Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin
Abstract
1. Introduction
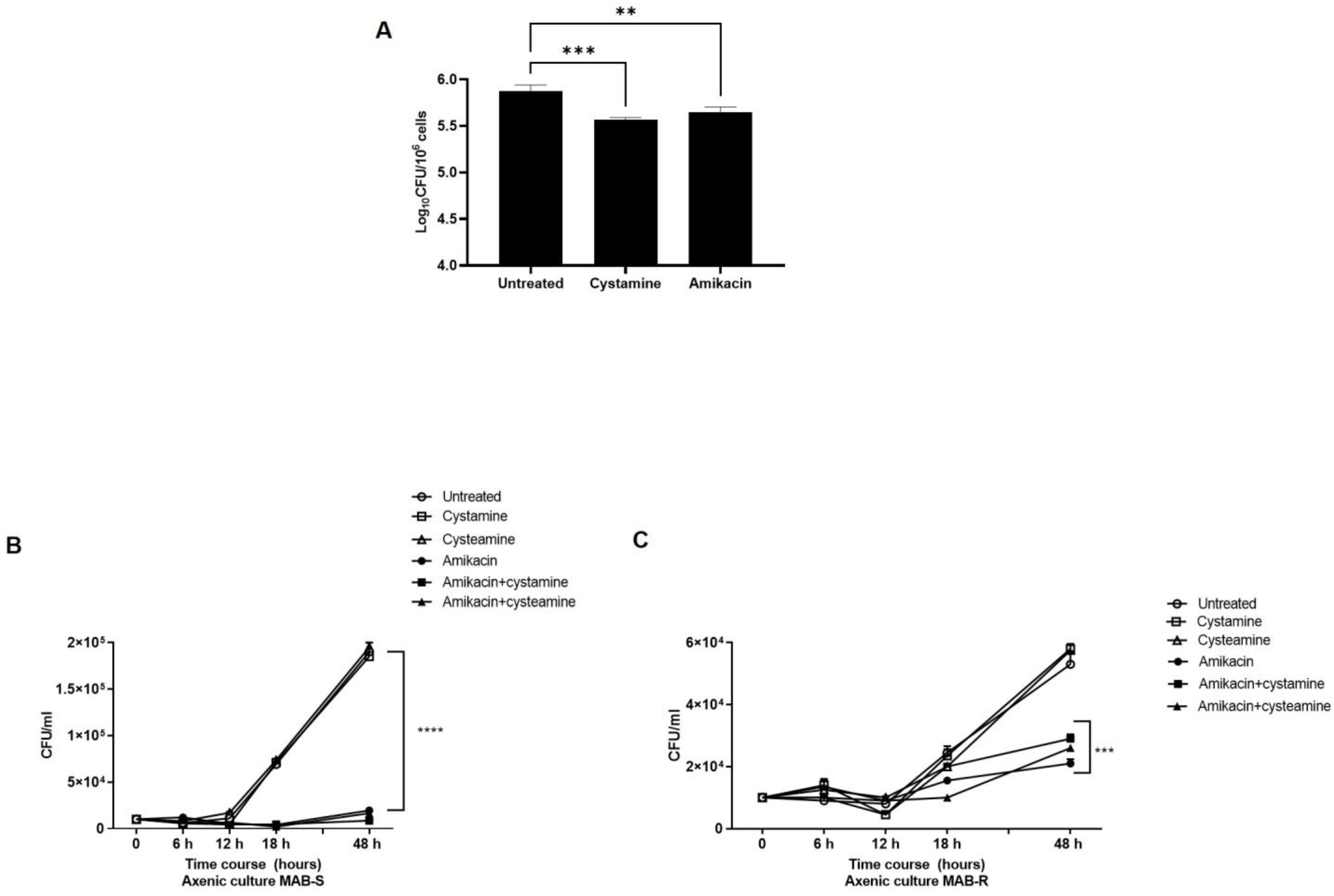
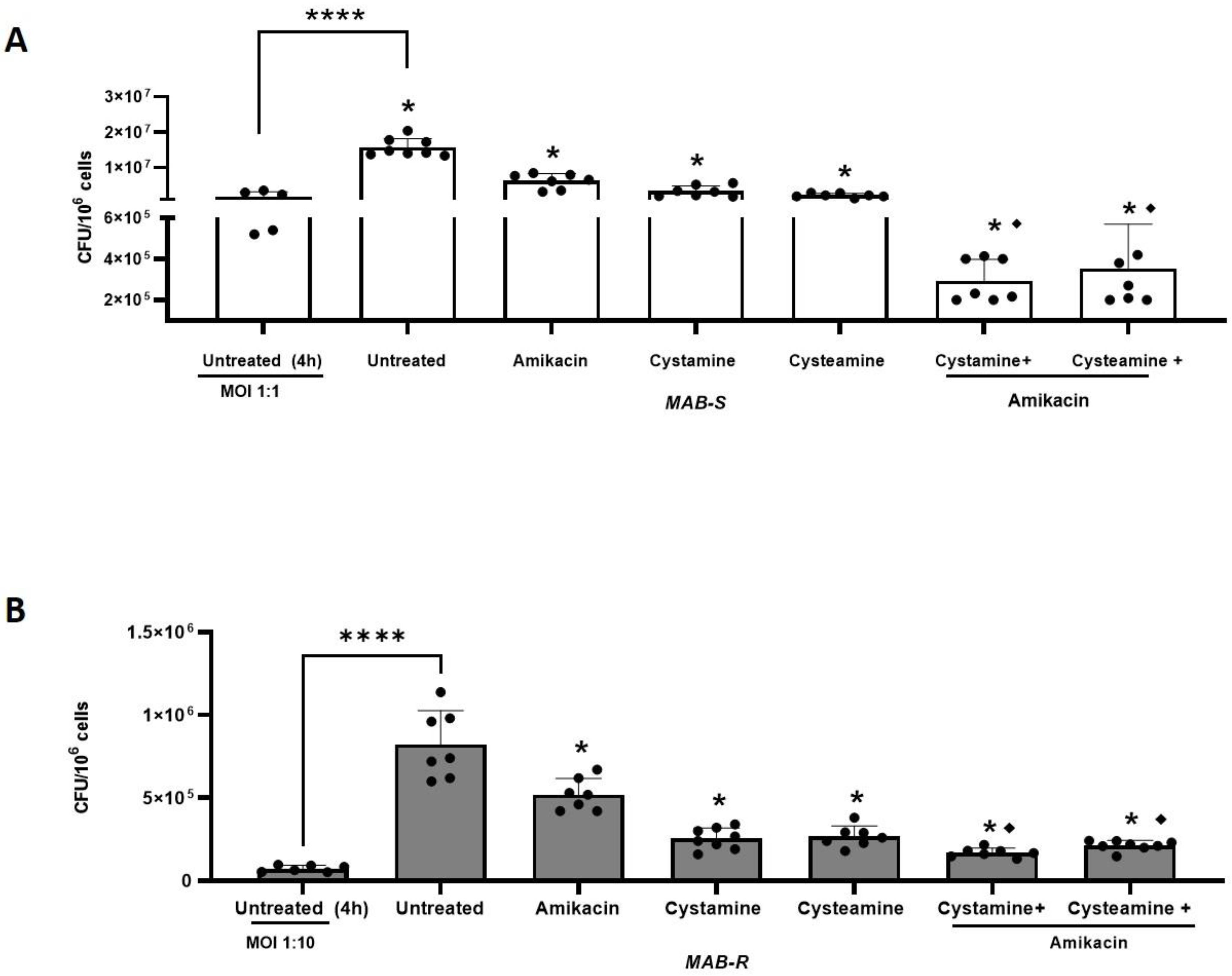
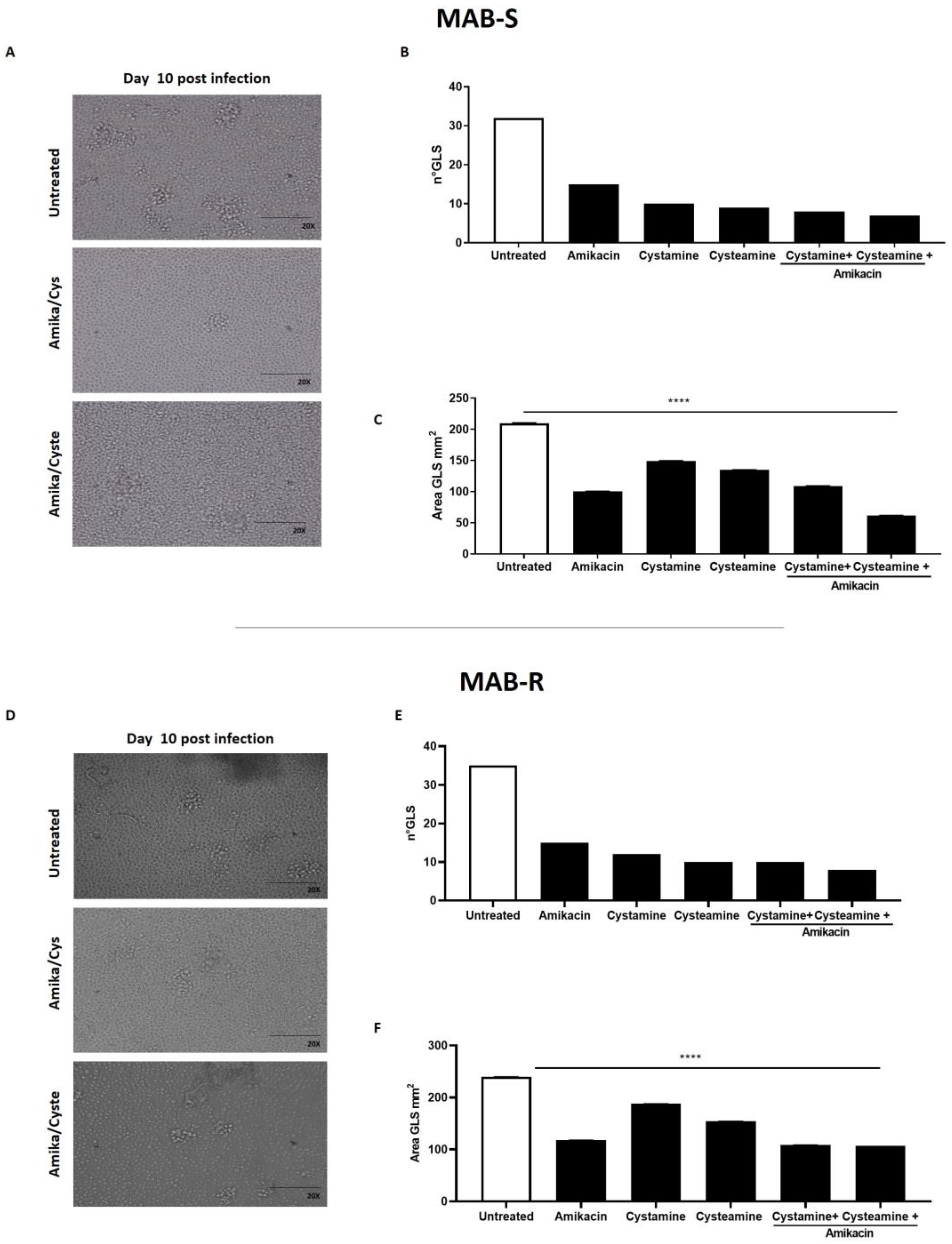
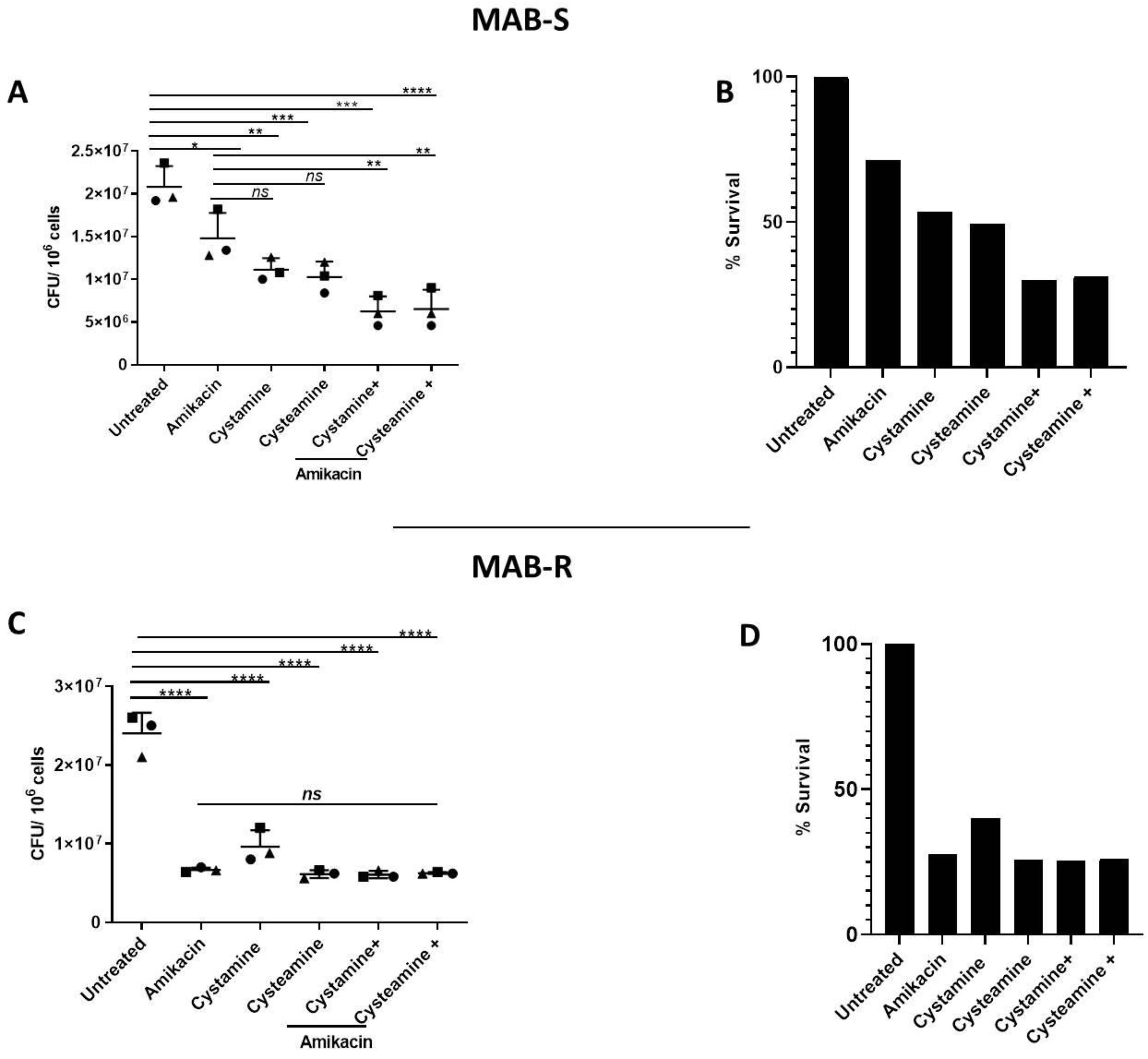
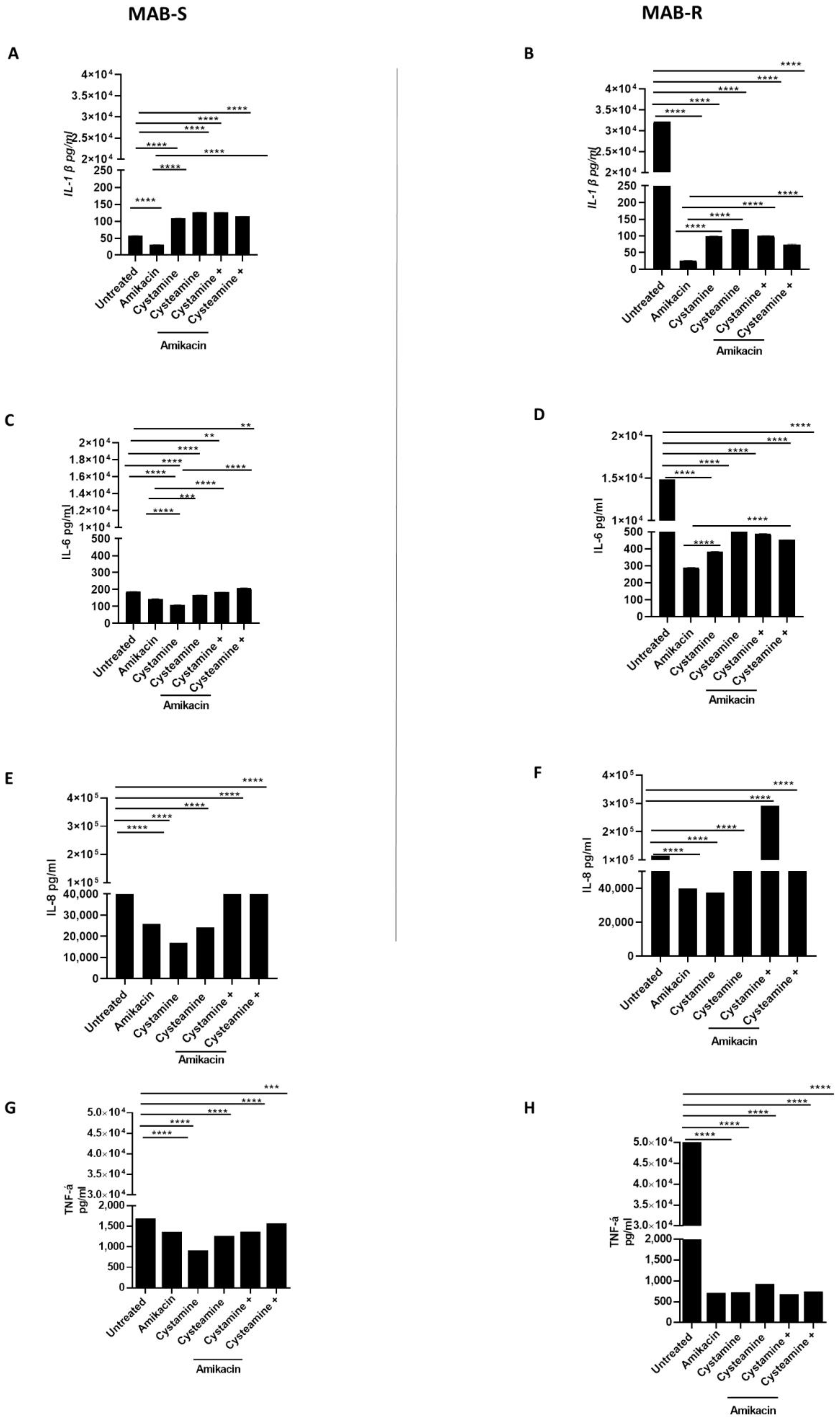
2. Results
3. Discussion
4. Material and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 2014, 27, 727–752. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Wagner, D.; Gallagher, J.; Morimoto, K.; Lange, C.; Haworth, C.S.; Floto, R.A.; Adjemian, J.; Prevots, D.R.; Griffith, D.E.; et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur. Respir. J. 2017, 49, 1601855. [Google Scholar] [CrossRef] [PubMed]
- Seaworth, B.J.; Griffith, D.E. Therapy of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Microbiol. Spectr. 2017, 5, 129–158. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Dukart, G.; Brown-Elliott, B.A.; Griffith, D.E.; Scerpella, E.G.; Marshall, B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 2014, 69, 1945–1953. [Google Scholar] [CrossRef]
- Roux, A.L.; Viljoen, A.; Bah, A.; Simeone, R.; Bernut, A.; Laencina, L.; Deramaudt, T.; Rottman, M.; Gaillard, J.L.; Majlessi, L.; et al. The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 2016, 6, 160185. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef]
- Raaijmakers, J.; Schildkraut, J.A.; Hoefsloot, W.; van Ingen, J. The role of amikacin in the treatment of nontuberculous mycobacterial disease. Expert Opin. Pharmacother. 2021, 22, 1961–1974. [Google Scholar] [CrossRef]
- Koh, W.J.; Jeong, B.H.; Kim, S.Y.; Jeon, K.; Park, K.U.; Jhun, B.W.; Lee, H.; Park, H.Y.; Kim, D.H.; Huh, H.J.; et al. Mycobacterial Characteristics and Treatment Outcomes in Mycobacterium abscessus Lung Disease. Clin. Infect. Dis. 2017, 64, 309–316. [Google Scholar] [CrossRef]
- Wu, M.L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Wallis, R.S.; Maeurer, M.; Mwaba, P.; Chakaya, J.; Rustomjee, R.; Migliori, G.B.; Marais, B.; Schito, M.; Churchyard, G.; Swaminathan, S.; et al. Tuberculosis—Advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect. Dis. 2016, 16, e34–e46. [Google Scholar] [CrossRef]
- Bergman, P.; Raqib, R.; Rekha, R.S.; Agerberth, B.; Gudmundsson, G.H. Host Directed Therapy Against Infection by Boosting Innate Immunity. Front. Immunol. 2020, 11, 1209. [Google Scholar] [CrossRef]
- Palucci, I.; Delogu, G. Host Directed Therapies for Tuberculosis: Futures Strategies for an Ancient Disease. Chemotherapy 2018, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.M.; Roca, F.J.; Oh, S.F.; McFarland, R.; Vickery, T.W.; Ray, J.P.; Ko, D.C.; Zou, Y.; Bang, N.D.; Chau, T.T.; et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 2012, 148, 434–446. [Google Scholar] [CrossRef]
- D’Eletto, M.; Farrace, M.G.; Rossin, F.; Strappazzon, F.; Giacomo, G.D.; Cecconi, F.; Melino, G.; Sepe, S.; Moreno, S.; Fimia, G.M.; et al. Type 2 transglutaminase is involved in the autophagy-dependent clearance of ubiquitinated proteins. Cell Death Differ. 2012, 19, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Palucci, I.; Matic, I.; Falasca, L.; Minerva, M.; Maulucci, G.; De, S.M.; Petruccioli, E.; Goletti, D.; Rossin, F.; Piacentini, M.; et al. Transglutaminase type 2 plays a key role in the pathogenesis of Mycobacterium tuberculosis infection. J. Intern. Med. 2018, 283, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Palucci, I.; Maulucci, G.; De Maio, F.; Sali, M.; Romagnoli, A.; Petrone, L.; Fimia, G.M.; Sanguinetti, M.; Goletti, D.; De Spirito, M.; et al. Inhibition of Transglutaminase 2 as a Potential Host-Directed Therapy Against Mycobacterium tuberculosis. Front. Immunol. 2019, 10, 3042. [Google Scholar] [CrossRef]
- Occhigrossi, L.; Rossin, F.; D’Eletto, M.; Farrace, M.G.; Ciccosanti, F.; Petrone, L.; Sacchi, A.; Nardacci, R.; Falasca, L.; Del Nonno, F.; et al. Transglutaminase 2 Regulates Innate Immunity by Modulating the STING/TBK1/IRF3 Axis. J. Immunol. 2021, 206, 2420–2429. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Pinto, J.T.; Cooper, A.J.L. Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo. Biosci. Rep. 2018, 38, BSR20180691. [Google Scholar] [CrossRef]
- Gallego-Villar, L.; Hannibal, L.; Haberle, J.; Thony, B.; Ben-Omran, T.; Nasrallah, G.K.; Dewik, A.N.; Kruger, W.D.; Blom, H.J. Cysteamine revisited: Repair of arginine to cysteine mutations. J. Inherit. Metab. Dis. 2017, 40, 555–567. [Google Scholar] [CrossRef]
- Ferrari, E.; Monzani, R.; Villella, V.R.; Esposito, S.; Saluzzo, F.; Rossin, F.; D’Eletto, M.; Tosco, A.; De, G.F.; Izzo, V.; et al. Cysteamine re-establishes the clearance of Pseudomonas aeruginosa by macrophages bearing the cystic fibrosis-relevant F508del-CFTR mutation. Cell Death Dis. 2017, 8, e2544. [Google Scholar] [CrossRef]
- Alonzi, T.; Aiello, A.; Petrone, L.; Najafi Fard, S.; D’Eletto, M.; Falasca, L.; Nardacci, R.; Rossin, F.; Delogu, G.; Castilletti, C.; et al. Cysteamine with In Vitro Antiviral Activity and Immunomodulatory Effects Has the Potential to Be a Repurposing Drug Candidate for COVID-19 Therapy. Cells 2021, 11, 52. [Google Scholar] [CrossRef]
- Alonzi, T.; Aiello, A.; Repele, F.; Falasca, L.; Francalancia, M.; Garbuglia, A.R.; Delogu, G.; Nicastri, E.; Piacentini, M.; Goletti, D. Cysteamine exerts in vitro antiviral activity against the SARS-CoV-2 Delta and Omicron variants. Cell Death Discov. 2022, 8, 288. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis Granuloma—The Critical Battlefield in Host Immunity and Disease. Front. Immunol. 2013, 4, 98. [Google Scholar] [CrossRef]
- Chatterjee, D.; Khoo, K.H. The surface glycopeptidolipids of mycobacteria: Structures and biological properties. Cell. Mol. Life Sci. 2001, 58, 2018–2042. [Google Scholar] [CrossRef]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152, 1581–1590. [Google Scholar] [CrossRef]
- Jönsson, B.E.; Gilljam, M.; Lindblad, A.; Ridell, M.; Wold, A.E.; Welinder-Olsson, C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 2007, 45, 1497–1504. [Google Scholar] [CrossRef]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jain, P.; Weisbrod, T.R.; Leung, L.W.; Freundlich, J.S.; Jacobs, W.R., Jr. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 4495–4500. [Google Scholar] [CrossRef]
- Devereux, G.; Wrolstad, D.; Bourke, S.J.; Daines, C.L.; Doe, S.; Dougherty, R.; Franco, R.; Innes, A.; Kopp, B.T.; Lascano, J.; et al. Oral cysteamine as an adjunct treatment in cystic fibrosis pulmonary exacerbations: An exploratory randomized clinical trial. PLoS ONE 2020, 15, e0242945. [Google Scholar] [CrossRef]
- Guirado, E.; Mbawuike, U.; Keiser, T.L.; Arcos, J.; Azad, A.K.; Wang, S.H.; Schlesinger, L.S. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. mBio 2015, 6, e02537-14. [Google Scholar] [CrossRef]
- Roux, A.L.; Ray, A.; Pawlik, A.; Medjahed, H.; Etienne, G.; Rottman, M.; Catherinot, E.; Coppée, J.Y.; Chaoui, K.; Monsarrat, B.; et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 2011, 13, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, E.R.; Archambault, A.S.; Greendyke, R.; Hsu, F.F.; Streeter, C.; Byrd, T.F. Mycobacterium abscessus Glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J. Immunol. 2009, 183, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.M.; Zahid, M.S.H.; Bachelder, E.M.; Ainslie, K.M. Formulation of host-targeted therapeutics against bacterial infections. Transl. Res. 2020, 220, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, G.; Saris, A.; Ottenhoff, T.H.M.; Haks, M.C. Host-directed therapy to combat mycobacterial infections. Immunol. Rev. 2021, 301, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Lopeman, R.C.; Harrison, J.; Desai, M.; Cox, J.A.G. Mycobacterium abscessus: Environmental Bacterium Turned Clinical Nightmare. Microorganisms 2019, 7, 90. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Bottger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef]
- Gutiérrez, A.V.; Baron, S.A.; Sardi, F.S.; Saad, J.; Coltey, B.; Reynaud-Gaubert, M.; Drancourt, M. Beyond phenotype: The genomic heterogeneity of co-infecting Mycobacterium abscessus smooth and rough colony variants in cystic fibrosis patients. J. Cyst. Fibros. 2021, 20, 421–423. [Google Scholar] [CrossRef]
- Pawlik, A.; Garnier, G.; Orgeur, M.; Tong, P.; Lohan, A.; Le Chevalier, F.; Sapriel, G.; Roux, A.L.; Conlon, K.; Honoré, N.; et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol. Microbiol. 2013, 90, 612–629. [Google Scholar] [CrossRef]
- Charrier, C.; Rodger, C.; Robertson, J.; Kowalczuk, A.; Shand, N.; Fraser-Pitt, D.; Mercer, D.; O’Neil, D. Cysteamine (Lynovex(R)), a novel mucoactive antimicrobial & antibiofilm agent for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 2014, 9, 189. [Google Scholar] [CrossRef]
- Devereux, G.; Fraser-Pitt, D.; Robertson, J.; Devlin, E.; Mercer, D.; O’Neil, D. Cysteamine as a Future Intervention in Cystic Fibrosis Against Current and Emerging Pathogens: A Patient-based ex vivo Study Confirming its Antimicrobial and Mucoactive Potential in Sputum. eBioMedicine 2015, 2, 1507–1512. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.L.; Kissa, K.; Dubremetz, J.F.; Gaillard, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef]
- Bernut, A.; Nguyen-Chi, M.; Halloum, I.; Herrmann, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus-Induced Granuloma Formation Is Strictly Dependent on TNF Signaling and Neutrophil Trafficking. PLoS Pathog. 2016, 12, e1005986. [Google Scholar] [CrossRef]
- Roach, D.R.; Bean, A.G.; Demangel, C.; France, M.P.; Briscoe, H.; Britton, W.J. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 2002, 168, 4620–4627. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Ruangkiattikul, N.; Rys, D.; Abdissa, K.; Rohde, M.; Semmler, T.; Tegtmeyer, P.K.; Kalinke, U.; Schwarz, C.; Lewin, A.; Goethe, R. Type I interferon induced by TLR2-TLR4-MyD88-TRIF-IRF3 controls Mycobacterium abscessus subsp. abscessus persistence in murine macrophages via nitric oxide. Int. J. Med. Microbiol. 2019, 309, 307–318. [Google Scholar] [CrossRef]
- Cruz-Aguilar, M.; Castillo-Rodal, A.I.; Arredondo-Hernández, R.; López-Vidal, Y. Non-tuberculous mycobacteria immunopathogenesis: Closer than they appear. a prime of innate immunity trade-off and NTM ways into virulence. Scand. J. Immunol. 2021, 94, e13035. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Chao, W.C.; Yen, C.L.; Wu, C.H.; Shieh, C.C. How mycobacteria take advantage of the weakness in human immune system in the modern world. J. Microbiol. Immunol. Infect. 2020, 53, 209–215. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef]
- Friedland, J.S. Targeting the inflammatory response in tuberculosis. N. Engl. J. Med. 2014, 371, 1354–1356. [Google Scholar] [CrossRef]
- Chao, W.C.; Yen, C.L.; Hsieh, C.Y.; Huang, Y.F.; Tseng, Y.L.; Nigrovic, P.A.; Shieh, C.C. Mycobacterial infection induces higher interleukin-1β and dysregulated lung inflammation in mice with defective leukocyte NADPH oxidase. PLoS ONE 2017, 12, e0189453. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, E.; Urbano, P.; Marcelli, F.; Simonetti, T.M.; Cirillo, D.M. Is real-time PCR better than conventional PCR for Mycobacterium tuberculosis complex detection in clinical samples? J. Clin. Microbiol. 2012, 50, 2810–2813. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Marchesi, F.; Balz, M.; Bally, F.; Böttger, E.C.; Bodmer, T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993, 31, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Sali, M.; Di, S.G.; Cascioferro, A.; Zumbo, A.; Nicolo, C.; Dona, V.; Rocca, S.; Procoli, A.; Morandi, M.; Ria, F.; et al. Surface expression of MPT64 as a fusion with the PE domain of PE_PGRS33 enhances Mycobacterium bovis BCG protective activity against Mycobacterium tuberculosis in mice. Infect. Immun. 2010, 78, 5202–5213. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Brambilla, C.; Llorens-Fons, M.; Julián, E.; Noguera-Ortega, E.; Tomàs-Martínez, C.; Pérez-Trujillo, M.; Byrd, T.F.; Alcaide, F.; Luquin, M. Mycobacteria Clumping Increase Their Capacity to Damage Macrophages. Front. Microbiol. 2016, 7, 1562. [Google Scholar] [CrossRef]
| MAB R | MAB S | |||||
|---|---|---|---|---|---|---|
| Antibiotic | - | +Cystamine | +Cysteamine | - | +Cystamine | +Cysteamine |
| Clarithromycin | 32 | 32 | 32 | 32 | 32 | 32 |
| Rifabutin | 8 | 8 | 8 | 8 | 8 | 8 |
| Ethambutol | 16 | 16 | 16 | 16 | 16 | 16 |
| Isoniazid | 8 | 8 | 8 | 8 | 8 | 8 |
| Moxifloxacin | 8 | 8 | 8 | 8 | 8 | 8 |
| Rifampin | 8 | 8 | 8 | 8 | 8 | 8 |
| Trimethoprim/Sulfamet. | 8/152 | 8/152 | 8/152 | 8/152 | 8/152 | 8/152 |
| Amikacin | 4 | 4 | 4 | 4 | 4 | 4 |
| Linezolid | 64 | 64 | 64 | 32 | 64 | 64 |
| Cirpofloxacin | 8 | 8 | 8 | 4 | 4 | 4 |
| Streptomycin | 64 | 64 | 64 | 64 | 64 | 64 |
| Doxycyckine | 16 | 16 | 16 | 16 | 16 | 16 |
| Ethionamide | 20 | 20 | 20 | 20 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palucci, I.; Salustri, A.; De Maio, F.; Pereyra Boza, M.d.C.; Paglione, F.; Sali, M.; Occhigrossi, L.; D’Eletto, M.; Rossin, F.; Goletti, D.; et al. Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin. Int. J. Mol. Sci. 2023, 24, 1203. https://doi.org/10.3390/ijms24021203
Palucci I, Salustri A, De Maio F, Pereyra Boza MdC, Paglione F, Sali M, Occhigrossi L, D’Eletto M, Rossin F, Goletti D, et al. Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin. International Journal of Molecular Sciences. 2023; 24(2):1203. https://doi.org/10.3390/ijms24021203
Chicago/Turabian StylePalucci, Ivana, Alessandro Salustri, Flavio De Maio, Maria del Carmen Pereyra Boza, Francesco Paglione, Michela Sali, Luca Occhigrossi, Manuela D’Eletto, Federica Rossin, Delia Goletti, and et al. 2023. "Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin" International Journal of Molecular Sciences 24, no. 2: 1203. https://doi.org/10.3390/ijms24021203
APA StylePalucci, I., Salustri, A., De Maio, F., Pereyra Boza, M. d. C., Paglione, F., Sali, M., Occhigrossi, L., D’Eletto, M., Rossin, F., Goletti, D., Sanguinetti, M., Piacentini, M., & Delogu, G. (2023). Cysteamine/Cystamine Exert Anti-Mycobacterium abscessus Activity Alone or in Combination with Amikacin. International Journal of Molecular Sciences, 24(2), 1203. https://doi.org/10.3390/ijms24021203








