Effectiveness of Flattening-Filter-Free versus Flattened Beams in V79 and Glioblastoma Patient-Derived Stem-like Cells
Abstract
1. Introduction
2. Results
2.1. Cell Killing and Lethal Mutations in Chinese Hamster V79 cells
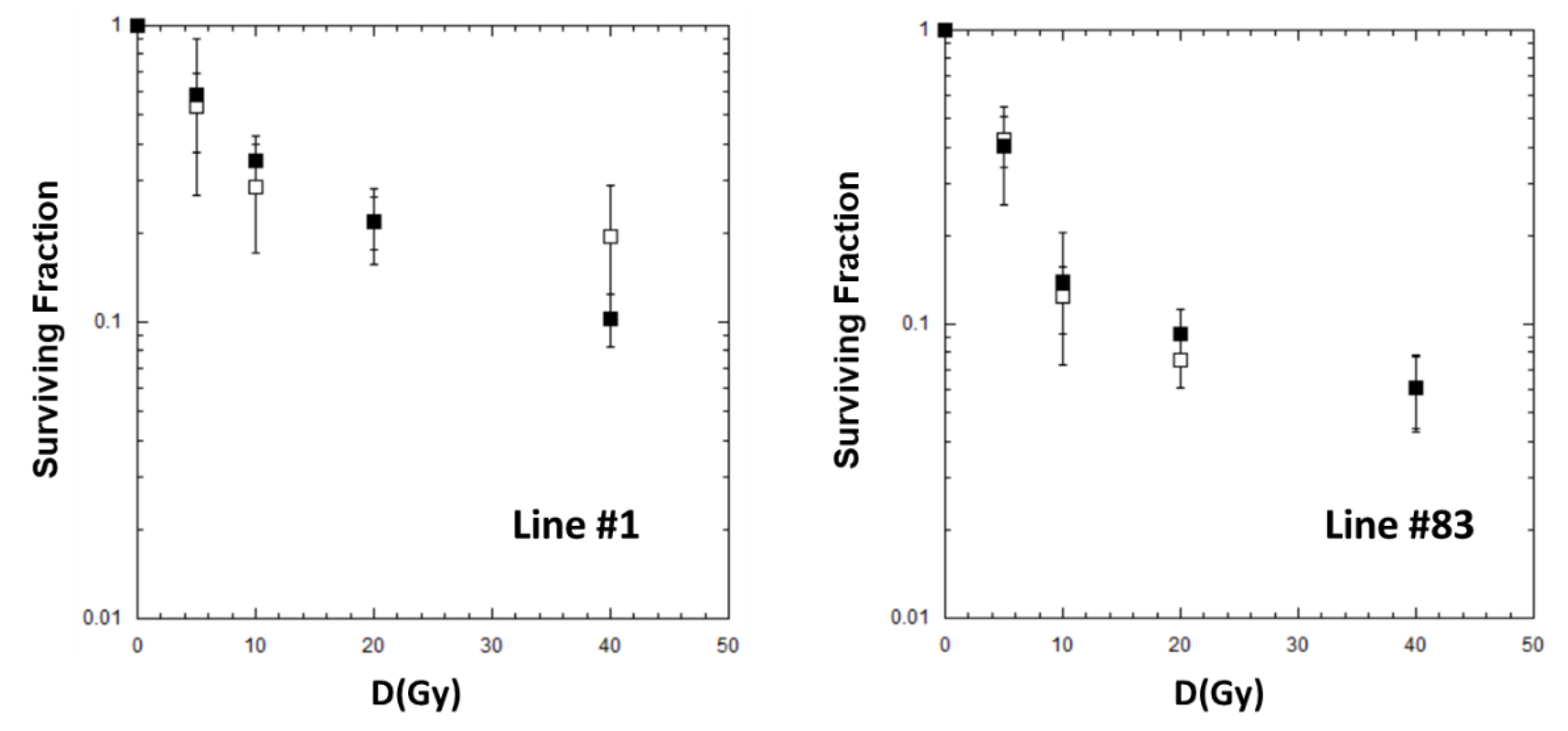
2.2. Cell Killing for GSCs
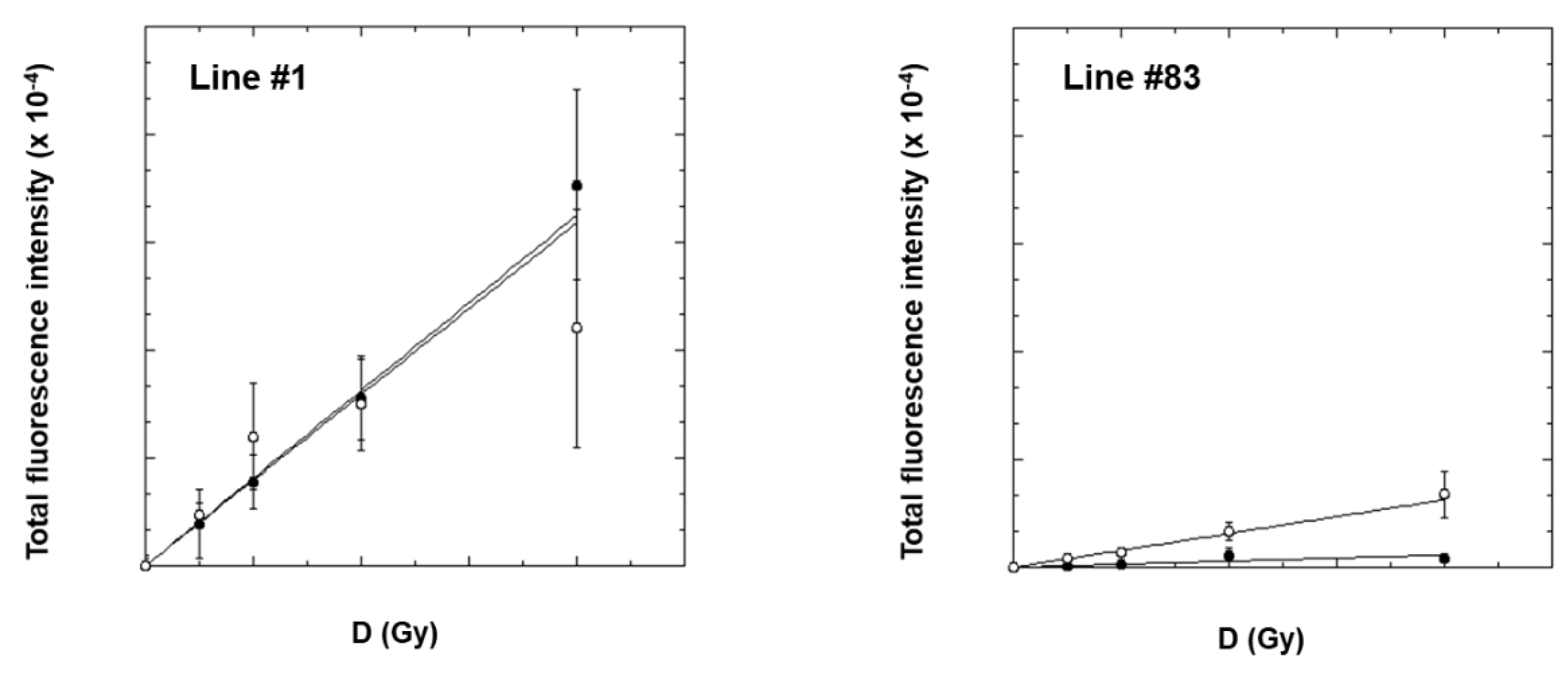
2.3. γ-H2AX Analysis by Flow-Cytometry
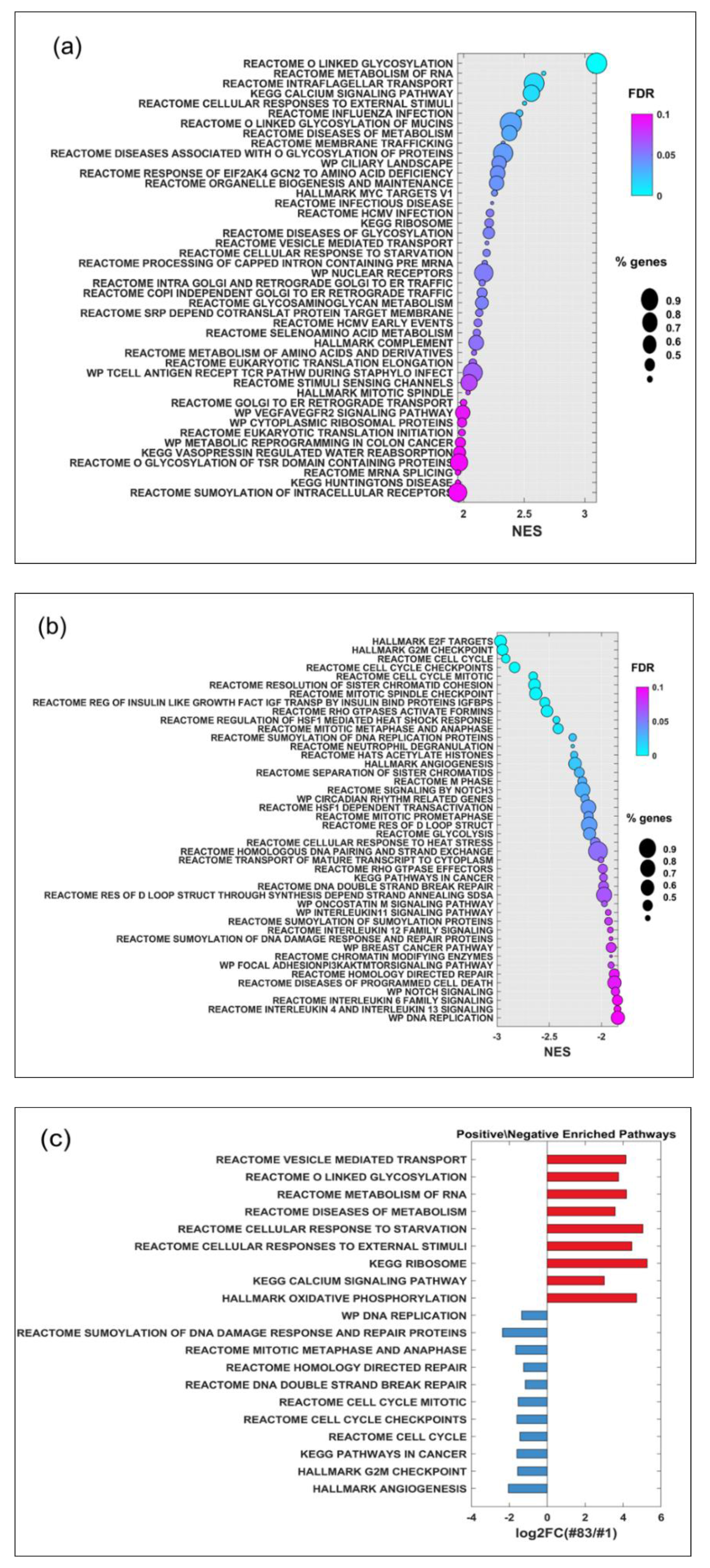
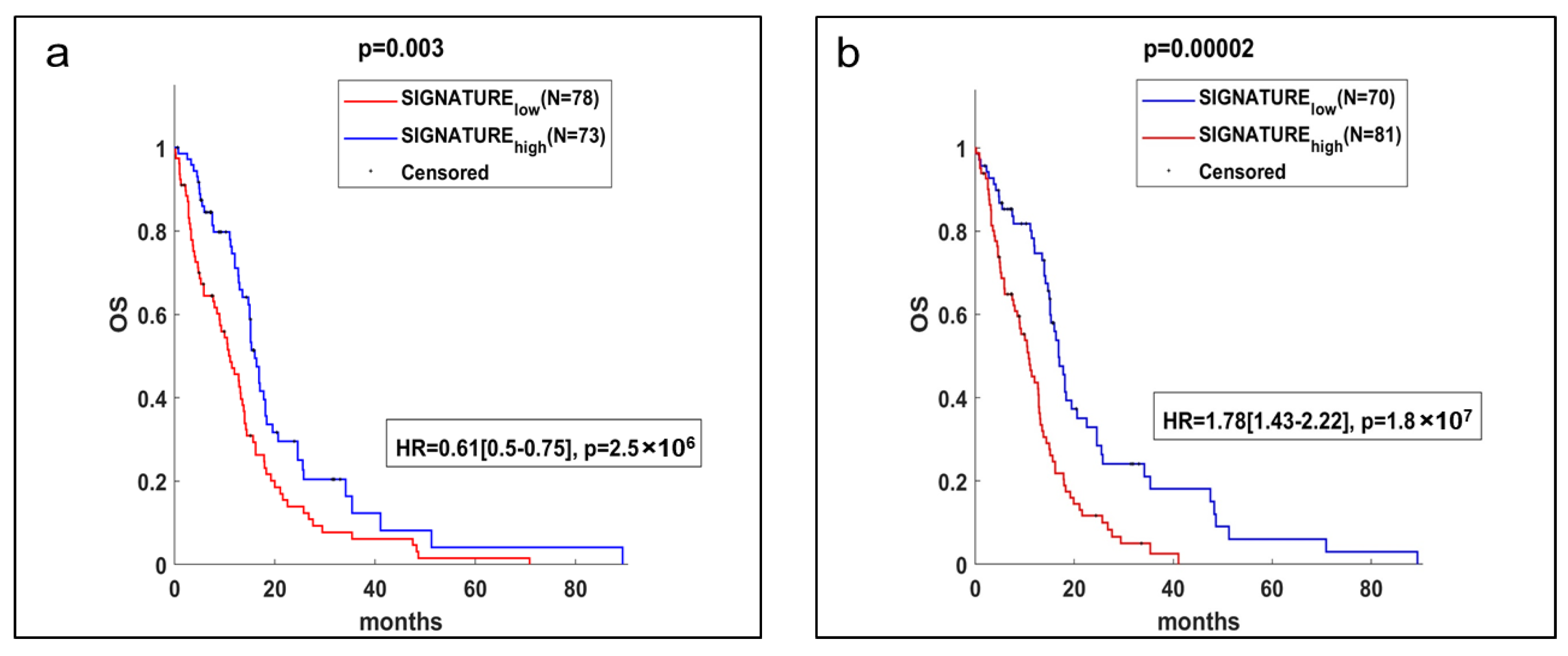
2.4. Gene Expression Analysis and Potential Prognostic Factors for ‘Patients’ Survival
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Irradiation Geometry, Treatment Planning, and Dose Measurements
4.3. Dose Measurements—Dosimetry
4.4. Cell Killing and Genomic Instability in V79 Cells
4.5. Cell Killing in GSCs
4.6. Flow Cytometry Measurements for γ-H2AX Detection in GSCs
4.7. Gene Expression Profiling in GSCs
4.7.1. The Cancer Genome Atlas (TCGA)
4.7.2. Target Prediction and Gene Set Enrichment Analysis (GSEA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCOA4 | Nuclear Receptor Coactivator 4 |
| NBN | Nibrin |
| STAG2 | Stromal Antigen 2 |
| MAPRE1 | Microtubule Associated Protein RP/EB Family Member 1 |
| E2F5 | E2F Transcription Factor 5 |
| PTEN | Phosphatase and tensin homolog |
| CEP57 | centrosomal protein 57 |
| GALNT12 | Polypeptide N-Acetylgalactosaminyltransferase 12 |
| POR | Cytochrome P450 Oxidoreductase |
| RPL39L | Ribosomal Protein L39 Like |
| CUL1 | Cullin 1 |
| MAP2K3 | Mitogen-Activated Protein Kinase Kinase 3 |
| CAMK4 | Calcium/Calmodulin Dependent Protein Kinase IV |
| DENND2A | DENN Domain Containing 2D |
| HPS1 | Hermansky-Pudlak syndrome 1 |
| ADAMTSL1 | a disintegrin and metalloproteinase with thrombospondin like protein 1 |
| SCO1 | Synthesis Of Cytochrome C Oxidase 1 |
| GPC5 | Glypican 5 |
| GALNT6 | Polypeptide N-Acetylgalactosaminyltransferase 6 |
| MYO1C | Myosin IC |
| RT-qPCR | quantitative real time PCR |
| NMR | Nuclear Magnetic Resonance |
| HP1-b | heterochromatin protein 1-beta |
| CK2 | casein kinase 2 |
| LET | Linear Energy Transfer |
| KAP-1 | Transcription intermediary factor 1-beta |
| ROS | Reactive oxygen species |
| EGF | Epidermal Growth Factor |
| b-FGF | basic Fibroblast Growth Factor |
| SSD | Source Surface Distance |
| CT | Computed Tomography |
| DPBS | Dulbecco’phosphate-buffered saline |
| BSA | Bovine Serum Albumina |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Anton, K.; Baehring, J.M.; Mayer, T. Glioblastoma multiforme: Overview of current treatment and future perspectives. Hematol. Oncol. Clin. N. Am. 2012, 26, 825–853. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Regev, O.; Merkin, V.; Blumenthal, D.T.; Melamed, I.; Kaisman-Elbaz, T. Tumor-Treating Fields for the treatment of glioblastoma: A systematic review anmeta-analysis. Neurooncol. Pract. 2021, 8, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Mendonca, M.S. News FLASH-RT: To Treat GBM and Spare Cognition, Fraction Size and Total Dose Matter. Clin. Cancer. Res. 2021, 27, 662–664. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Jorge, P.G.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer. Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Bewes, J.M.; Suchowerska, N.; Jackson, M.; Zhang, M.; McKenzie, D.R. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT). Phys. Med. Biol. 2008, 53, 3567–3578. [Google Scholar] [CrossRef]
- Keall, P.J.; Chang, M.; Benedict, S.; Thames, H.; Vedam, S.S.; Lin, P.S. Investigating the temporal effects of respiratory-gated and intensity-modulated radiotherapy treatment delivery on in vitro survival: An experimental and theoretical study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1547–1552. [Google Scholar] [CrossRef]
- Dang, T.M.; Peters, M.J.; Hickey, B.; Semciw, A. Efficacy of flattening-filter-free beam in stereotactic body radiation therapy planning and treatment: A systematic review with meta-analysis. J. Med. Imaging. Radiat. Oncol. 2017, 61, 379–387. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Vestergaard, A.; Overgaard, J.; Præstegaard, L.H. Dependence of cell survival on instantaneous dose rate of a linear accelerator. Radiother. Oncol. 2011, 101, 223–225. [Google Scholar] [CrossRef]
- Verbakel, W.F.; van den Berg, J.; Slotman, B.J.; Sminia, P. Comparable cell survival between high dose rate flattening filter free and conventional dose rate irradiation. Acta Oncol. 2013, 52, 652–657. [Google Scholar] [CrossRef]
- Ling, C.C.; Gerweck, L.E.; Zaider, M.; Yorke, E. Dose-rate effects in external beam radiotherapy redux. Radiother. Oncol. 2010, 95, 261–268. [Google Scholar] [CrossRef]
- Karan, T.; Moiseenko, V.; Gill, B.; Horwood, R.; Kyle, A.; Minchinton, A.I. Radiobiological effects of altering dose rate in filter-free photon beams. Phys. Med. Biol. 2013, 58, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Cerviño, L.I.; Soultan, D.; Advani, S.J.; Cornell, M.; Yock, A.; Pettersson, N.; Song, W.Y.; Aguilera, J.; Murphy, J.; Hoh, C.; et al. An in vitro study for the dosimetric and radiobiological validation of respiratory gating in conventional and hypofractionated radiotherapy of the lung: Effect of dose, dose rate, and breathing pattern. Phys. Med. Biol. 2019, 64, 135009. [Google Scholar] [CrossRef] [PubMed]
- Lasio, G.; Guerrero, M.; Goetz, W.; Lima, F.; Baulch, J.E. Effect of varying dose-per-pulse and average dose rate in X-ray beam irradiation on cultured cell survival. Radiat. Environ. Biophys. 2014, 53, 671–676. [Google Scholar] [CrossRef]
- King, R.B.; Hyland, W.B.; Cole, A.J.; Butterworth, K.T.; McMahon, S.J.; Redmond, K.M.; Trainer, C.; Prise, K.M.; McGarry, C.K.; Hounsell, A.R. An in vitro study of the radiobiological effects of flattening filter free radiotherapy treatments. Phys. Med. Biol. 2013, 58, N83–N94. [Google Scholar] [CrossRef] [PubMed]
- Lohse, I.; Lang, S.; Hrbacek, J.; Scheidegger, S.; Bodis, S.; Macedo, N.S.; Feng, J.; Lütolf, U.M.; Zaugg, K. Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiother. Oncol. 2011, 101, 226–232. [Google Scholar] [CrossRef]
- Sarojini, S.; Pecora, A.; Milinovikj, N.; Barbiere, J.; Gupta, S.; Hussain, Z.M.; Tuna, M.; Jiang, J.; Adrianzen, L.; Jun, J.; et al. A combination of high dose rate (10X FFF/2400 MU/min/10 MV X-rays) and total low dose (0.5 Gy) induces a higher rate of apoptosis in melanoma cells in vitro and superior preservation of normal melanocytes. Melanoma Res. 2015, 25, 376–389. [Google Scholar] [CrossRef]
- Belli, M.; Cera, F.; Cherubini, R.; Haque, A.M.; Ianzini, F.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A.; Tiveron, P. Inactivation and mutation induction in V79 cells by low energy protons: Re-evaluation of the results at the LNL facility. Int. J. Radiat. Biol. 1993, 63, 331–337. [Google Scholar] [CrossRef]
- Chang, W.P.; Little, J.B. Evidence that DNA double-strand breaksinitiate the phenotype of delayed reproductive death in Chinese hamster ovary cells. Radiat. Res. 1992, 131, 53–59. [Google Scholar] [CrossRef]
- Suzuki, K.; Ojima, M.; Kodama, S.; Watanabe, M. Radiation-induced DNA damage and delayed induced genomic instability. Oncogene 2003, 22, 6988–6993. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Eberhart, C.G.; Kai, M. RNA binding protein RBM14 promotes radio-resistance in glioblastoma by regulating DNA repair and cell differentiation. Oncotarget 2014, 5, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Koval, T.M. Multiphasic survival response of a radioresistant lepidopteran insect cell line. Radiat Res. 1984, 98, 642–648. [Google Scholar] [CrossRef]
- Xiong, R.; Xie, G.; Edmondson, A.E.; Sheard, M.A. A mathematical model for bacterial inactivation. Int. J. Food. Microbiol. 1999, 46, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Schettino, G.; Folkard, M.; Prise, K.M.; Vojnovic, B.; Bowey, A.G.; Michael, B.D. Low-dose hypersensitivity in Chinese hamster V79 cells targeted with counted protons using a charged-particle microbeam. Radiat. Res. 2001, 156, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Li, X.A.; D’Souza, W.D.; Stewart, R.D. Impact of prolonged fraction delivery times on tumor control: A note of caution for intensity-modulated radiation therapy (IMRT). Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mackonis, E.C.; Suchowerska, N.; Zhang, M.; Ebert, M.; McKenzie, D.R.; Jackson, M. Cellular response to modulated radiation fields. Phys. Med. Biol. 2007, 52, 5469–5482. [Google Scholar] [CrossRef]
- Ogino, H.; Shibamoto, Y.; Sugie, C.; Ito, M. Biological effects of intermittent radiation in cultured tumor cells: Influence of fraction number and dose per fraction. J. Radiat. Res. 2005, 46, 401–406. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Ito, M.; Sugie, C.; Ogino, H.; Hara, M. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: Implications for dose modification in radiosurgery and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1484–1490. [Google Scholar] [CrossRef]
- Mu, X.; Löfroth, P.O.; Karlsson, M.; Zackrisson, B. The effect of fraction time in intensity modulated radiotherapy: Theoretical and experimental evaluation of an optimisation problem. Radiother. Oncol. 2003, 68, 181–187. [Google Scholar] [CrossRef]
- Moiseenko, V.; Duzenli, C.; Durand, R.E. In vitro study of cell survival following dynamic MLC intensity-modulated radiation therapy dose delivery. Med. Phys. 2007, 34, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F.; Welsh, J.S.; Howard, S.P. Loss of biological effect in prolonged fraction delivery. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Biemans, R.; Reniers, B.; Bosmans, G.; Trani, D.; Podesta, M.; Kollaard, R.; ma Rouschop, K.; Theys, J.; Vooijs, M. High dose rate and flattening filter free irradiation can be safely implemented in clinical practice. Int. J. Radiat. Biol. 2015, 91, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Godley, A.; Shoemake, J.D.; Han, Z.; Magnelli, A.; Yu, J.S. The effects of extra high dose rate irradiation on glioma stem-like cells. PLoS ONE 2018, 13, e0202533. [Google Scholar] [CrossRef]
- Laurent, P.A.; Kownacka, A.; Boidot, R.; Richard, C.; Limagne, E.; Morgand, V.; Froidurot, L.; Bonin, C.; Aubignac, L.; Ghiringhelli, F.; et al. In-vivo and in-vitro impact of high-dose rate radiotherapy using flattening-filter-free beams on the anti-tumor immune response. Clin. Transl. Radiat. Oncol. 2020, 24, 116–122. [Google Scholar] [CrossRef]
- Belli, M.; Bettega, D.; Calzolari, P.; Cherubini, R.; Cuttone, G.; Durante, M.; Esposito, G.; Furusawa, Y.; Gerardi, S.; Gialanella, G.; et al. Effectiveness of monoenergetic and spread-out bragg peak carbon-ions for inactivation of various normal and tumour human cell lines. J. Radiat. Res. 2008, 49, 597–607. [Google Scholar] [CrossRef]
- Belli, M.; Cera, F.; Cherubini, R.; Dalla Vecchia, M.; Haque, A.M.; Ianzini, F.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A.; et al. RBE-LET relationships for cell inactivation and mutation induced by low energy protons in V79 cells: Further results at the LNL facility. Int. J. Radiat. Biol. 1998, 74, 501–509. [Google Scholar] [CrossRef]
- Günther, H.S.; Schmidt, N.O.; Phillips, H.S.; Kemming, D.; Kharbanda, S.; Soriano, R.; Modrusan, Z.; Meissner, H.; Westphal, M.; Lamszus, K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 2008, 27, 2897–2909. [Google Scholar] [CrossRef]
- Schulte, A.; Günther, H.S.; Phillips, H.S.; Kemming, D.; Martens, T.; Kharbanda, S.; Soriano, R.H.; Modrusan, Z.; Zapf, S.; Westphal, M. A distinct subset of glioma cell lines with stem cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia 2011, 59, 590–602. [Google Scholar] [CrossRef]
- Marziali, G.; Signore, M.; Buccarelli, M.; Grande, S.; Palma, A.; Biffoni, M.; Rosi, A.; D’Alessandris, Q.G.; Martini, M.; Larocca, L.M.; et al. Metabolic/Proteomic Signature Defines Two Glioblastoma Subtypes With Different Clinical Outcome. Sci. Rep. 2016, 6, 21557. [Google Scholar] [CrossRef]
- Gobin, M.; Nazarov, P.V.; Warta, R.; Timmer, M.; Reifenberger, G.; Felsberg, J.; Vallar, L.; Chalmers, A.J.; Herold-Mende, C.C.; Goldbrunner, R.; et al. A DNA repair and cell-cycle gene expression signature in primary and recurrent glioblastoma: Prognostic value and clinical implications. Cancer Res. 2019, 79, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Pece, S.; Tosoni, D.; Confalonieri, S.; Mazzarol, G.; Vecchi, M.; Ronzoni, S.; Bernard, L.; Viale, G.; Pelicci, P.G.; Di Fiore, P.P. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010, 140, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Jeyasekharan, A.D.; Bernal, J.A.; Venkitaraman, A.R. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 2008, 453, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Jaberaboansari, A.; Nelson, G.B.; Roti, J.L.; Wheeler, K.T. Postirradiation alterations of neuronal chromatin structure. Radiat. Res. 1988, 114, 94–104. [Google Scholar] [CrossRef]
- Kruhlak, M.J.; Celeste, A.; Dellaire, G.; Fernandez-Capetillo, O.; Müller, W.G.; McNally, J.G.; Bazett-Jones, D.P.; Nussenzweig, A. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J. Cell. Biol. 2006, 172, 823–834. [Google Scholar] [CrossRef]
- Li, Y.; Cucinotta, F.A. Mathematical Model of ATM Activation and Chromatin Relaxation by Ionizing Radiation. Int. J. Mol. Sci. 2020, 21, 1214. [Google Scholar] [CrossRef]
- Timm, S.; Lorat, Y.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Clustered DNA damage concentrated in particle trajectories causes persistent large-scale rearrangements in chromatin architecture. Radiother. Oncol. 2018, 129, 600–610. [Google Scholar] [CrossRef]
- Pallini, R.; Ricci-Vitiani, L.; Banna, G.L.; Signore, M.; Lombardi, D.; Todaro, M.; Stassi, G.; Martini, M.; Maira, G.; Larocca, L.M. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 2008, 14, 8205–8212. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; De Maria, R. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro. Oncol. 2017, 19, 1097–1108. [Google Scholar] [CrossRef]
- Pecchia, I.; Dini, V.; Ricci-Vitiani, L.; Biffoni, M.; Balduzzi, M.; Fratini, E.; Belli, M.; Campa, A.; Esposito, G.; Cirrone, G. Glioblastoma stem cells: Radiobiological response to ionising radiations of different qualities. Radiat. Prot. Dosim. 2015, 166, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Grande, S.; Ricci-Vitiani, L.; Luciani, A.M.; Buccarelli, M.; Biffoni, M.; Dini, V.; Esposito, G.; Cirrone, A.P.; Ciocca, M.; et al. Different Mechanisms Underlie the Metabolic Response of GBM Stem-Like Cells to Ionizing Radiation: Biological and MRS Studies on Effects of Photons and Carbon Ions. Int. J. Mol. Sci. 2020, 21, 5167. [Google Scholar] [CrossRef] [PubMed]
- Micke, A.; Lewis, D.F.; Yu, X. Multichannel film dosimetry with nonuniformity correction. Med. Phys. 2011, 38, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Niroomand-Rad, A.; Chiu-Tsao, S.T.; Grams, M.P.; Lewis, D.F.; Soares, C.G.; Van Battum, L.J.; Das, I.J.; Trichter, S.; Kissick, M.W.; Massillon-Jl, G. Report of AAPM Task Group 235 Radiochromic Film Dosimetry: An Update to TG-55. Med. Phys. 2020, 47, 5986–6025. [Google Scholar] [CrossRef] [PubMed]
- Puck, T.T.; Marcus, P.I. Action of x-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef]
- Thilly, W.G.; DeLuca, J.G.; Furth, E.E.; Hoppe, H.; Kaden, D.A.; Krolewski, J.J.; Liber, H.L.; Skopek, T.R.; Slapikoff, S.A.; Tizard, R.J.; et al. Gene-Locus Mutation Assays in Diploid Human Lymphoblast Lines. In Chemical Mutagens: Principles and Methods for Their Detection, 1st ed.; de Serres, F.J., Hollaender, A., Eds.; Springer: Boston, MA, USA, 1980; Volume 6, pp. 331–364. [Google Scholar] [CrossRef]
- Hamasaki, K.; Imai, K.; Nakachi, K.; Takahashi, N.; Kodama, Y.; Kusunoki, Y. Short-term culture and gammaH2AX flow cytometry determine differences in individual radiosensitivity in human peripheral T lymphocytes. Environ. Mol. Mutagen. 2007, 48, 38–47. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Guidoni, L.; Ricci-Vitiani, L.; Rosi, A.; Palma, A.; Grande, S.; Luciani, A.M.; Pelacchi, F.; di Martino, S.; Colosimo, C.; Biffoni, M.; et al. 1H NMR detects different metabolic profiles in glioblastoma stem-like cells. NMR Biomed. 2014, 27, 129–145. [Google Scholar] [CrossRef]

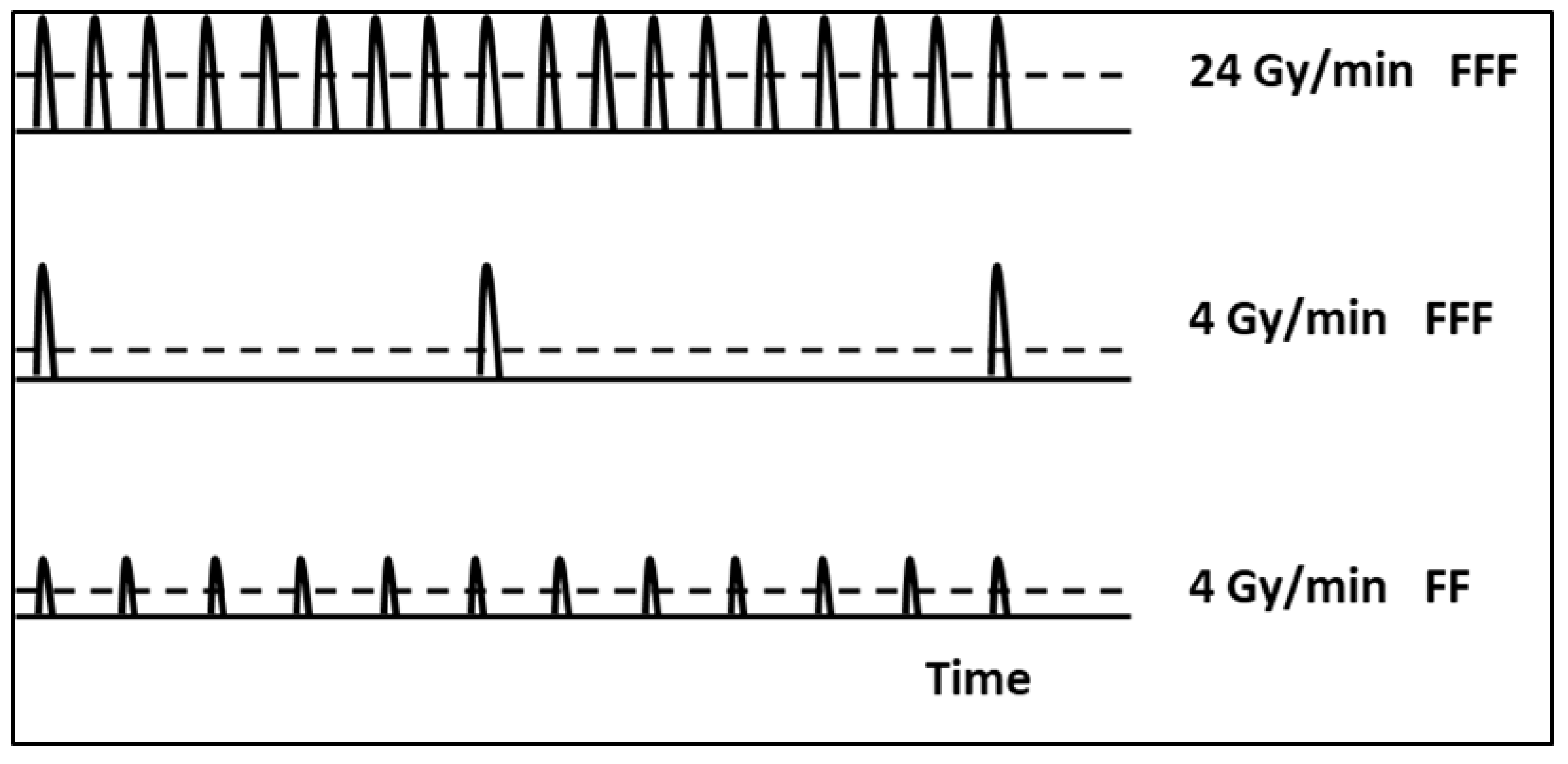
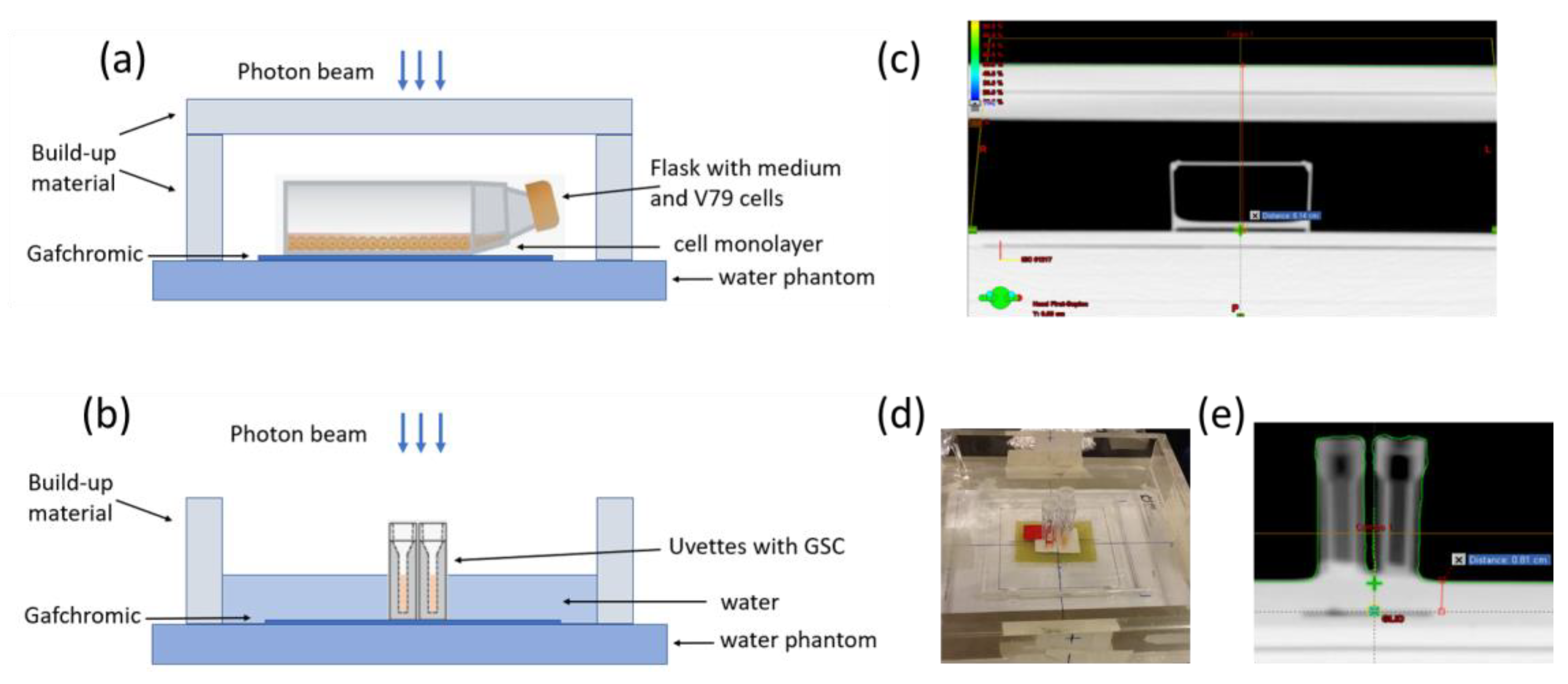
| Early Survival | Late Survival | |||
|---|---|---|---|---|
| Beam | Dose Rate (Gy/min) | α (Gy−1) | β (Gy−2) | α (Gy−2) |
| 10 MV FF | 4 | 0.084 ± 0.037 | 0.025 ± 0.006 | 0.084 ± 0.015 |
| 10 MV FFF | 4 | 0.124 ± 0.036 | 0.019 ± 0.005 | 0.080 ± 0.021 |
| 10 MV FFF | 24 | 0.166 ± 0.035 | 0.017 ± 0.005 | 0.034 ± 0.013 |
| Beam | Dose Rate (Gy/min) | Line | f | αs (Gy−1) | αr (Gy−1) |
|---|---|---|---|---|---|
| 10 MV FFF | 4 | #1 | 0.56 ± 0.30 | 0.24 ± 0.29 | 0.04 ± 0.02 |
| 10 MV FFF | 24 | #1 | 0.80 ± 0.23 | 0.19 ± 0.13 | 0.00 ± 0.04 |
| 10 MV FFF | 4 | #83 | 0.88 ± 0.08 | 0.27 ± 0.11 | 0.02 ± 0.02 |
| 10 MV FFF | 24 | #83 | 0.93 ± 0.05 | 0.24 ± 0.05 | 0.00 ± 0.02 |
| Beam | DR (Gy/min) | Line | PR (%) @ 4 h | PR (%) @ 24 h |
|---|---|---|---|---|
| 10 MV FFF | 4 | #1 | 45 ± 17 | 4 ± 3 |
| 10 MV FFF | 24 | #1 | 45 ± 18 | 24 ± 14 |
| 10 MV FFF | 4 | #83 | 50 ± 27 | 6 ± 4 |
| 10 MV FFF | 24 | #83 | 64 ± 20 | 14 ± 2 |
| Cells | End-Point | E (MV) | Dose Rate (Gy/min) | Dose (Gy) | Modulated Beam | Observed Effect | Reference |
|---|---|---|---|---|---|---|---|
| HNCa, FaDuDD; Chinese hamster lung fibroblasts, V79 | Clonogenic cell survival | 6 FFF | 5.01, 9.99, 29.91 | 1–10 | No | No | Sørensen et al. [9] |
| 6 FF | |||||||
| GBM cell lines: T98G (mut-p53) and U87MG (wt-p53) | Clonogenic cell survival | 10 FFF | 4, 24 | 5, 10 | No | Yes * | Lohse et al., 2011 [16] |
| 10 FF | 0.2, 4, 6 | ||||||
| Cervical carcinoma SiHa; NSCLC H460; V79 | Clonogenic cell Survival, γ-H2AX induction | 6 FFF | 3.6, 10 | 2, 5, 10 | Yes (compensator) | No | Karan et al., 2013 [12] |
| 6 FF | 3.6 | No | |||||
| SCLC SW1573; GBM cell lines T98 (mut-p53); astrocytoma D348 | Clonogenic cell survival | 10 FFF | ~24 | 2–12 | Yes (IMRT) | No | Verbakel et al., 2013 [10] |
| 6 FF | ~5.8 | ||||||
| GBM cell lines T98G (mut-p53); U87MG (wt-p53), V79 | Clonogenic cell survival | 10 FFF | 4, 24 | 5, 10 | No | No | Lasio et al., 2014 [14] |
| 6 FF | 4 | ||||||
| 6 FFF | 4, 14 | ||||||
| Human Ca lung A549; Ca breast (MCF and Ca brain U373 MG, Ca colon HCT116 and DLD-1; normal human lung NL20 and breast MCF10A; CHO9 | Clonogenic cell survival | 10 FFF | 4, 24 | 2, 5, 10 | No | No | Dubois et al., 2015 [33] |
| 10 FF | |||||||
| GSCs | Several # | 6 FFF | 0.2, 4, 4.2, 21.2 | 2–6 | No | No | Hao et al., 2018 [34] |
| 6 FF | |||||||
| Colon Ca CT26 murine | Several § | 10 FFF | 4, 12, 24 | 2–12 | No | No | Laurent et al., 2020 [35] |
| 10 FF | |||||||
| Melanoma cell lines (WC00046, WC00060, and WC00081) | Clonogenic cell survival | 10 FFF | 4, 24 | 0.25–8 | No | Yes | Sarojini et al. [17] |
| Energy | Q-Index | DPP (cGy/Pulse) | Pulse Length (µs) | PRF (Hz) | Average Dose Rate (Gy/min) | Delivery Time for 5 Gy (s) |
|---|---|---|---|---|---|---|
| 10 MV FF | 0.735 | 0.028 | 4.5 | 240 | 4 | 75 |
| 10 MV FFF | 0.691 | 0.111 | 4.5 | 360 | 24 | 12.5 |
| 4.5 | 60 | 4 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, V.; Esposito, G.; Sacconi, A.; D’Andrea, M.; Tabocchini, M.A.; Anello, P.; Ricci-Vitiani, L.; Buccarelli, M.; Pallini, R.; Strigari, L. Effectiveness of Flattening-Filter-Free versus Flattened Beams in V79 and Glioblastoma Patient-Derived Stem-like Cells. Int. J. Mol. Sci. 2023, 24, 1107. https://doi.org/10.3390/ijms24021107
Dini V, Esposito G, Sacconi A, D’Andrea M, Tabocchini MA, Anello P, Ricci-Vitiani L, Buccarelli M, Pallini R, Strigari L. Effectiveness of Flattening-Filter-Free versus Flattened Beams in V79 and Glioblastoma Patient-Derived Stem-like Cells. International Journal of Molecular Sciences. 2023; 24(2):1107. https://doi.org/10.3390/ijms24021107
Chicago/Turabian StyleDini, Valentina, Giuseppe Esposito, Andrea Sacconi, Marco D’Andrea, Maria Antonella Tabocchini, Pasquale Anello, Lucia Ricci-Vitiani, Mariachiara Buccarelli, Roberto Pallini, and Lidia Strigari. 2023. "Effectiveness of Flattening-Filter-Free versus Flattened Beams in V79 and Glioblastoma Patient-Derived Stem-like Cells" International Journal of Molecular Sciences 24, no. 2: 1107. https://doi.org/10.3390/ijms24021107
APA StyleDini, V., Esposito, G., Sacconi, A., D’Andrea, M., Tabocchini, M. A., Anello, P., Ricci-Vitiani, L., Buccarelli, M., Pallini, R., & Strigari, L. (2023). Effectiveness of Flattening-Filter-Free versus Flattened Beams in V79 and Glioblastoma Patient-Derived Stem-like Cells. International Journal of Molecular Sciences, 24(2), 1107. https://doi.org/10.3390/ijms24021107







