | 409 (69.3) | 186 (67.1) | 0.531 | 247 (75.8) | 383 (70.8) | 0.116 |
| NYHA class, n (%): | 0.488 | 0.001 * | ||||
| I NYHA class | / | / | 7 (2.1) | 35 (6.5) | ||
| II NYHA class | 130 (22.0) | 67 (24.2) | 69 (21.2) | 266 (49.2) | ||
| III NYHA class | 460 (78) | 210 (75.8) | 219 (67.2) | 223 (41.2) | ||
| IV NYHA class | / | / | 31 (9.5) | 17 (3.1) | ||
| QRS duration (ms) | 137.8 ±9.2 | 138.0 ± 9.5 | 0.160 | 127.2 ±6.2 | 120.6 ± 9.6 | 0.001 * |
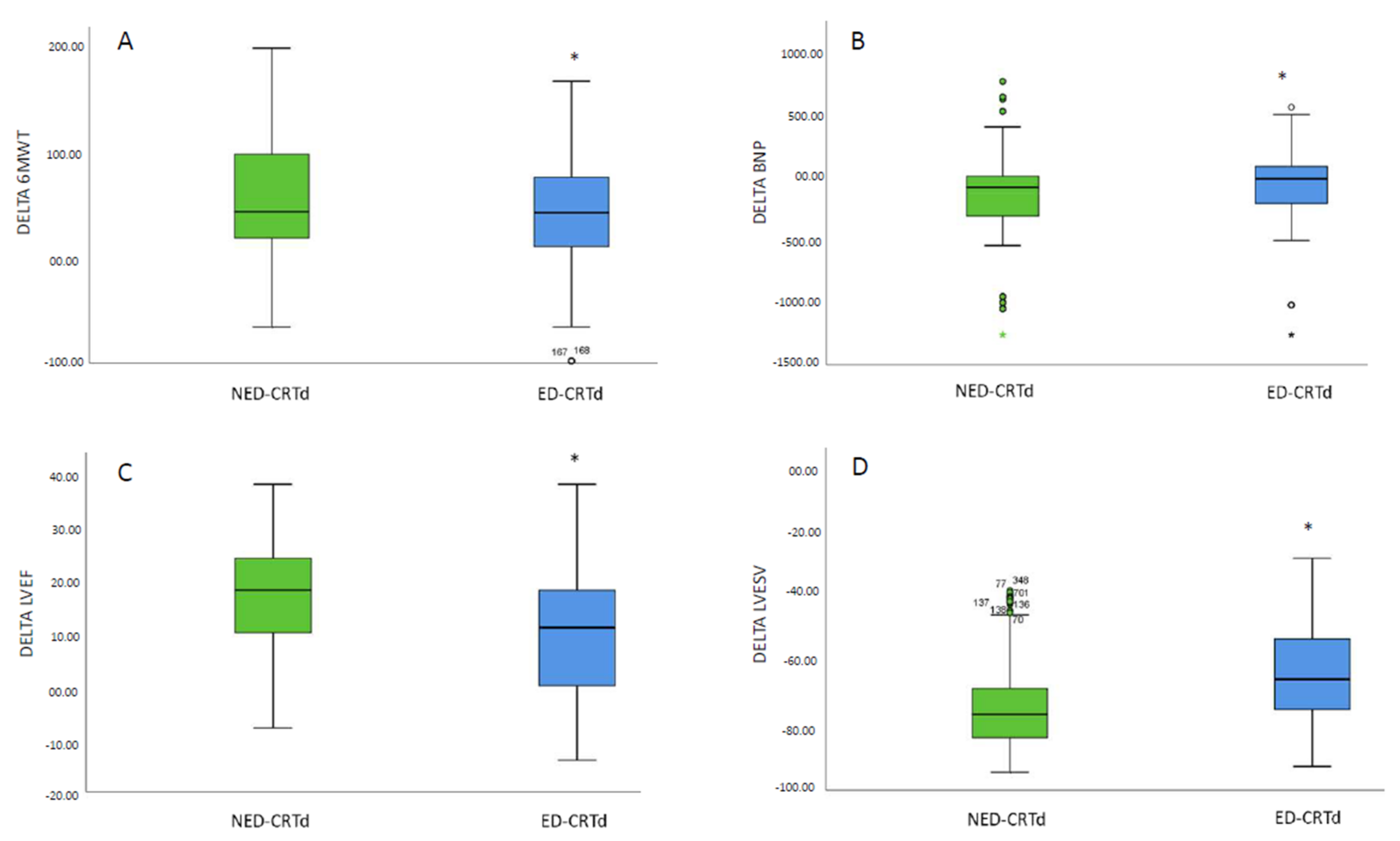
| 6MWT | 209.56 ± 44.15 | 208.16 ± 44.53 | 0.118 | 218.17 ± 44.15 | 247.17 ± 44.52 | 0.018 * |
| BNP (pg/mL) | 390.95 ± 29.34 | 402.33 ± 23.01 | 0.570 | 297.43 ± 16.22 | 266.25 ± 10.8 | 0.042 * |
| Endothelin-1, pmol/L | 6.49 ± 0.18 | 5.63 ± 0.25 | 0.007* | 5.41 ± 0.24 | 4.57 ± 0.17 | 0.003 * |
| miR-130a-5p, A.U. | 0.28 ± 0.014 | 0.27 ± 0.025 | 0.688 | 0.41 ± 0.034 | 0.51 ± 0.029 | 0.037 * |
| Inflammatory biomarkers | ||||||
| Lymphocytes | 7.13 ± 1.36 | 7.46 ± 1.52 | 0.438 | 7.93± 1.83 | 6.93± 1.12 | 0.001 * |
| Neutrophiles | 5.83 ± 1.06 | 5.70 ± 1.23 | 0.071 | 5.73 ± 0.92 | 5.24 ± 1.20 | 0.001 * |
| CRP (pg/l) x 10 | 9.26 ± 0. 41 | 8.96 ± 0.51 | 0.676 | 9.86 ± 0. 48 | 6.59 ± 0.38 | 0.001 * |
| IL6 (pg/mL) | 6.48 ± 0.02 | 6.52 ± 0.03 | 0.462 | 6.30 ± 0.06 | 6.10 ± 0.06 | 0.036 * |
| TNFα (pg/mL) x 10 | 6.43 ± 0.02 | 6.47 ± 0.02 | 0.144 | 6.38 ± 0.02 | 6.16 ± 0.02 | 0.001 * |
| Echocardiographic parameters | ||||||
| LVEF (%) | 26.8 ± 5.4 | 26.3 ± 4.9 | 0.126 | 36.7 ± 6.9 | 42.6 ± 4.5 | 0.001 * |
| LVEDd (mm) | 68.2 ± 4.1 | 69.1 ± 3.7 | 0.968 | 71.7 ± 5.8 | 68.1 ± 3.9 | 0.001 * |
| LVESd (mm) | 42.6 ± 5.3 | 43.2 ± 6.0 | 0.786 | 41.5 ± 3.8 | 38.6 ± 4.8 | 0.001 * |
| LVEDv (ml) | 224.8 ± 22.1 | 227.1 ± 24.3 | 0.335 | 228.4 ± 19.7 | 219.2 ± 14.1 | 0.001 * |
| LVESv (ml) | 140.2 ± 22.5 | 139.1 ± 23.8 | 0.328 | 137.25 ± 16.6 | 124.8 ± 17.2 | 0.001 * |
| Mitral insufficiency | 0.384 | 0.050 * | ||||
| + (%) | 272 (46.1) | 135 (48.7) | 130 (39.9) | 265 (49.0) | ||
| ++ (%) | 230 (38.9) | 108 (39.0) | 131 (40.2) | 219 (40.5) | ||
| +++ (%) | 88 (14.9) | 34 (12.3) | 62 (19.9) | 57 (10.5) | ||
| Medications | ||||||
| Beta blockers, n (%): | 405 (68.6) | 188 (67.9) | 0.876 | 237 (72.7) | 380 (70.2) | 0.487 |
| Carvedilol | 291 (71.9) | 137 (72.9) | 174 (73.4) | 281 (73.9) | ||
| Bisoprolol | 114 (28.1) | 51 (27.1) | 63 (26.6) | 99 (26.1) | ||
| Calcium antagonist, n (%) | 23 (3.9) | 9 (3.2) | 0.703 | 13 (4.0) | 22 (4.1) | 0.159 |
| Amiodarone, n (%) | 117 (19.8) | 60 (21.7) | 0.588 | 82 (25.1) | 108 (20.0) | 0.076 |
| ACE inhibitors, n (%) | 148 (25.1) | 68 (24.5) | 0.867 | 88 (27.0) | 143 (26.4) | 0.575 |
| ARS blockers, n (%) | 167 (28.3) | 84 (30.3) | 0.574 | 95 (29.1) | 165 (30.5) | 0.597 |
| Sacubitril/valsartan, n (%) | 188 (31.9) | 93 (33.6) | 0.641 | 132 (40.5) | 177 (32.7) | 0.023 * |
| Aspirin, n (%) | 224 (38.0) | 111 (40.1) | 0.601 | 134 (41.1) | 211 (39.0) | 0.424 |
| Warfarin, n (%) | 199 (33.7) | 102 (36.8) | 0.646 | 124 (38.0) | 185 (34.2) | 0.272 |
| NOAC, n (%) | 117 (19.8) | 55 (19.8) | 0.928 | 69 (21.2) | 112 (20.7) | 0.421 |
| Ticlopidine, n (%) | 10 (1.7) | 5 (1.8) | 0.826 | 8 (2.4) | 11 (2.0) | 0.496 |
| Ivabradine, n (%) | 183 (31.0) | 78 (28.2) | 0.473 | (30.9) | (28) | 0.822 |
| Digoxin, n (%) | 178 (30.2) | 91 (32.8) | 0.387 | (30.2) | (32.8) | 0.766 |
| Diuretics, n (%): | ||||||
| Loop diuretics | 526 (89.1) | 246 (88.8) | 0.602 | 303 (92.9) | 472 (87.2) | 0.041 * |
| Tiazides | 70 (11.9) | 30 (10.8) | 0.737 | 41 (12.6) | 60 (11.1) | 0.516 |
| Aldosterone Blockers | 384 (65.1) | 185 (66.8) | 0.433 | 228 (66.9) | 367(67.8) | 0.597 |
| Statins, n (%) | 416 (70.5) | 197 (71.1) | 0.810 | (72.1) | (72.4) | 0.875 |
| SGLT2-I, n (%) | 124 (21.0) | 61 (22.0) | 0.723 | 98 (30.1) | 124 (22.9) | 0.020 * |
| Study Outcomes at A 1 Year of Follow-Up | Overall Population | ED-CRTd | NED-CRTd | p Value |
|---|---|---|---|---|
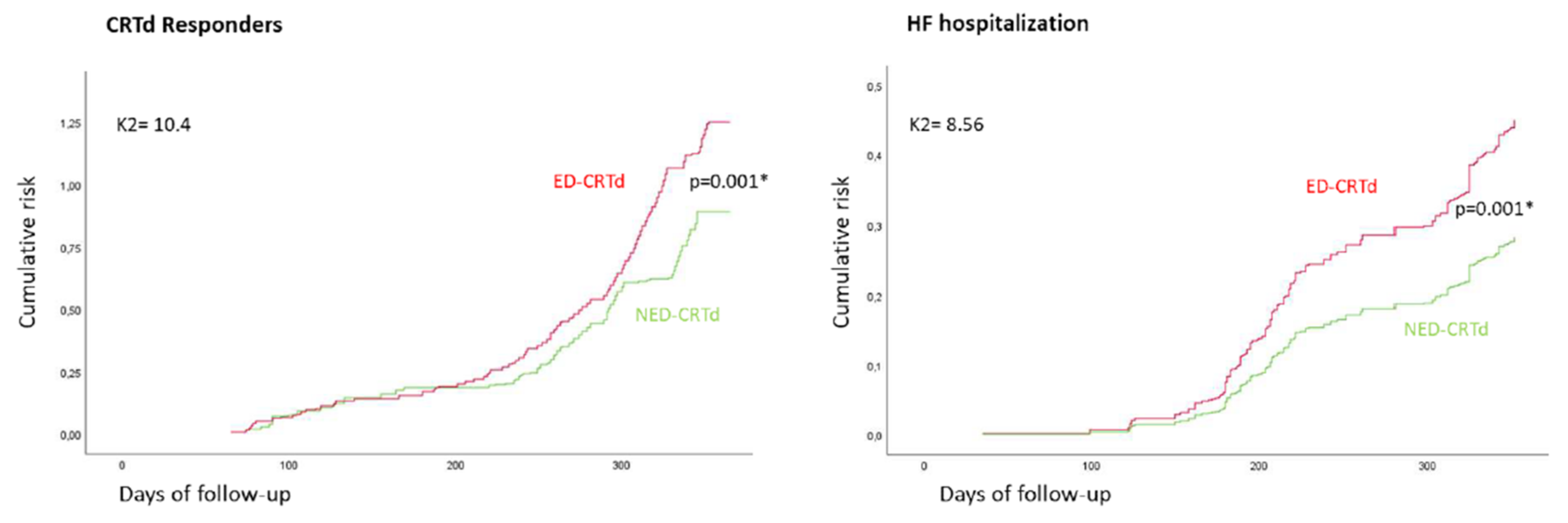
| CRTd responder rate, n (%) | 569 (65.6) | 189 (58) | 380 (70.2) | 0.001 * |
| Hospitalization for heart failure, n (%) | 269 (31.0) | 115 (35.3) | 154 (28.5) | 0.041 * |
| Cardiac deaths, n (%) | 51 (5.8) | 30 (9.2) | 21 (3.9) | 0.002 * |
| All-cause deaths, n (%) | 52 (5.9) | 25 (7.7) | 27 (5.0) | 0.139 |
| (A) | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||||
|---|---|---|---|---|---|---|
| CRTd Responders | CRTd Responders | |||||
| Risk Factors | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age | 0.998 | 0.984–1.012 | 0.737 | |||
| Hypertension | 1.380 | 1.145–1.664 | 0.001 * | 0.818 | 0.669–0.999 | 0.049 * |
| Obesity | 0.849 | 0.620–1.163 | 0.309 | |||
| T2DM | 0.682 | 0.577–1.807 | 0.185 | |||
| 6MWT | 0.989 | 0.907–1.001 | 0.165 | |||
| BNP | 1.012 | 0.999–1.100 | 0.120 | |||
| CRP | 1.012 | 1.004–1.020 | 0.004 * | 1.007 | 0.998–1.015 | 0.133 |
| Lymphocytes | 0.922 | 0.863–0.985 | 0.016 * | 0.820 | 0.758–0.987 | 0.009 * |
| miR-130a-5p | 1.826 | 1.106–2.306 | 0.036 * | 1.490 | 1.014–2.188 | 0.042 * |
| Endothelin-1 | 0.981 | 0.743–0.995 | 0.043 * | 0.859 | 0.839–0.979 | 0.001 * |
| LVEF | 0.976 | 0.961–0.991 | 0.002 * | 0.876 | 0.760–0.992 | 0.004 * |
| ARNI | 1.034 | 0.866–1.235 | 0.710 | |||
| NYHA 3 | 0.812 | 0.685–0.963 | 0.017* | 0.844 | 0.672–1.059 | 0.143 |
| BB | 0.986 | 0.826–1.178 | 0.879 | |||
| ED | 0.362 | 0.153–0.609 | 0.001* | 0.751 | 0.624–0.905 | 0.003* |
| (B) | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||||
| HF Hospitalizations | HF Hospitalizations | |||||
| Risk Factors | HR | 95% CI | p value | HR | 95% CI | p value |
| Age | 0.989 | 0.969–1.019 | 0.900 | |||
| Hypertension | 1.738 | 1.575–1.947 | 0.017 * | 1.818 | 1.720–2.907 | 0.001 * |
| Obesity | 0.971 | 0.622–1.516 | 0.898 | |||
| T2DM | 1.010 | 1.000–1.101 | 0.001 * | |||
| 6MWT | 0.998 | 0.996–1.001 | 0.190 | |||
| BNP | 1.011 | 1.000–1.102 | 0.001 * | 1.210 | 1.000–1.401 | 0.047 * |
| CRP | 0.983 | 0.969–0.997 | 0.018 * | 1.007 | 0.978–1.008 | 0.345 |
| Lymphocytes | 1.083 | 0.986–1.190 | 0.097 | 0.987 | 1.022–1.266 | 0.180 |
| miR-130a-5p | 0.566 | 0.384–0.835 | 0.004 * | 0.332 | 0.347–0.804 | 0.003 * |
| Endothelin-1 | 1.006 | 0.979–1.034 | 0.668 | |||
| LVEF | 1.026 | 1.003–1.050 | 0.029 * | 0.992 | 0.986–1.038 | 0.394 |
| ARNI | 0.160 | 0.086–0.563 | 0.001 * | 0.319 | 0.310–0.572 | 0.001 * |
| NYHA 3 | 1.071 | 0.843–1.360 | 0.576 | |||
| BB | 0.828 | 0.645–1.063 | 0.139 | |||
| ED | 1.301 | 1.232–1.390 | 0.001 * | 1.905 | 1.238–2.241 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardu, C.; Santulli, G.; Savarese, G.; Trotta, M.C.; Sacra, C.; Santamaria, M.; Volpicelli, M.; Ruocco, A.; Mauro, C.; Signoriello, G.; et al. Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p. Int. J. Mol. Sci. 2023, 24, 1510. https://doi.org/10.3390/ijms24021510
Sardu C, Santulli G, Savarese G, Trotta MC, Sacra C, Santamaria M, Volpicelli M, Ruocco A, Mauro C, Signoriello G, et al. Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p. International Journal of Molecular Sciences. 2023; 24(2):1510. https://doi.org/10.3390/ijms24021510
Chicago/Turabian StyleSardu, Celestino, Gaetano Santulli, Gianluigi Savarese, Maria Consiglia Trotta, Cosimo Sacra, Matteo Santamaria, Mario Volpicelli, Antonio Ruocco, Ciro Mauro, Giuseppe Signoriello, and et al. 2023. "Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p" International Journal of Molecular Sciences 24, no. 2: 1510. https://doi.org/10.3390/ijms24021510
APA StyleSardu, C., Santulli, G., Savarese, G., Trotta, M. C., Sacra, C., Santamaria, M., Volpicelli, M., Ruocco, A., Mauro, C., Signoriello, G., Marfella, L., D’Amico, M., Marfella, R., & Paolisso, G. (2023). Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p. International Journal of Molecular Sciences, 24(2), 1510. https://doi.org/10.3390/ijms24021510