N,Nʹ-Diarylurea Derivatives (CTPPU) Inhibited NSCLC Cell Growth and Induced Cell Cycle Arrest through Akt/GSK-3β/c-Myc Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. N,Nʹ-Substituted Phenylurea Derivative Compounds
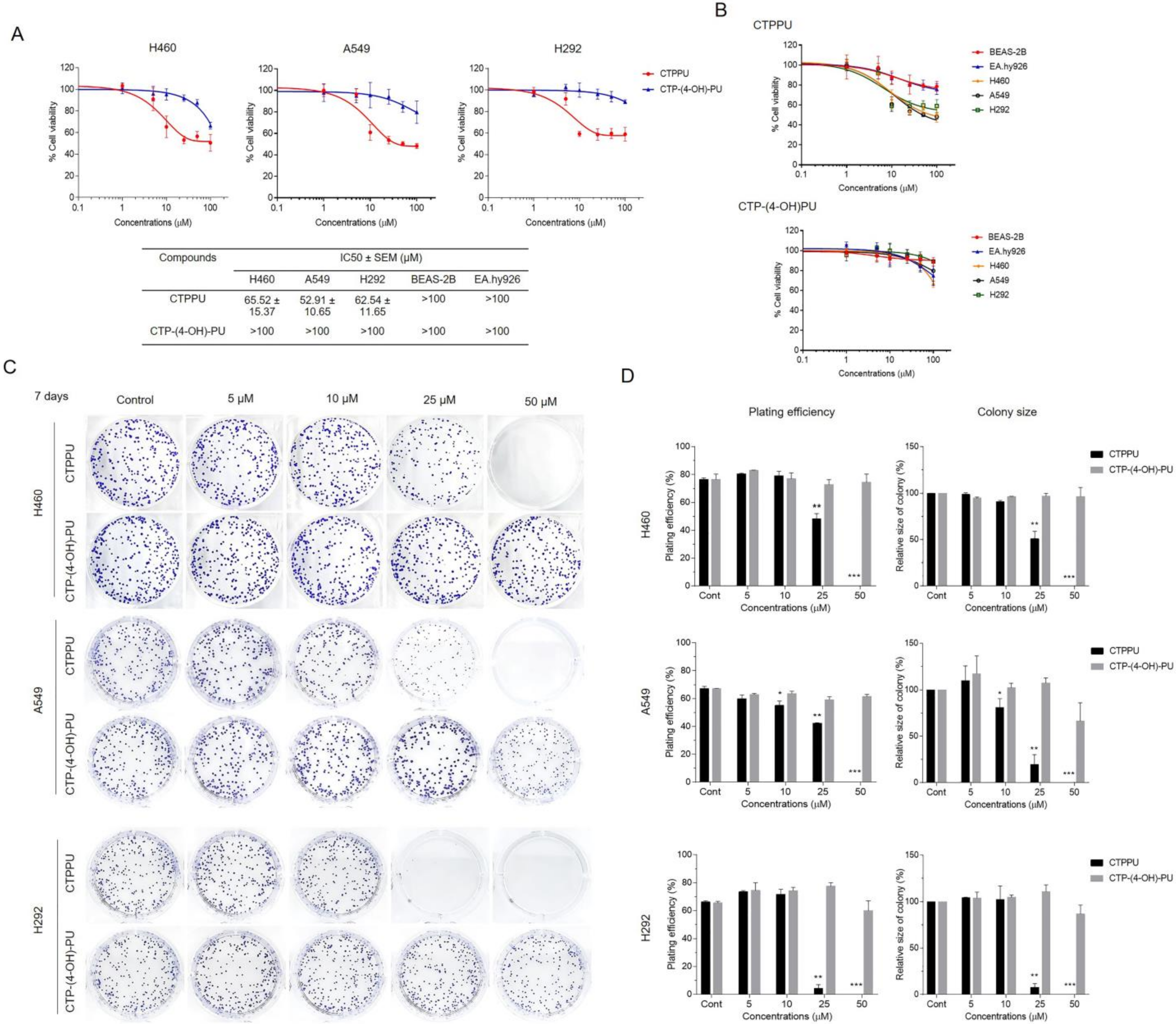
2.2. Cytotoxicity and Antiproliferative Effect of N,Nʹ-Substituted Phenylurea Derivatives on NSCLC Cells
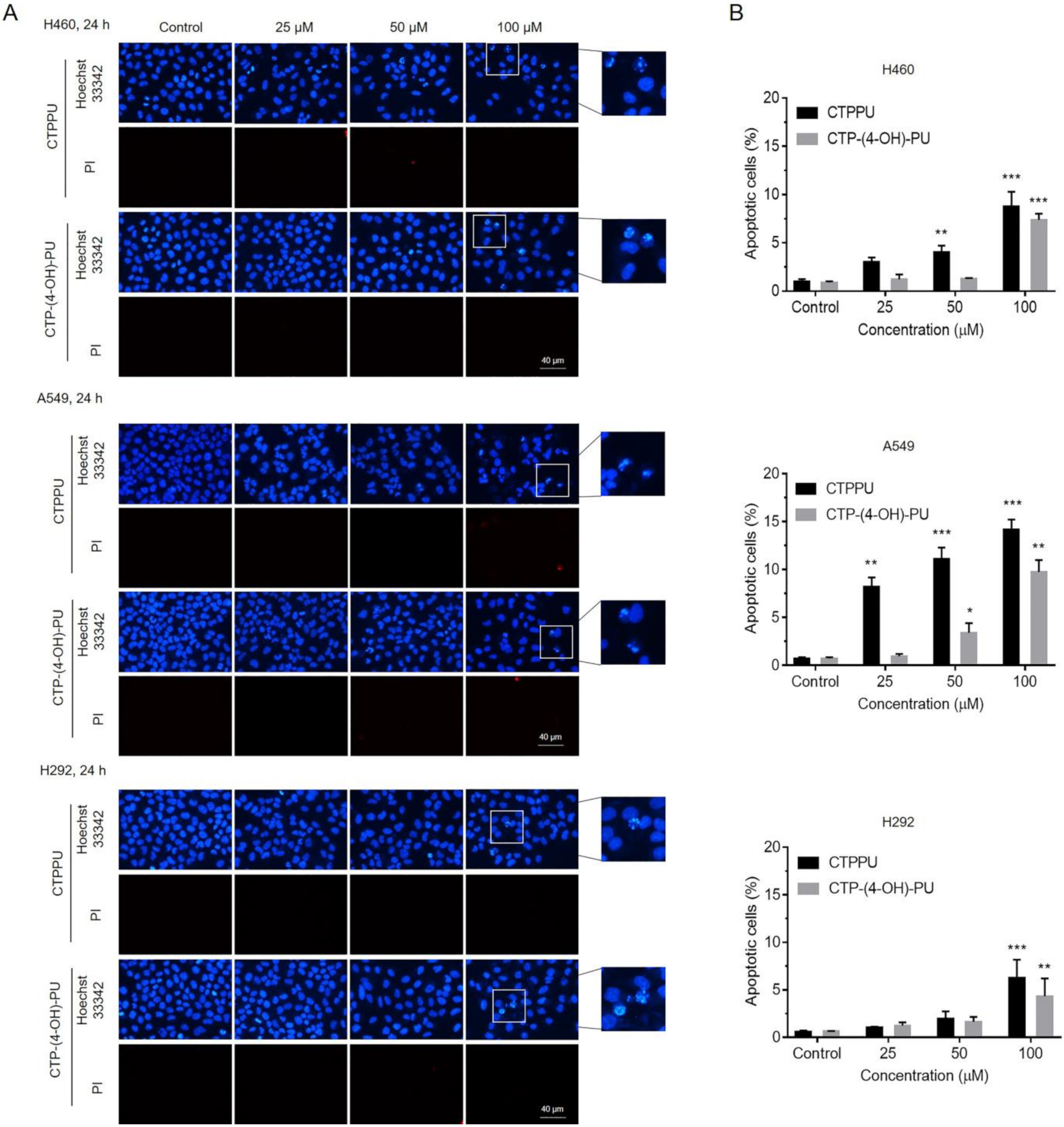
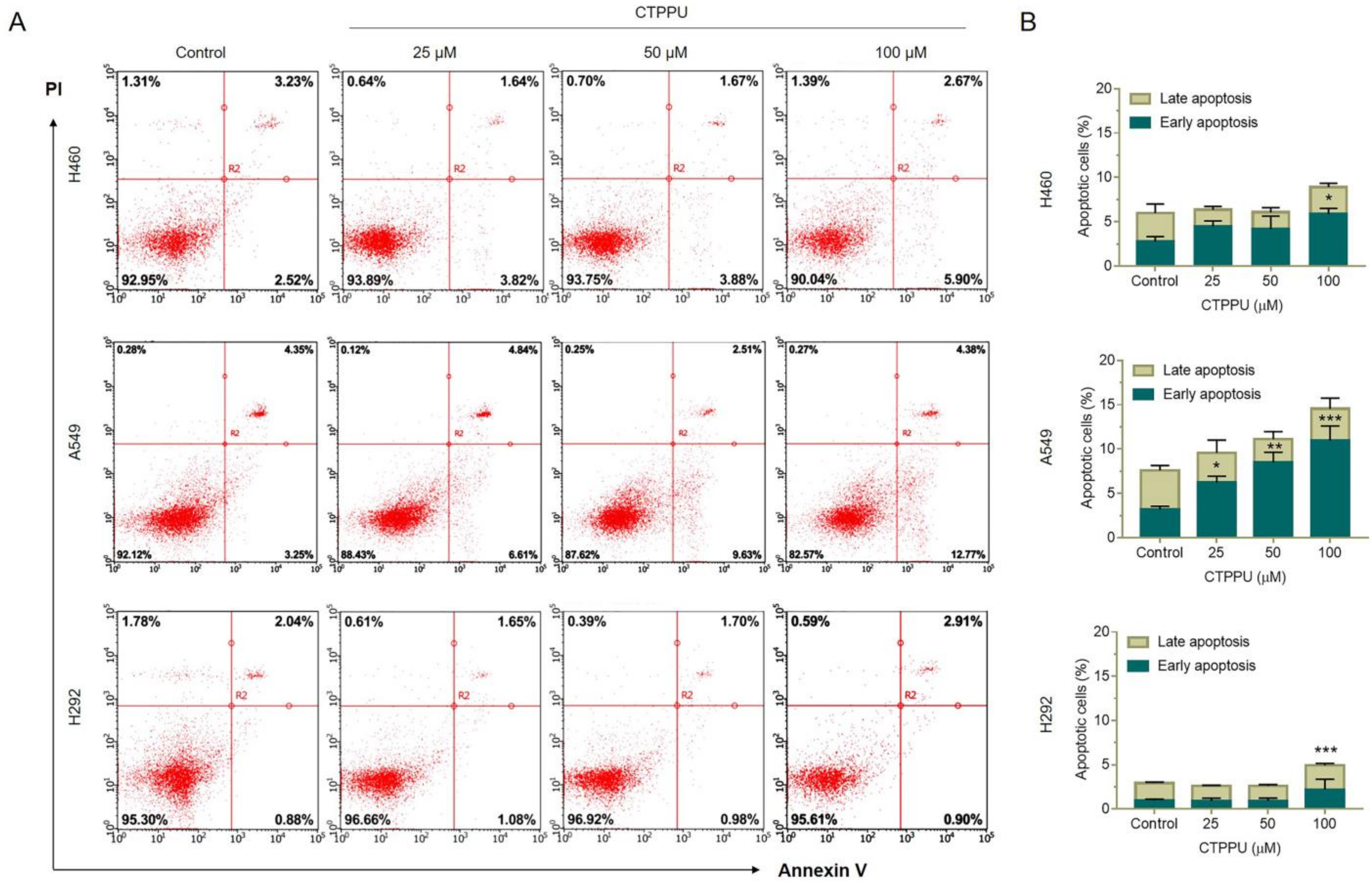
2.3. N,Nʹ-Substituted Phenylurea Derivatives Inhibit Cell Growth Rather Than Cause Cell Death through Apoptosis of NSCLC Cells
2.4. CTPPU Inhibits Cell Proliferation through Akt/glycogen Synthase Kinase (GSK)-3β/c-Myc Suppression Compared with CTP-(4-OH)-PU in NSCLC Cells
2.5. CTPPU Inhibits Cell Proliferation by Inducing G1/S Cell Cycle Arrest in NSCLC Cells
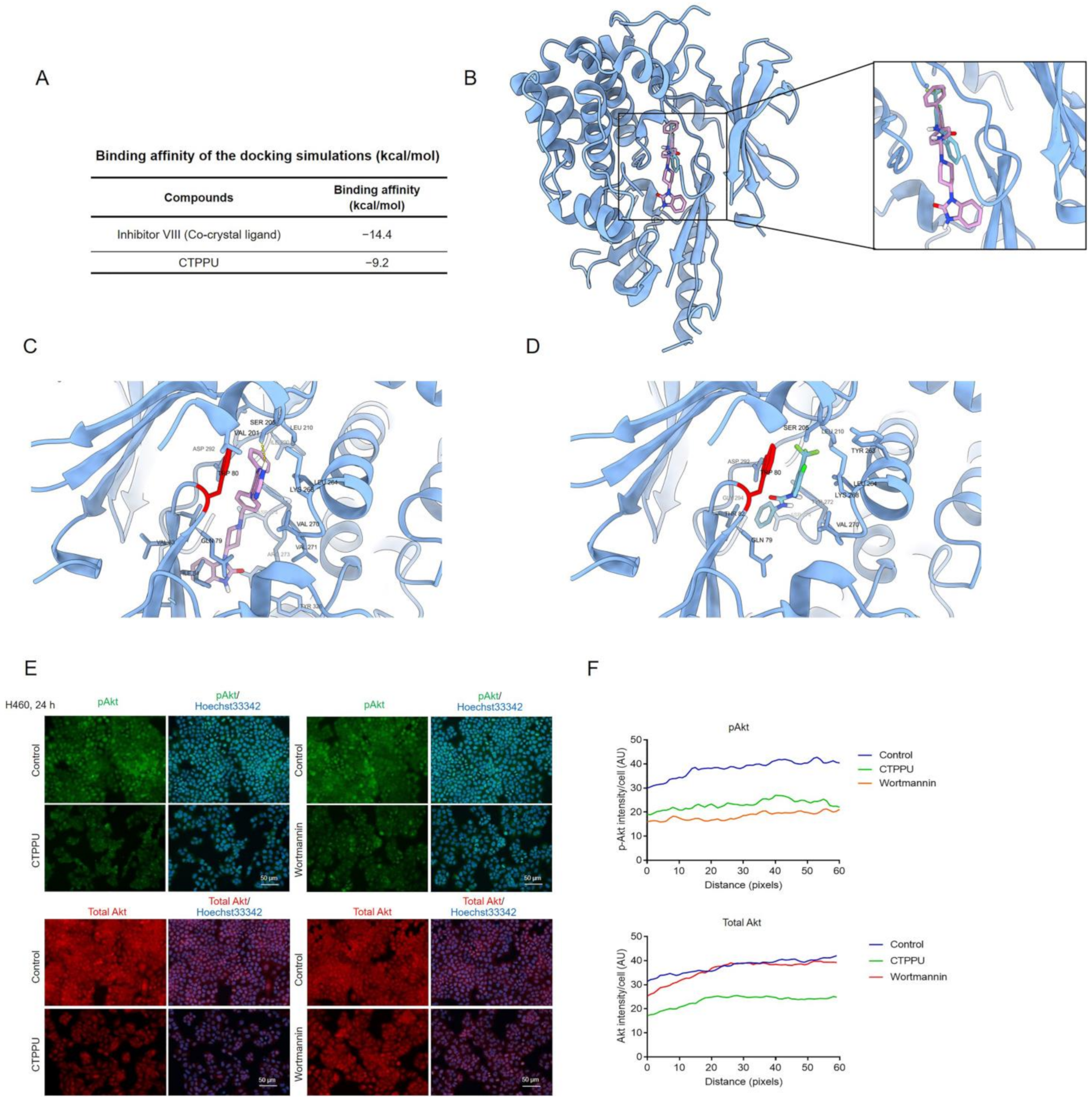
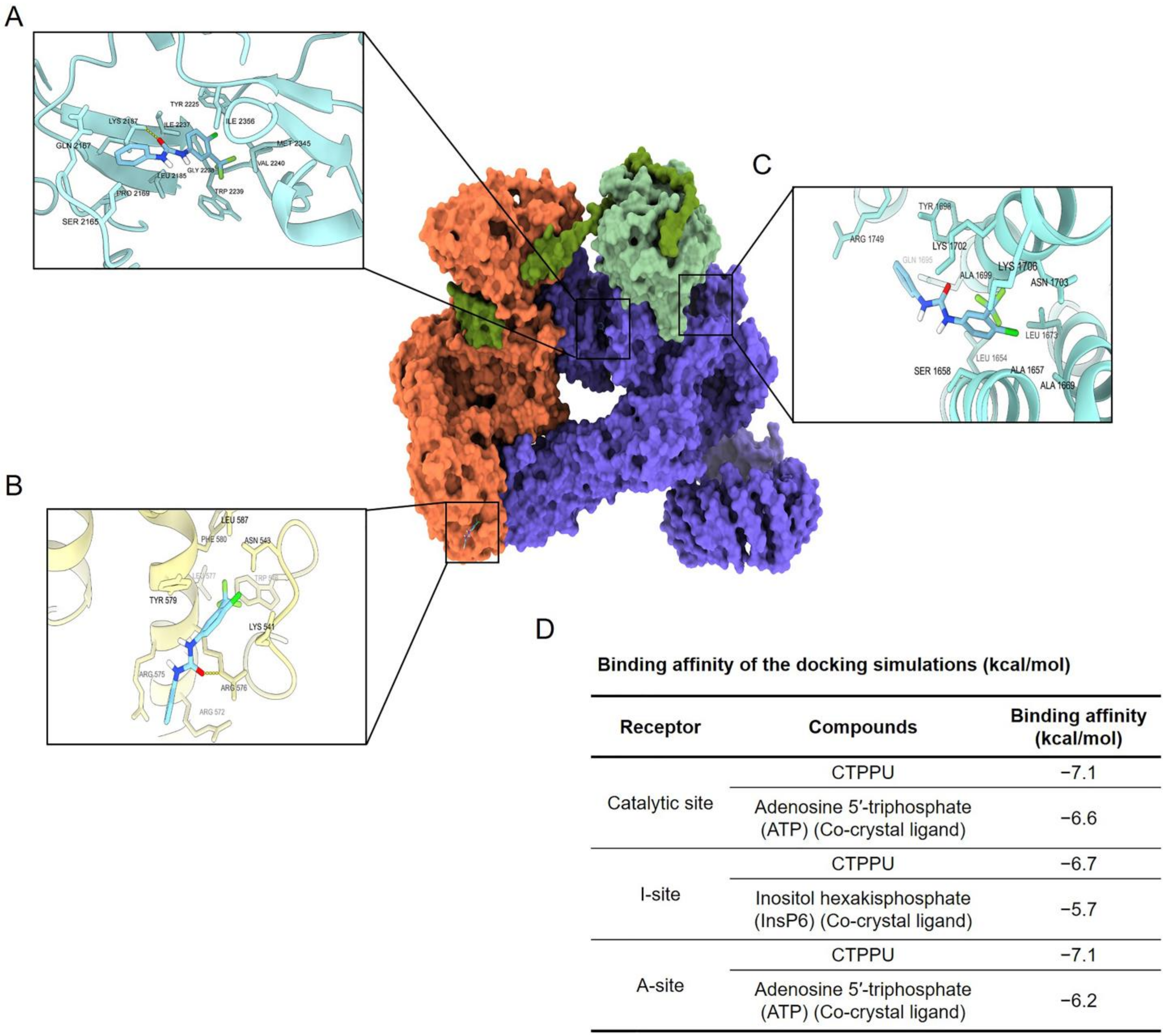
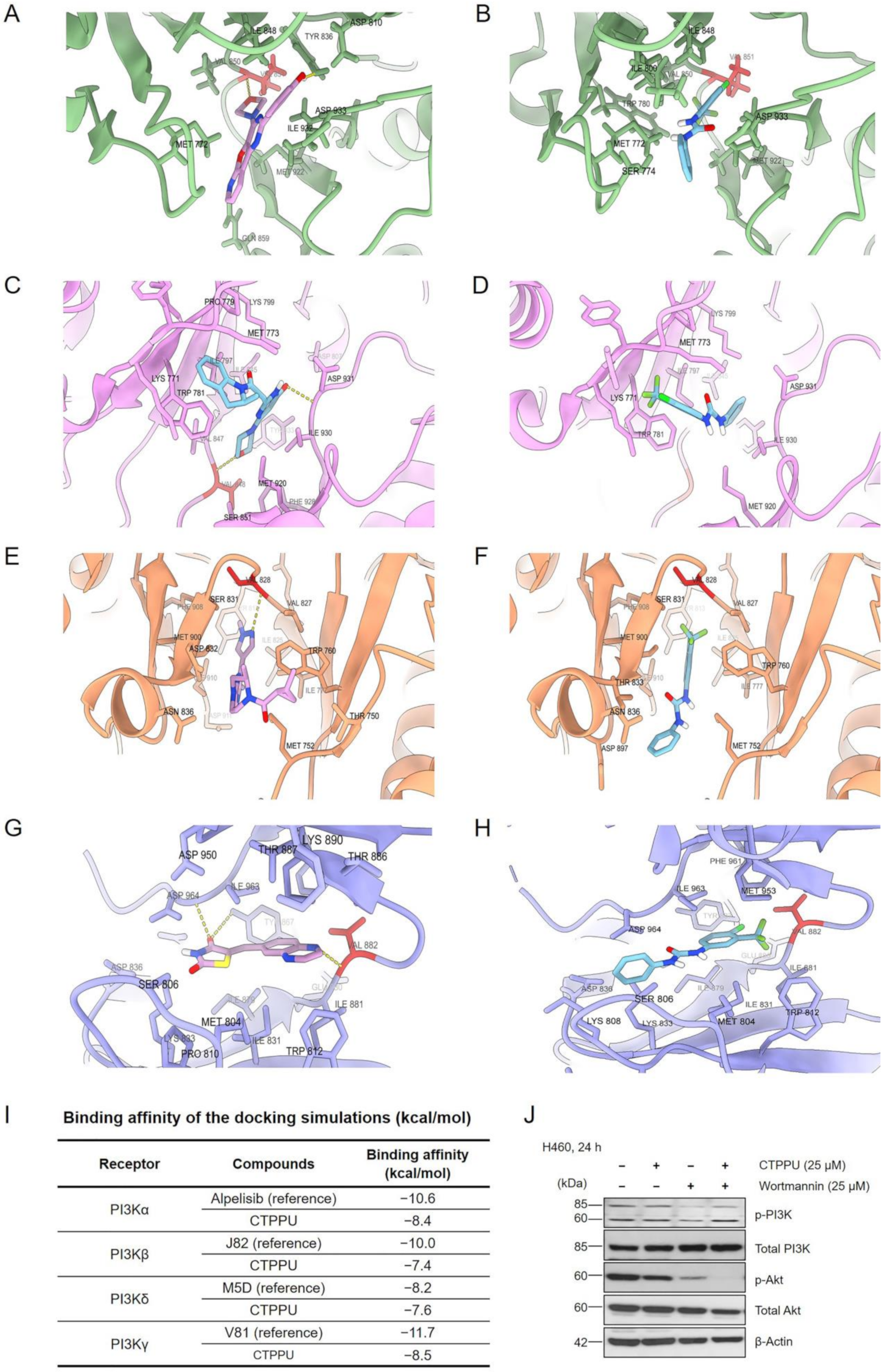
2.6. Molecular Docking Simulation Reveals the CTPPU Interaction with the Akt and Mammalian Target of Rapamycin Complex 2 (mTORC2) but Not PI3K Protein
3. Discussion
4. Materials and Methods
4.1. Synthesis of N,Nʹ-Substituted Phenylurea Derivatives
4.2. Reagents and Antibodies
4.3. Cell Culture
4.4. Preparation of Compounds Solution
4.5. Cytotoxicity Assay
4.6. Colony Formation Assay
4.7. Hoechst 33342 and PI Staining Assay
4.8. Apoptosis Analysis
4.9. Cell Cycle Analysis
4.10. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.11. Protein and Ligand Preparation
4.12. Computational Molecular Docking
4.13. Immunofluorescence Assay
4.14. Western Blot Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Casal-Mouriño, A.; Ruano-Ravina, A.; Lorenzo-González, M.; Rodríguez-Martínez, Á.; Giraldo-Osorio, A.; Varela-Lema, L.; Pereiro-Brea, T.; Barros-Dios, J.M.; Valdés-Cuadrado, L.; Pérez-Ríos, M. Epidemiology of stage III lung cancer: Frequency, diagnostic characteristics, and survival. Transl. Lung Cancer Res. 2021, 10, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Hung, J.J.; Hsu, W.H.; Hsieh, C.C.; Huang, B.S.; Huang, M.H.; Liu, J.S.; Wu, Y.C. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009, 64, 192–196. [Google Scholar] [CrossRef]
- Singal, G.; Miller, P.G.; Agarwala, V.; Li, G.; Kaushik, G.; Backenroth, D.; Gossai, A.; Frampton, G.M.; Torres, A.Z.; Lehnert, E.M.; et al. Association of Patient Characteristics and Tumor Genomics with Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019, 321, 1391–1399. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Balsara, B.R.; Pei, J.; Mitsuuchi, Y.; Page, R.; Klein-Szanto, A.; Wang, H.; Unger, M.; Testa, J.R. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis 2004, 25, 2053–2059. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Wu, D.W.; Lee, M.C.; Hsu, N.Y.; Wu, T.C.; Wu, J.Y.; Wang, Y.C.; Cheng, Y.W.; Chen, C.Y.; Lee, H. FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA reduction. Oncogene 2015, 34, 2505–2515. [Google Scholar] [CrossRef]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/mTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef]
- PI3K Inhibitor BKM120, Carboplatin, and Pemetrexed Disodium in Treating Patients with Stage IV Non-Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT01723800 (accessed on 9 November 2022).
- MK2206 and Erlotinib Hydrochloride in Treating Patients with Advanced Non-Small Cell Lung Cancer Who Have Progressed after Previous Response to Erlotinib Hydrochloride Therapy. Available online: https://ClinicalTrials.gov/show/NCT01294306 (accessed on 9 November 2022).
- A Phase 1/2 Trial of Perifosine in the Treatment of Non-Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT00399789 (accessed on 9 November 2022).
- Cheng, H.; Shcherba, M.; Pendurti, G.; Liang, Y.; Piperdi, B.; Perez-Soler, R. Targeting the PI3K/AKT/mTOR pathway: Potential for lung cancer treatment. Lung Cancer Manag. 2014, 3, 67–75. [Google Scholar] [CrossRef]
- Nasirzadeh, M.; Rasmi, Y.; Rahbarghazi, R.; Kheradmand, F.; Karimipour, M.; Aramwit, P.; Astinfeshan, M.; Gholinejad, Z.; Daeihasani, B.; Saboory, E.; et al. Crocetin promotes angiogenesis in human endothelial cells through PI3K-Akt-eNOS signaling pathway. EXCLI J. 2019, 18, 936–949. [Google Scholar] [CrossRef]
- Chaisit, S.; Jianmongkol, S. Rhinacanthin-C enhances chemosensitivity of breast cancer cells via the downregulation of P-glycoprotein through inhibition of Akt/NF-kappa B signaling pathway. J. HerbMed Pharmacol. 2021, 11, 91–98. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Hayakawa, Y.; Pongrakhananon, V. Molecular mechanisms of natural compounds in cell death induction and sensitization to chemotherapeutic drugs in lung cancer. Phytother. Res. 2019, 33, 2531–2547. [Google Scholar] [CrossRef]
- Zhang, H.-q.; Xie, X.-f.; Li, G.-m.; Chen, J.-r.; Li, M.-t.; Xu, X.; Xiong, Q.-y.; Chen, G.-r.; Yin, Y.-p.; Peng, F.; et al. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother. Res. 2021, 35, 4511–4525. [Google Scholar] [CrossRef]
- Sriratanasak, N.; Petsri, K.; Laobuthee, A.; Wattanathana, W.; Vinayanuwattikun, C.; Luanpitpong, S.; Chanvorachote, P. Novel c-Myc-Targeting Compound N, N-Bis (5-Ethyl-2-Hydroxybenzyl) Methylamine for Mediated c-Myc Ubiquitin-Proteasomal Degradation in Lung Cancer Cells. Mol. Pharmacol. 2020, 98, 130–142. [Google Scholar] [CrossRef]
- Losuwannarak, N.; Maiuthed, A.; Kitkumthorn, N.; Leelahavanichkul, A.; Roytrakul, S.; Chanvorachote, P. Gigantol Targets Cancer Stem Cells and Destabilizes Tumors via the Suppression of the PI3K/AKT and JAK/STAT Pathways in Ectopic Lung Cancer Xenografts. Cancers 2019, 11, 2032. [Google Scholar] [CrossRef]
- Sikka, P.; Sahu, J.; Mishra, A.; Hashim, S. Role of aryl urea containing compounds in medicinal chemistry. Med. Chem. 2015, 5, 479–483. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as Antitumor Agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Kondekar, N.B.; Sadani, A.A.; Chern, J.W. Ureas: Applications in Drug Design. Curr. Med. Chem. 2017, 24, 622–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Choudhary, A. Kinase Inhibitor Drugs. In Successful Drug Discovery; Wiley-VCH: Weinheim, Germany, 2018; pp. 65–93. [Google Scholar] [CrossRef]
- Chamni, S.; Zhang, J.; Zou, H. Benign synthesis of unsymmetrical arylurea derivatives using 3-substituted dioxazolones as isocyanate surrogates. Green Chem. Lett. Rev. 2020, 13, 246–257. [Google Scholar] [CrossRef]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Foster, D.A.; Yellen, P.; Xu, L.; Saqcena, M. Regulation of G1 Cell Cycle Progression: Distinguishing the Restriction Point from a Nutrient-Sensing Cell Growth Checkpoint(s). Genes Cancer 2010, 1, 1124–1131. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Wu, W.I.; Voegtli, W.C.; Sturgis, H.L.; Dizon, F.P.; Vigers, G.P.; Brandhuber, B.J. Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS ONE 2010, 5, e12913. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Ouyang, W.; Lazorchak, A.S.; Liu, D.; Shen, H.-M.; Su, B. mTOR complex 2 targets Akt for proteasomal degradation via phosphorylation at the hydrophobic motif. J. Biol. Chem. 2011, 286, 14190–14198. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef]
- Albihn, A.; Johnsen, J.I.; Henriksson, M.A. MYC in oncogenesis and as a target for cancer therapies. Adv. Cancer Res. 2010, 107, 163–224. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Delgado, M.D.; León, J. MYC Oncogene Contributions to Release of Cell Cycle Brakes. Genes 2019, 10, 244. [Google Scholar] [CrossRef]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; Ratti, S.; Candido, S.; Libra, M.; Montalto, G.; et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef]
- Oh, W.J.; Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 2011, 10, 2305–2316. [Google Scholar] [CrossRef]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Oh, W.J.; Wu, C.C.; Kim, S.J.; Facchinetti, V.; Julien, L.A.; Finlan, M.; Roux, P.P.; Su, B.; Jacinto, E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010, 29, 3939–3951. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Chimplee, S.; Roytrakul, S.; Sukrong, S.; Srisawat, T.; Graidist, P.; Kanokwiroon, K. Anticancer Effects and Molecular Action of 7-alpha-Hydroxyfrullanolide in G2/M-Phase Arrest and Apoptosis in Triple Negative Breast Cancer Cells. Molecules 2022, 27, 407. [Google Scholar] [CrossRef] [PubMed]
- Tungsukruthai, S.; Reamtong, O.; Roytrakul, S.; Sukrong, S.; Vinayanwattikun, C.; Chanvorachote, P. Targeting AKT/mTOR and Bcl-2 for Autophagic and Apoptosis Cell Death in Lung Cancer: Novel Activity of a Polyphenol Compound. Antioxidants 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Nonpanya, N.; Sanookpan, K.; Joyjamras, K.; Wichadakul, D.; Sritularak, B.; Chaotham, C.; Chanvorachote, P. Norcycloartocarpin targets Akt and suppresses Akt-dependent survival and epithelial-mesenchymal transition in lung cancer cells. PLoS ONE 2021, 16, e0254929. [Google Scholar] [CrossRef] [PubMed]
- Silapech, A.; Racha, S.; Aksorn, N.; Lafauy, P.; Tungsukruthai, S.; Vinayanuwattikun, C.; Sritularak, B.; Chanvorachote, P. Pongol Methyl Ether Inhibits Akt and Suppresses Cancer Stem Cell Phenotypes in Lung Cancer Cells. Pharmaceuticals 2021, 14, 1085. [Google Scholar] [CrossRef]
- Park, Y.; Jee, S.; Kim, J.G.; Chang, S. Study of Sustainability and Scalability in the Cp*Rh(III)-Catalyzed Direct C–H Amidation with 1,4,2-Dioxazol-5-ones. Org. Process. Res. Dev. 2015, 19, 1024–1029. [Google Scholar] [CrossRef]
- Ei, Z.Z.; Choochuay, K.; Tubsuwan, A.; Pinkaew, D.; Suksomtip, M.; Vinayanuwattikun, C.; Chanvorachote, P.; Chunhacha, P. GRP78/BiP determines senescence evasion cell fate after cisplatin-based chemotherapy. Sci. Rep. 2021, 11, 22448. [Google Scholar] [CrossRef]
- Scaiola, A.; Mangia, F.; Imseng, S.; Boehringer, D.; Berneiser, K.; Shimobayashi, M.; Stuttfeld, E.; Hall, M.N.; Ban, N.; Maier, T. The 3.2-Å resolution structure of human mTORC2. Sci. Adv. 2020, 6, eabc1251. [Google Scholar] [CrossRef]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef]
- Certal, V.; Carry, J.-C.; Halley, F.; Virone-Oddos, A.; Thompson, F.; Filoche-Rommé, B.; El-Ahmad, Y.; Karlsson, A.; Charrier, V.; Delorme, C.; et al. Discovery and Optimization of Pyrimidone Indoline Amide PI3Kβ Inhibitors for the Treatment of Phosphatase and Tensin Homologue (PTEN)-Deficient Cancers. J. Med. Chem. 2014, 57, 903–920. [Google Scholar] [CrossRef]
- Zhou, H.; McGowan, M.A.; Lipford, K.; Christopher, M.; Fradera, X.; Witter, D.; Lesburg, C.A.; Li, C.; Methot, J.L.; Lampe, J.; et al. Discovery and optimization of heteroaryl piperazines as potent and selective PI3Kδ inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126715. [Google Scholar] [CrossRef]
- Drew, S.L.; Thomas-Tran, R.; Beatty, J.W.; Fournier, J.; Lawson, K.V.; Miles, D.H.; Mata, G.; Sharif, E.U.; Yan, X.; Mailyan, A.K.; et al. Discovery of Potent and Selective PI3Kγ Inhibitors. J. Med. Chem. 2020, 63, 11235–11257. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2020, 49, D437–D451. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Frisch, A. Gaussian 09W Reference; Gaussian Inc.: Wallingford, CT, USA, 2009; p. 25. [Google Scholar]
- Ravindranath, P.A.; Forli, S.; Goodsell, D.S.; Olson, A.J.; Sanner, M.F. AutoDockFR: Advances in Protein-Ligand Docking with Explicitly Specified Binding Site Flexibility. PLoS Comput. Biol. 2015, 11, e1004586. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongsom, S.; Racha, S.; Ei, Z.Z.; Petsri, K.; Aksorn, N.; Chamni, S.; Panpuang, V.; Zou, H.; Chanvorachote, P. N,Nʹ-Diarylurea Derivatives (CTPPU) Inhibited NSCLC Cell Growth and Induced Cell Cycle Arrest through Akt/GSK-3β/c-Myc Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 1357. https://doi.org/10.3390/ijms24021357
Thongsom S, Racha S, Ei ZZ, Petsri K, Aksorn N, Chamni S, Panpuang V, Zou H, Chanvorachote P. N,Nʹ-Diarylurea Derivatives (CTPPU) Inhibited NSCLC Cell Growth and Induced Cell Cycle Arrest through Akt/GSK-3β/c-Myc Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(2):1357. https://doi.org/10.3390/ijms24021357
Chicago/Turabian StyleThongsom, Sunisa, Satapat Racha, Zin Zin Ei, Korrakod Petsri, Nithikoon Aksorn, Supakarn Chamni, Vitsarut Panpuang, Hongbin Zou, and Pithi Chanvorachote. 2023. "N,Nʹ-Diarylurea Derivatives (CTPPU) Inhibited NSCLC Cell Growth and Induced Cell Cycle Arrest through Akt/GSK-3β/c-Myc Signaling Pathway" International Journal of Molecular Sciences 24, no. 2: 1357. https://doi.org/10.3390/ijms24021357
APA StyleThongsom, S., Racha, S., Ei, Z. Z., Petsri, K., Aksorn, N., Chamni, S., Panpuang, V., Zou, H., & Chanvorachote, P. (2023). N,Nʹ-Diarylurea Derivatives (CTPPU) Inhibited NSCLC Cell Growth and Induced Cell Cycle Arrest through Akt/GSK-3β/c-Myc Signaling Pathway. International Journal of Molecular Sciences, 24(2), 1357. https://doi.org/10.3390/ijms24021357






