Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure
Abstract
1. Introduction
2. The Association between Gut Microbiota and the Development and Progression of Hypertension
3. Molecular Basis of Hypertension
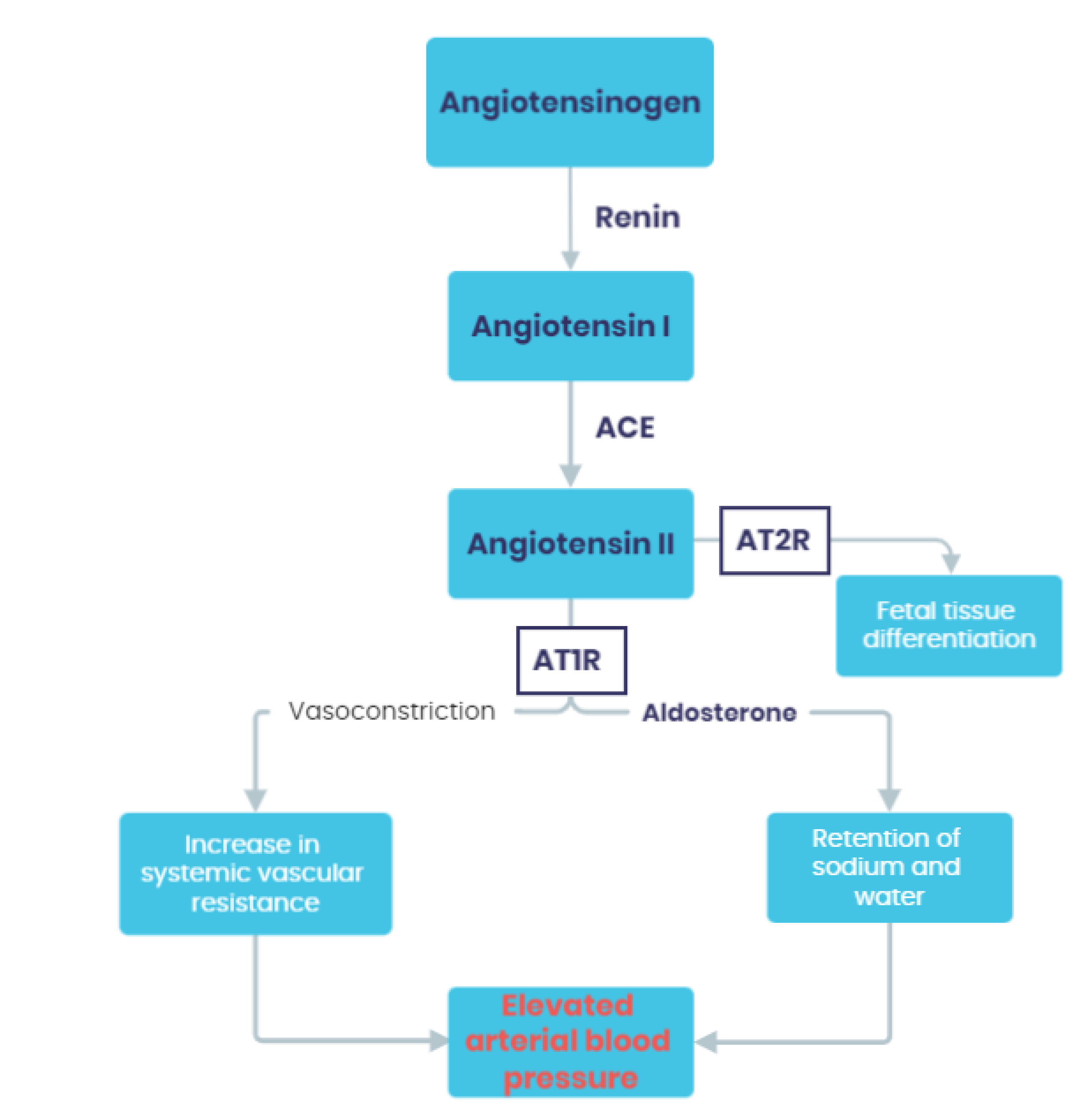
3.1. Activation of the Renin-Angiotensin-Aldosterone System (RAAS)
3.2. Baroreceptors
3.3. Adrenergic Receptors
3.4. Natriuretic Peptides
3.5. The Kinin–Kallikrein System (KKS)
3.6. Microbiota-Derived Metabolites
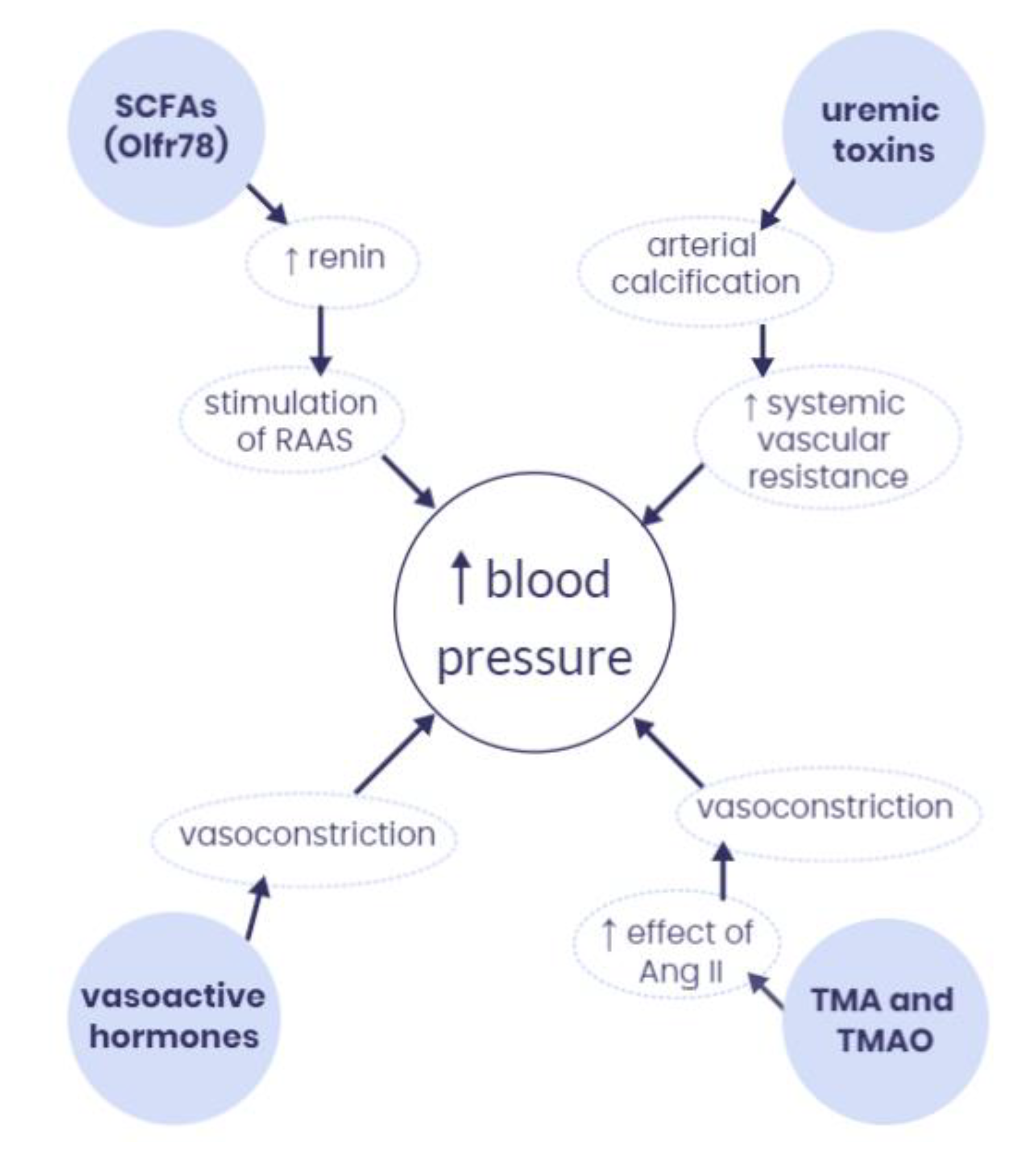
4. How Does the Microbiota-Derived Metabolites Interact with Hypertension at the Molecular Level?
4.1. Short-Chain Fatty Acids (SCFAs)
4.2. Vasoactive Hormones
4.3. Trimethylamine (TMA) and Trimethylamine N-Oxide (TMAO)
4.4. Uremic Toxins
5. Potential Use of Probiotics in Treatment of Hypertension
- ○
- Killing hostile microorganisms;
- ○
- Developing drugs that work as growth inhibitors of unwanted bacteria species;
- ○
- Creating substances that mimic function of SCFA so it could lower the price of probiotics and make it more accessible in regions where storing living organisms might be difficult (because of electricity deficiency or lower hygiene standards).
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kućmierz, J.; Frąk, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Molecular Interactions of Arterial Hypertension in Its Target Organs. Int. J. Mol. Sci. 2021, 22, 9669. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Pardell, H.; Armario, P.; Hernández, R. Pathogénie et épidémiologie de l’hypertension artérielle. Drugs 1998, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawes, C.M.; Hoorn, S.V.; Rodgers, A. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.X.; Zhou, B.; Chen, Z.; Ren, Q.; Lu, S.H.; Sawamura, T.; Han, Z.C. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J. Lipid Res. 2006, 47, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Subahpacker, C. Estrogen protection, oxidized LDL, endothelial dysfunction and vasorelaxation in cardiovascular disease: New insights into a complex issue. Cardiovasc. Res. 2007, 73, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Drosos, I.; Tavridou, A.; Kolios, G. New Aspects on the Metabolic role of Intestinal Microbiota in the Development of Atherosclerosis. Metabolism 2015, 64, 476–481. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 4592–4598. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R., Jr.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure: The CARDIA Study. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, M.; Wang, B.; Lin, H.; Wu, E.; Zhao, H.; Li, S. Differential Analysis of Hypertension-Associated Intestinal Microbiota. Int. J. Med. Sci. 2019, 16, 872–881. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Collard, D.; Prodan, A.; Levels, J.H.M.; Zwinderman, A.H.; Bäckhed, F.; Vogt, L.; Peters, M.J.L.; Muller, M.; Nieuwdorp, M.; et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: The HELIUS study. Eur. Heart J. 2020, 41, 4259–4267. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 63. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, R. Reflexes from the heart. Physiol. Rev. 1991, 71, 617–658. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Narkiewicz, K. Sympathetic nervous system and arterial hypertension: New perspectives, new data. Kardiol. Pol. 2013, 71, 441–446. [Google Scholar] [CrossRef]
- Sorota, S. The Sympathetic Nervous System as a Target for the Treatment of Hypertension and Cardiometabolic Diseases. J. Cardiovasc. Pharmacol. 2014, 63, 466–476. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic Peptides: Their Structures, Receptors, Physiologic Functions and Therapeutic Applications. In cGMP: Generators, Effectors and Therapeutic Implications; Schmidt, H.H.H.W., Hofmann, F., Stasch, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 191, pp. 341–366. [Google Scholar] [CrossRef]
- Rubattu, S.; Gallo, G. The Natriuretic Peptides for Hypertension Treatment. High Blood Press Cardiovasc. Prev. 2022, 29, 15–21. [Google Scholar] [CrossRef]
- Kashuba, E.; Bailey, J.; Allsup, D.; Cawkwell, L. The kinin–kallikrein system: Physiological roles, pathophysiology and its relationship to cancer biomarkers. Biomarkers 2013, 18, 279–296. [Google Scholar] [CrossRef]
- Sharma, J.N.; Narayanan, P. The kallikrein-kinin pathways in hypertension and diabetes. Prog. Drug Res. 2014, 69, 15–36. [Google Scholar] [PubMed]
- Robles-Vera, I.; Toral, M.; Duarte, J. Microbiota and Hypertension: Role of the Sympathetic Nervous System and the Immune System. Am. J. Hypertens. 2020, 33, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Raj, D. Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Thandassery, R.B.; Bhargava, N. Double pylorus: An optical illusion or reality? Gastroenterology 2012, 143, e7–e8. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin–angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Cardinale, J.P.; Sriramula, S.; Pariaut, R.; Guggilam, A.; Mariappan, N.; Elks, C.M.; Francis, J. HDAC Inhibition Attenuates Inflammatory, Hypertrophic, and Hypertensive Responses in Spontaneously Hypertensive Rats. Hypertension 2010, 56, 437–444. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef] [PubMed]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug. Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Stier, C.T. Serotonin and Dopamine in Essential Hypertension. Am. J. Hypertens. 2013, 26, 151. [Google Scholar] [CrossRef]
- Esler, M.; Jackman, G.; Bobik, A.; Leonard, P.; Kelleher, D.; Skews, H.; Jennings, G.; Korner, P. Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension 1981, 3, 149–156. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Wu, W.-K.; Chen, C.-C.; Liu, P.-Y.; Panyod, S.; Liao, B.-Y.; Chen, P.-C.; Kao, H.-L.; Kuo, H.-C.; Kuo, C.-H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef]
- Maksymiuk, K.M.; Szudzik, M.; Gawryś-Kopczyńska, M.; Onyszkiewicz, M.; Samborowska, E.; Mogilnicka, I.; Ufnal, M. Trimethylamine, a gut bacteria metabolite and air pollutant, increases blood pressure and markers of kidney damage including proteinuria and KIM-1 in rats. J. Transl. Med. 2022, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-Oxide: A Carnitine-Derived Metabolite That Prolongs the Hypertensive Effect of Angiotensin II in Rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Hartiala, J.; Bennett, B.J.; Tang, W.W.; Wang, Z.; Stewart, A.F.; Roberts, R.; McPherson, R.; Lusis, A.J.; Hazen, S.L.; Allayee, H. Comparative Genome-Wide Association Studies in Mice and Humans for Trimethylamine N-Oxide, a Proatherogenic Metabolite of Choline and l-Carnitine. Arter. Thromb. Vasc. Biol. 2014, 34, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Barri, Y.M. Hypertension and kidney disease: A deadly connection. Curr. Cardiol. Rep. 2006, 8, 411–417. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure with Aging in Mice and Humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef]
- Kumar, T.; Dutta, R.R.; Velagala, V.R.; Ghosh, B.; Mudey, A. Analyzing the Complicated Connection between Intestinal Microbiota and Cardiovascular Diseases. Cureus 2022, 14, e28165. [Google Scholar] [CrossRef]
- Wang, A.; Bolen, D.W. A Naturally Occurring Protective System in Urea-Rich Cells: Mechanism of Osmolyte Protection of Proteins against Urea Denaturation. Biochemistry 1997, 36, 9101–9108. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Pączek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar] [CrossRef]
- Opdebeeck, B.; D’Haese, P.C.; Verhulst, A. Molecular and Cellular Mechanisms that Induce Arterial Calcification by Indoxyl Sulfate and P-Cresyl Sulfate. Toxins 2020, 12, 58. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Mishima, E.; Abe, T. Role of the microbiota in hypertension and antihypertensive drug metabolism. Hypertens. Res. 2022, 45, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Pevsner-Fischer, M.; Blacher, E.; Tatirovsky, E.; Ben-Dov, I.Z.; Elinav, E. The gut microbiome and hypertension. Curr. Opin. Nephrol. Hypertens. 2017, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bordin, M.; D’Atri, F.; Guillemot, L.; Citi, S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2004, 2, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Utrilla, P.; Comalada, M.; Zorrilla, P.; Garrido-Mesa, J.; Zarzuelo, A.; Rodríguez-Cabezas, M.E.; Gálvez, J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem. Pharmacol. 2011, 82, 1891–1900. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Camuesco, D.; Arribas, B.; Comalada, M.; Bailón, E.; Cueto-Sola, M.; Utrilla, P.; Nieto, A.; Zarzuelo, A.; Rodríguez-Cabezas, M.E.; et al. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol. Res. 2011, 63, 308–319. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol.-Ren. Physiol. 2013, 305, F439–F444. [Google Scholar] [CrossRef]
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, J.L.; Ferrannini, E.; Grundy, S.M.; Haffner, S.M.; Heine, R.J.; Horton, E.S.; Kawamori, R. Primary Prevention of Cardiovascular Disease and Type 2 Diabetes in Patients at Metabolic Risk: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 3671–3689. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Sharafedtinov, K.K.; Sun, J.; Buys, N.; Jayasinghe, R. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—A randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Utrilla, M.P.; Garrido-Mesa, N.; Rodríguez-Cabezas, M.E.; Olivares, M.; Gálvez, J.; et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014, 127, 33–45. [Google Scholar] [CrossRef]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef]
- Huart, J.; Leenders, J.; Taminiau, B.; Descy, J.; Saint-Remy, A.; Daube, G.; Krzesinski, J.-M.; Melin, P.; de Tullio, P.; Jouret, F. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension 2019, 74, 1005–1013. [Google Scholar] [CrossRef]
- Wu, D.; Tang, X.; Ding, L.; Cui, J.; Wang, P.; Du, X.; Yin, J.; Wang, W.; Chen, Y.; Zhang, T. Candesartan attenuates hypertension-associated pathophysiological alterations in the gut. Biomed. Pharmacother. 2019, 116, 109040. [Google Scholar] [CrossRef]
- Matsutomo, T. Potential benefits of garlic and other dietary supplements for the management of hypertension (Review). Exp. Ther. Med. 2019, 19, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Mikhael, A.-M.; Davoodvandi, A.; Jafarnejad, S. Effect of Lactobacillusplantarum containing probiotics on blood pressure: A systematic review and meta-analysis. Pharmacol. Res. 2020, 153, 104663. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Class | Order | Family | Genus | Gram Classification | Presence in Hypertension |
|---|---|---|---|---|---|---|
| Actinomycetota | Coriobacteriia | Eggerthellales | Eggerthellaceae | Eggerthella | Positive | Higher [22] |
| Bacillota | Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | Positive | Higher [22] |
| Bacillota | Clostridia | Clostridiales | Eubacteriaceae | Anaerovorax | Positive | Higher [20] |
| Bacillota | Clostridia | Eubacteriales | Eubacteriaceae | Mogibacterium | Positive | Higher [20] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Butyricicoccus | Positive | Higher [20] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Parabacteroides | Negative | Higher [22] |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | Alistipes | Negative | Higher [18,21] |
| Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter | Negative | Higher [20] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Cellulosibacter | Positive | Higher [20] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Sporobacter | Positive | Higher [20] |
| Proteobacteria | Deltaproteobacteria | Bdellovibrionales | Bdellovibrionaceae | Vampirovibrio | Negative | Higher [20] |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | Negative | Higher [17,21] |
| Pseudomonadota | Betaproteobacteria | Burkholderiales | Oxalobacteraceae | Oxalobacter | Negative | Higher [20] |
| Pseudomonadota | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Klebsiella | Negative | Higher [17,22] |
| Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Salmonella | Negative | Higher [22] |
| Phylum | Class | Order | Family | Genus | Gram Classification | Presence in Hypertension |
|---|---|---|---|---|---|---|
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Enterorhabdus | Positive | Lower [21] |
| Actinomycetota | Actinomycetia | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | Positive | Lower [17] |
| Actinomycetota | Actinomycetia | Micrococcales | Micrococcaceae | Rothia | Positive | Lower [20] |
| Actinomycetota | Coriobacteriia | Coriobacteriales | Atopobiaceae | Atopobium | Positive | Lower [20] |
| Bacillota | Clostridia | Clostridiales | Eubacteriaceae | Eubacterium | Positive | Lower/Higher * [18,21] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Butyrivibrio | Positive | Lower [17] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Coprococcus | Positive | Lower [17] |
| Bacillota | Clostridia | Eubacteriales | Lachnospiraceae | Roseburia | Positive | Lower [17,22,23] |
| Bacillota | Clostridia | Eubacteriales | Oscillospiraceae | Faecalibacterium | Positive | Lower [17,22,23] |
| Bacillota | Clostridia | Eubacteriales | Peptostreptococcaceae | Romboutsia | Positive | Lower [21] |
| Bacillota | Negativicutes | Veillonellales | Veillonellaceae | Anaeroglobus | Negative | Lower [20] |
| Bacillota | Negativicutes | Veillonellales | Veillonellaceae | Megaspheara | Negative | Lower [20] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Acetobacteroides | Negative | Lower [21] |
| Bacteroidota | Bacteroidia | Bacteroidales | Porphyromonadaceae | Coprobacter | Negative | Lower [21] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Paraprevotella | Negative | Lower [21] |
| Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | Positive | Lower [21] |
| Firmicutes | Clostridia | Clostridiales | Christensenellaceae | Christensenella | Negative | Lower [21] |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiracea | Positive | Lower [21] |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | Positive | Lower [20,23] |
| Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Pseudocitrobacter | Negative | Lower [20] |
| Phylum | Class | Order | Family | Genus | Gram Classification | Presence in Hypertension |
|---|---|---|---|---|---|---|
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Oscillibacter | Positive | Higher [21]/Lower [17] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | Negative | Higher [17,20,23]/Lower [20] |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Negative | Higher [21]/Lower [18] |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium | Positive | Higher [17,20,23]/Lower [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarek, J.; Budny, E.; Saar, M.; Kućmierz, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. Int. J. Mol. Sci. 2023, 24, 1377. https://doi.org/10.3390/ijms24021377
Tokarek J, Budny E, Saar M, Kućmierz J, Młynarska E, Rysz J, Franczyk B. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. International Journal of Molecular Sciences. 2023; 24(2):1377. https://doi.org/10.3390/ijms24021377
Chicago/Turabian StyleTokarek, Julita, Emilian Budny, Maciej Saar, Joanna Kućmierz, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2023. "Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure" International Journal of Molecular Sciences 24, no. 2: 1377. https://doi.org/10.3390/ijms24021377
APA StyleTokarek, J., Budny, E., Saar, M., Kućmierz, J., Młynarska, E., Rysz, J., & Franczyk, B. (2023). Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. International Journal of Molecular Sciences, 24(2), 1377. https://doi.org/10.3390/ijms24021377







