Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats
Abstract
1. Introduction
2. Results
2.1. Morphology and Biocompatibility
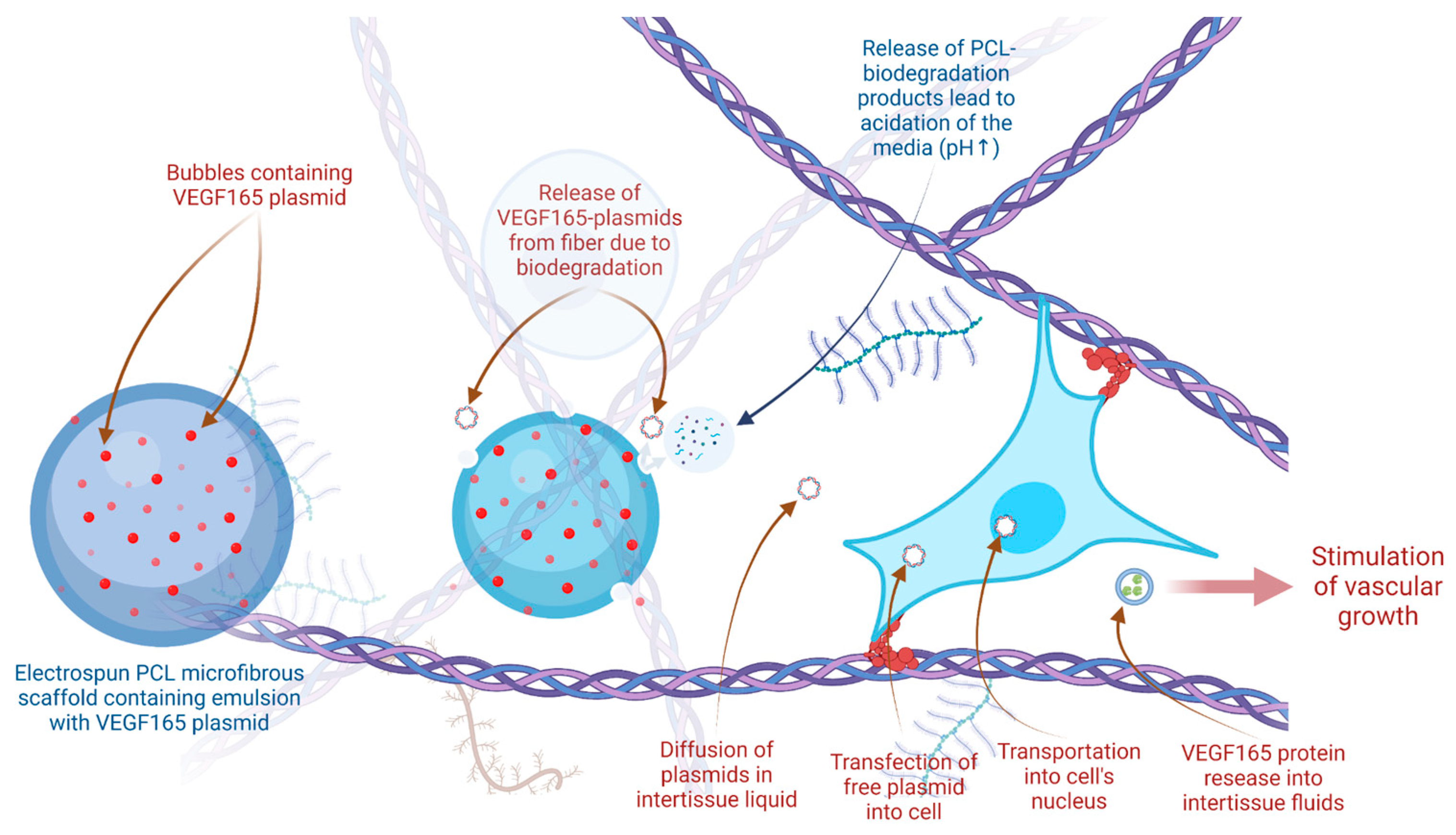
2.2. Angiogenesis
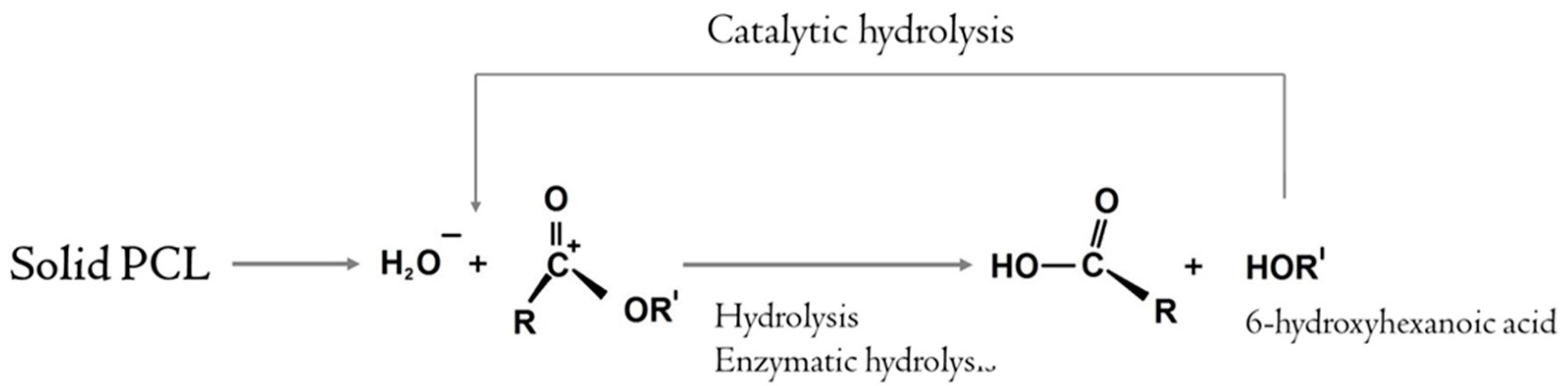
2.3. Biodegradation
3. Discussion
4. Materials and Methods
4.1. VEGF165-Modified Implants
4.2. Animals
4.3. Histology
4.4. Biocompatibility
4.5. Angiogenesis
4.6. Quantitative Assessment of the Bioresorption Dynamics of the Material
4.7. Statistics
4.8. Ethics
5. Conclusions
- (1)
- Microfibrous polycaprolactone scaffolds can be obtained by emulsion electrospinning and enriched with the gene-therapeutic drug Neovasculgen containing the pCMV-VEGF165 plasmid.
- (2)
- After subcutaneous implantation in rats, an increase in the vascular density was observed in microfibrous scaffolds enriched by the plasmid. Scaffolds with high concentration of pCMV-VEGF165 plasmid (0.05 ng of plasmid per 1 mg of PCL) accelerated vascularization compared to scaffolds with low plasmid concentration and control scaffolds. The vascular density on day 33 was 42% higher compared to the Control group (p-value = 0.0344).
- (3)
- The dose-dependent effect was shown between the concentration of the pCMV-VEGF165 plasmid and the vascularization on day 33. The effect was reversible and on day 46 the values in experimental groups did not differ from the control group. The dynamics of vascularization show that gene-activated materials may participate in delayed and prolonged physiological responses.
- (4)
- Microfiber bioresorption rate was higher in scaffolds with a High concentration of the VEGF165 plasmid compared to scaffolds with a Low plasmid concentration on days 33 and 46. However, we have not found a statistically significant correlation between scaffold degradation rate and vessel growth.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandre, N.; Amorim, I.; Caseiro, A.R.; Pereira, T.; Alvites, R.; Rêma, A.; Gonçalves, A.; Valadares, G.; Costa, E.; Santos-Silva, A.; et al. Long term performance evaluation of small-diameter vascular grafts based on polyvinyl alcohol hydrogel and dextran and MSCs-based therapies using the ovine pre-clinical animal model. Int. J. Pharm. 2017, 523, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Qian, E.; Kang, Y. Effects of macro-/micro-channels on vascularization and immune response of tissue engineering scaffolds. Cells 2021, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Control. Release 2021, 332, 460–492. [Google Scholar] [CrossRef] [PubMed]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the wound-healing process by curcumin-loaded chitosan/collagen blend electrospun nanofibers: In vitro and in vivo studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Örgül, D.; Eroğlu, H.; Tiryaki, M.; Pınarlı, F.A.; Hekimoglu, S. In-vivo evaluation of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers and mesenchymal stem cells in diabetic wound healing. J. Drug Deliv. Sci. Technol. 2021, 61, 102140. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Ahmed, M.G.; Gowda, B.H.J.; Shetty, P.K.; Nasrine, A.; Thriveni, M.; Noushida, N.; Sanjana, A. Recent advances in ocular drug delivery systems and targeting VEGF receptors for management of ocular angiogenesis: A comprehensive review. Future J. Pharm. Sci. 2021, 7, 186. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD peptides and incorporation of vascular endothelial growth factor are equally efficient for biofunctionalization of tissue-engineered vascular grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef]
- Kulakov, A.; Kogan, E.; Brailovskaya, T.; Vedyaeva, A.; Zharkov, N.; Krasilnikova, O.; Krasheninnikov, M.; Baranovskii, D.; Rasulov, T.; Klabukov, I. Mesenchymal stromal cells enhance vascularization and epithelialization within 7 days after gingival augmentation with collagen matrices in rabbits. Dent. J. 2021, 9, 101. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. Hif-1α and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikova, O.A.; Baranovskii, D.S.; Lyundup, A.V.; Shegay, P.V.; Kaprin, A.D.; Klabukov, I.D. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev. Rep. 2022, 18, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, C.; Baiguera, S.; Ajalloueian, F.; Bianco, A.; Macchiarini, P. Are synthetic scaffolds suitable for the development of clinical tissue-engineered tubular organs? J. Biomed. Mater. Research. Part A 2014, 102, 2427–2447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shen, Z.; Liang, X.; He, Y.; Kong, D.; Midgley, A.C.; Wang, K. Progress and Current Limitations of Materials for Artificial Bile Duct Engineering. Materials 2021, 14, 7468. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; King, M.W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 2011, 58, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Lemon, G.; Howard, D.; Tomlinson, M.; Buttery, L.; Rose, F.; Waters, S.; King, J.R. Mathematical modelling of tissue-engineered angiogenesis. Math. Biosci. 2009, 221, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mironov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(e-caprolactone) small-diameter vascular grafts in vivo. Front. Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef]
- Klabukov, I.D.; Balyasin, M.V.; Lyundup, A.V.; Krasheninnikov, M.E.; Titov, A.S.; Mudryak, D.L.; Shepelev, A.D.; Tenchurin, T.K.; Chvalun, S.N.; Dyuzheva, T.G. Angiogenic vitalization of biocompatible and biodegradable scaffold (in vivo experimental study). Patol. Fiziol. I Èksperimental’naia Ter. 2018, 62, 53–60. [Google Scholar]
- Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Krivkina, E.O.; Shabaev, A.R.; Rezvova, M.A.; Tkachenko, V.O.; Khanova, M.Y.; Sergeeva, T.Y.; Krutitskiy, S.S.; et al. A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals 2020, 13, 101. [Google Scholar] [CrossRef]
- Simón-Yarza, T.; Formiga, F.R.; Tamayo, E.; Pelacho, B.; Prosper, F.; Blanco-Prieto, M.J. Vascular Endothelial Growth Factor-Delivery Systems for Cardiac Repair: An Overview. Theranostics 2012, 2, 541–552. [Google Scholar] [CrossRef]
- Chen, S.; Galusková, D.; Kaňková, H.; Zheng, K.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis. Materials 2020, 13, 4010. [Google Scholar] [CrossRef] [PubMed]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.; Smits, A.I. Biomaterial-driven in situ cardiovascular tissue engineering—A multi-disciplinary perspective. npj Regen. Med. 2017, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- James, E.N.; Van Doren, E.; Li, C.; Kaplan, D.L. Silk biomaterials-mediated miRNA functionalized orthopedic devices. Tissue Eng. Part A 2019, 25, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tarantul, V.Z.; Gavrilenko, A.V. Gene Therapy for Critical Limb Ischemia: Per Aspera ad Astra. Curr. Gene Ther. 2022, 22, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, V.V.; Chulpanova, D.S.; Tazetdinova, L.G.; Salafutdinov, I.I.; Bozo, I.Y.; Isaev, A.A.; Deev, R.V.; Rizvanov, A.A. In vitro angiogenic properties of plasmid DNA encoding SDF-1α and VEGF165 genes. Appl. Biochem. Biotechnol. 2020, 190, 773–788. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Drobyshev, A.Y.; Redko, N.A.; Komlev, V.S.; Isaev, A.A.; Deev, R.V. Bringing a gene-activated bone substitute into clinical practice: From bench to bedside. Front. Bioeng. Biotechnol. 2021, 9, 599300. [Google Scholar] [CrossRef]
- Eremin, P.S.; Deev, R.V.; Bozo, I.Y.; Deshevoi, Y.B.; Lebedev, V.G.; Eremin, I.I.; Anisimova, S.A.; Nasonova, T.A.; Gil’Mutdinova, I.R.; Moroz, B.B. Tissue Healing after Severe Cutaneous Local Radiation Injuries under Gene-Mediated Induction of Angiogenesis Using “Neovasculgen”. J. Anat. Histopathol. 2020, 9, 26–34. [Google Scholar] [CrossRef]
- Gatina, D.Z.; Garanina, E.E.; Zhuravleva, M.N.; Synbulatova, G.E.; Mullakhmetova, A.F.; Solovyeva, V.V.; Kiyasov, A.P.; Rutland, C.S.; Rizvanov, A.A.; Salafutdinov, I.I. Proangiogenic Effect of 2A-Peptide Based Multicistronic Recombinant Constructs Encoding VEGF and FGF2 Growth Factors. Int. J. Mol. Sci. 2021, 22, 5922. [Google Scholar] [CrossRef]
- Klabukov, I.D.; Krasilnikova, O.A.; Baranovskii, D.S.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Comment on: ‘Regenerative medicine, organ bioengineering and transplantation’. Br. J. Surg. 2021, 108, e386. [Google Scholar] [CrossRef]
- Slobodkina, E.; Boldyreva, M.; Karagyaur, M.; Eremichev, R.; Alexandrushkina, N.; Balabanyan, V.; Akopyan, Z.; Parfyonova, Y.; Tkachuk, V.; Makarevich, P. Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle. Pharmaceutics 2020, 12, 1231. [Google Scholar] [CrossRef]
- Lopes, S.V.; Collins, M.N.; Reis, R.L.; Oliveira, J.M.; Silva-Correia, J. Vascularization approaches in tissue engineering: Recent developments on evaluation tests and modulation. ACS Appl. Bio Mater. 2021, 4, 2941–2956. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches—An overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef]
- Tenchurin, T.; Lyundup, A.; Demchenko, A.; Krasheninnikov, M.; Balyasin, M.; Klabukov, I.; Shepelev, A.; Mamagulashvili, V.; Orehov, A.; Chvalun, S.; et al. Modification of biodegradable fibrous scaffolds with Epidermal Growth Factor by emulsion electrospinning for promotion of epithelial cells proliferation. Genes Cells 2017, 12, 47–52. [Google Scholar] [CrossRef]
- De la Vega, R.E.; Atasoy-Zeybek, A.; Panos, J.A.; Van Griensven, M.; Evans, C.H.; Balmayor, E.R. Gene therapy for bone healing: Lessons learned and new approaches. Transl. Res. 2021, 236, 1–16. [Google Scholar] [CrossRef]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Vasanthan, K.S.; Srinivasan, V.; Mathur, V.; Agarwal, P.; Negi, N.; Kumari, S. 3D Bioprinting for esophageal tissue regeneration: A review. J. Mater. Res. 2022, 37, 88–113. [Google Scholar] [CrossRef]
- Kitpipatkun, P.; Sutummaporn, K.; Kato, K.; Murakami, T.; Kobayashi, K.; Nakazawa, Y.; Tanaka, R. Silk fibroin/polyurethane patch implantation in hyperglycemic rat model. J. Biomater. Appl. 2021, 36, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Konan, S.; Haddad, F.S. A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery. Knee 2009, 16, 6–13. [Google Scholar] [CrossRef]
- Katunin, A.; Wronkowicz, A.; Bilewicz, M.; Wachla, D. Criticality of self-heating in degradation processes of polymeric composites subjected to cyclic loading: A multiphysical approach. Arch. Civ. Mech. Eng. 2017, 17, 806–815. [Google Scholar] [CrossRef]
- Ahadian, S.; Khademhosseini, A. Smart scaffolds in tissue regeneration. Regen. Biomater. 2018, 5, 125–128. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Han, X.; Pan, J.; Buchanan, F.; Weir, N.; Farrar, D. Analysis of degradation data of poly(l-lactide–co-l,d-lactide) and poly(l-lactide) obtained at elevated and physiological temperatures using mathematical models. Acta Biomater. 2010, 6, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J. A model for simultaneous crystallisation and biodegradation of biodegradable polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Isovolemic Degradation of Polycaprolactone Particles and Calculation of Their Original Size from Human Biopsy. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2866. [Google Scholar] [CrossRef]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.M.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Zhu, S.; Liu, Z.; Luo, B.; Lu, L.; Zhou, C. Sustained release of plasmid DNA from PLLA/POSS nanofibers for angiogenic therapy. Chem. Eng. J. 2019, 365, 270–281. [Google Scholar] [CrossRef]
- Inoue, S.; Hakamata, Y.; Kaneko, M.; Kobayashi, E. Gene therapy for organ grafts using rapid injection of naked DNA: Application to the rat liver. Transplantation 2004, 77, 997–1003. [Google Scholar] [CrossRef]
- Wolff, J.A.; Budker, V. The mechanism of naked DNA uptake and expression. Adv Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef]
- Hughes, T.S.; Langer, S.J.; Johnson, K.W.; Chavez, R.A.; Watkins, L.R.; Milligan, E.D.; Leinwand, L.A. Intrathecal injection of naked plasmid DNA provides long-term expression of secreted proteins. Mol. Ther. 2009, 17, 88–94. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Sadick, H.; Naim, R.; Gössler, U.; Hörmann, K.; Riedel, F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int. J. Mol. Med. 2005, 15, 15–19. [Google Scholar] [CrossRef]
- Holzbach, T.; Vlaskou, D.; Neshkova, I.; Konerding, M.A.; Wörtler, K.; Mykhaylyk, O.; Gänsbacher, B.; Machens, H.; Plank, C.; Giunta, R.E. Non-viral VEGF165 gene therapy–magnetofection of acoustically active magnetic lipospheres (‘magnetobubbles’) increases tissue survival in an oversized skin flap model. J. Cell. Mol. Med. 2010, 14, 587–599. [Google Scholar] [PubMed]
- Zhou, J.; Zhao, Y.; Wang, J.; Zhang, S.; Liu, Z.; Zhen, M.; Liu, Y.; Liu, P.; Yin, Z.; Wang, X. Therapeutic Angiogenesis Using Basic Fibroblast Growth Factor in Combination with a Collagen Matrix in Chronic Hindlimb Ischemia. Sci. World J. 2012, 2012, 652794. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Kedem, A.; Ruvinov, E.; Levy, O.; Freeman, I.; Landa, N.; Holbova, R.; Feinberg, M.S.; Dror, S.; Etzion, Y.; et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA 2009, 106, 14990–14995. [Google Scholar] [CrossRef]
- Amaral, J.; Lee, J.W.; Chou, J.; Campos, M.M.; Rodríguez, I.R. 7-Ketocholesterol induces inflammation and angiogenesis in vivo: A novel rat model. PLoS ONE 2013, 8, e56099. [Google Scholar] [CrossRef] [PubMed]
- Shaterian, A.; Borboa, A.; Sawada, R.; Costantini, T.; Potenza, B.; Coimbra, R.; Baird, A.; Eliceiri, B.P. Real-time analysis of the kinetics of angiogenesis and vascular permeability in an animal model of wound healing. Burns 2009, 35, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Lenard, A.; Daetwyler, S.; Betz, C.; Ellertsdottir, E.; Belting, H.G.; Huisken, J.; Affolter, M. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 2015, 13, e1002126. [Google Scholar] [CrossRef]
- Martin, J.R.; Gupta, M.K.; Page, J.M.; Yu, F.; Davidson, J.M.; Guelcher, S.A.; Duvall, C.L. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials 2014, 35, 3766–3776. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, A.J. Pathology of cemented low-friction arthroplasties in autopsy specimens. In Implant Bone Interface; Springer: London, UK, 1990; pp. 77–82. [Google Scholar]
- Araco, A.; Caruso, R.; Araco, F.; Overton, J.; Gravante, G. Capsular contractures: A systematic review. Plast. Reconstr. Surg. 2009, 124, 1808–1819. [Google Scholar] [CrossRef]
- Malahias, M.; Jordan, D.J.; Hughes, L.C.; Hindocha, S.; Juma, A. A literature review and summary of capsular contracture: An ongoing challenge to breast surgeons and their patients. Int. J. Surg. Open 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Brode, G.L.; Koleske, J.V. Lactone polymerization and polymer properties. J. Macromol. Sci.—Chem. 1972, 6, 1109–1144. [Google Scholar] [CrossRef]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 90, 906–919. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- Barone, G.D.; Ferizović, D.; Biundo, A.; Lindblad, P. Hints at the Applicability of Microalgae and Cyanobacteria for the Biodegradation of Plastics. Sustainability 2020, 12, 10449. [Google Scholar] [CrossRef]
- Piperno, A.; Sciortino, M.T.; Giusto, E.; Montesi, M.; Panseri, S.; Scala, A. Recent advances and challenges in gene delivery mediated by polyester-based nanoparticles. Int. J. Nanomed. 2021, 16, 5981. [Google Scholar] [CrossRef]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Versypt, A.N.F.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Walejewska, E.; Idaszek, J.; Heljak, M.; Chlanda, A.; Choinska, E.; Hasirci, V.; Swieszkowski, W. The effect of introduction of filament shift on degradation behaviour of PLGA-and PLCL-based scaffolds fabricated via additive manufacturing. Polym. Degrad. Stab. 2020, 171, 109030. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, H.; Ye, S.H.; Yoshizumi, T.; Wagner, W.R. Tailoring the degradation rates of thermally responsive hydrogels designed for soft tissue injection by varying the autocatalytic potential. Biomaterials 2015, 53, 484–493. [Google Scholar] [CrossRef] [PubMed]



| Day | Type of Sample | Concentration of Neovasculgen (VEGF165 Plasmid) in Buffer (Ratio of the Mass of VEGF165 Plasmid per Mass of PCL Scaffold) | Porosity, % | Mean Pore Size, μm | Mass of Sample (Mean ± SD), mg | Mass of VEGF165 Plasmid per Sample, ng |
|---|---|---|---|---|---|---|
| 1 | Control | No | 91.1 | 54 | 7.9 ± 2.2 | 0 |
| 2 | Low concentration | 0.005 μg/mL (0.005 ng/mg) | 90.8 | 46.5 | 12.5 ± 2.9 | 0.063 ± 0.015 |
| 3 | High concentration | 0.05 μg/mL (0.05 ng/mg) | 93.5 | 36 | 14.7 ± 3.7 | 0.74 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399. https://doi.org/10.3390/ijms24021399
Klabukov I, Balyasin M, Krasilnikova O, Tenchurin T, Titov A, Krasheninnikov M, Mudryak D, Sulina Y, Shepelev A, Chvalun S, et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. International Journal of Molecular Sciences. 2023; 24(2):1399. https://doi.org/10.3390/ijms24021399
Chicago/Turabian StyleKlabukov, Ilya, Maksim Balyasin, Olga Krasilnikova, Timur Tenchurin, Alexander Titov, Mikhail Krasheninnikov, Daniil Mudryak, Yana Sulina, Alexey Shepelev, Sergei Chvalun, and et al. 2023. "Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats" International Journal of Molecular Sciences 24, no. 2: 1399. https://doi.org/10.3390/ijms24021399
APA StyleKlabukov, I., Balyasin, M., Krasilnikova, O., Tenchurin, T., Titov, A., Krasheninnikov, M., Mudryak, D., Sulina, Y., Shepelev, A., Chvalun, S., Dyuzheva, T., Yakimova, A., Sosin, D., Lyundup, A., Baranovskii, D., Shegay, P., & Kaprin, A. (2023). Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. International Journal of Molecular Sciences, 24(2), 1399. https://doi.org/10.3390/ijms24021399








