Tricarboxylic Acid Metabolite Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery
Abstract
1. Introduction
2. Results
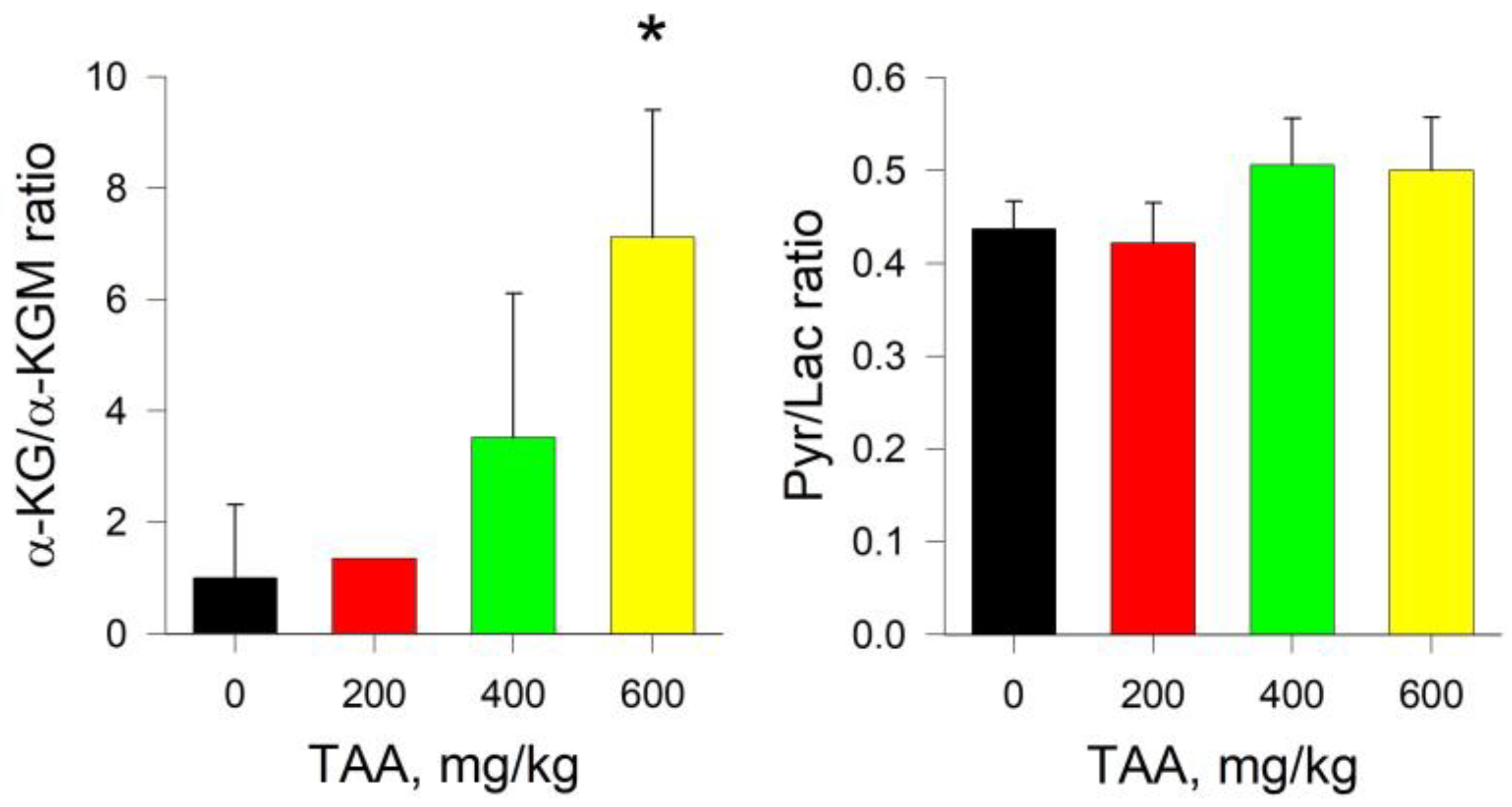
2.1. Rat Blood Plasma
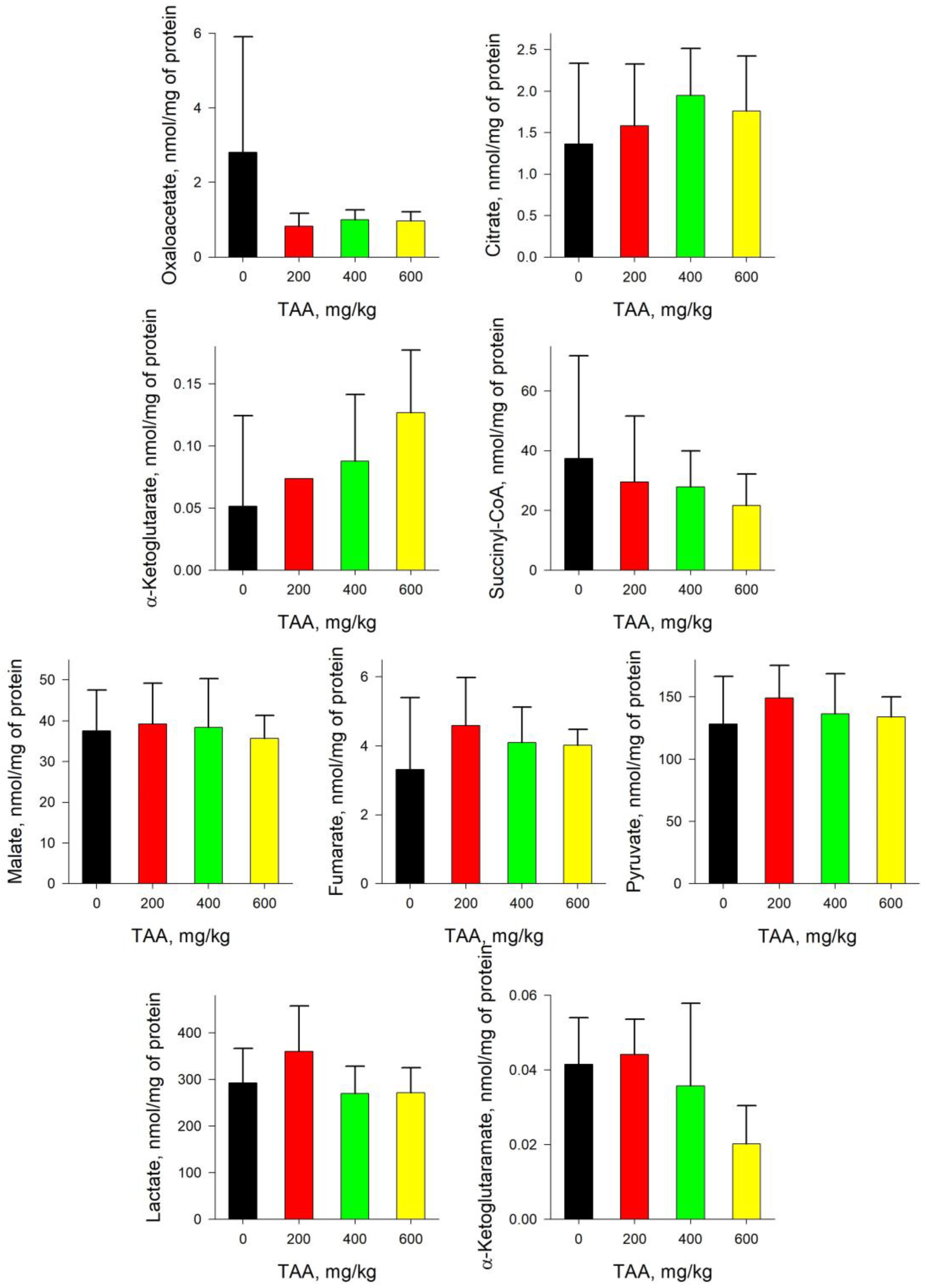
2.2. Rat Liver
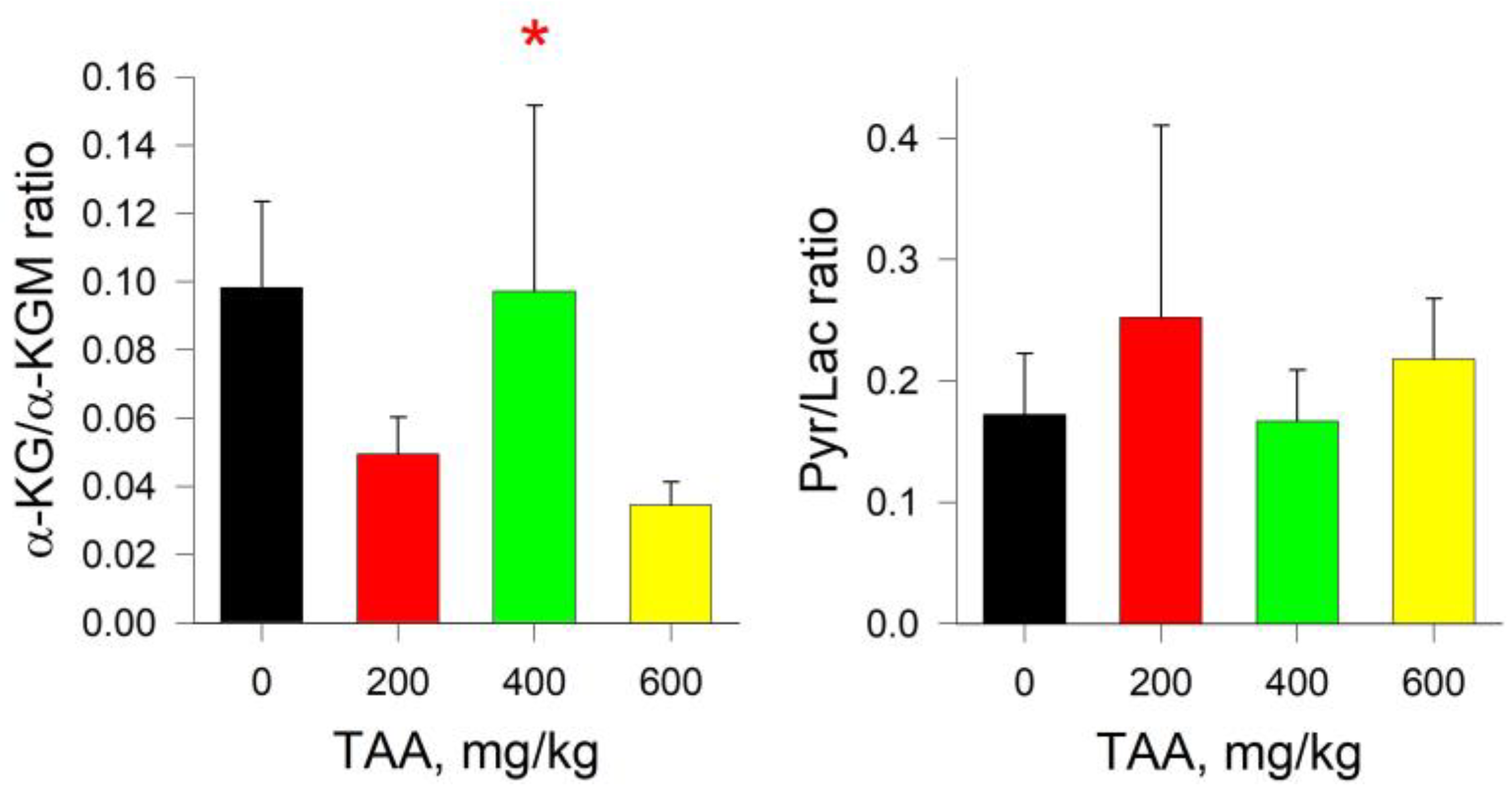
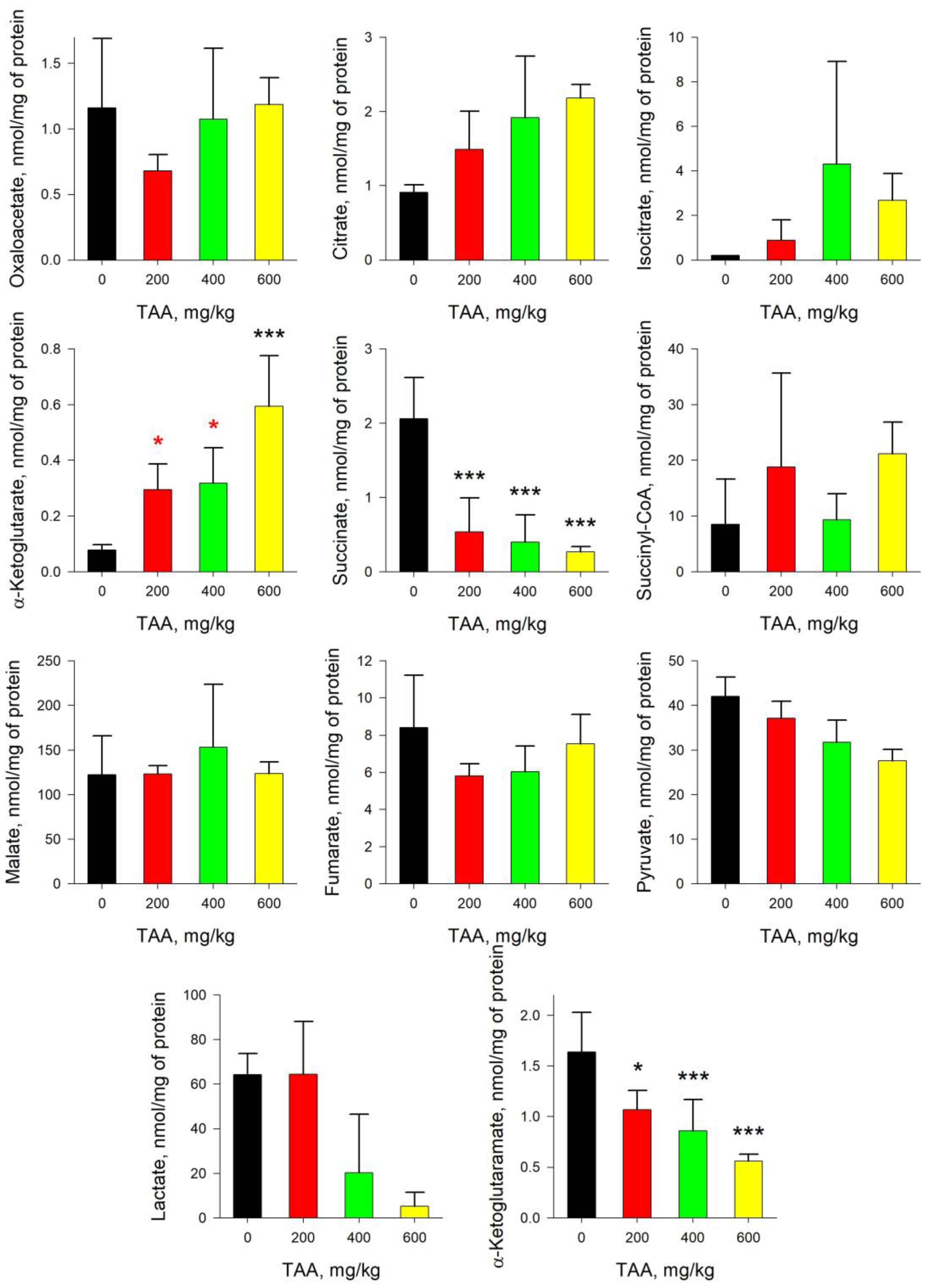
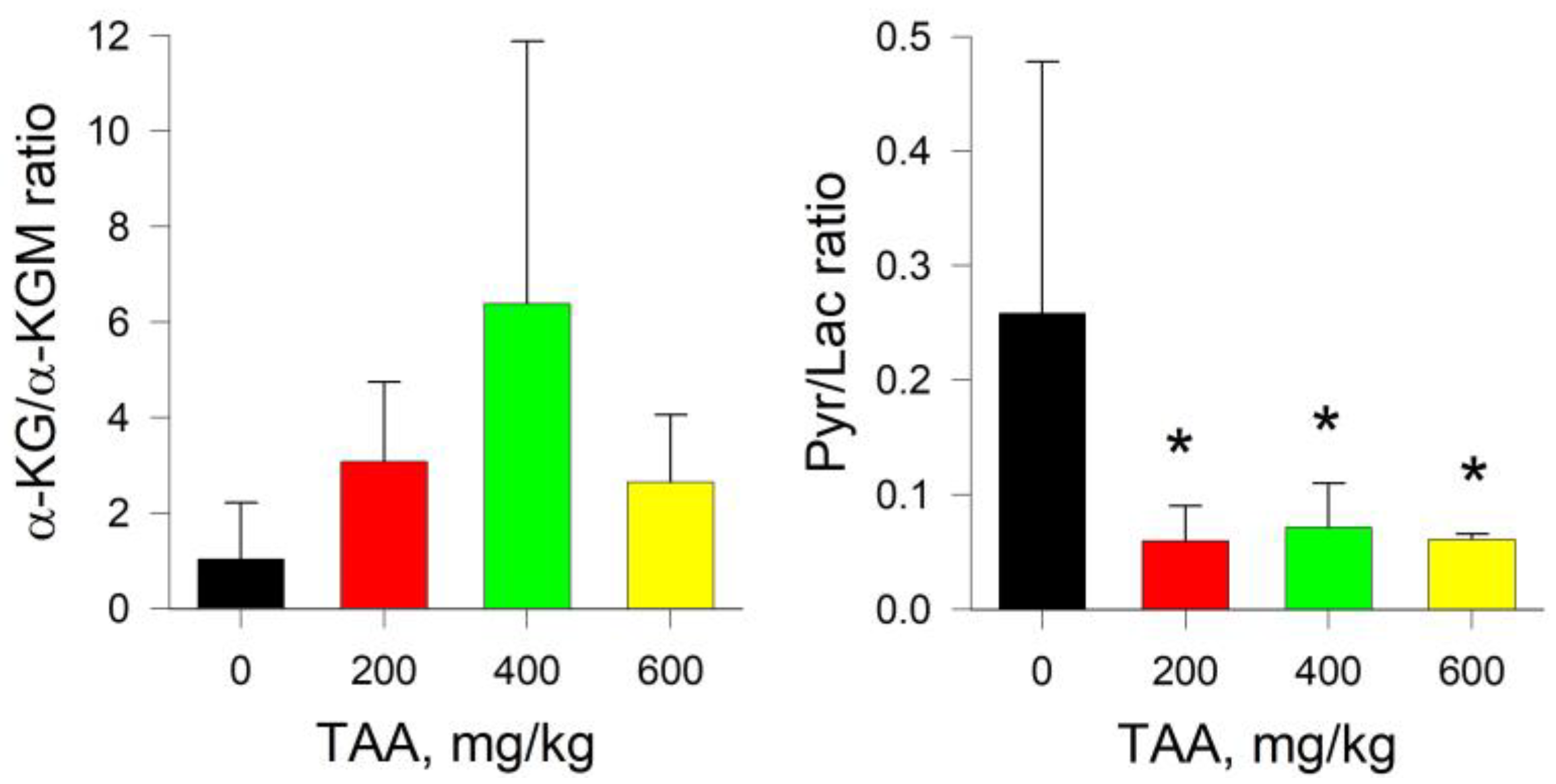
2.3. Rat Kidney
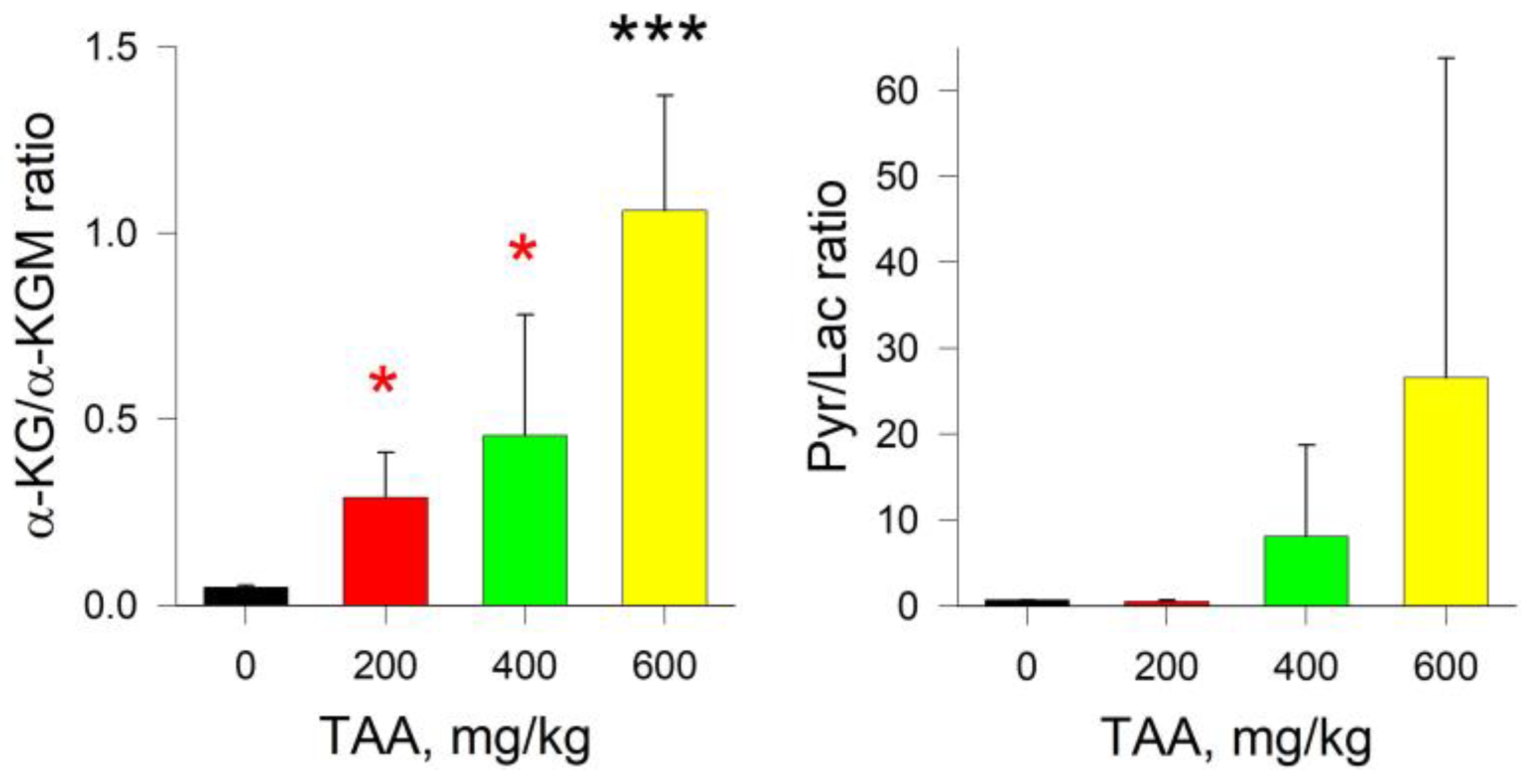
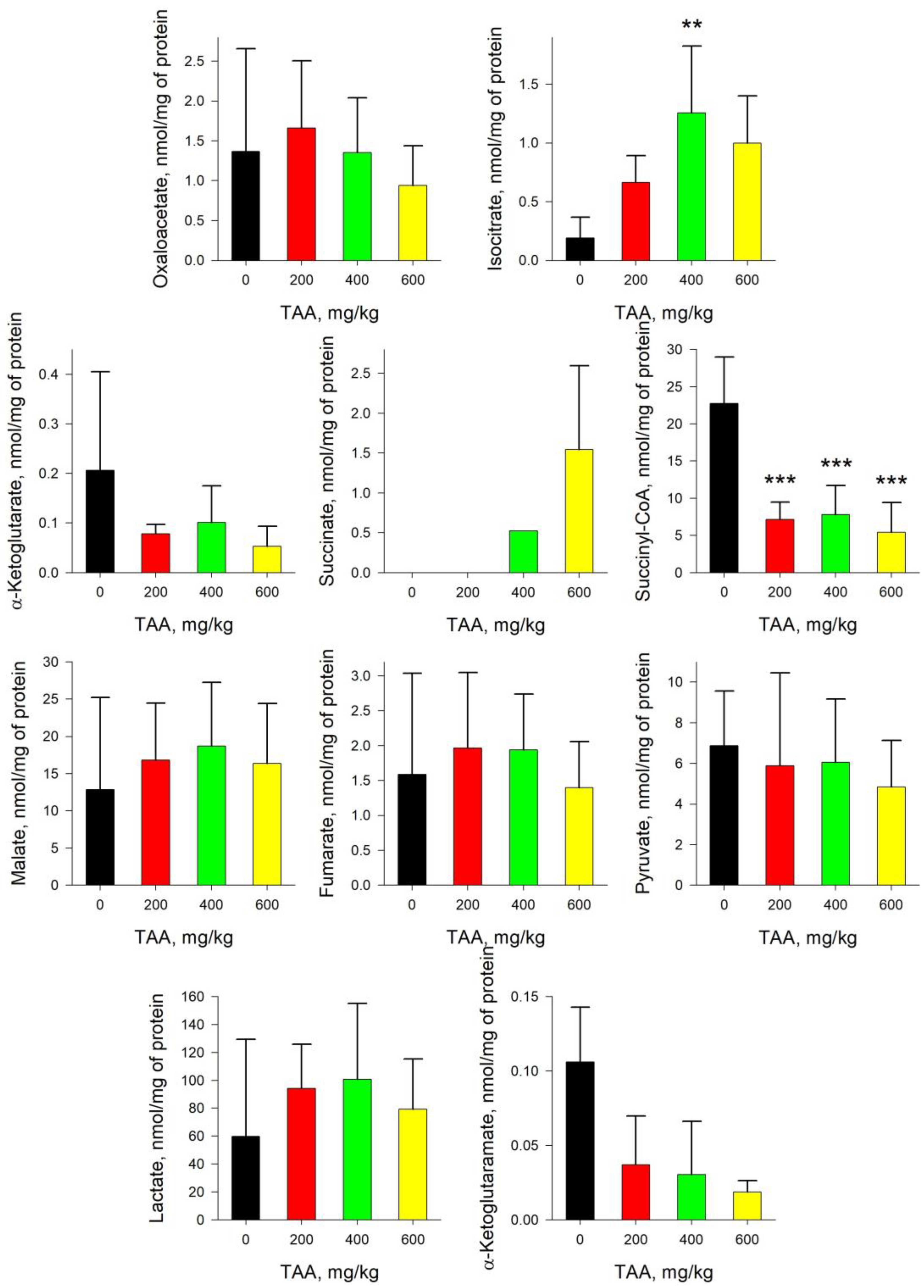
2.4. Rat Brain
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Rat Model of Liver Failure
4.3. Preparation of Samples for HPLC Analysis
4.4. Preparation of Tissue Samples for Analysis
4.5. Preparation of Plasma Samples for Analysis
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy-Definition, nomenclature, diagnosis, and quantification: Final report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P. Hepatic encephalopathy: Diagnosis and management. Liver Int. 2018, 38, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Kala, G. Energy metabolism in brain cells: Effects of elevated ammonia concentrations. Metab. Brain Dis. 2007, 22, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Cooper, A.J. Brain α-ketoglutarate dehydrogenase complex: Kinetic properties, regional distribution, and effects of inhibitors. J. Neurochem. 1986, 47, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Montagnese, S.; Jalan, R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J. Hepatol. 2015, 62, 437–447. [Google Scholar] [CrossRef]
- Shmarakov, I.O.; Borschovetska, V.L.; Marchenko, M.M.; Blaner, W.S. Retinoids modulate thioacetamide-induced acute hepatotoxicity. Toxicol. Sci. 2014, 139, 284–292. [Google Scholar] [CrossRef]
- Mladenović, D.; Krstić, D.; Colović, M.; Radosavljević, T.; Rasić-Marković, A.; Hrncić, D.; Macut, D.; Stanojlović, O. Different sensitivity of various brain structures to thioacetamide-induced lipid peroxidation. Med. Chem. 2012, 8, 52–58. [Google Scholar] [CrossRef]
- Bruck, R.; Weiss, S.; Traister, A.; Zvibel, I.; Aeed, H.; Halpern, Z.; Oren, R. Induced hypothyroidism accelerates the regression of liver fibrosis in rats. J. Gastroenterol. Hepatol. 2007, 22, 2189–2194. [Google Scholar] [CrossRef]
- Koblinova, E.; Mrazova, I.; Vernerova, Z.; Ryska, M. Acute Liver Failure Induced by Thioacetamide: Selection of Optimal Dosage in Wistar and Lewis Rats. Physiol. Res. 2014, 63, 491–503. [Google Scholar] [CrossRef]
- Díez-Fernández, C.; Boscá, L.; Fernández-Simón, L.; Alvarez, A.; Cascales, M. Relationship between genomic DNA ploidy and parameters of liver damage during necrosis and regeneration induced by thioacetamide. Hepatology 1993, 18, 912–918. [Google Scholar] [CrossRef]
- Murphy, M.G.; Crocker, J.F.; Lee, S.H.; Acott, P.; Her, H. Sequestration of coenzyme A by the industrial surfactant, Toximul MP8. A possible role in the inhibition of fatty-acid β-oxidation in a surfactant/influenza B virus mouse model for acute hepatic encephalopathy. Biochim. Biophys. Acta 1997, 1361, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Pistone, G.; Elvira, R.; Leotta, C.; Scarpello, L.; Liborio, R. Effects of L-carnitine in patients with hepatic encephalopathy. World J. Gastroenterol. 2005, 11, 7197–7202. [Google Scholar] [CrossRef] [PubMed]
- Shurubor, Y.I.; Cooper, A.J.L.; Krasnikov, A.B.; Isakova, E.P.; Deryabina, Y.I.; Beal, M.F.; Krasnikov, B.F. Changes of Coenzyme A and Acetyl-Coenzyme A Concentrations in Rats After a Single-Dose Intraperitoneal Injection of Hepatotoxic Thioacetamide are not Consistent with Rapid Recovery. Int. J. Mol. Sci. 2020, 21, 8918. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Lai, J.C.K.; Gelbard, A.S. Ammonia in Liver and Extrahepatic Tissues: An Overview of Metabolism and Toxicity in Mammals. Hepatic Enceph. 1989, 22, 27–48. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Kuhara, T. α-Ketoglutaramate: An overlooked metabolite of glutamine and a biomarker for hepatic encephalopathy and inborn errors of the urea cycle. Metab. Brain Dis. 2014, 29, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Shurubor, Y.; Cooper, A.J.; Isakova, E.P.; Deryabina, Y.I.; Beal, M.F.; Krasnikov, B.F. HPLC determination of α-ketoglutaramate [5-amino-2,5-dioxopentanoate] in biological samples. Anal. Biochem. 2016, 494, 52–54. [Google Scholar] [CrossRef]
- Nikulin, M.; Drobot, V.; Švedas, V.; Krasnikov, B.F. Preparative Biocatalytic Synthesis of α-Ketoglutaramate. Int. J. Mol. Sci. 2021, 22, 12748. [Google Scholar] [CrossRef] [PubMed]
- Shurubor, Y.; Cooper, A.J.; Isakova, E.P.; Deryabina, Y.I.; Beal, M.F.; Krasnikov, B.F. Simultaneous determination of tricarboxylic acid cycle metabolites by high-performance liquid chromatography with ultraviolet detection. Anal. Biochem. 2016, 503, 8–10. [Google Scholar] [CrossRef]
- Butterworth, R.F. Pathophysiology of hepatic encephalopathy: A new look at ammonia. Metab. Brain Dis. 2002, 17, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hindfelt, B.; Plum, F.; Duffy, T.E. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J. Clin. Investig. 1977, 59, 386–396. [Google Scholar] [CrossRef]
- Ratnakumari, L.; Murthy, C.R. Response of rat cerebral glycolytic enzymes to hyperammonemic states. Neurosci. Lett. 1993, 161, 37–40. [Google Scholar] [CrossRef]
- Zwingmann, C.; Chatauret, N.; Leibfritz, D.; Butterworth, R.F. Selective increase of brain lactate synthesis in experimental acute liver failure: Results of a [1H-13C] nuclear magnetic resonance study. Hepatology 2003, 37, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Katanuma, N.; Okada, M.; Nishii, Y. Regulation of urea cycle and TCA cycle by ammonia. Adv. Enzym. Regul. 1966, 4, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Tofteng, F.; Larsen, F.S. The effect of indomethacin on intracranial pressure, cerebral perfusion and extracellular lactate and glutamate concentrations in patients with fulminant hepatic failure. J. Cereb. Blood Flow Metab. 2004, 24, 798–804. [Google Scholar] [CrossRef]
- Kala, G.; Hertz, L. Ammonia effects on pyruvate/lactate production in astrocytes—Interaction with glutamate. Neurochem. Int. 2005, 47, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Donaldson, N.; Wyncoll, D.; Wendon, J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: A cohort study. Lancet 2002, 359, 558–563. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Larsen, F.S. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit. Care Med. 2006, 34, 337–343. [Google Scholar] [CrossRef]
- Bessman, S.P.; Bessman, A.N. The cerebral and peripheral uptake of ammonia in liver disease with an hypothesis for the mechanism of hepatic coma. J. Clin. Investig. 1955, 34, 622–628. [Google Scholar] [CrossRef]
- Lai, J.C.K.; Cooper, A.J.L. Neurotoxicity of ammonia and fatty acids: Differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme a derivatives. Neurochem. Res. 1991, 16, 795–803. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.; Grau, E.; Minana, M.D.; Marcaida, G.; Grisolia, S.; Felipo, V. Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of the NMDA receptor and Na+,K+-ATPase. J. Neurochem. 1994, 63, 2172–2178. [Google Scholar] [CrossRef]
- Xue, Z.; Li, B.; Gu, L.; Hu, X.; Li, M.; Butterworth, R.F.; Peng, L. Increased Na, K-ATPase α2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem. Int. 2010, 57, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.R.; Reddy, P.V.; Tong, X.; Norenberg, M.D. Brain edema in acute liver failure: Inhibition by L-histidine. Am. J. Pathol. 2010, 176, 1400–1408. [Google Scholar]
- Kuhara, T.; Inoue, Y.; Ohse, M.; Krasnikov, B.F.; Cooper, A.J.L. Urinary 2-hydroxy-5-oxoproline, the lactam form of α-ketoglutaramate, is markedly increased in urea cycle disorders. Anal. Bioanal. Chem. 2011, 400, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Shurubor, Y.I.; Matson, W.R.; Willett, W.C.; Hankinson, S.E.; Kristal, B.S. Biological variability dominates and influences analytical variance in HPLC-ECD studies of the human plasma metabolome. BMC Clin. Pathol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurubor, Y.I.; Rogozhin, A.E.; Isakova, E.P.; Deryabina, Y.I.; Krasnikov, B.F. Tricarboxylic Acid Metabolite Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery. Int. J. Mol. Sci. 2023, 24, 1384. https://doi.org/10.3390/ijms24021384
Shurubor YI, Rogozhin AE, Isakova EP, Deryabina YI, Krasnikov BF. Tricarboxylic Acid Metabolite Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery. International Journal of Molecular Sciences. 2023; 24(2):1384. https://doi.org/10.3390/ijms24021384
Chicago/Turabian StyleShurubor, Yevgeniya I., Alexander E. Rogozhin, Elena P. Isakova, Yulia I. Deryabina, and Boris F. Krasnikov. 2023. "Tricarboxylic Acid Metabolite Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery" International Journal of Molecular Sciences 24, no. 2: 1384. https://doi.org/10.3390/ijms24021384
APA StyleShurubor, Y. I., Rogozhin, A. E., Isakova, E. P., Deryabina, Y. I., & Krasnikov, B. F. (2023). Tricarboxylic Acid Metabolite Imbalance in Rats with Acute Thioacetamide-Induced Hepatic Encephalopathy Indicates Incomplete Recovery. International Journal of Molecular Sciences, 24(2), 1384. https://doi.org/10.3390/ijms24021384






