Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases
Abstract
1. Introduction
2. Results
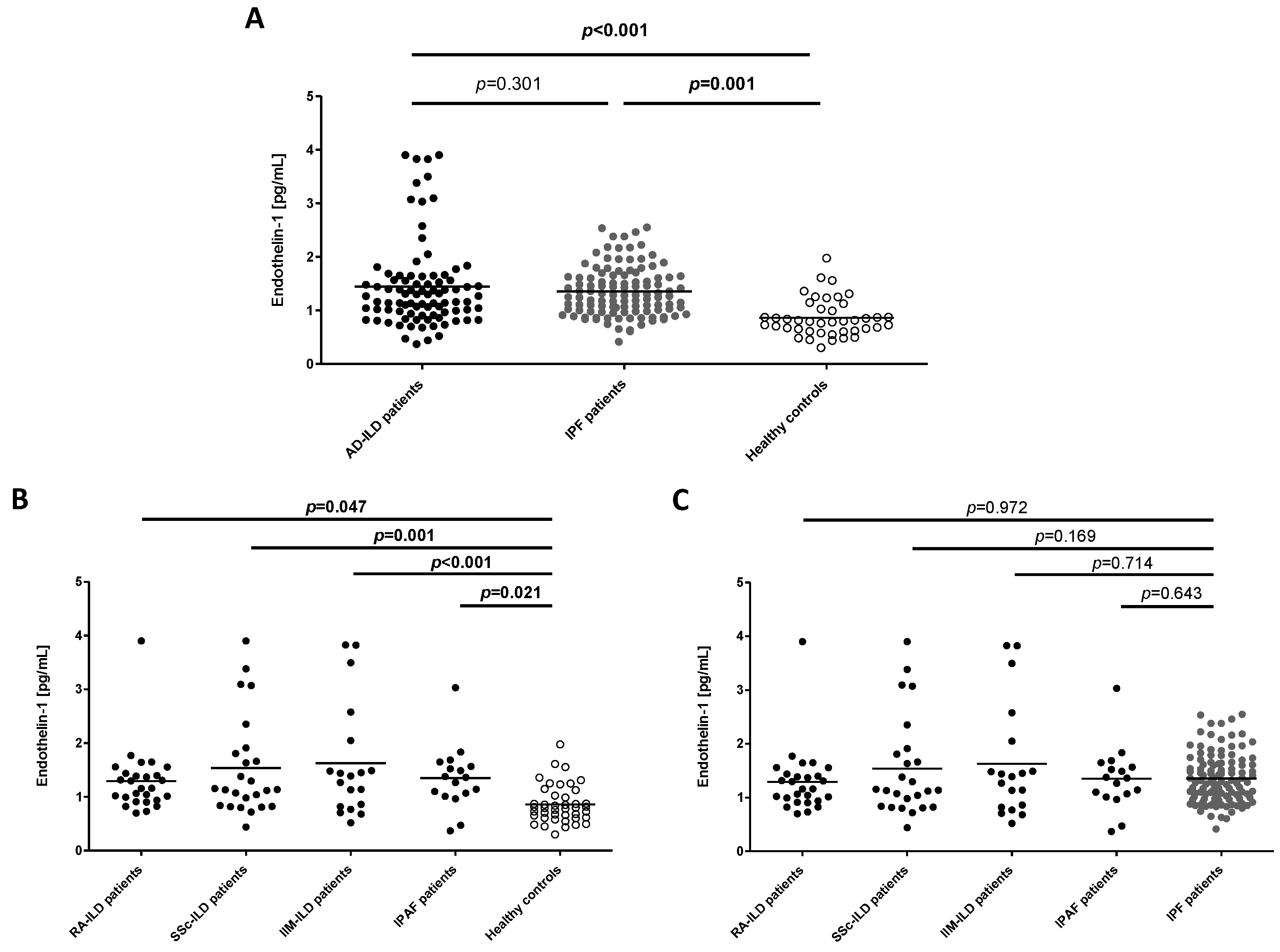
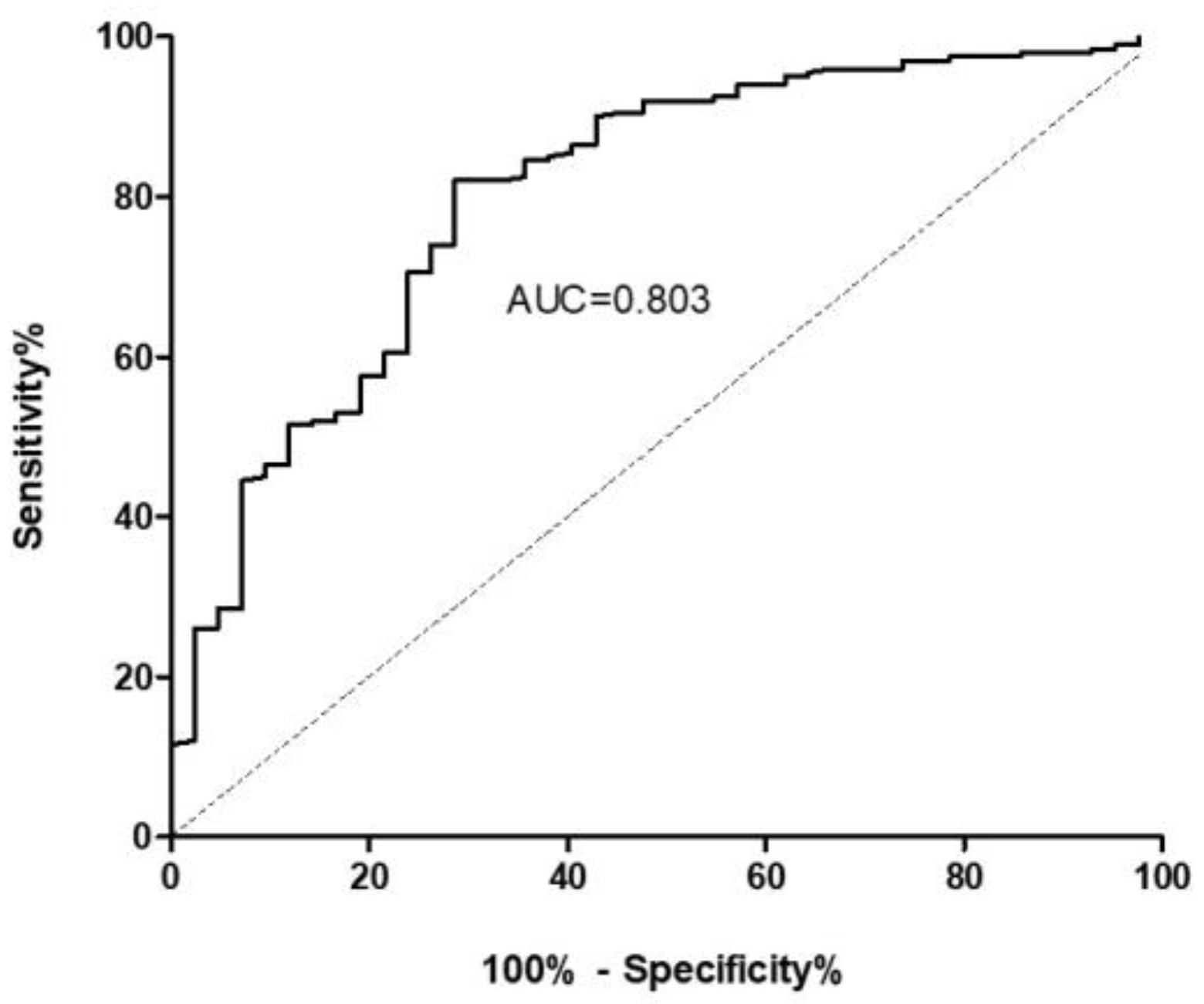
2.1. ET-1 as a Biomarker of ILD
2.2. ET-1 for the Differential Diagnosis between ILD Patients
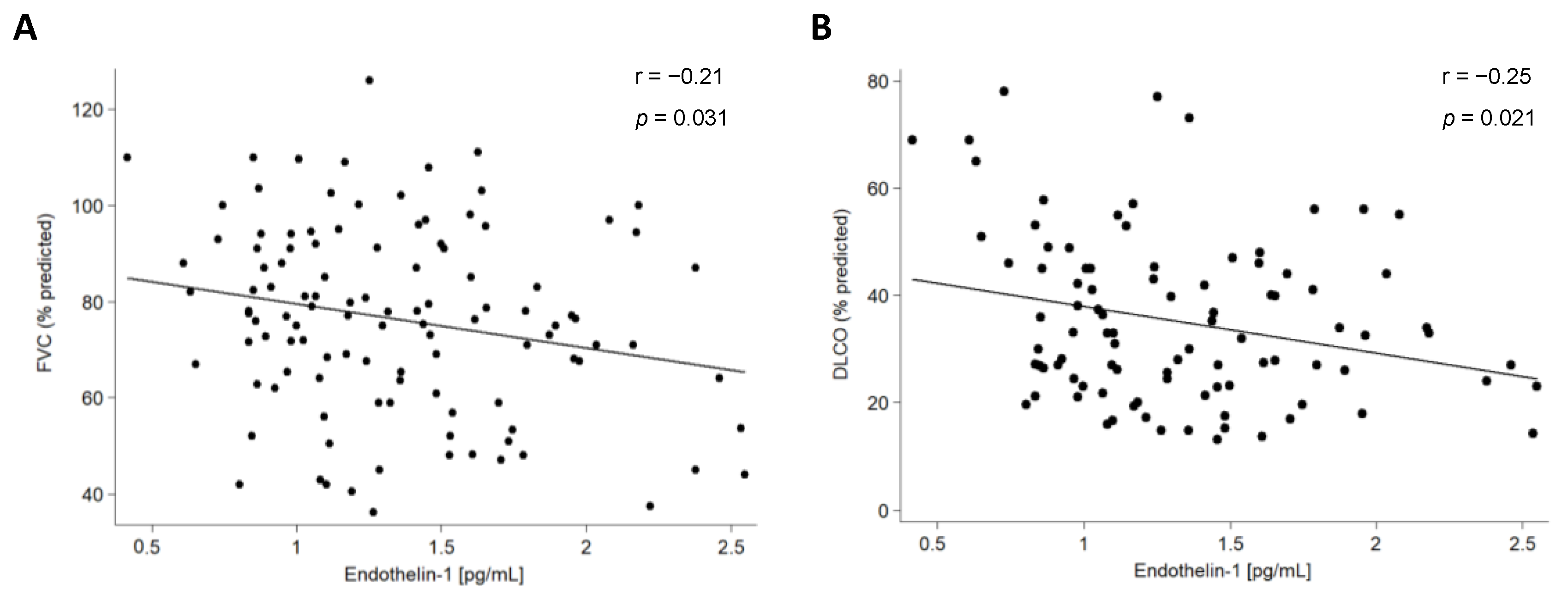
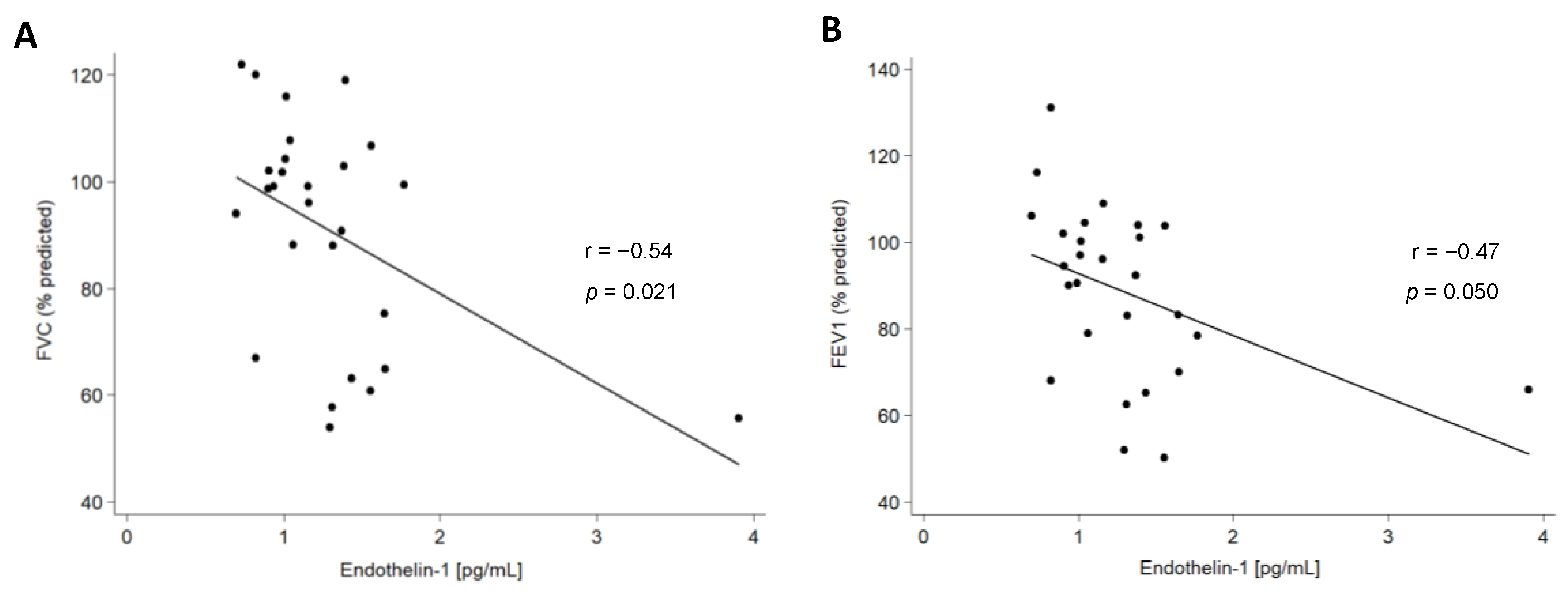
2.3. Association of ET-1 with Clinical Characteristics of Patients with ILD
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. ET-1 Serum Levels Determination
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W.; et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Mateo, B.; Remuzgo-Martínez, S.; Mora Cuesta, V.M.; Iturbe-Fernández, D.; Fernández-Rozas, S.; Prieto-Peña, D.; Calderón-Goercke, M.; Corrales, A.; Blanco Rodríguez, G.B.; Gómez-Román, J.J.; et al. The Spectrum of Interstitial Lung Disease Associated with Autoimmune Diseases: Data of a 3.6-Year Prospective Study from a Referral Center of Interstitial Lung Disease and Lung Transplantation. J. Clin. Med. 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, P.; Goules, A.; Hoffmann-Vold, A.M.; Matteson, E.L.; Tzioufas, A. Natural history and screening of interstitial lung disease in systemic autoimmune rheumatic disorders. Ther. Adv. Musculoskelet Dis. 2021, 13, 1759720X211037519. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Atienza-Mateo, B.; Remuzgo-Martínez, S.; Prieto-Peña, D.; Mora Cuesta, V.M.; Iturbe-Fernández, D.; Llorca, J.; Sánchez-Bilbao, L.; Corrales, A.; Blanco Rodríguez, G.; Gómez-Román, J.J.; et al. Rituximab in the Treatment of Interstitial Lung Disease Associated with Autoimmune Diseases: Experience from a Single Referral Center and Literature Review. J. Clin. Med. 2020, 9, 3070. [Google Scholar] [CrossRef]
- Flores Valdez, N. Endothelin-1: Intrinsic vasoconstrictor vascular endothelial. Rev. Med. 2013, 21, 42–56. [Google Scholar] [CrossRef]
- D’Orléans-Juste, P.; Plante, M.; Honoré, J.C.; Carrier, E.; Labonté, J. Synthesis and degradation of endothelin-1. Can. J. Physiol. Pharmacol. 2003, 81, 503–510. [Google Scholar] [CrossRef]
- Fagan, K.A.; McMurtry, I.F.; Rodman, D.M. Role of endothelin-1 in lung disease. Respir. Res. 2001, 2, 90–101. [Google Scholar] [CrossRef]
- Mayes, M.D. Endothelin and endothelin receptor antagonists in systemic rheumatic disease. Arthritis Rheum. 2003, 48, 1190–1199. [Google Scholar] [CrossRef]
- Sticherling, M. The role of endothelin in connective tissue diseases. Rheumatology 2006, 45 (Suppl. S3), iii8–iii10. [Google Scholar] [CrossRef]
- Ross, B.; D’Orléans-Juste, P.; Giaid, A. Potential role of endothelin-1 in pulmonary fibrosis: From the bench to the clinic. Am. J. Respir. Cell Mol. Biol. 2010, 42, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Brown, K.K. The role of endothelin-1 in the pathogenesis of idiopathic pulmonary fibrosis. BioDrugs 2010, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, M.; Pulsatelli, L.; Grigolo, B.; Facchini, A.; Fasano, L.; Cinti, C.; Fabbri, M.; Gasbarrini, G.; Meliconi, R. Endothelin-1 in idiopathic pulmonary fibrosis. J. Clin. Pathol. 1995, 48, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Furukawa, K.; Tsao, M.S.; Maghazachi, A.; Corrin, B.; Yanagisawa, M.; Barnes, P.J.; Giaid, A. Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: Possible involvement of proinflammatory cytokines. Am. J. Respir. Cell Mol. Biol. 1997, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bonella, F.; Costabel, U. Biomarkers in connective tissue disease-associated interstitial lung disease. Semin. Respir. Crit. Care Med. 2014, 35, 181–200. [Google Scholar] [CrossRef]
- Silver, R.M. Endothelin and scleroderma lung disease. Rheumatology 2008, 47 (Suppl. S5), v25–v26. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Klimiuk, P.A.; Sierakowski, S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: Relationship to organ systemic involvement. Clin. Rheumatol. 2005, 24, 111–116. [Google Scholar] [CrossRef]
- Abraham, D.J.; Vancheeswaran, R.; Dashwood, M.R.; Rajkumar, V.S.; Pantelides, P.; Xu, S.W.; du Bois, R.M.; Black, C.M. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am. J. Pathol. 1997, 151, 831–841. [Google Scholar]
- Vancheeswaran, R.; Magoulas, T.; Efrat, G.; Wheeler-Jones, C.; Olsen, I.; Penny, R.; Black, C.M. Circulating endothelin-1 levels in systemic sclerosis subsets—A marker of fibrosis or vascular dysfunction? J. Rheumatol. 1994, 21, 1838–1844. [Google Scholar] [PubMed]
- Remuzgo-Martínez, S.; Genre, F.; Pulito-Cueto, V.; Atienza-Mateo, B.; Mora Cuesta, V.M.; Iturbe Fernández, D.; Fernández Rozas, S.M.; Lera-Gómez, L.; Alonso Lecue, P.; Ussetti, M.P.; et al. Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease. Biomedicines 2021, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Rodriguez-Carrio, J.; Prieto-Peña, D.; Portilla, V.; et al. Angiogenic T Cells: Potential Biomarkers for the Early Diagnosis of Interstitial Lung Disease in Autoimmune Diseases? Biomedicines 2022, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Moermans, C.; Henket, M.; Corhay, J.L.; Louis, R. Blood Biomarkers in Idiopathic Pulmonary Fibrosis. Lung 2017, 195, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Wujak, L.; Wygrecka, M.; Markart, P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018, 68–69, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Fernández-Rozas, S.; Atienza-Mateo, B.; Lera-Gómez, L.; Alonso-Lecue, P.; Rodríguez-Carrio, J.; et al. Endothelial Progenitor Cells as a Potential Biomarker in Interstitial Lung Disease Associated with Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 4098. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, O.; André, B.; Gester, F.; de Seny, D.; Moermans, C.; Struman, I.; Louis, R.; Malaise, M.; Guiot, J. Biomarkers in systemic sclerosis-associated interstitial lung disease: Review of the literature. Rheumatology 2019, 58, 1534–1546. [Google Scholar] [CrossRef]
- Satoh, M.; Tanaka, S.; Ceribelli, A.; Calise, S.J.; Chan, E.K. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin. Rev. Allergy Immunol. 2017, 52, 1–19. [Google Scholar] [CrossRef]
- Miądlikowska, E.; Rzepka-Wrona, P.; Miłkowska-Dymanowska, J.; Białas, A.J.; Piotrowski, W.J. Review: Serum Biomarkers of Lung Fibrosis in Interstitial Pneumonia with Autoimmune Features-What Do We Already Know? J. Clin. Med. 2021, 11, 79. [Google Scholar] [CrossRef]
- Fonseca, C.; Abraham, D.; Renzoni, E.A. Endothelin in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Molet, S.; Furukawa, K.; Maghazechi, A.; Hamid, Q.; Giaid, A. Chemokine- and cytokine-induced expression of endothelin 1 and endothelin-converting enzyme 1 in endothelial cells. J. Allergy Clin. Immunol. 2000, 105, 333–338. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tracleer (accessed on 23 December 2022).
- Oelsner, E.C.; Pottinger, T.D.; Burkart, K.M.; Allison, M.; Buxbaum, S.G.; Hansel, N.N.; Kumar, R.; Larkin, E.K.; Lange, L.A.; Loehr, L.R.; et al. Adhesion molecules, endothelin-1 and lung function in seven population-based cohorts. Biomarkers 2013, 18, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Goldberg, H.; Dellaripa, P.F. The impact of rheumatological evaluation in the management of patients with interstitial lung disease. Rheumatology 2011, 50, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Cesar, E.; Lopez-Lopez, L.; Lara, E.; Hidalgo-San Juan, M.V.; Parrado Romero, C.; Palencia, J.L.R.S.; Martín-Montañez, E.; Garcia-Fernandez, M. Serum biomarkers in differential diagnosis of idiopathic pulmonary fibrosis and connective tissue disease-associated interstitial lung disease. J. Clin. Med. 2021, 10, 3167. [Google Scholar] [CrossRef]
- Aoshima, Y.; Enomoto, Y.; Muto, S.; Meguro, S.; Kawasaki, H.; Kosugi, I.; Fujisawa, T.; Enomoto, N.; Inui, N.; Nakamura, Y.; et al. Gremlin-1 for the differential diagnosis of idiopathic pulmonary fibrosis versus other interstitial lung diseases: A clinical and pathophysiological analysis. Lung. 2021, 199, 289–298. [Google Scholar] [CrossRef] [PubMed]
- van Batenburg, A.A.; Kazemier, K.M.; van Oosterhout, M.F.M.; van der Vis, J.J.; Grutters, J.C.; Goldschmeding, R.; van Moorsel, C.H.M. Telomere shortening and DNA damage in culprit cells of different types of progressive fibrosing interstitial lung disease. ERJ Open Res. 2021, 7, 00691–2020. [Google Scholar] [CrossRef] [PubMed]
- Mulet, A.; Signes-Costa, J. Idiopathic pulmonary fibrosis and telomeres. J. Clin. Med. 2022, 11, 6893. [Google Scholar] [CrossRef]
- Snetselaar, R.; van Moorsel, C.H.M.; Kazemier, K.M.; van der Vis, J.J.; Zanen, P.; van Oosterhout, M.F.M.; Grutters, J.C. Telomere length in interstitial lung diseases. Chest 2015, 148, 1011–1018. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar]
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung disease. Clin. Med. 2017, 17, 146–153. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, K.; Granger, B.; Amelin, D.; Guiguet, M.; Hachulla, E.; Maurier, F.; Meyer, A.; Tohmé, A.; Charuel, J.L.; Musset, L.; et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol. 2018, 75, 1528–1537. [Google Scholar] [CrossRef]
- Remuzgo-Martínez, S.; Atienza-Mateo, B.; Ocejo-Vinyals, J.G.; Genre, F.; Pulito-Cueto, V.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Pérez-Fernández, R.; Prieto-Peña, D.; et al. Role of MUC1 rs4072037 polymorphism and serum KL-6 levels in patients with antisynthetase syndrome. Sci. Rep. 2021, 11, 22574. [Google Scholar] [CrossRef]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
| IPF Patients | AD-ILD Patients | ||||
|---|---|---|---|---|---|
| Variable | r | p | r | p | |
| FVC (% predicted) | −0.21 | 0.031 | −0.35 | 0.002 | |
| FEV1 (% predicted) | −0.15 | 0.119 | −0.30 | 0.010 | |
| DLCO (% predicted) | −0.25 | 0.021 | −0.16 | 0.257 | |
| Variable | Category | Mean ± SD [pg/mL] | p | Mean ± SD [pg/mL] | p |
| HRCT pattern | UIP | 1.35 ± 0.46 | - | 1.49 ± 0.83 | 0.354 |
| NSIP | - | 1.30 ± 0.65 | |||
| Characteristic | IPF Patients (n = 112) | AD-ILD Patients (n = 91) |
|---|---|---|
| Sex (men/women), n (%) | 93/19 (83.0/17.0) | 48/43 (52.7/47.3) |
| Age at the time of the study (years), mean ± SD | 64.5 ± 6.3 | 61.1 ± 9.2 |
| Smoking history, n (%) | 94 (83.9) | 60 (65.9) |
| Packs of cigarettes per year, mean ± SD | 32.6 ± 21.0 | 28.1 ± 24.2 |
| Pulmonary function tests | ||
| FVC (% predicted), mean ± SD | 76.2 ± 19.3 | 80.4 ± 25.7 |
| FEV1 (% predicted), mean ± SD | 77.9 ± 19.5 | 79.1 ± 24.9 |
| DLCO (% predicted), mean ± SD | 35.2 ± 15.3 | 39.6 ± 17.6 |
| HRCT pattern | ||
| UIP, n (%) | 112 (100.0) | 33 (39.8) |
| Probable UIP, n (%) | - | 10 (12.0) |
| Indeterminate for UIP pattern, n (%) | - | 2 (2.4) |
| Features most consistent with an alternative diagnosis | ||
| NSIP, n (%) | - | 34 (41.0) |
| Non-NSIP, n (%) | - | 4 (4.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulito-Cueto, V.; Genre, F.; López-Mejías, R.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Portilla, V.; Sebastián Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 1275. https://doi.org/10.3390/ijms24021275
Pulito-Cueto V, Genre F, López-Mejías R, Mora-Cuesta VM, Iturbe-Fernández D, Portilla V, Sebastián Mora-Gil M, Ocejo-Vinyals JG, Gualillo O, Blanco R, et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. International Journal of Molecular Sciences. 2023; 24(2):1275. https://doi.org/10.3390/ijms24021275
Chicago/Turabian StylePulito-Cueto, Verónica, Fernanda Genre, Raquel López-Mejías, Víctor Manuel Mora-Cuesta, David Iturbe-Fernández, Virginia Portilla, María Sebastián Mora-Gil, Javier Gonzalo Ocejo-Vinyals, Oreste Gualillo, Ricardo Blanco, and et al. 2023. "Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases" International Journal of Molecular Sciences 24, no. 2: 1275. https://doi.org/10.3390/ijms24021275
APA StylePulito-Cueto, V., Genre, F., López-Mejías, R., Mora-Cuesta, V. M., Iturbe-Fernández, D., Portilla, V., Sebastián Mora-Gil, M., Ocejo-Vinyals, J. G., Gualillo, O., Blanco, R., Corrales, A., Ferraz-Amaro, I., Castañeda, S., Cifrián Martínez, J. M., Atienza-Mateo, B., Remuzgo-Martínez, S., & González-Gay, M. Á. (2023). Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. International Journal of Molecular Sciences, 24(2), 1275. https://doi.org/10.3390/ijms24021275










