A Multifunctional Dental Resin Composite with Sr-N-Doped TiO2 and n-HA Fillers for Antibacterial and Mineralization Effects
Abstract
1. Introduction
2. Results
2.1. Characterization of Sr-N-TiO2 Powders
2.2. Properties of DRCs
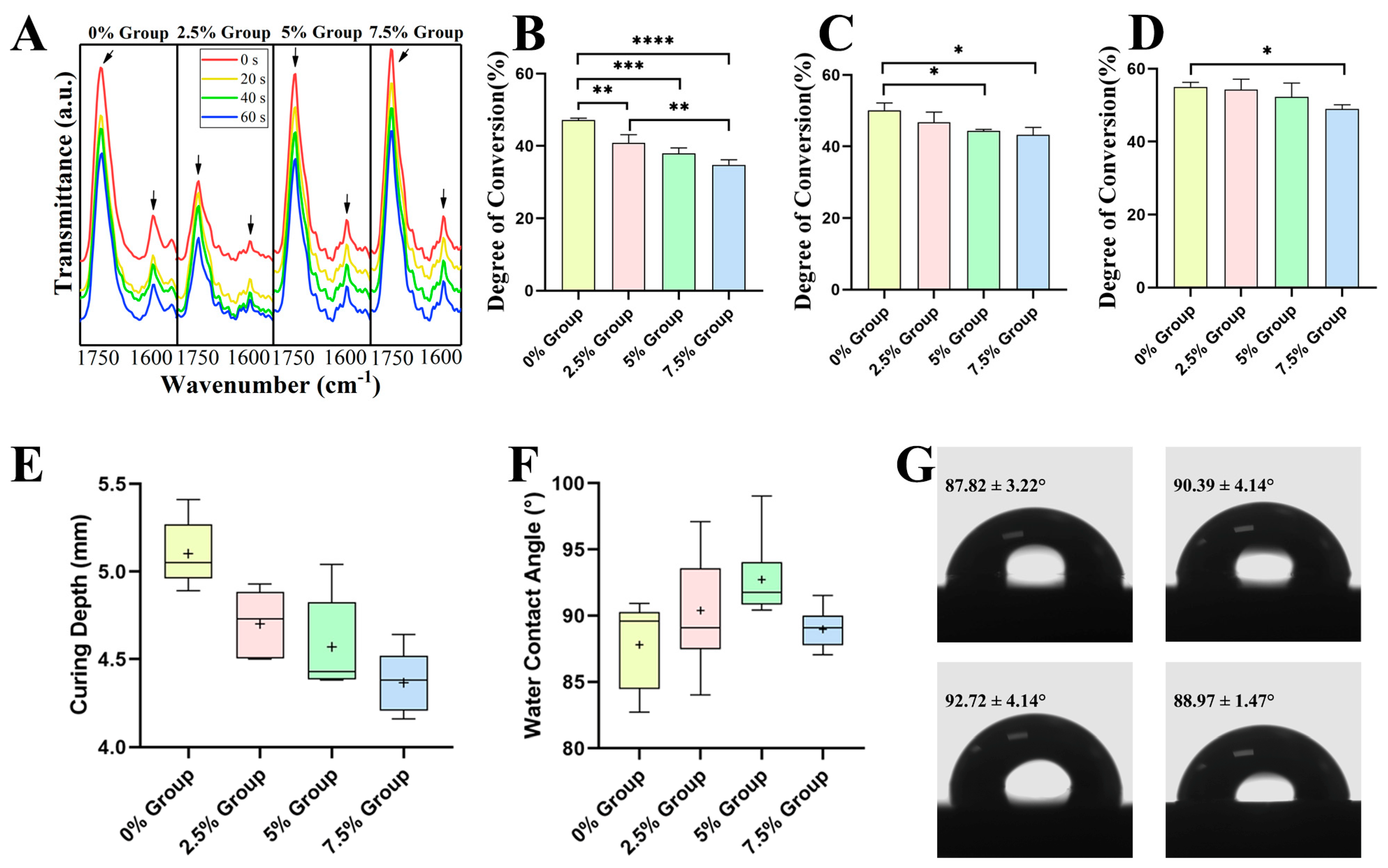
2.2.1. Physicochemical Properties
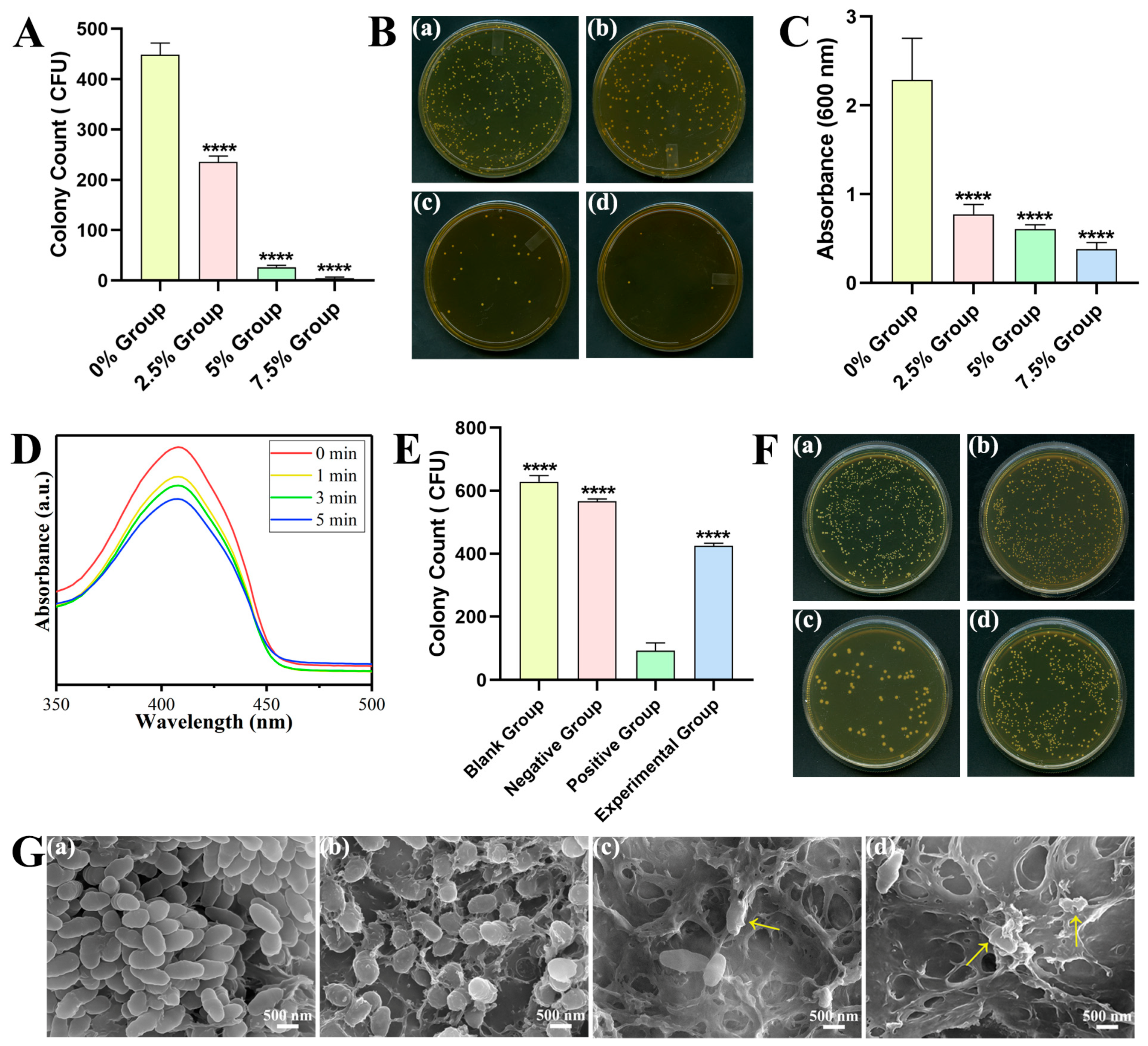
2.2.2. Antibacterial Properties and Antibacterial Mechanism
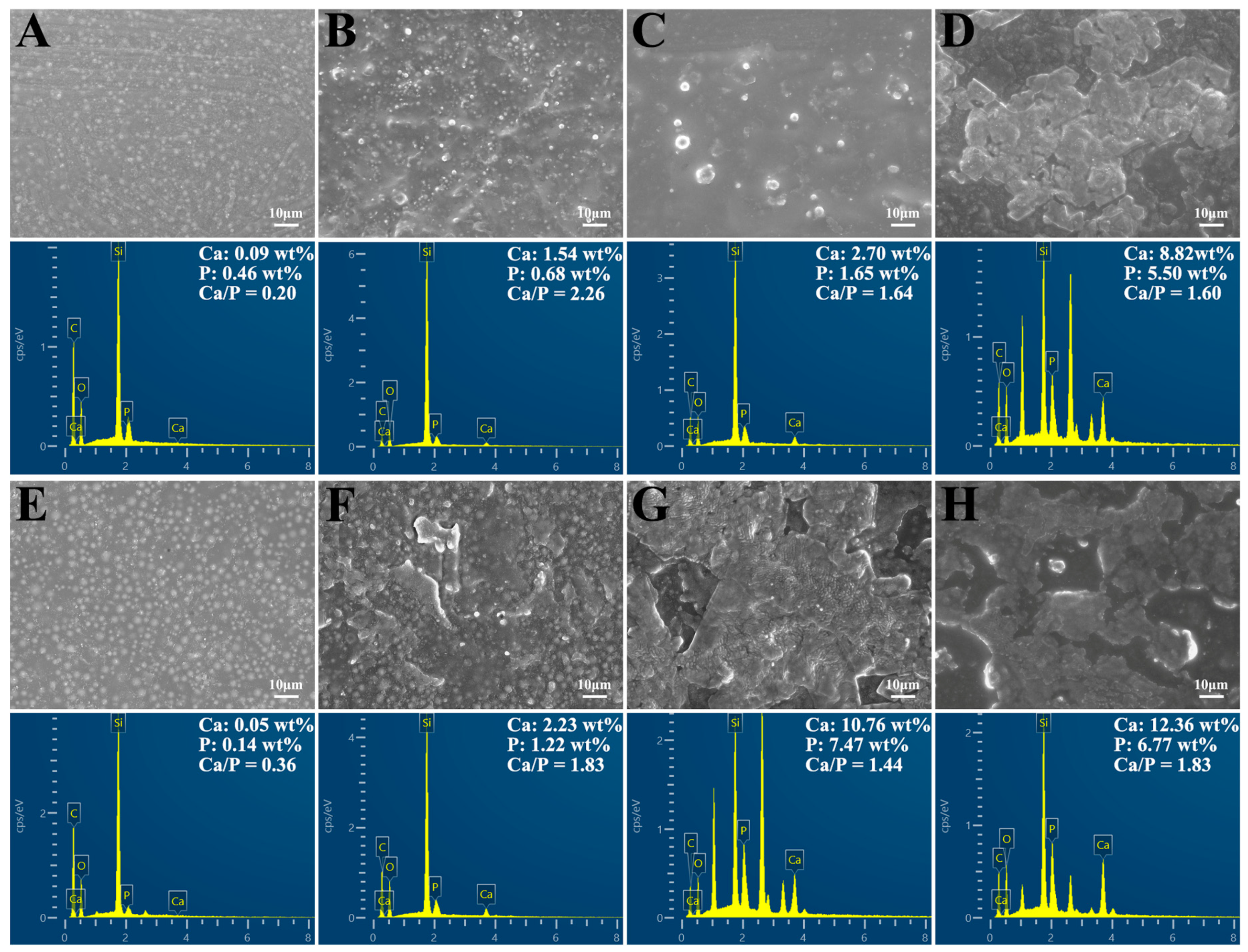
2.2.3. Bioactive Properties
2.3. Cell Compatibility
3. Discussion
3.1. Characterization of Sr-N-TiO2 Powders
3.2. Physicochemical Properties of DRCs
3.3. Antibacterial Properties and Antibacterial Mechanism of DRCs
3.4. Mineralization Properties of DRCs
3.5. Cell Compatibility of DRCs
4. Materials and Methods
4.1. Synthesis and Characterization of the Modified Nanoparticles
4.2. Preparation of DRCs
4.3. Physicochemical Properties
4.3.1. Degree of Conversion
4.3.2. Curing Depth
4.3.3. Water Contact Angle
4.4. Antibacterial Properties and Antibacterial Mechanism
4.4.1. Bacterial Culture
4.4.2. Colony-Forming Units (CFU) Counting
4.4.3. Crystal Violet Staining Assay
4.4.4. Detection of ROS Release
4.4.5. CFU Counting with NAC
4.4.6. SEM of the Bacteria Attached to the Surface
4.4.7. Bacterial Live/Dead Staining
4.5. Remineralization Properties
4.6. Cytotoxic Properties
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nobre, M.A.; Sezinando, A.; Fernandes, I.; Maló, P. Risk Score to Predict Dental Caries in Adult Patients for Use in the Clinical Setting. J. Clin. Med. 2019, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Xue, J.; Wang, J.; Feng, D.; Huang, H.; Wang, M. Application of Antimicrobial Polymers in the Development of Dental Resin Composite. Molecules 2020, 25, 4738. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Z.; Jiang, H.; Duan, Y.; Li, J.; Liu, X.; Hong, L.; Zhao, C. Preparation and evaluation of novel bio-based Bis-GMA-free dental composites with low estrogenic activity. Dent. Mater. 2022, 38, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Krois, J.; Gostemeyer, G.; Schwendicke, F. Secondary caries risk of different adhesive strategies and restorative materials in permanent teeth: Systematic review and network meta-analysis. J. Dent. 2020, 104, 103541. [Google Scholar] [CrossRef]
- Beck, F.; Lettner, S.; Graf, A.; Bitriol, B.; Dumitrescu, N.; Bauer, P.; Moritz, A.; Schedle, A. Survival of direct resin restorations in posterior teeth within a 19-year period (1996–2015): A meta-analysis of prospective studies. Dent. Mater. 2015, 31, 958–985. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Silvestrin, L.B.; Garcia, I.M.; Visioli, F.; Collares, F.M.; Leitune, V.C.B. Physicochemical and biological properties of experimental dental adhesives doped with a guanidine-based polymer: An in vitro study. Clin. Oral Investig. 2022, 26, 3627–3636. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, H.; Li, Z.; Huang, X. Autopolymerizing acrylic repair resin containing low concentration of dimethylaminohexadecyl methacrylate to combat saliva-derived bacteria. J. Mater. Sci. Mater. Med. 2022, 33, 49. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Sun, H.; Liu, Y.; Liu, W.; Su, B.; Li, S. The Development of Filler Morphology in Dental Resin Composites: A Review. Materials 2021, 14, 5612. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, S.S.; Bonan, R.F.; Mota, M.F.; Farias, R.; Menezes, R.R.; Bonan, P.R.F.; Maciel, P.P.; Ramos-Perez, F.M.M.; Batista, A.U.D.; da Cruz Perez, D.E. Effect of the incorporation of silica blow spun nanofibers containing silver nanoparticles (SiO2/Ag) on the mechanical, physicochemical, and biological properties of a low-viscosity bulk-fill composite resin. Dent. Mater. 2021, 37, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.B.; Bernardi, M.I.B.; Marangoni, V.S.; de Abreu Bernardi, A.C.; de Souza Rastelli, A.N.; Hernandes, A.C. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater. Sci. Eng. C 2018, 96, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Cui, Y.N.; Sun, Q.; Liu, M.; Niu, H.; Wang, J.X. Antibacterial activity and reinforcing effect of SiO2-ZnO complex cluster fillers for dental resin composites. Biomater. Sci. 2020, 9, 1795–1804. [Google Scholar] [CrossRef]
- Nam, K.Y.; Lee, C.H.; Lee, C.J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, e413–e419. [Google Scholar] [CrossRef]
- Sorkhdini, P.; Gregory, R.L.; Crystal, Y.O.; Tang, Q.; Lippert, F. Effectiveness of in vitro primary coronal caries prevention with silver diamine fluoride—Chemical vs biofilm models. J. Dent. 2020, 99, 103418. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic Applications of Antimicrobial Silver-Based Biomaterials in Dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2020, 6, 12–25. [Google Scholar] [CrossRef]
- Guo, X.; Rao, L.; Wang, P.; Wang, C.; Ao, Y.; Jiang, T.; Wang, W. Photocatalytic properties of P25-doped TiO2 composite film synthesized via sol-gel method on cement substrate. J. Environ. Sci. 2018, 66, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zang, Y.; Xu, L.; Wang, J.; Jia, H.; Miao, F. Synthesis of high-performance conjugated microporous polymer/TiO2 photocatalytic antibacterial nanocomposites. Mater. Sci. Eng. C 2021, 126, 112121. [Google Scholar] [CrossRef]
- Kuroiwa, A.; Nomura, Y.; Ochiai, T.; Sudo, T.; Nomoto, R.; Hayakawa, T.; Kanzaki, H.; Nakamura, Y.; Hanada, N. Antibacterial, Hydrophilic Effect and Mechanical Properties of Orthodontic Resin Coated with UV-Responsive Photocatalyst. Materials 2018, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Esteban Florez, F.L.; Trofimov, A.A.; Ievlev, A.; Qian, S.; Rondinone, A.J.; Khajotia, S.S. Advanced characterization of surface-modified nanoparticles and nanofilled antibacterial dental adhesive resins. Sci. Rep. 2020, 10, 9811. [Google Scholar] [CrossRef] [PubMed]
- Cinar, Z. The Role of Molecular Modeling in TiO2 Photocatalysis. Molecules 2017, 22, 556. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, C.; Ren, Z.; Fu, X.; Qian, G.; Wang, Z. N-doped TiO2/C nanocomposites and N-doped TiO2 synthesised at different thermal treatment temperatures with the same hydrothermal precursor. Dalton Trans. 2014, 43, 13783–13791. [Google Scholar] [CrossRef]
- Kawashita, M.; Endo, N.; Watanabe, T.; Miyazaki, T.; Furuya, M.; Yokota, K.; Abiko, Y.; Kanetaka, H.; Takahashi, N. Formation of bioactive N-doped TiO2 on Ti with visible light-induced antibacterial activity using NaOH, hot water, and subsequent ammonia atmospheric heat treatment. Colloids Surf. B Biointerfaces 2016, 145, 285–290. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Boonto, Y.; Kajitvichyanukul, P. Visible light photocatalytic antibacterial activity of Ni-doped and N-doped TiO2 on Staphylococcus aureus and Escherichia coli bacteria. Environ. Sci. Pollut. Res. 2015, 23, 4111–4119. [Google Scholar] [CrossRef]
- Bakhshayesh, A.M.; Bakhshayesh, N. Enhanced performance of dye-sensitized solar cells aided by Sr, Cr co-doped TiO2 xerogel films made of uniform spheres. J. Colloid Interface Sci. 2015, 460, 18–28. [Google Scholar] [CrossRef]
- Costa, A.I.; Gemini-Piperni, S.; Alves, A.C.; Costa, N.A.; Checca, N.R.; Leite, P.E.; Rocha, L.A.; Pinto, A.M.P.; Toptan, F.; Rossi, A.L.; et al. TiO2 bioactive implant surfaces doped with specific amount of Sr modulate mineralization. Mater. Sci. Eng. C 2020, 120, 111735. [Google Scholar] [CrossRef]
- Zhao, Z.; Omer, A.A.; Qin, Z.; Osman, S.; Xia, L.; Singh, R.P. Cu/N-codoped TiO2 prepared by the sol-gel method for phenanthrene removal under visible light irradiation. Environ. Sci. Pollut. Res. 2020, 27, 17530–17540. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Wan, Z.; Yang, X.; Cai, Q. Dental resin composites with improved antibacterial and mineralization properties via incorporating zinc/strontium-doped hydroxyapatite as functional fillers. Biomed. Mater. 2022, 17, 045002. [Google Scholar] [CrossRef]
- AlGhannam, M.I.; AlAbbas, M.S.; AlJishi, J.A.; AlRuwaili, M.A.; AlHumaid, J.; Ibrahim, M.S. Remineralizing Effects of Resin-Based Dental Sealants: A Systematic Review of In Vitro Studies. Polymers 2022, 14, 779. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Taubock, T.T. Ion release and hydroxyapatite precipitation of resin composites functionalized with two types of bioactive glass. J. Dent. 2022, 118, 103950. [Google Scholar] [CrossRef] [PubMed]
- Murgolo, S.; Moreira, I.S.; Piccirillo, C.; Castro, P.M.L.; Ventrella, G.; Cocozza, C.; Mascolo, G. Photocatalytic Degradation of Diclofenac by Hydroxyapatite–TiO2 Composite Material: Identification of Transformation Products and Assessment of Toxicity. Materials 2018, 11, 1779. [Google Scholar] [CrossRef]
- Márquez Brazón, E.; Piccirillo, C.; Moreira, I.S.; Castro, P.M.L. Photodegradation of pharmaceutical persistent pollutants using hydroxyapatite-based materials. J. Environ. Manag. 2016, 182, 486–495. [Google Scholar] [CrossRef]
- Zheng, L.; Li, K.; Ning, C.; Sun, J. Study on antibacterial and fluoride-releasing properties of a novel composite resin with fluorine-doped nano-zirconia fillers. J. Dent. 2021, 113, 103772. [Google Scholar] [CrossRef]
- Chroszcz, M.W.; Barszczewska-Rybarek, I.M.; Kazek-Kesik, A. Novel Antibacterial Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues and Triethylene Glycol Dimethacrylate. Int. J. Mol. Sci. 2022, 23, 4954. [Google Scholar] [CrossRef]
- Alansy, A.S.; Saeed, T.A.; Al-Attab, R.; Guo, Y.; Yang, Y.; Liu, B.; Fan, Z. Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite. Dent. Mater. 2022, 38, e266–e274. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londono, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional Dental Composite with Piezoelectric Nanofillers for Combined Antibacterial and Mineralization Effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. Part B Eng. 2021, 230, 109495. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Swamiappan, S. Biomineralization, mechanical, antibacterial and biological investigation of larnite and rankinite bioceramics. Mater. Sci. Eng. C 2020, 118, 111466. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Sajjad, S.; Leghari, S.A.K.; Flox, C.; Ahmad, S. Competitive role of nitrogen functionalities of N doped GO and sensitizing effect of Bi2O3 QDs on TiO2 for water remediation. J. Environ. Sci. 2021, 108, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Fauzi, N.A.; Ireland, A.J.; Sherriff, M.; Bandara, H.; Su, B. Nitrogen doped titanium dioxide as an aesthetic antimicrobial filler in dental polymers. Dent. Mater. 2022, 38, 147–157. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti biofilm filler content for composite resins. Dent. Mater. 2019, 35, e36–e46. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, S.; Yu, B.; Liu, Z.; Wang, D. Surface engineering for an enhanced photoelectrochemical response of TiO2 nanotube arrays by simple surface air plasma treatment. Chem. Commun. 2015, 51, 16940–16943. [Google Scholar] [CrossRef]
- Huang, B.; Yang, W.; Wen, Y.; Shan, B.; Chen, R. Co3O4-Modified TiO2 Nanotube Arrays via Atomic Layer Deposition for Improved Visible-Light Photoelectrochemical Performance. ACS Appl. Mater. Interfaces 2014, 7, 422–431. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Collares, F.M.; Ogliari, F.A.; Zanchi, C.H.; Petzhold, C.L.; Piva, E.; Samuel, S.M. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J. Adhes. Dent. 2011, 13, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Dickens, S.H. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent. Mater. 2000, 17, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Osorio, R.; Toledano, M.; Cabrerizo-Vílchez, M.A.; Nunes, T.G.; Kalachandra, S. Novel light-cured resins and composites with improved physicochemical properties. Dent. Mater. 2007, 23, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Gristina, R.; D’Aloia, E.; Senesi, G.S.; Milella, A.; Nardulli, M.; Sardella, E.; Favia, P.; d’Agostino, R. Increasing cell adhesion on plasma deposited fluorocarbon coatings by changing the surface topography. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 139–149. [Google Scholar] [CrossRef]
- Wang, T.; Matinlinna, J.P.; He, J.; Ahmed, K.E.; Burrow, M.F. Biomechanical and biological evaluations of novel BPA-free fibre-reinforced composites for biomedical applications. Mater. Sci. Eng. C 2020, 117, 111309. [Google Scholar] [CrossRef]
- da Silva, A.F.V.; Cesca, K.; Ambrosi, A.; Zin, G.; Di Luccio, M.; Oliveira, J.V. An expedite facile method for modification of PVDF membranes with polydopamine and TiO2 to improve water permeation. Mater. Lett. 2022, 324, 132611. [Google Scholar] [CrossRef]
- Nodeh, Z.P.; Beni, A.A.; Moghadam, A.J. Development of evaporation technique for concentrating lead acid wastewater from the battery recycling plant, by nanocomposite ceramic substrates and solar/wind energy. J. Environ. Manag. 2023, 328, 116980. [Google Scholar] [CrossRef]
- Loesche, W.J. Microbiology of Dental Decay and Periodontal Disease. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 99. [Google Scholar]
- Whiley, R.A.; Beighton, D. Current classification of the oral streptococci. Oral Microbiol. Immunol. 1998, 13, 195–216. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef]
- Fan, W.; Qi, Y.; Wang, R.; Xu, C.; Zhao, N.; Xu, F.J. Calcium carbonate-methylene blue nanohybrids for photodynamic therapy and ultrasound imaging. Sci. China Life Sci. 2018, 61, 483–491. [Google Scholar] [CrossRef]
- Mvango, S.; Mashazi, P. Synthesis, characterization of copper oxide-gold nanoalloys and their peroxidase-like activity towards colorimetric detection of hydrogen peroxide and glucose. Mater. Sci. Eng. C 2018, 96, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yin, J.; Cui, L.; Yang, Y.; Chen, X. Apatite-forming ability of bioactive poly (l-lactic acid)/grafted silica nanocomposites in simulated body fluid. Colloids Surf. B Biointerfaces 2011, 86, 218–224. [Google Scholar] [CrossRef]
- Lööf, J.; Svahn, F.; Jarmar, T.; Engqvist, H.; Pameijer, C.H. A comparative study of the bioactivity of three materials for dental applications. Dent. Mater. 2008, 24, 653–659. [Google Scholar] [CrossRef]
- Al-Odayni, A.B.; Alotaibi, D.H.; Saeed, W.S.; Al-Kahtani, A.; Assiri, A.; Alkhtani, F.M.; Alrahlah, A. Eugenyl-2-Hydroxypropyl Methacrylate-Incorporated Experimental Dental Composite: Degree of Polymerization and In Vitro Cytotoxicity Evaluation. Polymers 2022, 14, 277. [Google Scholar] [CrossRef]
- Skubalova, Z.; Michalkova, H.; Michalek, P.; Strmiska, V.; Guran, R.; Merlos Rodrigo, M.A.; Castkova, K.; Hynek, D.; Pekarik, V.; Zitka, O.; et al. Prevalent anatase crystalline phase increases the cytotoxicity of biphasic titanium dioxide nanoparticles in mammalian cells. Colloids Surf. B Biointerfaces 2019, 182, 110391. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Q.; Zhu, Y.; Yu, S.; Hou, Y.; Cui, Z.; Zhu, S. Construction and properties of the antibacterial epitaxial transition layer on a zirconia ceramic surface. RSC Adv. 2021, 11, 34699–34709. [Google Scholar] [CrossRef]


| Group | Reinforcing Fillers (wt%) | Traditional Fillers (wt%) | |
|---|---|---|---|
| Control Group | 0% Group | 0 | 60 |
| Experimental Groups | 2.5% Group | 2.5 | 57.5 |
| 5% Group | 5 | 55 | |
| 7.5% Group | 7.5 | 52.5 | |
| Groups | DC (%) | CD (mm) | ||
|---|---|---|---|---|
| 20 s | 40 s | 60 s | ||
| 0% Group | 47.12 ± 0.56 | 50.13 ± 2.06 | 55.04 ± 1.21 | 5.10 ± 0.19 |
| 2.5% Group | 40.84 ± 2.30 | 46.71 ± 2.93 | 54.30 ± 2.83 | 4.70 ± 0.19 |
| 5% Group | 37.99 ± 1.50 | 44.39 ± 0.37 | 52.27 ± 3.80 | 4.57 ± 0.28 |
| 7.5% Group | 34.75 ± 1.44 | 43.21 ± 2.13 | 48.98 ± 1.13 | 4.36 ± 0.18 |
| Group | RGR (%) | Cytotoxicity Grades | ||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | |
| 0% Group | 85.88 | 93.25 | 89.22 | I | I | I |
| 2.5% Group | 90.28 | 95.75 | 92.24 | I | I | I |
| 5% Group | 78.52 | 87.31 | 86.28 | I | I | I |
| 7.5% Group | 76.25 | 85.45 | 87.48 | I | I | I |
| Group | RGR (%) | Cytotoxicity Grades | ||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | |
| 0% Group | 109.14 | 109.17 | 111.82 | 0 | 0 | 0 |
| 2.5% Group | 84.88 | 90.91 | 91.24 | I | I | I |
| 5% Group | 81.08 | 100.69 | 98.63 | I | 0 | I |
| 7.5% Group | 79.39 | 94.95 | 91.73 | I | I | I |
| Group | RGR (%) | Cytotoxicity Grades | ||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | |
| 0% Group | 142.31 | 123.28 | 117.04 | 0 | 0 | 0 |
| 2.5% Group | 135.44 | 106.72 | 101.51 | 0 | 0 | 0 |
| 5% Group | 139.10 | 128.92 | 117.79 | 0 | 0 | 0 |
| 7.5% Group | 120.83 | 106.61 | 101.05 | 0 | 0 | 0 |
| RGR (%) | Toxicity Grade | Safety Standards |
|---|---|---|
| ≥100 | 0 | Safe |
| 75–99 | I | Safe |
| 50–74 | II | Insecurity |
| 25–49 | III | Insecurity |
| 1–24 | IV | Insecurity |
| <1 | V | Insecurity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, H.; Hong, L.; Zou, X.; Song, J.; Han, R.; Chen, J.; Yu, Y.; Liu, X.; Zhao, H.; et al. A Multifunctional Dental Resin Composite with Sr-N-Doped TiO2 and n-HA Fillers for Antibacterial and Mineralization Effects. Int. J. Mol. Sci. 2023, 24, 1274. https://doi.org/10.3390/ijms24021274
Zhao Y, Zhang H, Hong L, Zou X, Song J, Han R, Chen J, Yu Y, Liu X, Zhao H, et al. A Multifunctional Dental Resin Composite with Sr-N-Doped TiO2 and n-HA Fillers for Antibacterial and Mineralization Effects. International Journal of Molecular Sciences. 2023; 24(2):1274. https://doi.org/10.3390/ijms24021274
Chicago/Turabian StyleZhao, Yuanhang, Hong Zhang, Lihua Hong, Xinying Zou, Jiazhuo Song, Rong Han, Jiawen Chen, Yiyan Yu, Xin Liu, Hong Zhao, and et al. 2023. "A Multifunctional Dental Resin Composite with Sr-N-Doped TiO2 and n-HA Fillers for Antibacterial and Mineralization Effects" International Journal of Molecular Sciences 24, no. 2: 1274. https://doi.org/10.3390/ijms24021274
APA StyleZhao, Y., Zhang, H., Hong, L., Zou, X., Song, J., Han, R., Chen, J., Yu, Y., Liu, X., Zhao, H., & Zhang, Z. (2023). A Multifunctional Dental Resin Composite with Sr-N-Doped TiO2 and n-HA Fillers for Antibacterial and Mineralization Effects. International Journal of Molecular Sciences, 24(2), 1274. https://doi.org/10.3390/ijms24021274






