The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment
Abstract
:1. Introduction
2. Results
2.1. High Concentrations of Melatonin Significantly Affected the Expression of Clock Genes Bmal1 and Per2 in HNSCC Cells
2.2. The expression of Bmal1 Is Not Affected by Low Doses of Melatonin in HNSCC Cells
2.3. High Doses of Melatonin Induce the Circadian Expression of Sirtuin-1 in HNSCC Cells
2.4. High Doses of Melatonin Have No Effect on the Induction of the Circadian Rhythm of Mitochondrial Respiratory Activity in Cal-27 Cells
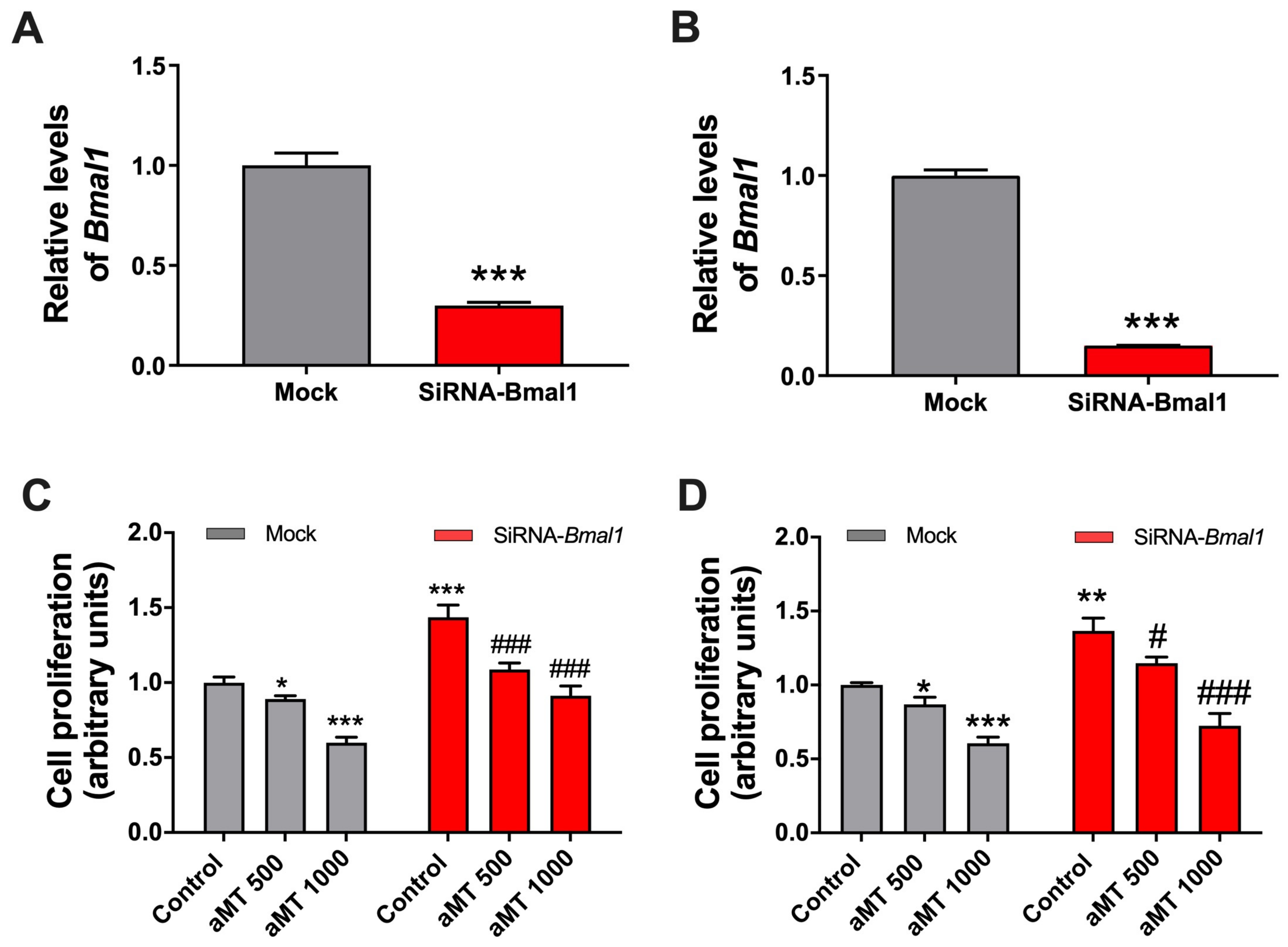
2.5. Bmal1 Knockdown Increases Cell Proliferation While Melatonin’s Antiproliferative Effects Are Maintained
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Proliferation Assay
4.3. Quantitative RT-PCR
4.4. Respirometric Measurements
4.5. BMAL1-Specific siRNA Transfection in Cal-27 Cells
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisani, P.; Airoldi, M.; Allais, A.; Aluffi Valletti, P.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic Disease in Head & Neck Oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Fu, X.-J.; Yang, K.; Chen, D.; Tang, H.; Zhao, Q. The Clock Gene PER1 Suppresses Expression of Tumor-Related Genes. in Human. Oral. Squamous Cell Carcinoma. Oncotarget 2016, 7, 20574–20583. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, X.; Yang, K. Overexpression of the Clock Gene Per2 Suppresses Oral Squamous Cell Carcinoma Progression by Activating Autophagy via the PI3K/AKT/MTOR Pathway. J. Cancer 2020, 11, 3655–3666. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Lin, S.F.; Lu, C.T.; Lin, P.M.; Yang, M.Y. Altered Expression of Circadian Clock Genes in Head and Neck Squamous Cell Carcinoma. Tumor Biol. 2012, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Scrima, R.; Cela, O.; Merla, G.; Augello, B.; Rubino, R.; Quarato, G.; Fugetto, S.; Menga, M.; Fuhr, L.; Relógio, A.; et al. Clock-Genes and Mitochondrial Respiratory Activity: Evidence of a Reciprocal Interplay. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1344–1351. [Google Scholar] [CrossRef]
- Cela, O.; Scrima, R.; Pazienza, V.; Merla, G.; Benegiamo, G.; Augello, B.; Fugetto, S.; Menga, M.; Rubino, R.; Fuhr, L.; et al. Clock Genes-Dependent Acetylation of Complex I Sets Rhythmic Activity of Mitochondrial OxPhos. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 596–606. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Rodríguez-Santana, C.; Florido, J.; Martínez-Ruiz, L.; López-Rodríguez, A.; Acuña-Castroviejo, D.; Escames, G. Role of Melatonin in Cancer: Effect on Clock Genes. Int. J. Mol. Sci. 2023, 24, 1919. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.; Chen, H.; Ritz, B. Circulating Melatonin Levels: Possible Link between Parkinson’s Disease and Cancer Risk? Cancer Causes Control. 2006, 17, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between Light at Night, Melatonin Secretion, Sleep Deprivation, and the Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, B.; Li, D.; Reiter, R.J.; Bai, Y.; Abay, B.; Chen, G.; Lin, S.; Zheng, T.; Ren, Y.; et al. Melatonin Inhibits Lung Cancer Development by Reversing the Warburg Effect via Stimulating the SIRT3/PDH Axis. J. Pineal Res. 2021, 71, e12755. [Google Scholar] [CrossRef] [PubMed]
- Florido, J.; Martinez-Ruiz, L.; Rodriguez-Santana, C.; López-Rodríguez, A.; Hidalgo-Gutiérrez, A.; Cottet-Rousselle, C.; Lamarche, F.; Schlattner, U.; Guerra-Librero, A.; Aranda-Martínez, P.; et al. Melatonin Drives Apoptosis in Head and Neck Cancer by Increasing Mitochondrial ROS Generated via Reverse Electron Transport. J. Pineal Res. 2022, 73, e12824. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin Synergizes the Chemotherapeutic Effect of 5-Fluorouracil in Colon Cancer by Suppressing PI3K/AKT and NF-ΚB/INOS Signaling Pathways. J. Pineal Res. 2017, 62, e12380. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Artime, A.; Cernuda-Cernuda, R.; Artime-Naveda, F.; Cepas, V.; Gonzalez-Menendez, P.; Fernadez-Vega, S.; Quiros-Gonzalez, I.; Sainz, R.M.; Mayo, J.C. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and Pro-Apoptotic Activity of Melatonin Analogues on Melanoma and Breast Cancer Cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a Novel Sirt1 Inhibitor, Imparts Antiproliferative Effects against Prostate Cancer in Vitro in Culture and in Vivo in TRAMP Model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef]
- Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.Q.; García-Verdugo, J.M.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin Targets Metabolism in Head and Neck Cancer Cells by Regulating Mitochondrial Structure and Function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef]
- Benna, C.; Helfrich-Förster, C.; Rajendran, S.; Monticelli, H.; Pilati, P.; Nitti, D.; Mocellin, S. Genetic Variation of Clock Genes and Cancer Risk: A Field Synopsis and Meta-Analysis. Oncotarget 2017, 8, 23978–23995. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Katchy, C.A.; Fu, L. Circadian Gene Variants in Cancer. Ann. Med. 2014, 46, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Tropea, S.; Benna, C.; Rossi, C.R. Circadian Pathway Genetic Variation and Cancer Risk: Evidence from Genome-Wide Association Studies. BMC Med. 2018, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A.; Damiola, F.; Schibler, U. A Serum Shock Induces Circadian Gene Expression in Mammalian Tissue Culture Cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.S.; Kim, J.Y.; Ahn, Y.T.A.E.; Lee, I.W.; Choi, S.W.; Jang, H.B.; Lee, J.C.; An, W.G. Melatonin Exerts Anticancer Effects in Human Tongue Squamous Cell Carcinoma Cells by Promoting Autophagy. Anticancer. Res. 2020, 40, 6295–6303. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Huang, W.; Reiter, R.J.; Ahmad, N. Melatonin Resynchronizes Dysregulated Circadian Rhythm Circuitry in Human Prostate Cancer Cells. J. Pineal Res. 2010, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Redondo, J.A.; Bibes, R.; Drubbel, A.V.; Dassy, B.; Bisteau, X.; Maury, E.; Beck, B. Per2 Circadian Oscillation Sensitizes Esophageal Cancer Cells to Chemotherapy. Biology 2021, 10, 266. [Google Scholar] [CrossRef]
- Guo, F.; Tang, Q.; Chen, G.; Sun, J.; Zhu, J.; Jia, Y.; Zhang, W. Aberrant Expression and Subcellular Localization of PER2 Promote the Progression of Oral Squamous Cell Carcinoma. Biomed. Res. Int. 2020, 2020, 8587458. [Google Scholar] [CrossRef]
- Tang, Q.; Cheng, B.; Xie, M.; Chen, Y.; Zhao, J.; Zhou, X.; Chen, L. Circadian Clock Gene Bmal1 Inhibits Tumorigenesis and Increases Paclitaxel Sensitivity in Tongue Squamous Cell Carcinoma. Cancer Res. 2017, 77, 532–544. [Google Scholar] [CrossRef]
- Soták, M.; Sumová, A.; Pácha, J. Cross-Talk between the Circadian Clock and the Cell Cycle in Cancer. Ann. Med. 2014, 46, 221–232. [Google Scholar] [CrossRef]
- Matsumoto, C.S.; Almeida, L.O.; Guimarães, D.M.; Martins, M.D.; Papagerakis, P.; Papagerakis, S.; Leopoldino, A.M.; Castilho, R.M.; Squarize, C.H. PI3K-PTEN Dysregulation Leads to MTOR-Driven Upregulation of the Core Clock Gene BMAL1 in Normal and Malignant Epithelial Cells. Oncotarget 2016, 7, 42393–42407. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lesicka, M.; Jabłońska, E.; Wieczorek, E.; Seroczyńska, B.; Siekierzycka, A.; Skokowski, J.; Kalinowski, L.; Wąsowicz, W.; Reszka, E. Altered Circadian Genes Expression in Breast Cancer Tissue According to the Clinical Characteristics. PLoS ONE 2018, 13, e0199622. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Wang, Y.; Wan, C.; Liu, Y.; Zhu, B.; Yang, C.; Wang, X.; Wang, Z.; Cornelissen-Guillaume, G.; Halberg, F. Circadian Gene MPer2 Overexpression Induces Cancer Cell Apoptosis. Cancer Sci. 2006, 97, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Coppi, L.; Ligorio, S.; Mitro, N.; Caruso, D.; De Fabiani, E.; Crestani, M. Pgc1s and beyond: Disentangling the Complex Regulation of Mitochondrial and Cellular Metabolism. Int. J. Mol. Sci. 2021, 22, 6913. [Google Scholar] [CrossRef] [PubMed]
- Dhara, A.; Aier, I.; Paladhi, A.; Varadwaj, P.K.; Hira, S.K.; Sen, N. PGC1 Alpha Coactivates ERG Fusion to Drive Antioxidant Target Genes under Metabolic Stress. Commun. Biol. 2022, 5, 416. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.; Parsons, R.; Garner, N.; Oster, H.; Rawashdeh, O. CircaCompare: A Method to Estimate and Statistically Support Differences in Mesor, Amplitude and Phase, between Circadian Rhythms. Bioinformatics 2020, 36, 1208–1212. [Google Scholar] [CrossRef]
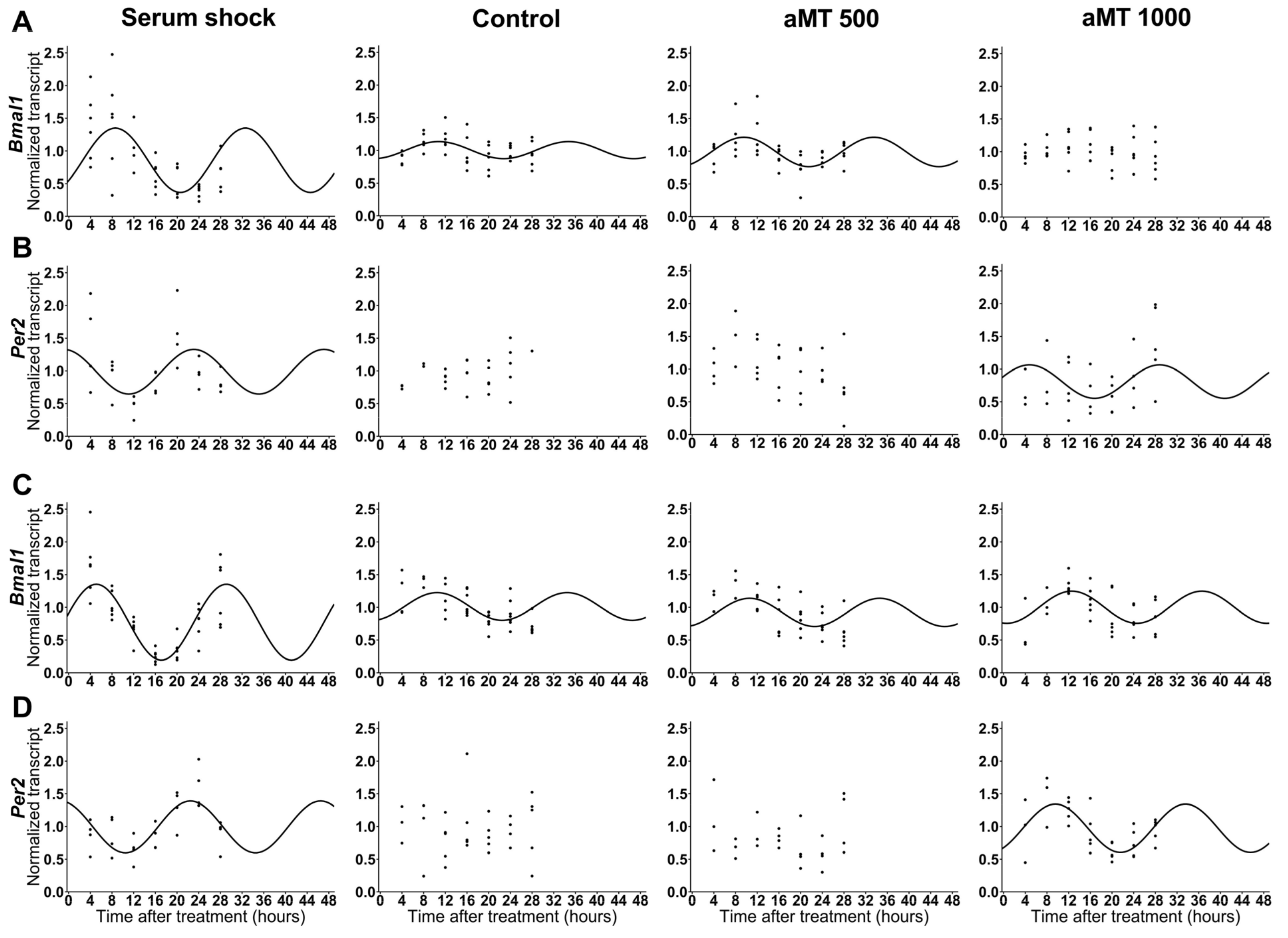
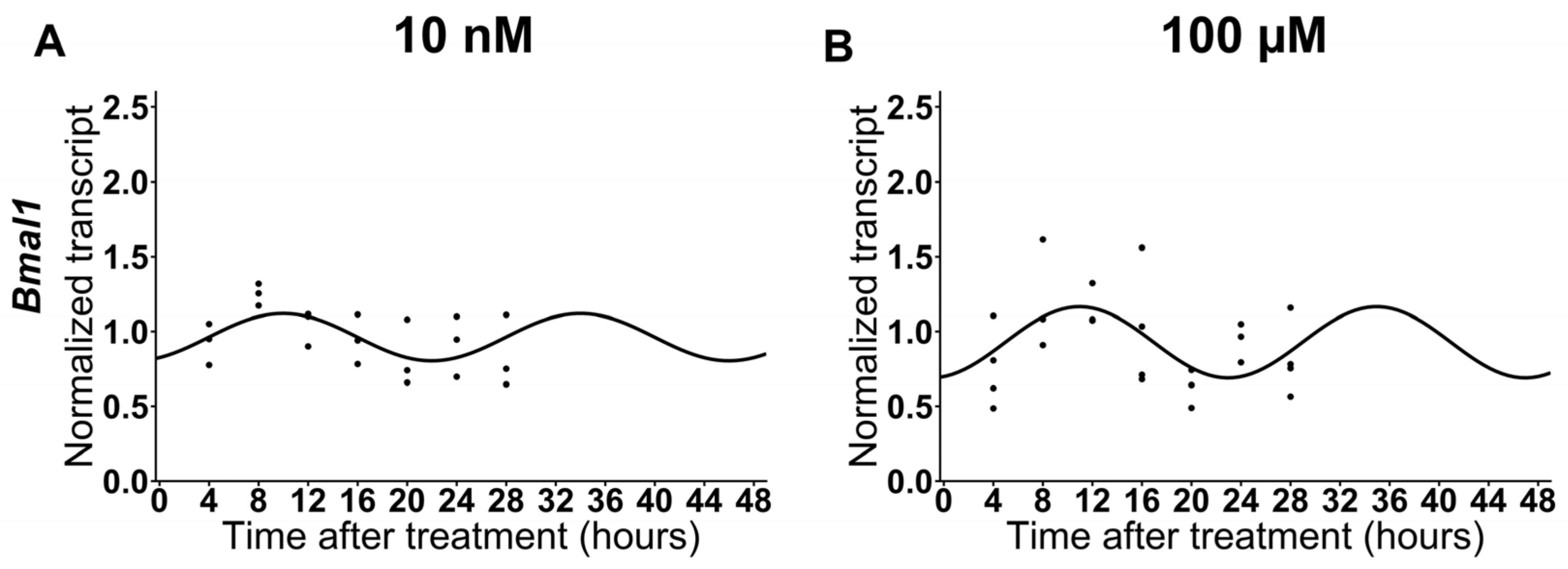

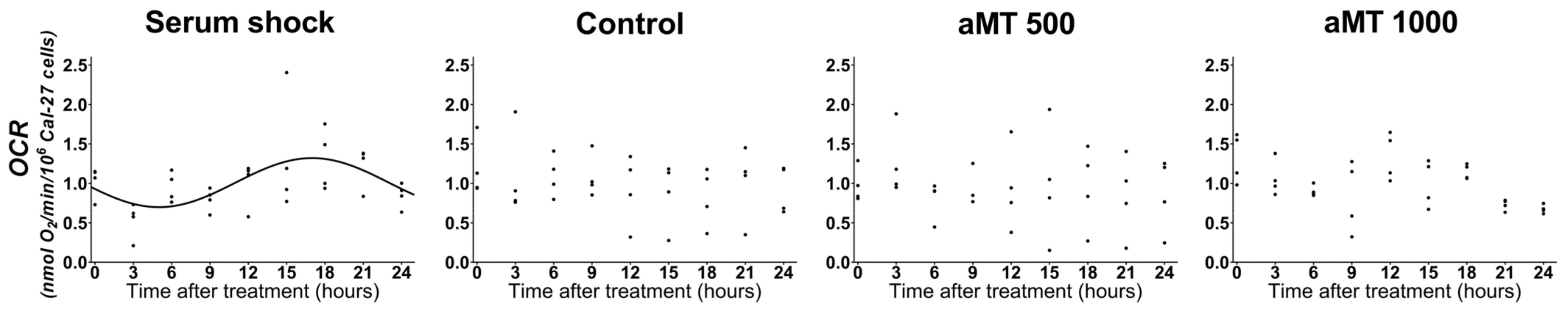

| Cell Line | Gene | Treatment | Presence of Rhythmicity (p-Value) | Acrophase | Amplitude |
|---|---|---|---|---|---|
| Cal-27 | Bmal1 | Serum shock | <0.001 | 8.570589 | 0.492039 |
| Control | <0.01 | 10.76038 | 0.13079 | ||
| aMT 500 μM | <0.001 | 9.514851 | 0.225551 | ||
| aMT 1000 μM | Ns | - | - | ||
| Per2 | Serum shock | <0.01 | 23.05335 | 0.341575 | |
| Control | Ns | - | - | ||
| aMT 500 μM | Ns | - | - | ||
| aMT 1000 μM | <0.05 | 4.777426 | 0.256121 | ||
| SCC9 | Bmal1 | Serum shock | <0.001 | 5.105071 | 0.580572 |
| Control | <0.001 | 10.4789 | 0.211801 | ||
| aMT 500 μM | <0.01 | 10.58747 | 0.215423 | ||
| aMT 1000 μM | <0.001 | 12.57654 | 0.245783 | ||
| Per2 | Serum shock | <0.001 | 22.4778 | 0.396895 | |
| Control | Ns | - | - | ||
| aMT 500 μM | Ns | - | - | ||
| aMT 1000 μM | <0.001 | 9.562281 | 0.369996 |
| Cell Line | Gene | Comparison | p-Value for Acrophase Difference | p-Value for Amplitude Difference |
|---|---|---|---|---|
| Cal-27 | Bmal1 | Serum shock vs. Control | ns (0.328288) | ** (0.002598) |
| Serum shock vs. aMT 500 μM | ns (0.506968) | * (0.029825) | ||
| Control vs. aMT 500 μM | ns (0.407893) | ns (0.182816) | ||
| Per2 | Serum shock vs. aMT 1000 μM | ns (0.012848) | ns (0.613012) | |
| SCC9 | Bmal1 | Serum shock vs. Control | *** (2.95 × 10−5) | *** (0.000234) |
| Serum shock vs. aMT 500 μM | *** (7.57 × 10−5) | *** (0.000461) | ||
| Serum shock vs. aMT 1000 μM | *** (3.8 × 10−8) | *** (0.00063) | ||
| Control vs. aMT 500 μM | ns (0.940019) | ns (0.968606) | ||
| Control vs. aMT 1000 μM | ns (0.125217) | ns (0.693895) | ||
| aMT 500 μM vs. aMT 1000 μM | ns (0.176089) | ns (0.738686) | ||
| Per2 | Serum shock vs. aMT 1000 μM | ns (1.25 × 10−15) | ns (0.653382) |
| Cell Line | Gene | Treatment | Presence of Rhythmicity (p-Value) | Acrophase | Amplitude |
|---|---|---|---|---|---|
| Cal-27 | Sirt1 | Serum shock | <0.05 | 8.240747 | 0.18581 |
| Control | Ns | - | - | ||
| aMT 500 μM | <0.01 | 10.27415 | 0.208889 | ||
| aMT 1000 μM | <0.01 | 12.19064 | 0.193523 | ||
| SCC9 | Sirt1 | Serum shock | <0.01 | 7.629646 | 0.193267 |
| Control | Ns | - | - | ||
| aMT 500 μM | <0.05 | 11.1633 | 0.154587 | ||
| aMT 1000 μM | <0.05 | 9.559829 | 0.174447 |
| Cell Line | Gene | Comparison | p-Value for Acrophase Difference | p-Value for Amplitude Difference |
|---|---|---|---|---|
| Cal-27 | Sirt1 | Serum shock vs. aMT 500 μM | ns (0.303475) | ns (0.832268) |
| Serum shock vs. aMT 1000 μM | ns (0.073515) | ns (0.943527) | ||
| aMT 500 μM vs. aMT 1000 μM | ns (0.271717) | ns (0.874045) | ||
| SCC9 | Sirt1 | Serum shock vs. aMT 500 μM | ns (0.087927) | ns (0.695747) |
| Serum shock vs. aMT 1000 μM | ns (0.290038) | ns (0.842563) | ||
| aMT 500 μM vs. aMT 1000 μM | ns (0.449046) | ns (0.844595) |
| Cell Line | Treatment | Presence of Rhythmicity (p-Value) | Acrophase | Amplitude |
|---|---|---|---|---|
| Cal-27 | Serum shock | <0.001 | 17.00138 | 0.311756 |
| Control | Ns | - | - | |
| aMT 500 μM | Ns | - | - | |
| aMT 1000 μM | Ns | - | - | |
| Serum shock after control | <0.05 | 14.65534 | 0.143189 | |
| Serum shock after aMT 1000 μM | <0.01 | 15.15964 | 0.225529 |
| Cell Line | Comparison | p-Value for Acrophase Difference | p-Value for Amplitude Difference |
|---|---|---|---|
| Cal-27 | Serum Shock vs. Serum shock after vehicle | ns (0.391999) | Ns (0.14612) |
| Serum shock vs. Serum shock after 1000 μM aMT | ns (0.293366) | Ns (0.462536) |
| Gene | Gene Description | Forward Primer | Reverse Primer |
|---|---|---|---|
| Bmal1 | Brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1 | ATCCTCAACTACAGCCAGAATG | TCGTGCTCCAGAACATAATCG |
| Per2 | Period circadian clock 2 | CCCTTCCGCATGACGCCCTACCTG | GACCGCCCTTTCATCCACATCCTG |
| Sirt1 | Sirtuin 1 | ACAGGTTGCGGGAATCCAAA | GTTCATCAGCTGGGCACCTA |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GTACTACACTGAATTCACCCCCACTG | TGCGGCATCTTCAAACCTCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Santana, C.; López-Rodríguez, A.; Martinez-Ruiz, L.; Florido, J.; Cela, O.; Capitanio, N.; Ramírez-Casas, Y.; Acuña-Castroviejo, D.; Escames, G. The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment. Int. J. Mol. Sci. 2023, 24, 15030. https://doi.org/10.3390/ijms241915030
Rodríguez-Santana C, López-Rodríguez A, Martinez-Ruiz L, Florido J, Cela O, Capitanio N, Ramírez-Casas Y, Acuña-Castroviejo D, Escames G. The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment. International Journal of Molecular Sciences. 2023; 24(19):15030. https://doi.org/10.3390/ijms241915030
Chicago/Turabian StyleRodríguez-Santana, César, Alba López-Rodríguez, Laura Martinez-Ruiz, Javier Florido, Olga Cela, Nazzareno Capitanio, Yolanda Ramírez-Casas, Darío Acuña-Castroviejo, and Germaine Escames. 2023. "The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment" International Journal of Molecular Sciences 24, no. 19: 15030. https://doi.org/10.3390/ijms241915030
APA StyleRodríguez-Santana, C., López-Rodríguez, A., Martinez-Ruiz, L., Florido, J., Cela, O., Capitanio, N., Ramírez-Casas, Y., Acuña-Castroviejo, D., & Escames, G. (2023). The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment. International Journal of Molecular Sciences, 24(19), 15030. https://doi.org/10.3390/ijms241915030









