The Involvement of Cx43 in JNK1/2-Mediated Endothelial Mechanotransduction and Human Plaque Progression
Abstract
1. Introduction
2. Results
2.1. JNK1/2 Was Activated in HUVECs by Inflammatory and Mechanical Stimuli
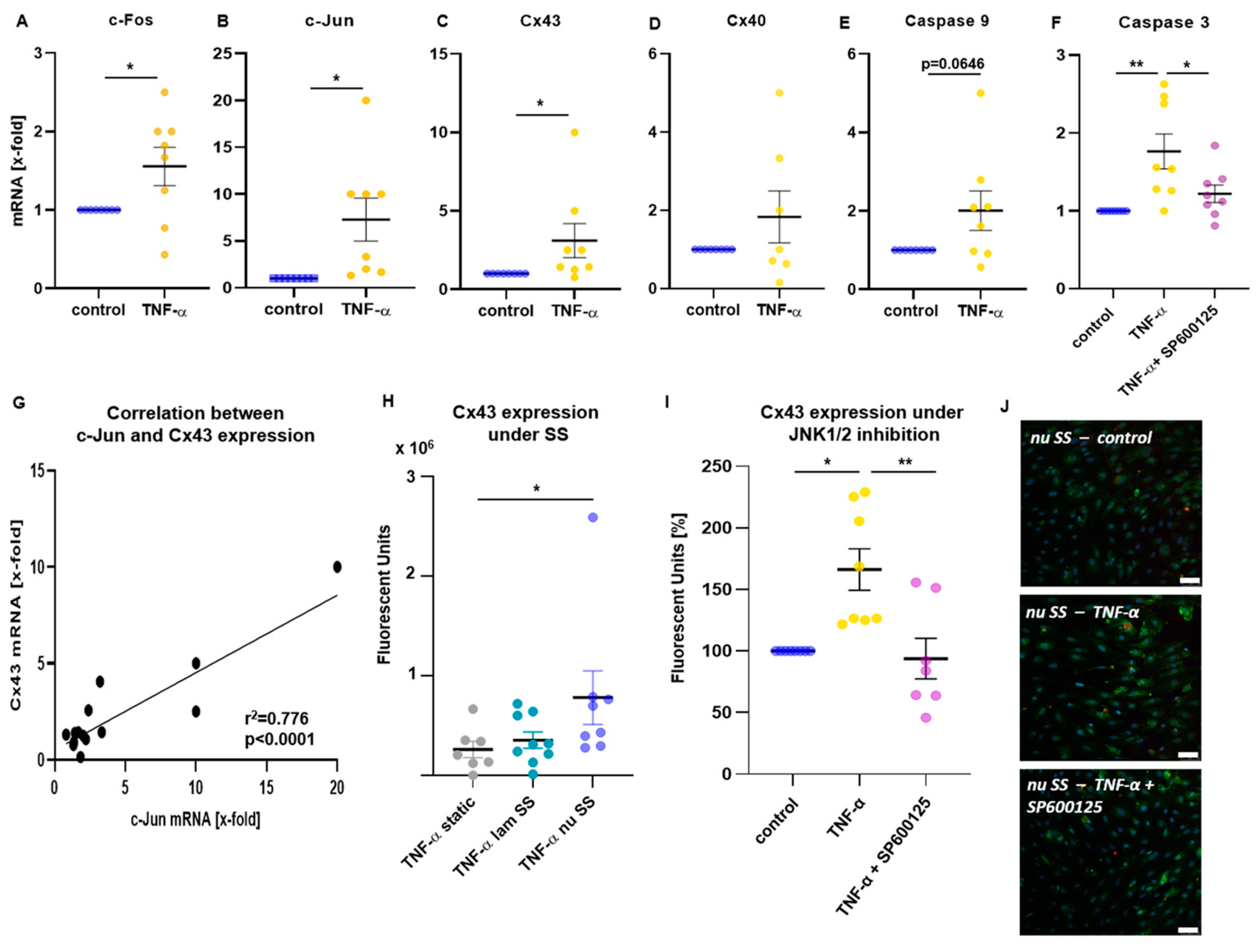
2.2. Inflammatory Mechanoactivation of JNK1/2 Was Associated with the Induction of Cx43 and Caspase 3
2.3. Time-Dependent Cx43 Induction by Mechanical Stimulation
2.4. Inflammatory Stimulation Acts as Booster of Cx43 Expression in Mechanoactivated HUVECs
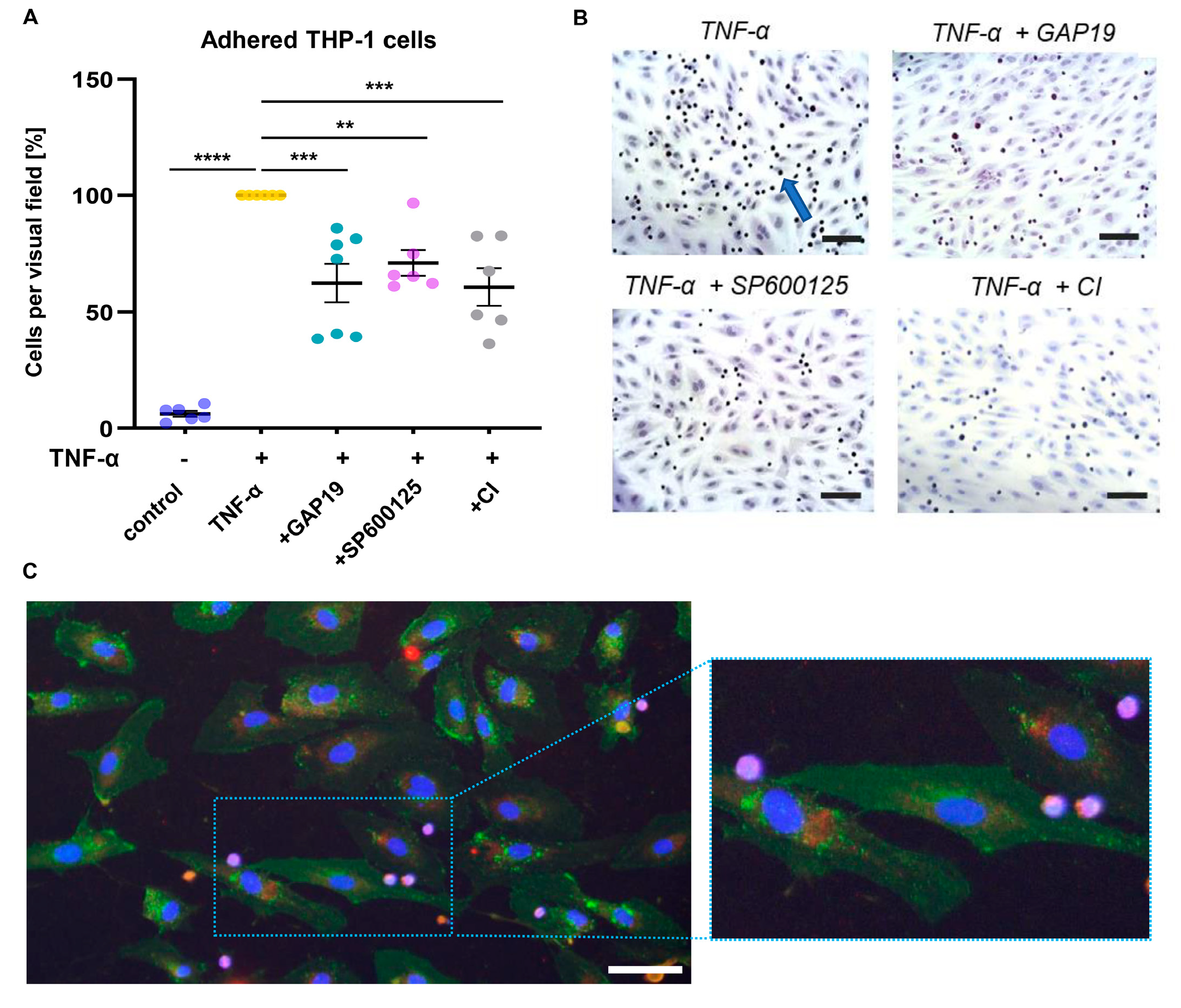
2.5. Cx43 Is Involved in Proatherogenic Cell Responses in HUVECs Exposed to Non-Uniform SS
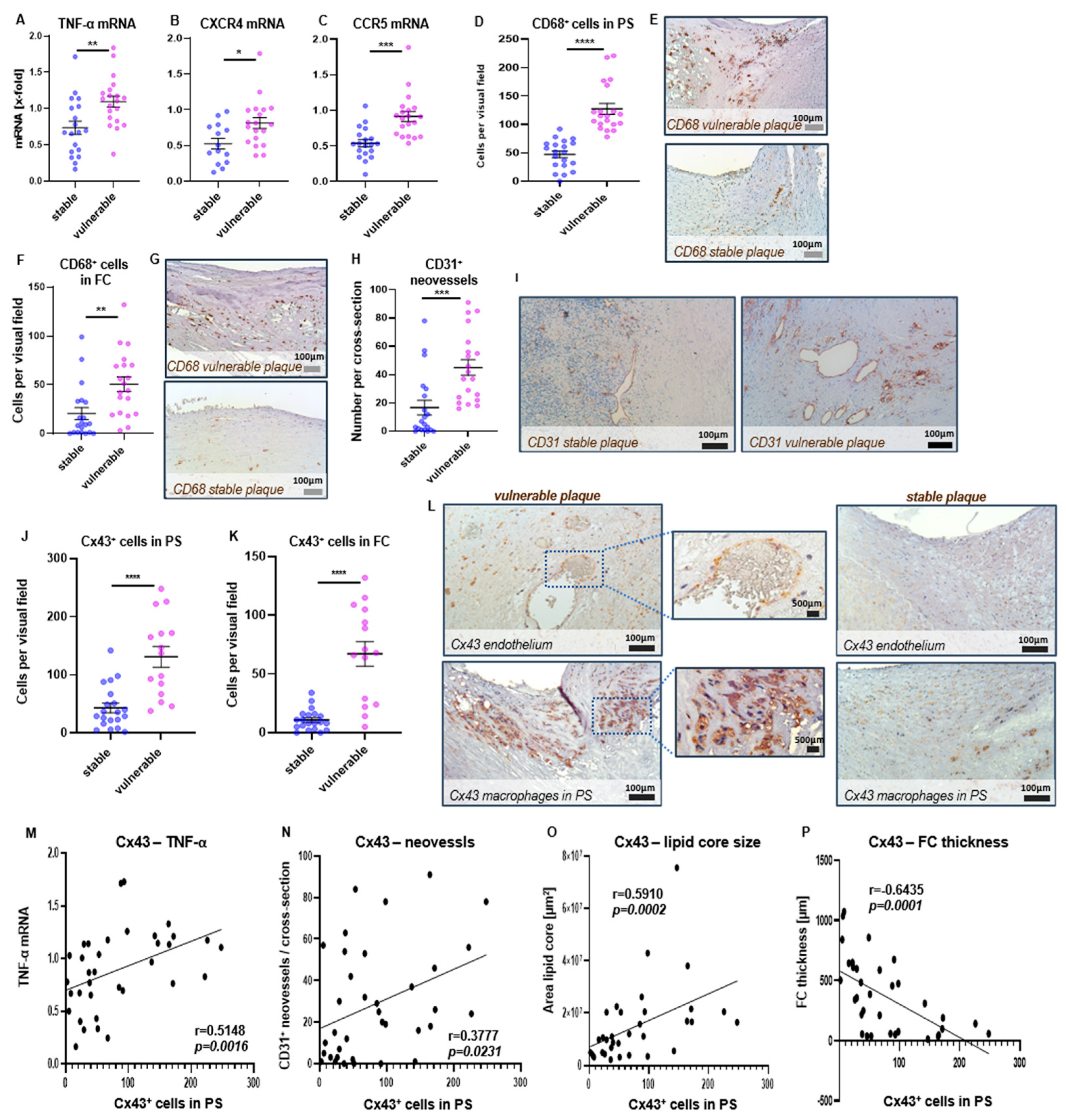
2.6. Expression of TNF-α and Cx43 in Human Carotid Plaque Specimens
3. Discussion
4. Materials and Methods
4.1. Patients
Ethics Statement
4.2. Isolation of Human Umbilical Vein ECs (HUVECs) from Umbilical Cord
4.3. Analysis of Endothelial Mechanoactivation by Dynamic Flow Experiments
4.4. Flow Cytometric Analysis of Endothelial Mechanoactivation
4.5. Immunofluorescent Staining
4.6. Adhesion Assay
4.7. Quantitative Real-Time PCR
4.8. Collection of Carotid Plaque Specimens of Patients Undergoing Endarterectomy
4.9. Histological Analysis of Plaque Specimens
4.10. Analysis of Cytokine Expression in Human Carotid Plaques
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 20 December 2022).
- Kasner, S.E.; Chimowitz, M.I.; Lynn, M.J.; Howlett-Smith, H.; Stern, B.J.; Hertzberg, V.S.; Frankel, M.R.; Levine, S.R.; Chaturvedi, S.; Benesch, C.G.; et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006, 113, 555–563. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Turan, T.N.; Derdeyn, C.P.; Fiorella, D.; Chimowitz, M.I. Treatment of atherosclerotic intracranial arterial stenosis. Stroke 2009, 40, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieslar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 1970. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Liu, H.; Ip, V.; Soo, Y.; Abrigo, J.; Fan, F.; Ma, S.H.; Ma, K.; Ip, B.; Liu, J.; et al. Regional High Wall Shear Stress Associated With Stenosis Regression in Symptomatic Intracranial Atherosclerotic Disease. Stroke 2020, 51, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Wang, X.; Ge, J. Haemodynamics of atherosclerosis: A matter of higher hydrostatic pressure or lower shear stress? Cardiovasc. Res. 2021, 117, e57–e59. [Google Scholar] [CrossRef]
- Mucka, S.; Miodonska, M.; Jakubiak, G.K.; Starzak, M.; Cieslar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef]
- Caro, C.G.; Fitz-Gerald, J.M.; Schroter, R.C. Arterial wall shear and distribution of early atheroma in man. Nature 1969, 223, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Giddens, D.P.; Bharadvaj, B.K.; Sottiurai, V.S.; Mabon, R.F.; Glagov, S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983, 53, 502–514. [Google Scholar] [CrossRef]
- Ajami, N.E.; Gupta, S.; Maurya, M.R.; Nguyen, P.; Li, J.Y.; Shyy, J.Y.; Chen, Z.; Chien, S.; Subramaniam, S. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl. Acad. Sci. USA 2017, 114, 10990–10995. [Google Scholar] [CrossRef] [PubMed]
- Levesque, M.J.; Nerem, R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef]
- Maurya, M.R.; Gupta, S.; Li, J.Y.; Ajami, N.E.; Chen, Z.B.; Shyy, J.Y.; Chien, S.; Subramaniam, S. Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2023236118. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Li, Y.S.; Sotoudeh, M.; Yuan, S.; Li, S.; Chien, S.; Shyy, J.Y. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 227–234. [Google Scholar] [CrossRef]
- Li, S.; Kim, M.; Hu, Y.L.; Jalali, S.; Schlaepfer, D.D.; Hunter, T.; Chien, S.; Shyy, J.Y. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 1997, 272, 30455–30462. [Google Scholar] [CrossRef]
- Tseng, H.; Peterson, T.E.; Berk, B.C. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ. Res. 1995, 77, 869–878. [Google Scholar] [CrossRef]
- Urschel, K.; Garlichs, C.D.; Daniel, W.G.; Cicha, I. VEGFR2 signalling contributes to increased endothelial susceptibility to TNF-alpha under chronic non-uniform shear stress. Atherosclerosis 2011, 219, 499–509. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- De Cesaris, P.; Starace, D.; Starace, G.; Filippini, A.; Stefanini, M.; Ziparo, E. Activation of Jun N-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor alpha leads to intercellular adhesion molecule-1 expression. J. Biol. Chem. 1999, 274, 28978–28982. [Google Scholar] [CrossRef]
- Jo, H.; Sipos, K.; Go, Y.M.; Law, R.; Rong, J.; McDonald, J.M. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J. Biol. Chem. 1997, 272, 1395–1401. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Breton, S. The MAPK/ERK signaling pathway regulates the expression and localization of Cx43 in mouse proximal epididymisdagger. Biol. Reprod. 2022, 106, 919–927. [Google Scholar] [CrossRef]
- Qi, M.; Elion, E.A. MAP kinase pathways. J. Cell Sci. 2005, 118, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Isakson, B.E.; Damon, D.N.; Day, K.H.; Liao, Y.; Duling, B.R. Connexin40 and connexin43 in mouse aortic endothelium: Evidence for coordinated regulation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1199–H1205. [Google Scholar] [CrossRef]
- De Bock, M.; Culot, M.; Wang, N.; Bol, M.; Decrock, E.; De Vuyst, E.; da Costa, A.; Dauwe, I.; Vinken, M.; Simon, A.M.; et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2011, 31, 1942–1957. [Google Scholar] [CrossRef]
- Blackburn, J.P.; Peters, N.S.; Yeh, H.I.; Rothery, S.; Green, C.R.; Severs, N.J. Upregulation of connexin43 gap junctions during early stages of human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1219–1228. [Google Scholar] [CrossRef]
- Chadjichristos, C.E.; Morel, S.; Derouette, J.P.; Sutter, E.; Roth, I.; Brisset, A.C.; Bochaton-Piallat, M.L.; Kwak, B.R. Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ. Res. 2008, 102, 653–660. [Google Scholar] [CrossRef]
- Yeh, H.I.; Lupu, F.; Dupont, E.; Severs, N.J. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3174–3184. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Jang, B.; Choi, H.S.; Kim, H.J.; Park, J.H.; Jeon, Y.C.; Carp, R.I.; Kim, Y.S.; Choi, E.K. Upregulation of Connexin 43 Expression Via C-Jun N-Terminal Kinase Signaling in Prion Disease. J. Alzheimers Dis. 2016, 49, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Luo, L.; Chen, Z.; Kim, H.S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef]
- Azuma, N.; Duzgun, S.A.; Ikeda, M.; Kito, H.; Akasaka, N.; Sasajima, T.; Sumpio, B.E. Endothelial cell response to different mechanical forces. J. Vasc. Surg. 2000, 32, 789–794. [Google Scholar] [CrossRef]
- Westwick, J.K.; Weitzel, C.; Minden, A.; Karin, M.; Brenner, D.A. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J. Biol. Chem. 1994, 269, 26396–26401. [Google Scholar] [CrossRef] [PubMed]
- Surapisitchat, J.; Hoefen, R.J.; Pi, X.; Yoshizumi, M.; Yan, C.; Berk, B.C. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc. Natl. Acad. Sci. USA 2001, 98, 6476–6481. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gerli, R.; Doria, A.; Matsuura, E.; Cerinic, M.M.; Ronda, N.; Jara, L.J.; Abu-Shakra, M.; Meroni, P.L.; Sherer, Y. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005, 112, 3337–3347. [Google Scholar] [CrossRef]
- McDonald, C.J.; Calabresi, P. Occlusive vascular disease in psoriatic patients. N. Engl. J. Med. 1973, 288, 912. [Google Scholar]
- Bosco, D.; Haefliger, J.A.; Meda, P. Connexins: Key mediators of endocrine function. Physiol. Rev. 2011, 91, 1393–1445. [Google Scholar] [CrossRef]
- Kameritsch, P.; Khandoga, N.; Pohl, U.; Pogoda, K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 2013, 4, e584. [Google Scholar] [CrossRef]
- Ma, J.W.; Ji, D.D.; Li, Q.Q.; Zhang, T.; Luo, L. Inhibition of connexin 43 attenuates oxidative stress and apoptosis in human umbilical vein endothelial cells. BMC Pulm. Med. 2020, 20, 19. [Google Scholar] [CrossRef]
- Sebbagh, M.; Renvoize, C.; Hamelin, J.; Riche, N.; Bertoglio, J.; Breard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001, 3, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Littlewood, T.D.; Bennett, M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006, 12, 1075–1080. [Google Scholar] [CrossRef]
- Rossig, L.; Dimmeler, S.; Zeiher, A.M. Apoptosis in the vascular wall and atherosclerosis. Basic. Res. Cardiol. 2001, 96, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, F.; Tang, L.; Bao, X.; Liu, Z.; Huang, C.; Zhang, T.; Yao, W. Distinct Roles For ROCK1 and ROCK2 in the Regulation of Oxldl-Mediated Endothelial Dysfunction. Cell Physiol. Biochem. 2018, 49, 565–577. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Asamoto, M.; Naiki, T.; Ogawa, K.; Takahashi, S.; Sato, S.; Shirai, T. Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol. Pathol. 2010, 38, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M.; Decrock, E.; Vanhaecke, T.; Leybaert, L.; Rogiers, V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol. Sci. 2012, 125, 175–186. [Google Scholar] [CrossRef]
- Uzu, M.; Sato, H.; Shimizu, A.; Shibata, Y.; Ueno, K.; Hisaka, A. Connexin 43 enhances Bax activation via JNK activation in sunitinib-induced apoptosis in mesothelioma cells. J. Pharmacol. Sci. 2017, 134, 101–107. [Google Scholar] [CrossRef]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, Z.Y.; Groopman, J.E. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood 1998, 92, 756–764. [Google Scholar] [CrossRef]
- Dietel, B.; Cicha, I.; Voskens, C.J.; Verhoeven, E.; Achenbach, S.; Garlichs, C.D. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis 2013, 230, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Beronov, K.; Ramirez, E.L.; Osterode, K.; Goppelt-Struebe, M.; Raaz, D.; Yilmaz, A.; Daniel, W.G.; Garlichs, C.D. Shear stress preconditioning modulates endothelial susceptibility to circulating TNF-alpha and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis 2009, 207, 93–102. [Google Scholar] [CrossRef]
- Fitzpatrick, M. Measuring Cell Fluorescence Using ImageJ. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 20 December 2022).
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.S.; Gomez-Paredes, C.; Mason, M.A.; Taxy, B.A.; Howland, D.; Bates, G.P. Extensive Expression Analysis of Htt Transcripts in Brain Regions from the zQ175 HD Mouse Model Using a QuantiGene Multiplex Assay. Sci. Rep. 2019, 9, 16137. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Cohort 1 = Stable Plaque | Cohort 2 = Vulnerable Plaque | p-Value |
|---|---|---|---|
| Age | 73.1 ± 2.1 | 76.9 ± 1.6 | n.s. |
| Sex, male, n (%) | 13 (65) | 15 (75) | n.s. |
| BMI | 27.1 ± 1.1 | 28.0 ± 1.1 | n.s. |
| Ischemic symptoms, n (%) | 2 (10) | 6 (30) | n.s. |
| Diabetes, n (%) | 3 (15) | 8 (40) | n.s. |
| Smoking, n (%) | 7 (35) | 9 (45) | n.s. |
| Hyperlipidemia, n (%) | 11 (55) | 12 (60) | n.s. |
| Hypertension, n (%) | 17 (85) | 17 (85) | n.s. |
| CRP (mg/dL) | 1.5 ± 0.5 | 1.0 ± 0.2 | n.s. |
| Creatinine (mg/dL) | 1.2 ± 0.2 | 1.0 ± 0.1 | n.s. |
| LDL-cholesterol (mg/dL) | 127.7 ± 12.7 | 128.7 ± 6.0 | n.s. |
| FC thinnest site (µm) | 421 ± 50 | 84 ± 23 | <0.0001 |
| Lipid core area (mm2) | 7.8 ± 1.4 | 23.9 ± 3.9 | <0.0001 |
| Parameter | Cx43+ Cells PS r | Cx43+ Cells PS p-Value | Cx43+ Cells FC r | Cx43+ Cells FC p-Value |
|---|---|---|---|---|
| TNF-α mRNA | 0.5148 | 0.0016 | 0.5405 | 0.0010 |
| CCR5 mRNA | 0.5526 | 0.0006 | 0.5483 | 0.0008 |
| CXCR4 mRNA | 0.3287 | n.s. | 0.3101 | n.s. |
| CD68+ cells PS/FC | 0.6864 | 0.0001 | 0.2037 | n.s. |
| CD3+ T cells PS/FC | 0.4485 | 0.0061 | 0.2917 | n.s. |
| CD31+ neovessels | 0.3777 | 0.0231 | 0.2717 | n.s. |
| Ratio Plaque/Lumen | 0.4365 | 0.0099 | 0.2998 | n.s. |
| Mean lipid core area | 0.5910 | 0.0002 | 0.6665 | <0.0001 |
| FC (thinnest site) | −0.5235 | 0.0015 | −0.5274 | 0.0016 |
| Lumen area | −0.2173 | n.s. | −0.0714 | n.s. |
| Target Gene | Primer Assay | GeneGlobe ID |
|---|---|---|
| Cx40 (gap junction protein alpha 1) | Hs_GJA1_1_SG | QT00012684 |
| Cx43 (gap junction protein alpha 5) | Hs_GJA5_va.1_SG | QT01001315 |
| c-Fos (FBJ murine osteosarcoma viral oncogene homolog) | Hs_FOS_1_SG | QT00007070 |
| c-Jun (jun proto-oncogene) | Hs_JUN_1_SG | QT00242956 |
| IL-18 (Interleukin 18) | Hs_IL18_1_SG | QT00014560 |
| IL-1 beta (Interleukin 1 beta) | Hs_IL1B_1_SG | QT00021385 |
| Fas (Fas cell surface death receptor) | Hs_FAS_1_SG | QT00030618 |
| Caspase 3 (apoptosis-related cysteine peptidase) | Hs_CASP3_1_SG | QT00023947 |
| Caspase 9 (apoptosis-related cysteine peptidase) | Hs_CASP9_1_SG | QT00036267 |
| HPRT1 (hypoxanthine phosphoribosyl-transferase 1) | Hs_HPRT1_1_SG | QT00059066 |
| YWHAZ (tyrosine 3-monooxygenase) | Hs_YWHAZ_1_SG | QT00087962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauchi, M.; Oshita, K.; Urschel, K.; Furtmair, R.; Kühn, C.; Stumpfe, F.M.; Botos, B.; Achenbach, S.; Dietel, B. The Involvement of Cx43 in JNK1/2-Mediated Endothelial Mechanotransduction and Human Plaque Progression. Int. J. Mol. Sci. 2023, 24, 1174. https://doi.org/10.3390/ijms24021174
Tauchi M, Oshita K, Urschel K, Furtmair R, Kühn C, Stumpfe FM, Botos B, Achenbach S, Dietel B. The Involvement of Cx43 in JNK1/2-Mediated Endothelial Mechanotransduction and Human Plaque Progression. International Journal of Molecular Sciences. 2023; 24(2):1174. https://doi.org/10.3390/ijms24021174
Chicago/Turabian StyleTauchi, Miyuki, Kensuke Oshita, Katharina Urschel, Roman Furtmair, Constanze Kühn, Florian M. Stumpfe, Balazs Botos, Stephan Achenbach, and Barbara Dietel. 2023. "The Involvement of Cx43 in JNK1/2-Mediated Endothelial Mechanotransduction and Human Plaque Progression" International Journal of Molecular Sciences 24, no. 2: 1174. https://doi.org/10.3390/ijms24021174
APA StyleTauchi, M., Oshita, K., Urschel, K., Furtmair, R., Kühn, C., Stumpfe, F. M., Botos, B., Achenbach, S., & Dietel, B. (2023). The Involvement of Cx43 in JNK1/2-Mediated Endothelial Mechanotransduction and Human Plaque Progression. International Journal of Molecular Sciences, 24(2), 1174. https://doi.org/10.3390/ijms24021174





