1. Introduction
Andrographolide (ANDRO) is a diterpenoid lactone and the main active constituent of the
Andrographis paniculata, a traditional Asian medicinal plant [
1]. Its described effects include anti-inflammatory, antiviral, antitumoral, and antioxidant properties, most occurring at the systemic level [
2]. However, the effect of ANDRO has also been evidenced in the central nervous system [
3].
Among its multiple effects, ANDRO protects and benefits brain function, refining memory mechanisms and cognitive performance tasks in rodent models [
3,
4,
5,
6]. ANDRO prevents neuroinflammation-associated neurodegeneration in the prefrontal cortex and hippocampus of mice, improving working memory performance and regulating the expression of synaptic plasticity markers [
7]. Due to these effects, several studies have been conducted with ANDRO in animal models of neurodegenerative diseases. In the mouse model of Alzheimer’s disease (AD), the double transgenic mouse model APP/PS-1, the treatment of 7-month-old but not 12-month-old mice with ANDRO for one month significantly decreased the Amyloid-β (Aβ) aggregates. These data suggest that ANDRO may prevent Aβ production and aggregation in the early stages of disease development [
6]. In contrast, ANDRO reduced tau phosphorylation at both ages. At the same time, the synaptic proteins, synaptic plasticity paradigms (long-term plasticity, LTP), and spatial memory performance were improved with the treatment with ANDRO. These effects correlate with the increment of β-catenin and the inactive form of GSK-3β, indicating that ANDRO activates Wnt signaling [
6]. In the same transgenic mice, ANDRO almost reverted their cognitive impairment, reduced oxidative stress, and protected mitochondria from instability [
8]. Studies with the early-onset J20 Tg AD mouse model showed that four months of ANDRO treatment, applied at presymptomatic stages, improved some deficient metabolic markers, such as the lower levels of glucose uptake, ATP, and glycolytic rate, whereas by acting in the presynaptic region, it modulated the machinery to optimize the strength of the evoked response and improved cognitive performance [
9]. These data suggest that developmental and disease progression times are relevant when using ANDRO as a treatment; timing is the essence of ANDRO treatment.
Exciting results have been obtained using ANDRO in the
Octodon degus, an endemic Chilean rodent that develops metabolic, sleep, social-effective, and age disorders, including neurodegeneration, as in humans [
5]. Degus is a diurnal, long-lived social animal that is an excellent model for studying high cognitive tasks [
10,
11]. Degus suffer from the effects of aging on several physiological aspects, including reduced cognitive function and deficient synaptic activity that start at around three years old and worsen over time. We recently reported sex differences in a study that involves age progression in degus [
12]. Interestingly, we found that female degus are strongly affected by age. Young females (12–24 months old) have more efficient synaptic transmission than young males; however, aged females (60–84 months old) become several times less efficient than aged males. Studying LTP, we observed that males and females displayed the same amount of LTP, but aged females had to recruit more axonal terminals to produce the same evoked response. Instead, males recruited the same axonal terminals when young or aged, indicating that their synaptic mechanisms were not affected during age progression. These data showed a strong aging impact on the synaptic mechanisms of female degus [
12].
Previous studies showed that aged female degus (56 months old) treated with ANDRO for three months increased memory recognition, learning performance, and the level of some postsynaptic proteins, reducing the aggregation of oligomeric Aβ species [
5]. Although ANDRO also improved their basal synaptic activity, it was insufficient to reestablish LTP in these aged animals [
5], suggesting that a more prolonged ANDRO treatment might be necessary for females in this species.
The evidence suggests that dose, treatment duration, and the animal’s age at the onset of treatment are fundamental in determining the potential effect of ANDRO in the brain. Therefore, knowing that ANDRO might benefit several physiological aspects during age progression in degus, we asked whether longer treatments could improve the deleterious effects of age in both adult (36 months old) and aged (72 months old) female degus. Females are traditionally misrepresented in scientific studies; assuming that the main physiological features are common to all sexes and evolve in similar ways, males are supra-represented when they are used as representative of both sexes. Nevertheless, the evolution of psychosomatic features shows animal lifetime differences depending on sex. Given our previous observations in which age had a more detrimental effect on females than males, we used adult and aged female degus to study ANDRO administration, suggesting that it would improve cognitive impairment and increase the synaptic strength associated with aging. To our knowledge, this is the first time a long-term ANDRO administration study in a natural long-lived animal model has been conducted.
3. Discussion
This study aimed to evaluate and compare the effect of a long-term ANDRO treatment (12 months) on behavioral, electrophysiological, and molecular features obtained from adult and aged female degus. Our data demonstrated that ANDRO improves memory and preference for novel experiences in both ages. Neither age nor treatment affected the socialization of degus; however, ANDRO restored the defects in social recognition and long-term memory in aged female degus. On synaptic physiology, ANDRO showed different effects, either reducing the synaptic efficacy of basal neurotransmission in adult females or increasing it in aged females compared to their age-conspecific control. However, in both cases, ANDRO increases LTP, apparently through different mechanisms. Meanwhile, the analysis of synaptic proteins showed age-dependent changes in SYT, NR2B, and PSD95, which could explain some of the cognitive and physiological changes observed in females.
The use of
Octodon degus as a neurological model has been extensively reported [
5,
10,
11,
12,
14,
15,
16,
17,
18,
19,
20]. The broad range of degus’ behavioral complexities, the similarities with the human aging process, and the biological mechanisms underlying synaptic deficiencies make degus a multifaceted model. Furthermore, degus have demonstrated strong sex-dependent specificities in the aging process and the natural adaptation to respond to ambient variations [
10,
12,
18].
Studies with females in scientific publications are commonly underrepresented. Most scientific research uses males, but some relevant differences have been missed in looking for similar features. In that context, we recently demonstrated that female degus, not males, suffer age-dependent synaptic detrimental effects [
12,
16]. Compared to males, young females (12–24 months old) showed more efficient synaptic transmission [
10,
12], but aged females (60–84 months old) displayed a significant reduction in synaptic efficacy compared to males. Moreover, aged females have fewer functional axonal terminals, needing the recruitment of twice as many axons to reach equivalent LTP levels as aged males [
12]. These data demonstrate that age affects mainly females, despite no significant differences between male and female degus in brain asymmetry, size, and morphology of hippocampal areas having been found [
20]. In addition, the cognitive evaluation of behavioral tasks showed that young females with high short-term memory performance exhibited a poor display of long-term memory tasks. In contrast, aged females with poor short-term memory displayed high performance in long-term memory tasks [
16]. These data indicate that the mechanisms for short- or long-term memories are not preserved equally in females.
Here, we compared behavioral, physiological, and molecular aspects of adults and aged female degus. We studied whether some of the changes observed in aged females were evident in a previous adult stage, and how these could be modulated with long-term ANDRO administration. The reason for longer treatments is that shorter ones partially did not succeed in complete response. ANDRO improved synaptic strength in aged male degus and reduced several AD-related hallmarks, such as Aβ or tau protein [
5,
21,
22], but did not increase LTP [
5]. In that study, ANDRO was applied for only three months, perhaps not enough time to modify the mechanisms involved in LTP generation. In the AD-presymptomatic J20 mice model, four months of ANDRO was indeed enough to improve LTP in adult mice [
9], the comparison between transgenic mice and the natural long-lived degus may not be appropriate, since the mechanisms involved could not be similar. In the present work, female degus were injected with ANDRO for over 12 months, and we observed that LTP increased over the control measurements in both adult and aged females. To our knowledge, this is the first long-term study of a long-lived animal. These data show that when working with a long-living animal model, the treatment duration and the age of the animals are the main factors to be considered when using ANDRO.
Multiple neurophysiological effects have been associated with the treatment of ANDRO, and controlling these during long-term use is far from easy. A summary of clinical studies using Andrographolide showed that more investigation is required considering dosage and secondary effects, including toxicity [
23]. Some studies have reported side effects associated with high doses (4–6 mg/kg) causing allergic reactions, tiredness, headache, pruritus/rash, diarrhea, nausea, metallic taste, bitter taste, dry tongue, eyes sensitive to light, and decreased short-term memory [
24,
25]. However, the ANDRO dose used in the present study (2 mg/kg) was previously reported on other animal models [
6], even in degus [
5]. During its administration, we did take care to strictly follow the guidelines of animal supervision and care established by the bioethics committee. We observed no side effects throughout the study; thus, no animal was removed, indicating that our female degus tolerated ANDRO treatment with no evident toxic effects.
We used different behavioral paradigms to measure specific aspects of memory processing. As shown, tests such as NOR/NOL and the three-chamber social interaction test, which measure short-term recognition memory and social memory, respectively, demonstrated that ANDRO improved the exploration of new behaviors in adult and aged females, while enhancing the social recognition deficit in aged females. These aspects are considered relevant in the biological life of the animals: the recognition of changing environmental conditions and familiar conspecifics is essential for animal survival in natural situations. Studies from Uekita and Okanova suggested that the degu hippocampus, a key brain area being affected by aging, plays an important role not only in spatial recognition but also in social recognition [
26]. The authors showed that changes in social behavior of degus resulting from hippocampal lesions, were interpreted as the impairment of social recognition rather than in novelty detection. Our results follow these statements, since social and object recognition memory, but not sociability, was impaired by age. Interestingly, ANDRO improved social and object recognition memory in aged female degus, suggesting that it modifies memory mechanisms. On the other hand, we performed the Barnes maze test, a highly hippocampal-dependent spatial learning task used to measure long-term memory [
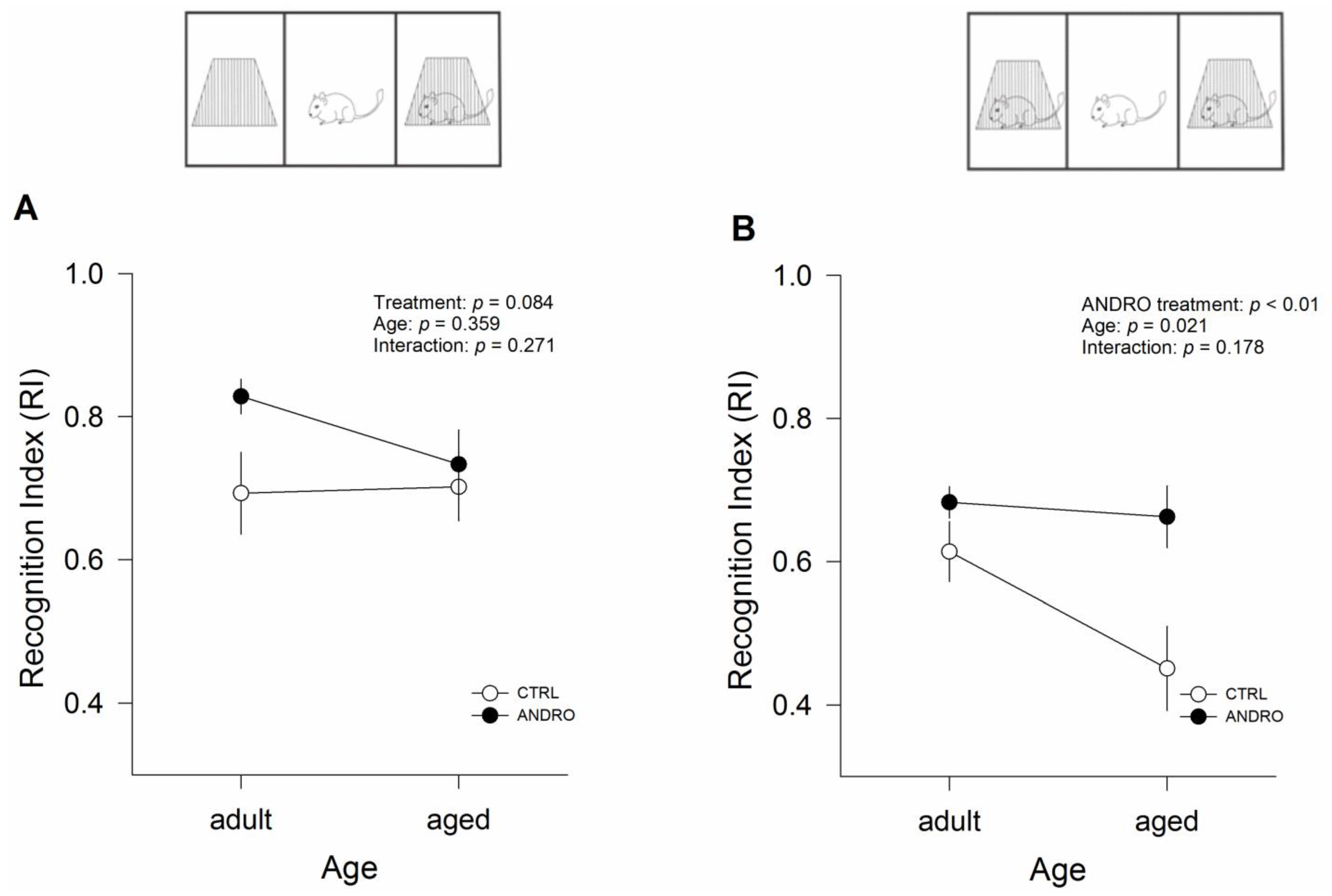
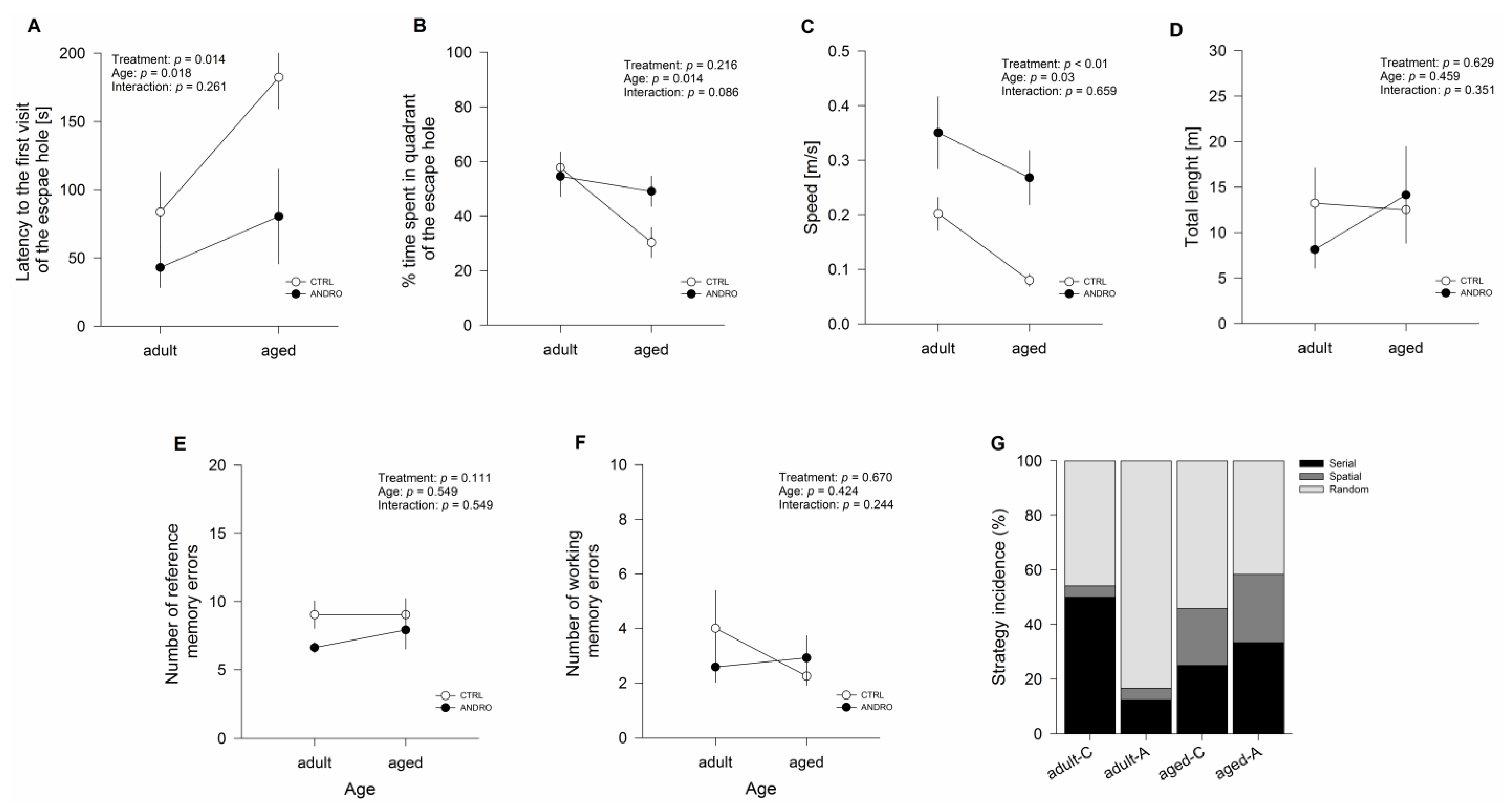
27]. During this test, we observed that the ANDRO-treated animals reduced the latency to the first visit and increased the time spent in the escape quadrant, showing that long-term memory processes were enhanced with the treatment. Altogether, these data indicate that ANDRO improves the performance of behavioral task-relevant aspects affected during the aging process.
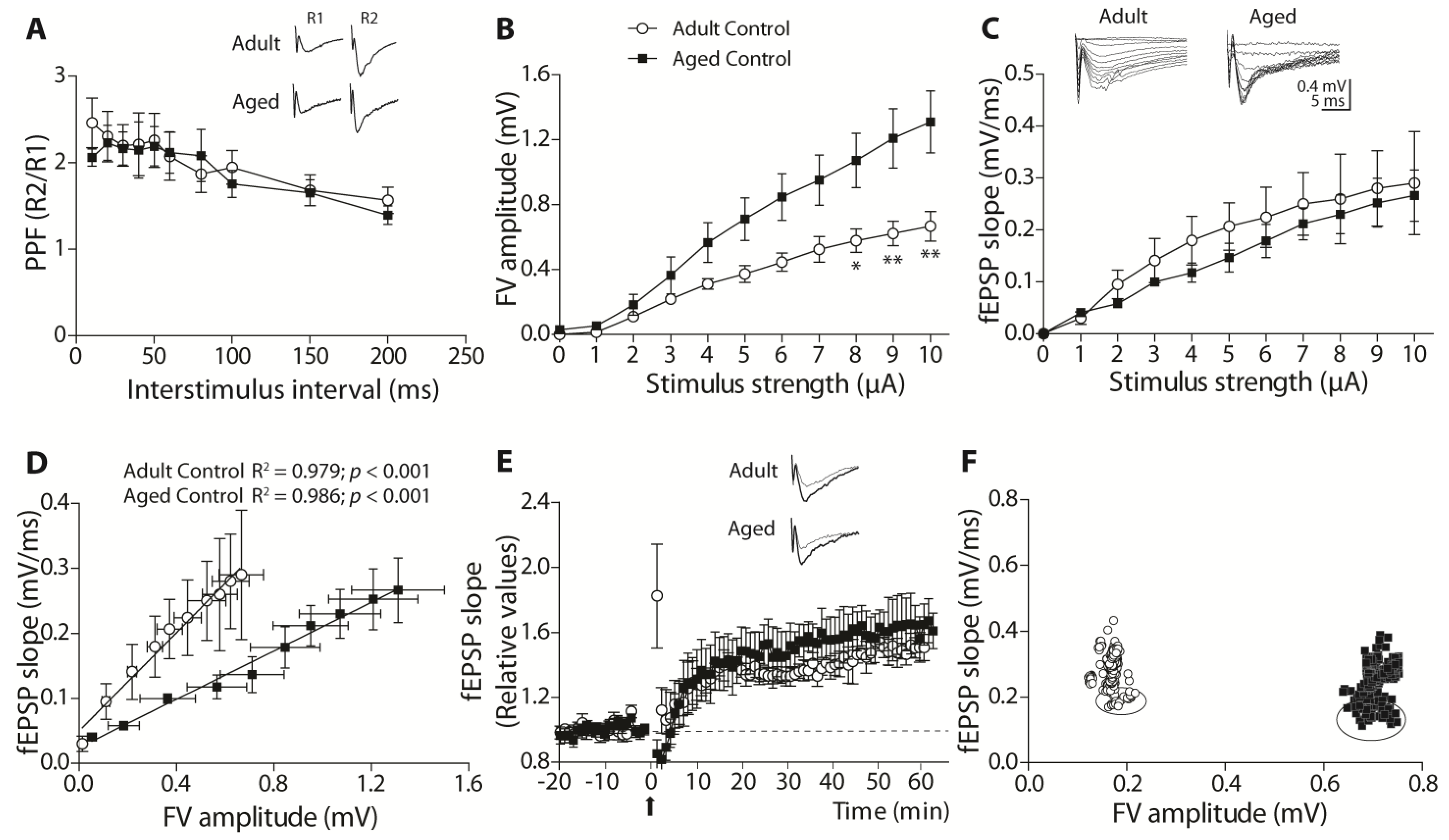
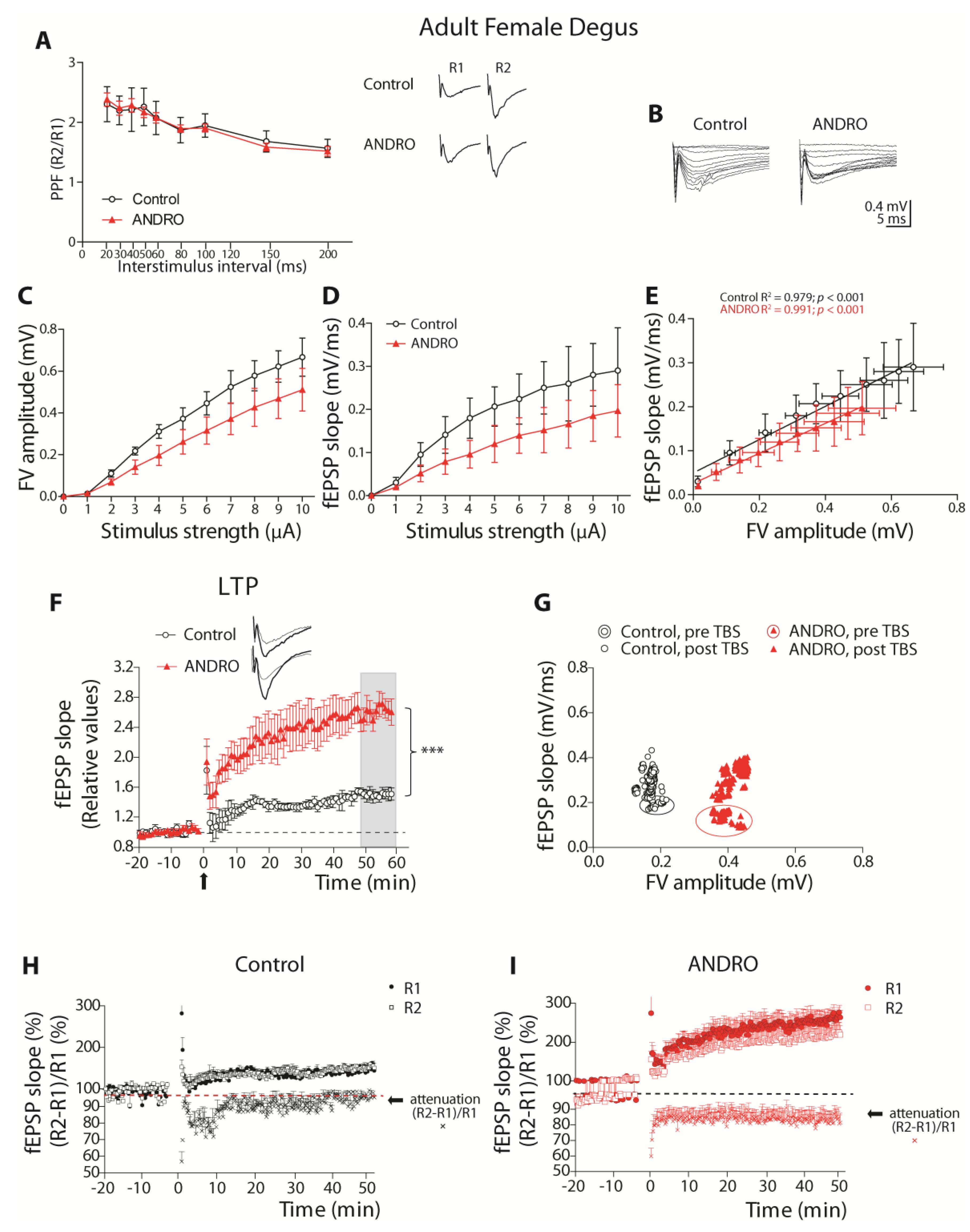
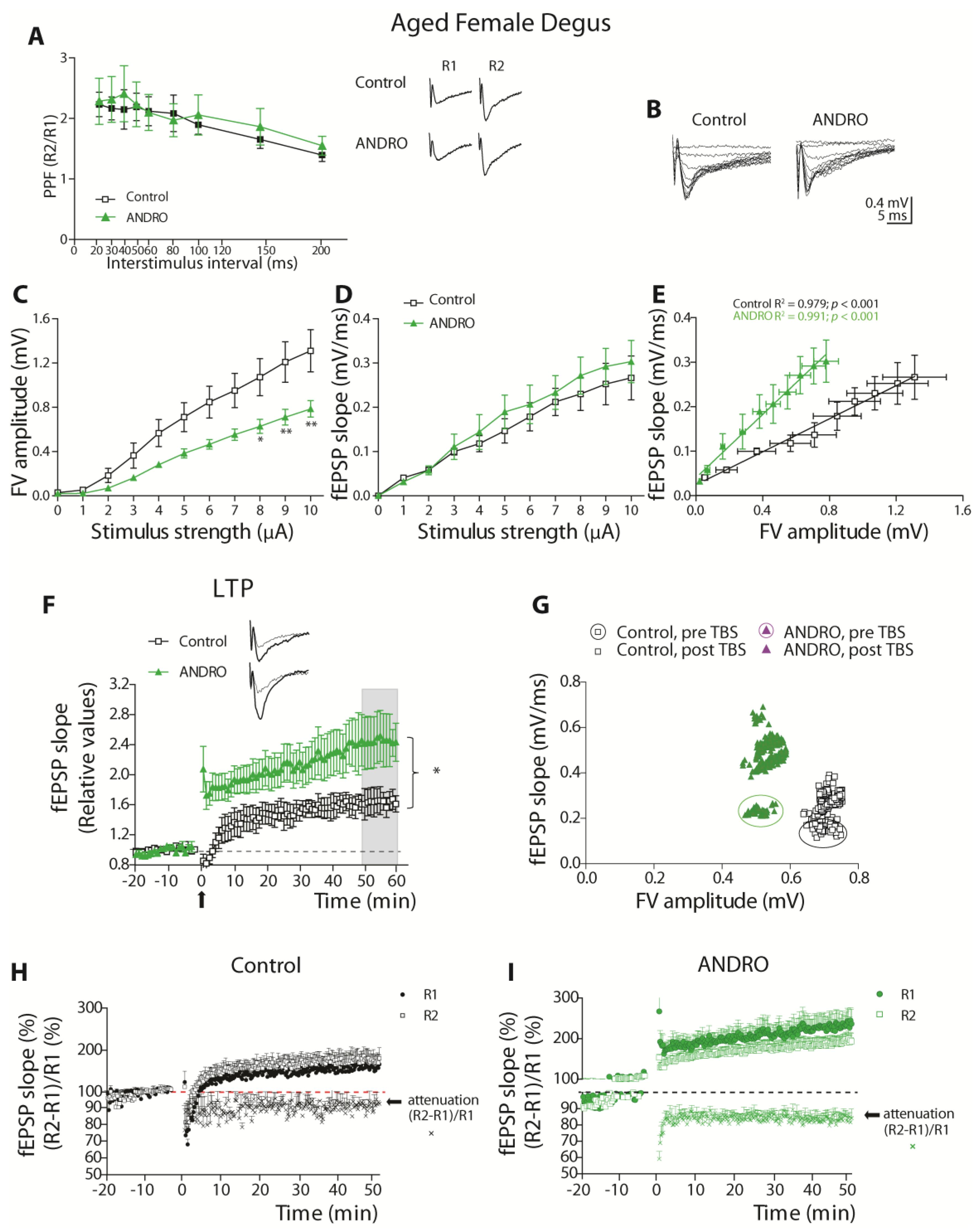
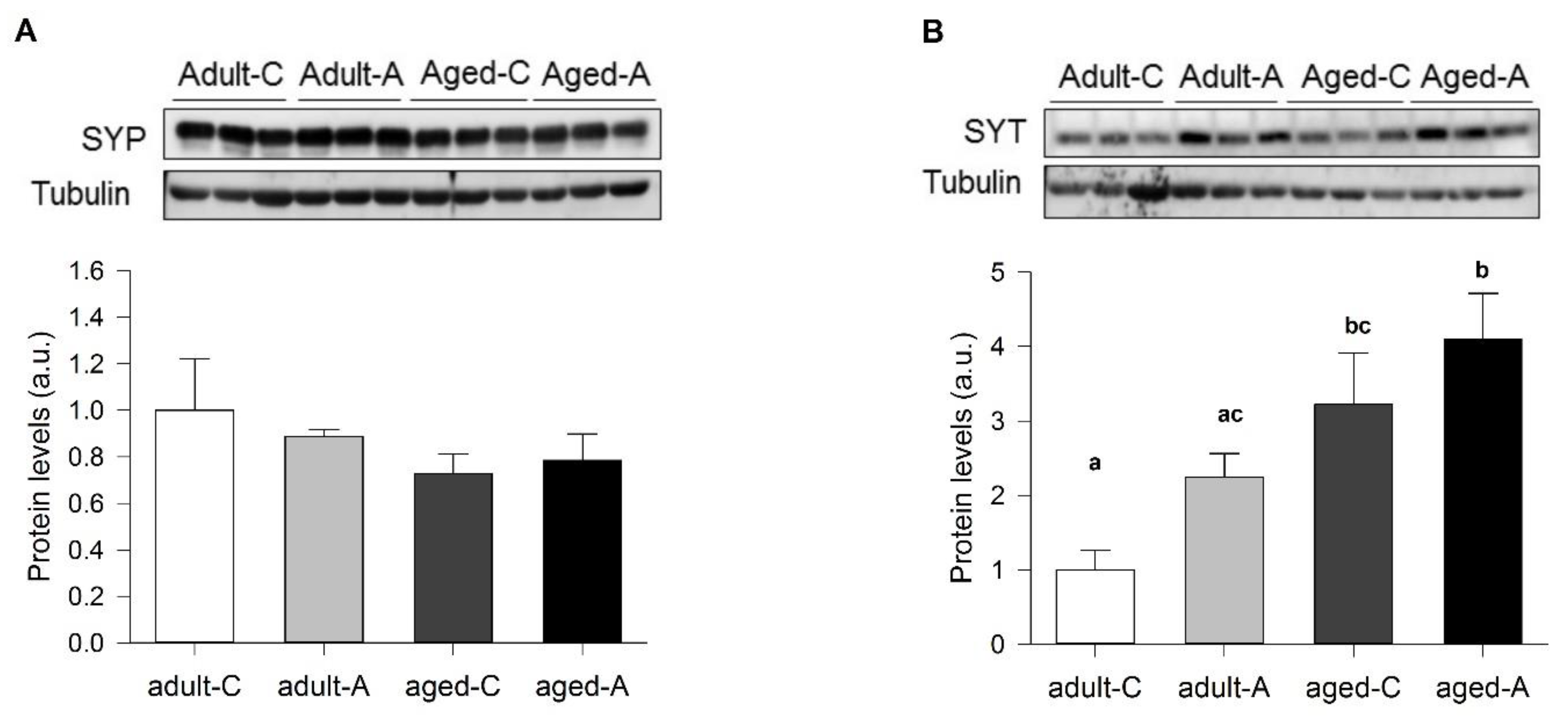
Using brain slices of the adult and aged females, those treated long-term with ANDRO or the controls, we found interesting physiological aspects of synaptic communication. The facilitatory synaptic transmission in this synapse depends on the calcium availability that triggers synaptic vesicle Ca
2+-dependent release. ANDRO did not affect this mechanism in adult or aged females during basal transmission. Noteworthy, the level of SYT, the presynaptic Ca
2+ sensor, was higher in aged females and probably increased the fast vesicle release. We also measured the facilitation during LTP generation, the so-called attenuation. During LTP development, modifications in synaptic transmission involved postsynaptic and presynaptic components. The facilitation of the two pulses (R2/R1) mainly decreases, because R1 increases beyond the increment of R2; this effect is called “attenuation” of the R2/R1 ratio and can be permanent or transient along the development of LTP. Surprisingly, in adult females, the attenuation was short and similar to that previously observed in young female degus [
12], suggesting that the attenuation mechanism in females is altered from very early in life. However, in adult females treated with ANDRO, the attenuation becomes stable, suggesting that the pre-and postsynaptic mechanisms involved in attenuation become ‘stabilized.’ This effect of ANDRO has been previously observed in mice [
9] and suggests that ANDRO induces permanent changes in the hippocampal synaptic apparatus. More studies are required to demonstrate this hypothesis.
We also observed significant improvement in the LTP of these animals’ brain slices, indicating that the processes that involve acquisition, consolidation, and long-term retrieval improved with ANDRO. Nevertheless, the protocol to generate LTP appears to activate different mechanisms in adults and aged females. In adult females, the TBS induced the recruitment of more axons in the ANDRO-treated degus that evoked similar responses to the adult control and could be the reason for the higher LTP. Instead, in the aged females, the TBS recruited fewer axons that evoked higher EPSPs than the aged control. These data suggest that in aged animals, ANDRO treatment led to structural changes that made the synaptic transmission more efficient, influencing the postsynaptic mechanisms that induce plasticity.
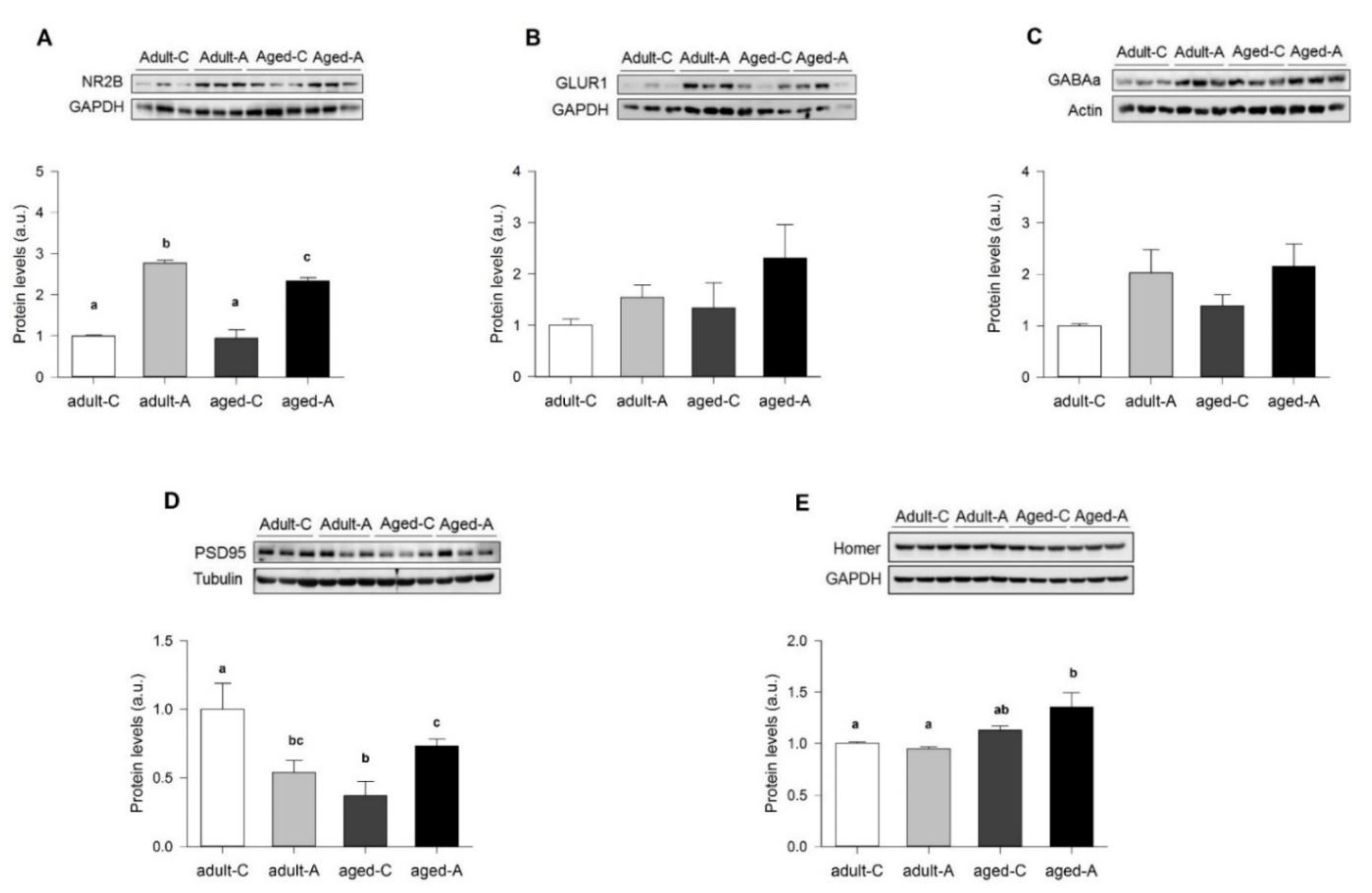
Measurements of basal synaptic activity reported that adult females with ANDRO showed less excitable and efficient synaptic transmission than controls. A clue that can help to explain these differences may be relay in synaptic proteins. The treatment with ANDRO significantly reduced the expression of PSD95 in adults, whereas it increased NR2B-containing NMDARs. As a scaffolding protein, PSD95 stabilizes postsynaptic receptors contributing to spine morphogenesis and maturation [
28,
29], and it is high in young female degus compared to aged ones [
5]. In the case of NR2B-type receptors, its increment correlates with the LTP increment [
30]. NR2Bs receptors, generally allocated in extrasynaptic sites, are more highly mobile than the less mobile NR2As at synaptic sites [
31]. Whether ANDRO regulates the lateral movement of these receptors is unknown, but as recently observed, the remotion of PSD95 from the synapses leads to an increase in the surface mobility of glutamate receptors [
32]. Here, we did not examine the expression of NR2A-type receptors, but previously, we did show that its level diminished in aged compared to younger females [
5]. The increment in the level of NR2B-type receptors suggests that this receptor could mobilize more to synaptic sites. Thus, lowering PSD95 expression could be a mechanism used by ANDRO to regulate the excessive NR2B expression that by itself can increase LTP [
30,
33]. This mechanism also could explain the lower basal activity in adult females. Instead, in aged females, ANDRO increased NR2B and PSD95, as well as NR2A, as was shown before [
5], suggesting that these molecules are needed to stabilize an aged synapse. The high expression of Homer1 supports this idea in aged females with ANDRO, a component of postsynaptic density proposed not only as a scaffold protein but as a crosslinker for different synapse-related proteins [
34].
The above-exposed antecedents show that ANDRO modulates several aspects of synaptic physiology. ANDRO is a non-ATP-competitive, substrate-competitive inhibitor of GSK-3β. The specific inhibition of GSK-3β by ANDRO induces the accumulation of β-catenin in the nucleus, causing the transcription of Wnt target genes [
35], which is the central mechanism of canonical Wnt pathway activation. Interestingly, in the brain, ANDRO does not activate other signaling pathways that also increase the levels of Ser9-GSK-3β when active, such as Akt and mTOR, suggesting that its principal mechanism is mediated by the activation of Wnt signaling [
35]. ANDRO also reduces astrogliosis, neuroinflammation caused by released cytokines, and oxidative stress in aged animals [
8,
21,
36]. We did not assay for specific hallmarks of neurodegeneration or neuroinflammation in the present work. However, considering the age of the animals, we cannot dismiss the effect these aspects could have on the animal's physiological status, as was previously reported. It remains to be determined whether those aspects differ in timing and intensity among the sexes.
Our results suggested that as a long-lived animal,
Octodon degus is an excellent model to visualize living social aspects, aging detrimental steps, and the sex component. On the one hand, young females appear to be more sensitive to social isolation stress lowering their synaptic efficacy, as compared to young males, despite their mechanisms of long-term memory appearing very stable, unlike males [
10]. In normal conditions, young females show more efficient synaptic activity than males but similar levels of plasticity [
12]. Compared to aged females, synapses in young females are highly efficient, but they generate similar LTP levels at both ages. Here, we showed that adult females are more similar to the younger ones in synaptic efficacy (compared to data in [
12]) and better than aged females but with a similar level of LTP. Thus, the synaptic efficacy determined by how many axons are recruited to produce a particular postsynaptic evoked response can be modified, but it is different from what determines the amount of synaptic plasticity. On the other hand, the effect of ANDRO in females suggested that both mechanisms are modulated independently; in adults, the efficacy of synaptic transmission diminishes, but LTP increases several times. Instead, in aged females, both the synaptic efficacy and plasticity increase. Does the adult age represent an intermediate state for some aging features? A previous report showed a reduction in the content of SYP protein in aged compared to young females [
5]. We did not find differences in adult and aged females, suggesting that lowering SYP is an earlier consequence of aging. In adult females, lower SYT and NR2B and higher PSD95 could induce a high-cost LTP, where two times as many axons are recruited with ANDRO but evoke the same postsynaptic response. In aged females, the interplay between high SYT and lower NR2B and PSD95 during basal activity may bring a place for plasticity: ANDRO can increase NR2B and PSD95, enhancing synaptic efficacy by reducing the recruited axons to evoke the maximum response. Therefore, long-term ANDRO administration could potentially improve females' cognitive impairment associated with normal aging.
4. Materials and Methods
4.1. Animals
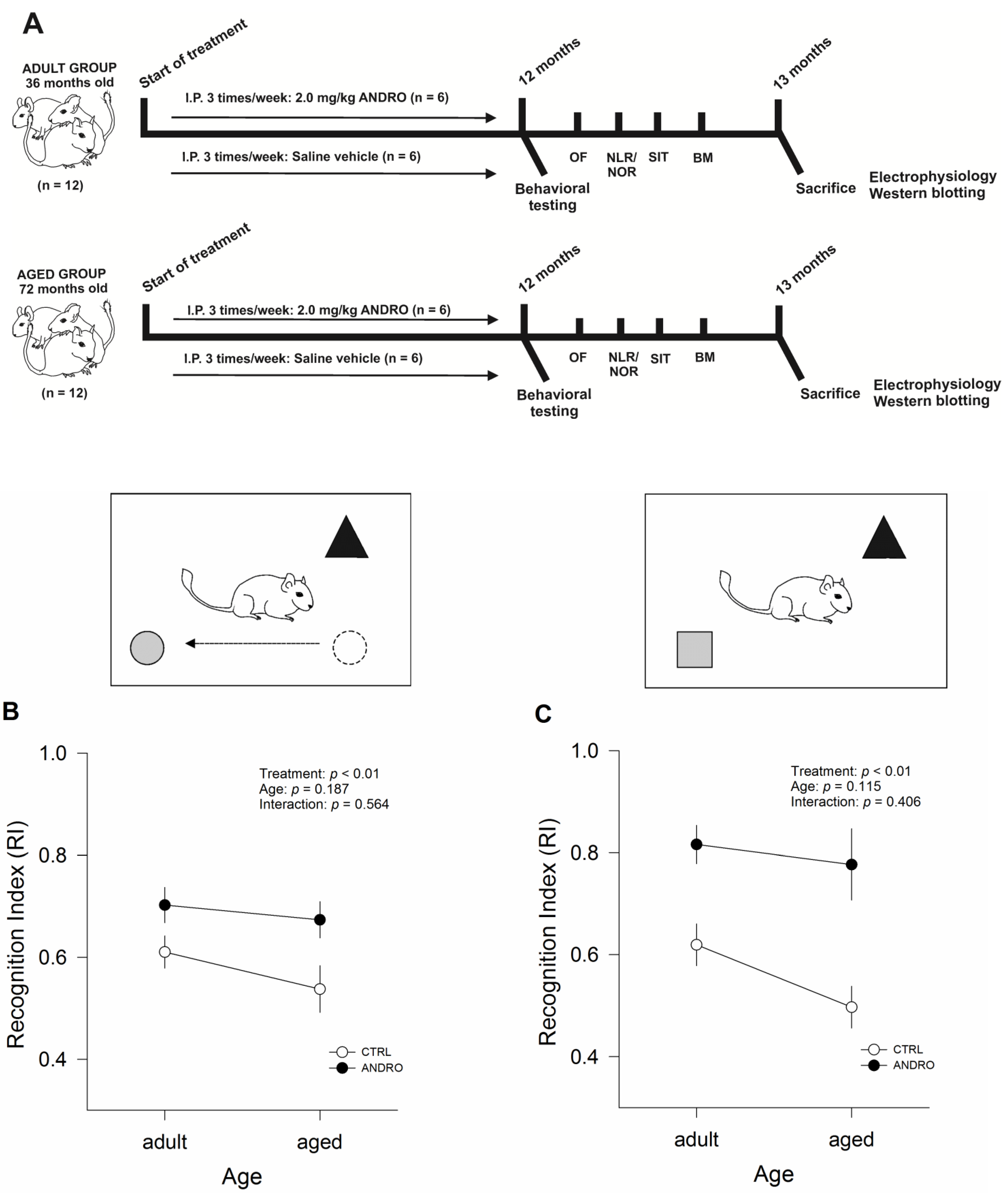
Adult female degus (36 months old, n = 12) and aged female degus (72 months old, n = 12) weighing 200 ± 25 g and 209 ± 23 g (mean ± SEM), respectively, were obtained from our colony. These animals were all derived from laboratory-bred lines. Twenty-four female degus were used in this study: 12 animals in the ANDRO group (n = 6 adults, n = 6 aged) and 12 animals in the control group (n = 6 adults, n = 6 aged). Degus were kept in pairs of related and unrelated females housed in clear acrylic aquaria (length × height × depth: 50 × 35 × 23 cm) with bedding of hardwood chips, water, and food (commercial rabbit pellet; Champion, Santiago, Chile) provided ad libitum. Each cage contained 1 nest box made of clear acrylic (22 × 12 × 15 cm). Animals were kept in a ventilated room exposed to a 12:12 h light: dark cycle with temperatures controlled (yearly minimum = 13.4 ± 0.2 °C; yearly maximum = 24.9 ± 0.2 °C).
Intraperitoneal (IP) injections of 2.0 mg/kg of ANDRO (Sigma-Aldrich, Merck, Santiago, Chile) in the saline vehicle were administered 3 times per week, as described in the literature [
5]. Control animals were injected with only vehicle solution. ANDRO and vehicle were given over 12 months. Each week, we measured body mass, and the doses for intraperitoneal injections were recalculated. To the best of our knowledge, this study is the first to evaluate the effects of ANDRO administered over a prolonged period in this long-lived rodent model.
Our design avoided the effect of hormonal fluctuation in 17–21-day regular cycling females by performing behavioral tests in the diestrus phase of the estrous cycle. All animal protocols followed the National Institutes of Health (NIH, Baltimore, MD, USA) guidelines and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The Bioethical and Biosafety Committee approved all procedures of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile (CBB-170113007-2017). The efforts were made to minimize animal suffering and reduce the number of animals used.
4.2. Behavioral Observations
As detailed below, adults and aged female degus, both control and ANDRO treated, were subjected to four behavioral tasks. The experiments were conducted from the least to the most intrusive, minimizing behavioral experiences' effect on the results. The experiments were as follows: (i) open field; (ii) the novel object recognition test; (iii) three-chambered social interaction test; (iv) Barnes maze test. Animals were subjected to one test daily (except the Barnes maze test, which is longer). All behavioral tests were performed during the active phase of the animals (between 09:00 to 16:00 h). At the end of each session, animals were returned to their home cages, and the area was cleaned with 70% ethanol solution.
4.2.1. The Open-Field Test
To discard locomotor differences between groups and specifically evaluate the exploratory behavior, animals were observed for 5 min in the open-field test, consisting of a white Plexiglas box (length x height x depth: 100 × 100 × 100 cm). The frequency of total ‘central crossings’ (with a four-paw criterion) was scored [
5]. In addition, the percentage of time in corners and in the middle arena, speed, and total length were assessed [
5].
4.2.2. Novel Object Recognition Test
The novel object recognition test is a double test used to evaluate cognition, particularly working memory and attention, and it can also test the preference for novelty in rodents [
37]. The test arena used an open box (length × height × depth: 63 × 40 × 30 cm) made of white Plexiglas. For this test, we followed the object recognition protocol previously used in degus [
5]. Briefly, animals were exposed to a 10 min familiarization assay and then tested in two consecutive five-minute assays, with a one-hour inter-trial interval. For Session 1 (Familiarization): two different objects (‘Object A’ and ‘Object B’) were placed in the corners of the home cage, and the animal was allowed to explore them for 10 min freely. Following this period, the test animal was returned to her home cage for one hour. Session 2 (‘Novel location recognition’ or NLR): one of the familiar objects (Object B) was moved to an adjacent unoccupied corner. The test animal was then free to interact with the objects A and B for five minutes. Following this period, the test animal was returned to her home cage for one hour. Session 3 (‘Novel Object Recognition’ or NOR): the familiar Object B was replaced by a new but similar object (i.e., similar texture and consistency). We recorded the time spent exploring each object (i.e., approaching within 1–3 cm of the object) and then calculated the recognition index (RI), which is the time spent with object B divided by the sum of the time spent with objects A and B, to quantify NLR and NOR.
4.2.3. Three-Chambered Social Interaction Test
We used the three-chamber test to assess (i) social affiliation/motivation by comparing the time degus spent interacting with an empty cage versus a cage containing an unfamiliar (novel) degu; and (ii) social memory/preference for social novelty by measuring the time the degus spent interacting with an unfamiliar versus a familiar degu. The open-field arena was subdivided into three equal compartments using transparent Plexiglas walls, each with a small opening (2.8 cm in diameter) that allows access into each compartment. The degu to be used as the social partner was sex-matched, unfamiliar, and unrelated. The test comprised three sessions of 20-min. The social test was performed following the protocol previously described [
38]. Briefly, three steps or phases were performed in the following order: Phase 1 or Habituation: the test animal was placed in the middle compartment and allowed to explore the apparatus. Phase 2 or Session 1 (‘Partner I’): the test animal was returned to the middle compartment; meanwhile, Partner I (a sex-matched, unfamiliar, and unrelated degu) was placed inside a wire cage in one of the lateral chambers. The test animal was free to interact with the social partner or an empty wire cage. At the end of Session 1, the test animal was returned to her home cage for one hour. Phase 3 or Session 2 (‘Partner II’): The test animal was returned to the middle compartment; a second unfamiliar, unrelated degu was placed inside an identical wire containment cage in the opposite side chamber, which was empty during Session 1. The test animal could freely explore between the first, already investigated, unfamiliar degu (Partner I) or the novel unfamiliar degu (Partner II). As measurements of social interaction, we recorded the time spent exploring the partner (i.e., the time the animal spent touching the containment cage housing or not housing the new partner for each chamber individually, with the forepaw or nose). The recognition index (RI) was calculated to evaluate social affiliation/motivation and social memory differences. The RI for Session 1 is the quotient of the time the degu spent with Partner I divided by the sum of the time spent with Partner I and the empty wire cage. For Session 2, the RI is the time spent with Partner II divided by the time spent with Partners I and II. A RI ≤ 0.50 indicates that degus lack social affiliation/motivation during Session 1 and social memory during Session 2.
4.2.4. Barnes Maze Test
This test has a strong hippocampal-dependent spatial component [
39], leading us to evaluate spatial navigation, learning, and memory [
18]. The Barnes maze consisted of an elevated circular platform of white Plexiglas of 160 cm in diameter surrounded by a 45 cm-high wall. Eighteen circular holes (8 cm in diameter) were bored through the platform equidistant from each other (16 cm) and 5.5 cm from the outer edge. Every hole was blocked except for the target one. A plastic escape box (length × height × depth: 31 × 13 × 16 cm) was positioned under the escape hole. Accurate performance requires subjects to learn and remember the location of the escape hole; therefore, spatial cues (a combination of different colors and shapes: a yellow star, a red square, and a green apple) were placed on the wall of the maze [
40]. Briefly, the procedure was divided into three phases: habituation, training, and a test phase, and it was performed as previously described [
38,
41]. Session 1 (Habituation): begins with the animal test into the escape box for two minutes. After that, the animal test was placed near the escape hole and left for one minute to escape. If the animal did not enter the escape box, it was gently picked up and put through the target hole into the escape box and left for two minutes. Finally, the animal was placed in the maze's center and left for four minutes to explore the platform and enter the escape box. If the animal did not enter the escape box, it was put into the escape box as mentioned above and left there for two minutes. Session 2 (Training): two days after Session 1, we trained each animal for 7 days. Session 3 (Test phase): seven days after Session 2, we exposed the test animals to a memory-retrieval session. Both the training and test phases consisted of four consecutive four-minute trials, separated by a five-minute resting phase in the animal home cage. At the beginning of each trial, the animal was confined for 30 s in a start box in the center of the maze. If the animal did not enter the escape box within the allotted time, it was manually picked up, placed in the escape box, and left undisturbed for 2 min. To discard locomotor differences between groups, we measured the speed and the distance (in meters) covered from the initiation of exploration of the escape hole to entrance into the escape hole. We registered the latency to the first visit of the escape hole, reference memory errors (every first visit to a non-escape hole in each trial), and working memory errors (repeated visits to the same non-escape hole in the same trial). 'Search strategies' used during retrieval trials were categorized into 3 groups: random, serial, and spatial, as previously described [
10,
42]. Briefly, searches were classified as ‘random’ when localized searches of escape holes were interrupted by central crosses, or when no systematic search pattern was discernible. ‘Serial searches’ were defined as searches of consecutive holes around the maze, and ‘spatial searches’ were defined as searches following a direct path to the escape hole. In all cases, a digital video camera (LifeCam Studio Full HD, Microsoft Corp, Redmond, WA, USA) was mounted above the test arena, and the performance of each animal was monitored with image tracking software (HVS Image, Hampton, UK).
4.3. Electrophysiology
Female degus were separated into two groups: adult (36 months old) and aged females (72 months old), control or treated with ANDRO. Every day, one animal was anesthetized and euthanized by decapitation. The brain was quickly removed and placed in a cold artificial cerebrospinal artificial (ACSF-)modified solution (sucrose replaces part of sodium), composed of the following (in mM): 85 NaCl, 75 sucrose, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 dextrose, 3.5 MgSO4, 0.5 CaCl2, 3 sodium pyruvate, 0.5 sodium L-ascorbate, and 3 Myo-inositol (305 mOsm, pH 7.4, and oxygenated 95% O2/5% CO2). The brain was cut with a vibratome in coronal slices of about 300–350 µm containing the hippocampal formation. The slices were allowed to recover for an hour in the same solution at room temperature. Before recording, the solution was changed to an oxygenated ‘recording solution’ composed of (in mM): 126 NaCl, 3.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 dextrose, 1 MgSO4, 2 CaCl2, 3 sodium pyruvate, 0.5 sodium L-ascorbate, and 3 Myo-inositol (305 mOsm, pH 7.4, and oxygenated 95% O2/5% CO2). At the time of the recording, the temperature was raised to 35–36 °C. Each slice was placed under an upright infrared-differential interference contrast (IR-DIC) fluorescence microscope (Eclipse FNI, Nikon Instruments Inc., Melville, NY, USA) to visualize the hippocampal circuit with a 40x water objective. The Schaffer collaterals between CA3 and CA1 were stimulated using a bipolar concentric electrode (World Precision Instruments, Sarasota, FL, USA) connected to an ISO-Flex stimulus generator (AMPI, Jerusalem, Israel). The recording of evoked field excitatory postsynaptic potentials (fEPSPs) was performed with a glass electrode (World Precision Instruments, United States) of 0.5–1 MΩ pulled on a p-97 Micropipette Puller (Sutter Instruments, Novato, CA, USA) and filled with recording solution. The signals were recorded using a MultiClamp 700B amplifier (Axon CNS, Molecular Devices LLC, San Jose, CA, USA) and digitally sampled at 30 kHz using a Digidata-1440A interface (Axon CNS, Molecular Devices LLC, San Jose, CA, USA). The analyses were performed offline using pClamp 10.3 (Molecular Devices LLC, San Jose, CA, USA). Several protocols were used to characterize the basal status of this synapse: (i) The input–output curve, where increasing levels of current intensity were successively applied, and the fEPSP slopes and fiber volley (FV) amplitude measurements were obtained. The amount of current to evoke 60% of the maximum slope value was used to perform other protocols in the same slice. (ii) Paired-pulse facilitation (PPF) protocol, where two pulses, R1 and R2, were applied, separated by different inter-stimulus intervals (ISI), and the R2/R1 ratio was obtained to estimate the degree of facilitation. (iii) Long-term plasticity (LTP) protocol, where two pulses (R1 and R2, separated by 50 ms each) were evoked every 15 s. The first pulse (R1) slope was averaged for 15 to 20 min to obtain a stable basal signal (pre-TBS). Theta-burst stimulation (TBS, 5 bursts at 100 Hz every 20 s) was applied to induce LTP. The post-TBS was evaluated using the identical two pulses separated by 50 ms for at least 60 min. (iv) The attenuation was calculated using R1 and R2 pulses (%) and the PPF (R2-R1)/R1(%).
4.4. Western Blotting
The hippocampi of control and ANDRO-treated degus were dissected on ice and immediately processed as previously described [
5,
20,
36]. Briefly, the hippocampal and cortical tissues were homogenized in RIPA buffer (10 mM Tris-Cl, pH 7.4, EDTA 5 mM, 1% NP-40, 1% sodium deoxycholate, and 1% SDS) supplemented with a protease inhibitor mixture and phosphatase inhibitors (25 mM NaF, 100 mM Na
3VO
4, and 30 μM Na
4P
2O
7) using a Potter homogenizer and then sequentially passed through syringes of different calibers. The protein samples were centrifuged twice at 14,000 rpm for 20 min at 4 °C. The protein concentrations were determined using the BCA Protein Assay Kit (Pierce). The samples were resolved by SDS-PAGE, followed by immunoblotting on PVDF membranes. The membranes were incubated with the primary antibodies: glutamate ionotropic receptor AMPA type subunit 1 (GLUR-1, Santa Cruz Biotechnology, sc-13152, Santa Cruz, CA, USA), glycogen synthase kinase-3β (GSK-3β, Santa Cruz Biotechnology, sc-81462, Santa Cruz, CA, USA), GSK-3β phosphorylated in Serine 9 (p-GSK-3β, Santa Cruz Biotechnology, sc-373800, Santa Cruz, CA, USA), Homer (Santa Cruz Biotechnology, sc-17842, CA, USA), N-methyl D-aspartate receptor subtype 2B (NR2B, Santa Cruz Biotechnology, sc-365597, Santa Cruz, CA, USA), postsynaptic density 95 (PSD95, Thermo scientific, MA1-045), synaptotagmin I/II (SYT, Santa Cruz Biotechnology, sc-393392, Santa Cruz, CA, USA), synaptophysin (SYP, Santa Cruz Biotechnology, sc-17750, Santa Cruz, CA, USA) and gamma-aminobutyric acid receptor (GABA
A receptor, G0545, Sigma-Aldrich, Merck, Santiago, Chile). They were then washed with phosphate-buffered saline (PBS)-Tween 0.1% and incubated with secondary antibodies conjugated with HRP: anti-mouse, anti-rabbit, and anti-goat IgG peroxidase-conjugated antibodies (Pierce, Rockford, IL, USA). Later, the membranes were visualized using an ECL kit (Luminata Forte Western HRP substrate, Merck, Millipore, MA, USA). To analyze the results, all target protein signals were normalized against the loading control (α-tubulin, β-actin or GAPDH).
4.5. Statistical Analysis
All data are presented as the mean ± standard error (SEM). We used a t-test to analyze the effect of age in control female degus across each variable of the behavioral, electrophysiological, and/or biochemistry measurements. We used two-way ANOVAs to determine the effects of treatment (control and ANDRO), age (adult and aged degus), and the interaction between these factors. Fisher's LSD post hoc comparisons were performed to examine the individual main effect of treatment and age. The RI was analyzed in the NLR/NOR and the three-chambered social interaction tests. The assumptions of normally distributed data and homogeneous variances were confirmed using Shapiro–Wilk and Levene's tests, respectively. Statistical analyses were performed using the Statistica (StatSoft, Tulsa, OK, USA) software package. For the Western blot analysis, we used one-way ANOVA to analyze the effect of ANDRO treatment in both adult and aged degus. For the electrophysiological measurements, we used n = 6 adult females, n = 6 adult females + ANDRO, n = 6 aged females, and n = 6 aged females + ANDRO. At least three slices from each animal were considered replicates, averaged together, and counted like n = 1. Input–output curves were analyzed via two-way ANOVA to determine the effect of treatment and stimulus intensity, or age and stimulus intensity, and the interaction between the two factors. We plotted the FV amplitude value and its corresponding fEPSP slope to determine the differences in synaptic transmission efficacy. The points were adjusted with linear regression. To further compare whether the regression lines were different, we used the analysis of covariance (ANCOVA). This analysis tests the null hypothesis that both regressions are identical in slope and intercept. We applied this analysis to our data to determine the difference between our experimental groups. The PPF protocol was analyzed with two-way ANOVA to determine the effect of treatment and inter-stimulus interval, or age and inter-stimulus interval, and the interaction between the two factors. Using Pearson’s correlation, we found the correlation between FV amplitude and fEPSP slope before or after TBS. Using Fisher ’r’ to ’z’ transformation, we used z-scores to compute the significance of the difference when we compared two correlation coefficients. For the attenuation plot, we plotted the R1 (%) and R2 (%) and used the formula (R2-R1)/R1 (%). The two-way ANOVA determined the differences between time course and treatment, or time course and age, and the interaction between both factors. Additionally, an unpaired t-test was performed over the average of the last 10 min recordings to assay differences between the two groups. Amplitudes and area were obtained using PClamp 10.2, ( Molecular Devices LLC, San Jose, CA, USA) and the statistical analyses were obtained using the GraphPad Prism 5.1 (GraphPad software Inc., San Diego, CA, USA). Differences were considered statistically significant at p < 0.05.