Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
| Samples | Dataset (Cases) | Deliverables | Reference |
|---|---|---|---|
| OSCC | H&E-stained whole-slide images (training set n = 20, validation set n = 60) | Simultaneous segmentation of microvessels and nerves | Neural Comput &Applic 32, 9915–9928 (2020) [16] |
| HNSCC | H&E-stained whole-slide images (n = 334) | Segmentation of nerves and PNI | PMID: 36496513 [17] |
| OSCC | H&E-stained whole-slide images (training set = 80, validation set = 10) | PNI classifier | PMID: 36353548 [18] |
| HNSCC | RNA-seq and clinical data (TCGA. n = 351), scRNA-seq (GSE103322, n = 18) | PNI-associated coexpression module with 12 hub genes | PMID: 31214495 [14] |
| HNSCC | Multiomics and clinical data (TCGA, n = 361) | PNI-related gene expression profile (263 genes) | PMID: 30409320 [13] |
| OSCC | H&E- an ICH- stained whole-slide images (n = 142), NanoString Spatial Profiling | Spatial and transcriptomic analysis | PMID: 35819260 [15] |
2. Results
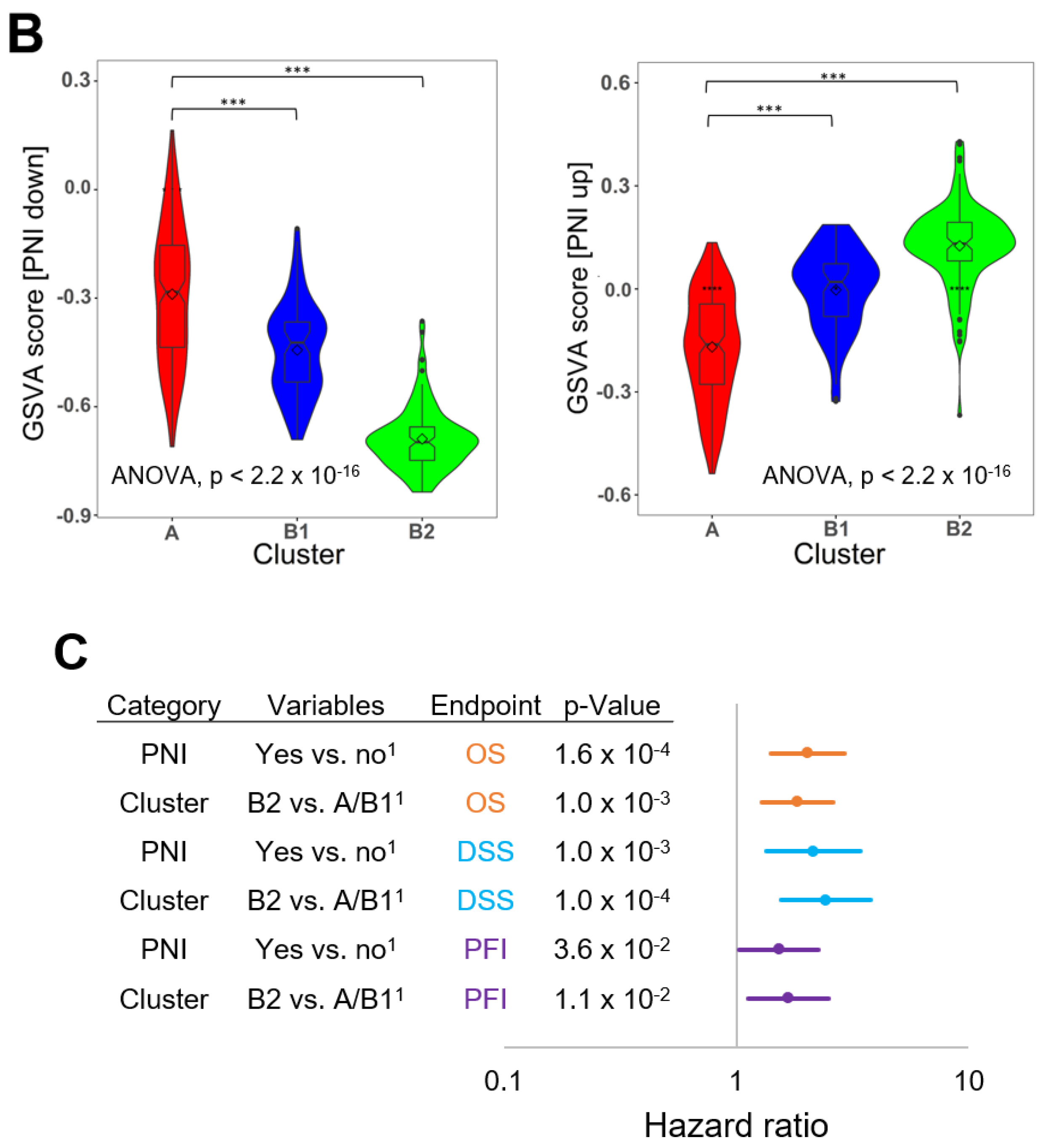
2.1. PNI as an Independent Prognostic Factor
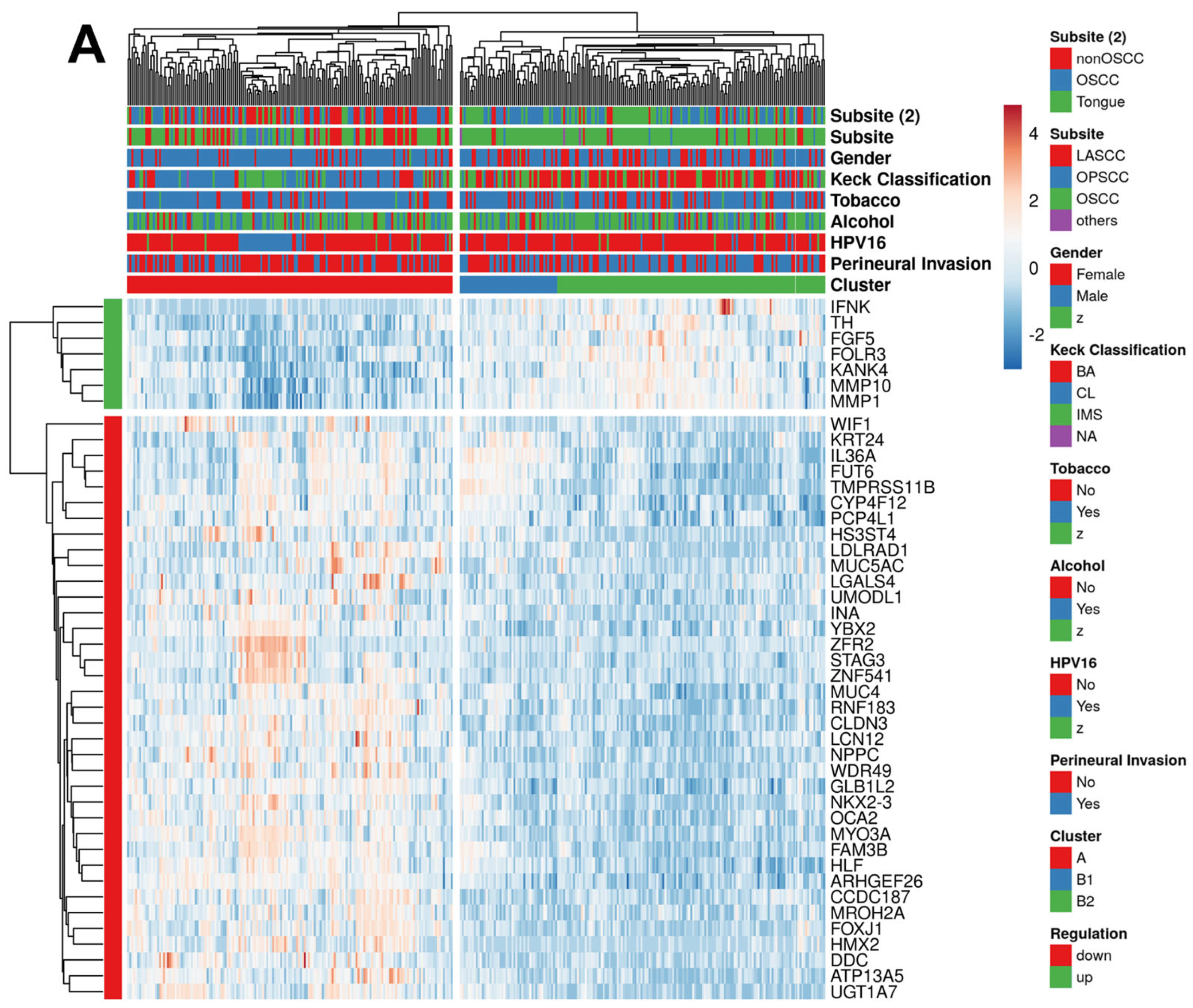
2.2. PNI-Related Gene Expression Signature
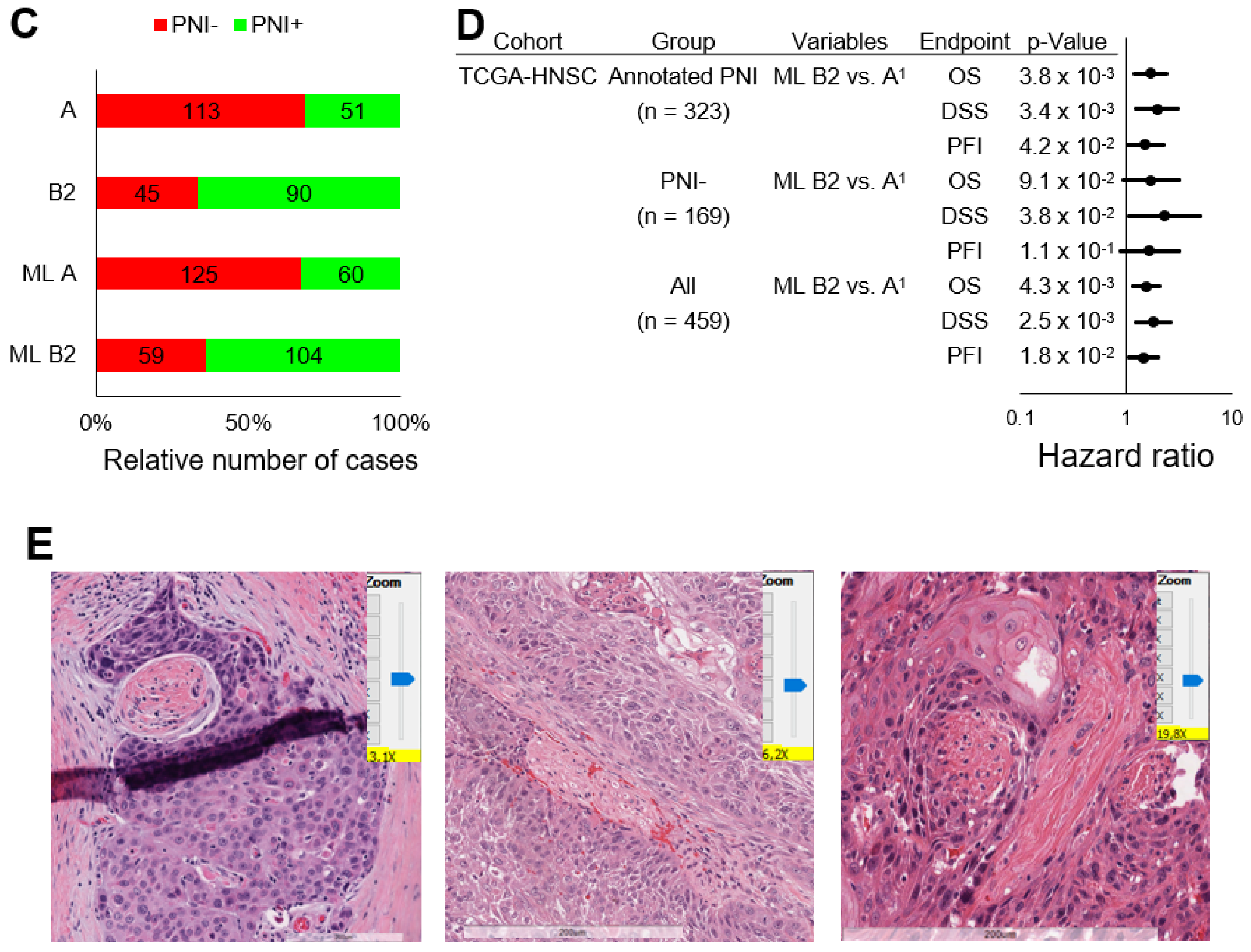
2.3. Validation in TCGA-HNSC without Annotated PNI Status and Independent HNSCC Cohorts
2.4. Single-Cell RNA-Sequencing Analysis of the PNI-Related 44-Gene Signature
2.5. Establishment of a PNI-Related Machine Learning Model for Occult PNI
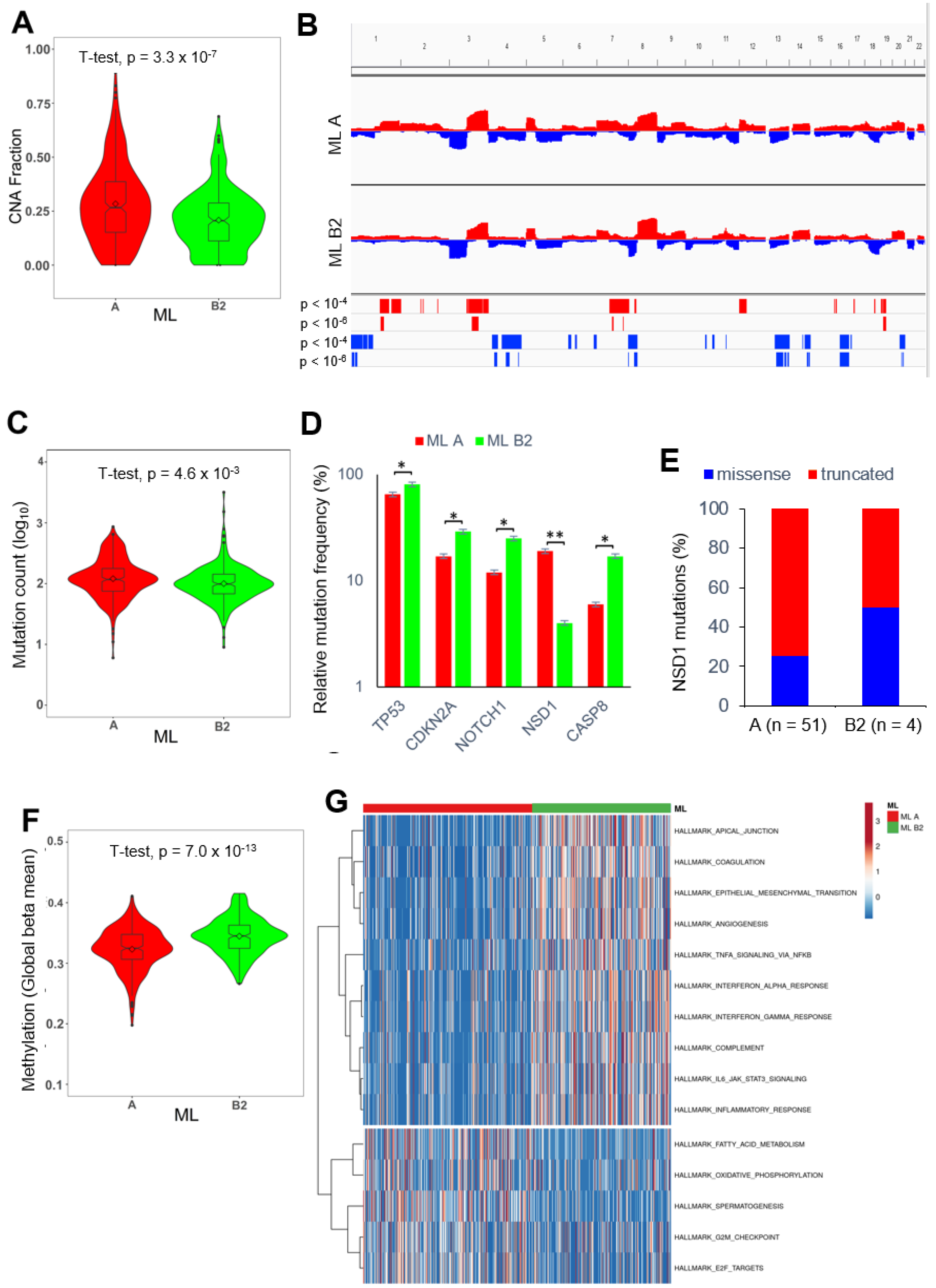
2.6. PNI-Related Alterations in the Mutational Landscape
2.7. PNI-Related Alterations in the Immune Landscape
2.8. PNI-Related Alterations in Gene Regulatory Networks and Pathway Activities
3. Discussion
4. Materials and Methods
4.1. Data Collection and Key Resources
4.2. Survival Analysis
4.3. Differential Gene Expression Analysis
4.4. Unsupervised Hierarchical Clustering
4.5. Single-Cell RNA-Sequencing Analysis
4.6. Machine Learning Models and Review of Digital Slides
4.7. Analysis of Multiomics Data, Epigenetic Alterations, and Immune Cell Deconvolution
4.8. Gene Set Variation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| CESC | Cervical squamous cell carcinoma |
| CNA | Copy number alteration |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| COAD | Adenocarcinoma of the colon |
| DEGs | Differentially expressed genes |
| DNA | Deoxyribonucleic acid |
| DSS | Disease-specific survival |
| EMT | Epithelial-to-mesenchymal transition |
| GSVA | Gene set variation analysis |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papilloma virus |
| HR | Hazard ratio |
| IFNK | Interferon kappa |
| LASCC | Laryngeal squamous cell carcinoma |
| LUAD | Adenocarcinoma of the lung |
| ML | Machine learning |
| MSigDB | Molecular Signatures Database |
| NSD1 | Nuclear receptor-binding SET domain protein 1 |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| PAAD | Adenocarcinoma of the pancreas |
| PDAC | Pancreatic ductal adenocarcinoma |
| PFI | Progression-free interval |
| PNI | Perineural invasion |
| RCT | Radiochemotherapy |
| RNA-seq | RNA-sequencing |
| ROC | Receiver-operating characteristic |
| SCC | Squamous cell carcinoma |
| scRNA-seq | Single-cell RNA sequencing |
| TCGA | The Cancer Genome Atlas |
| TCGA-HNSC | Head and neck squamous cell carcinoma of The Cancer Genome Atlas |
| UMAP | Uniform manifold approximation and projection |
References
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, R.; Wang, Y.; Yu, M. Perineural invasion as a prognostic factor in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Acta Oto-Laryngol. 2019, 139, 1038–1043. [Google Scholar] [CrossRef]
- Brandwein-Gensler, M.; Teixeira, M.S.; Lewis, C.M.M.; Lee, B.; Rolnitzky, L.M.; Hille, J.J.D.; Genden, E.; Urken, M.L.; Wang, B.Y. Oral Squamous Cell Carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am. J. Surg. Pathol. 2005, 29, 167–178. [Google Scholar] [CrossRef]
- Cha, D.E.; Yu, A.T.; Khajoueinejad, N.; Gleeson, E.; Shaltiel, T.; Berger, Y.; Macfie, R.; Golas, B.J.; Sarpel, U.; Labow, D.M.; et al. Perineural Invasion of Pancreatic Ductal Adenocarcinoma is Associated with Early Recurrence after Neoadjuvant Therapy Followed by Resection. World J. Surg. 2023, 1, 1–8. [Google Scholar] [CrossRef]
- Hosoya, K.; Wakahara, M.; Ikeda, K.; Umekita, Y. Perineural Invasion Predicts Unfavorable Prognosis in Patients With Invasive Breast Cancer. Cancer Diagn. Progn. 2023, 3, 208–214. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Misztal, C.I.; Green, C.; Mei, C.; Bhatia, R.; Torres, J.M.V.; Kamrava, B.; Moon, S.; Nicolli, E.; Weed, D.; Sargi, Z.; et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers 2021, 13, 6011. [Google Scholar] [CrossRef]
- Kurtz, K.A.; Hoffman, H.T.; Zimmerman, M.B.; Robinson, R.A. Perineural and Vascular Invasion in Oral Cavity Squamous Carcinoma: Increased Incidence on Re-review of Slides and by Using Immunohistochemical Enhancement. Arch. Pathol. Lab. Med. 2005, 129, 354–359. [Google Scholar] [CrossRef]
- Schmitd, L.; Scanlon, C.; D’silva, N. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750. [Google Scholar] [CrossRef]
- Elicin, O.; Giger, R. Comparison of Current Surgical and Non-Surgical Treatment Strategies for Early and Locally Advanced Stage Glottic Laryngeal Cancer and Their Outcome. Cancers 2020, 12, 732. [Google Scholar] [CrossRef]
- Jones, T.M.; De, M.; Foran, B.; Harrington, K.; Mortimore, S. Laryngeal cancer: United Kingdom National Multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S75–S82. [Google Scholar] [CrossRef]
- Lin, C.C.; Fedewa, S.A.; Prickett, K.K.; Higgins, K.A.; Chen, A.Y. Comparative effectiveness of surgical and nonsurgical therapy for advanced laryngeal cancer. Cancer 2016, 122, 2845–2856. [Google Scholar] [CrossRef]
- Saidak, Z.; Clatot, F.; Chatelain, D.; Galmiche, A. A gene expression profile associated with perineural invasion identifies a subset of HNSCC at risk of post-surgical recurrence. Oral Oncol. 2018, 86, 53–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Jin, R.; Fan, Y.; Li, T.; Shuai, Y.; Li, X.; Wang, X.; Luo, J. Integrating Clinical and Genetic Analysis of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 434. [Google Scholar] [CrossRef]
- Schmitd, L.B.; Perez-Pacheco, C.; Bellile, E.L.; Wu, W.; Casper, K.; Mierzwa, M.; Rozek, L.S.; Wolf, G.T.; Taylor, J.M.; D’Silva, N.J. Spatial and Transcriptomic Analysis of Perineural Invasion in Oral Cancer. Clin. Cancer Res. 2021, 28, 3557–3572. [Google Scholar] [CrossRef]
- Fraz, M.M.; Khurram, S.A.; Graham, S.; Shaban, M.; Hassan, M.; Loya, A.; Rajpoot, N.M. FABnet: Feature attention-based network for simultaneous segmentation of microvessels and nerves in routine histology images of oral cancer. Neural Comput. 2020, 32, 9915–9928. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Wang, C.; Yu, X.; Zhao, T.; Huang, C.; Gao, Y. Expectation-maximization algorithm leads to domain adaptation for a perineural invasion and nerve extraction task in whole slide digital pathology images. Med. Biol. Eng. Comput. 2023, 61, 457–473. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Yang, C.-H.; Lin, Y.-C.; Hsieh, Y.-H.; Chen, Y.-A.; Chang, M.D.-T.; Lin, Y.-Y.; Liao, C.-T. A domain knowledge enhanced yield based deep learning classifier identifies perineural invasion in oral cavity squamous cell carcinoma. Front. Oncol. 2022, 12, 951560. [Google Scholar] [CrossRef]
- Keck, M.K.; Zuo, Z.; Khattri, A.; Stricker, T.P.; Brown, C.D.; Imanguli, M.; Rieke, D.; Endhardt, K.; Fang, P.; Brägelmann, J.; et al. Integrative Analysis of Head and Neck Cancer Identifies Two Biologically Distinct HPV and Three Non-HPV Subtypes. Clin. Cancer Res. 2015, 21, 870–881. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef]
- Ghosh, D.; Cabrera, J. Enriched Random Forest for High Dimensional Genomic Data. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2817–2828. [Google Scholar] [CrossRef]
- Peri, S.; Izumchenko, E.; Schubert, A.D.; Slifker, M.J.; Ruth, K.; Serebriiskii, I.G.; Guo, T.; Burtness, B.A.; Mehra, R.; Ross, E.A.; et al. NSD1- and NSD2-damaging mutations define a subset of laryngeal tumors with favorable prognosis. Nat. Commun. 2017, 8, 1772. [Google Scholar] [CrossRef]
- Bui, N.; Huang, J.K.; Bojorquez-Gomez, A.; Licon, K.; Sanchez, K.S.; Tang, S.N.; Beckett, A.N.; Wang, T.; Zhang, W.; Shen, J.P.; et al. Disruption of NSD1 in Head and Neck Cancer Promotes Favorable Chemotherapeutic Responses Linked to Hypomethylation. Mol. Cancer Ther. 2018, 17, 1585–1594. [Google Scholar] [CrossRef]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve–Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Ittmann, M.; Li, R.; Powell, M.; Frolov, A.; Wheeler, T.M.; Thompson, T.C.; Rowley, D. Growth and Survival Mechanisms Associated with Perineural Invasion in Prostate Cancer. Cancer Res. 2004, 64, 6082–6090. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-Related Axonogenesis and Neurogenesis in Prostate Cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef]
- Silva, V.M.; Gomes, J.A.; Tenório, L.P.G.; Neta, G.C.D.O.; Paixão, K.D.C.; Duarte, A.K.F.; da Silva, G.C.B.; Ferreira, R.J.S.; Koike, B.D.V.; Marques, C.D.S.; et al. Schwann cell reprogramming and lung cancer progression: A meta-analysis of transcriptome data. Oncotarget 2019, 10, 7288–7307. [Google Scholar] [CrossRef]
- Lee, T.; Chiu, P.; Li, W.; Yang, M.; Wei, P.; Chu, P.; Wang, Y.; Tai, S. Nerve-tumour interaction enhances the aggressiveness of oral squamous cell carcinoma. Clin. Otolaryngol. 2019, 44, 1087–1095. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.V.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Cracchiolo, J.R.; Xu, B.; Migliacci, J.C.; Katabi, N.; Pfister, D.G.; Lee, N.Y.; Patel, S.G.; Ghossein, R.A.; Wong, R.J. Patterns of recurrence in oral tongue cancer with perineural invasion. Head Neck 2018, 40, 1287–1295. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Grzanka, D.; Gagat, M. The Role of Actin Dynamics and Actin-Binding Proteins Expression in Epithelial-to-Mesenchymal Transition and Its Association with Cancer Progression and Evaluation of Possible Therapeutic Targets. BioMed Res. Int. 2018, 2018, 4578373. [Google Scholar] [CrossRef]
- Jia, X.; Lu, M.; Rui, C.; Xiao, Y. Consensus-Expressed CXCL8 and MMP9 Identified by Meta-Analyzed Perineural Invasion Gene Signature in Gastric Cancer Microarray Data. Front. Genet. 2019, 10, 851. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Yan, Q.-L.; Wang, J.-W.; Chen, Y.; Ye, Q.-H.; Wang, Z.-J.; Huang, T. The Key Genes for Perineural Invasion in Pancreatic Ductal Adenocarcinoma Identified With Monte-Carlo Feature Selection Method. Front. Genet. 2020, 11, 554502. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kang, Y.; Im, N.; Kim, B.; Kwon, T.; Jung, K.; Baek, S. Actin-Associated Gene Expression is Associated with Early Regional Metastasis of Tongue Cancer. Laryngoscope 2021, 131, 813–819. [Google Scholar] [CrossRef]
- Koo, K.; Mouradov, D.; Angel, C.M.; Iseli, T.A.; Wiesenfeld, D.; McCullough, M.J.; Burgess, A.W.; Sieber, O.M. Genomic Signature of Oral Squamous Cell Carcinomas from Non-Smoking Non-Drinking Patients. Cancers 2021, 13, 1029. [Google Scholar] [CrossRef]
- Verdoodt, B.; Sommerer, F.; Palisaar, R.-J.; Noldus, J.; Vogt, M.; Nambiar, S.; Tannapfel, A.; Mirmohammadsadegh, A.; Neid, M. Inverse association of p16INK4a and p14ARF methylation of the CDKN2a locus in different Gleason scores of prostate cancer. Prostate Cancer Prostatic Dis. 2011, 14, 295–301. [Google Scholar] [CrossRef]
- Čelešnik, H.; Büdefeld, T.; Čizmarević, B.; Švagan, M.; Potočnik, U. MIR137/MIR2682 locus is associated with perineural invasiveness in head and neck cancer. J. Oral Pathol. Med. 2021, 50, 874–881. [Google Scholar] [CrossRef]
- Lounglaithong, K.; Bychkov, A.; Sampatanukul, P. Aberrant promoter methylation of the PAQR3 gene is associated with prostate cancer. Pathol. Res. Pract. 2018, 214, 126–129. [Google Scholar] [CrossRef]
- Bidar, N.; Rezaei, T.; Amini, M.; Jebelli, A.; Mokhtarzadeh, A.; Baradaran, B. ZNF677 downregulation by promoter hypermethylation as a driver event through gastric tumorigenesis. Exp. Mol. Pathol. 2021, 121, 104663. [Google Scholar] [CrossRef]
- Pugongchai, A.; Bychkov, A.; Sampatanukul, P. Promoter hypermethylation of SOX11 correlates with adverse clinicopathological features of human prostate cancer. Int. J. Exp. Pathol. 2017, 98, 341–346. [Google Scholar] [CrossRef]
- Lukosiute-Urboniene, A.; Mazeike, A.; Kazokaite, M.; Silkuniene, G.; Silkunas, M.; Barauskas, V.; Barauskas, G.; Gulbinas, A.; Dauksa, A.; Dambrauskas, Z. Epigenetic Regulation of APAF-1 Through DNA Methylation in Pancreatic Cancer. Anticancer Res. 2020, 40, 3765–3779. [Google Scholar] [CrossRef]
- Islam, F.; Gopalan, V.; Pillai, S.; Lu, C.-T.; Kasem, K.; Lam, A.K.-Y. Promoter hypermethylation inactivate tumor suppressor FAM134B and is associated with poor prognosis in colorectal cancer. Genes Chromosom. Cancer 2018, 57, 240–251. [Google Scholar] [CrossRef]
- Park, J.S.; Park, Y.N.; Lee, K.Y.; Kim, J.K.; Yoon, D.S. P16 Hypermethylation Predicts Surgical Outcome Following Curative Resection of Mid/Distal Bile Duct Cancer. Ann. Surg. Oncol. 2013, 20, 2511–2517. [Google Scholar] [CrossRef]
- Hurník, P.; Chyra, Z.; Ševčíková, T.; Štembírek, J.; Trtková, K.S.; Gaykalova, D.A.; Buchtová, M.; Hrubá, E. Epigenetic Regulations of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. Front. Genet. 2022, 13, 848557. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Y.; Jiang, W. Important Cells and Factors from Tumor Microenvironment Participated in Perineural Invasion. Cancers 2023, 15, 1360. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Guo, Y.; Zhang, Z.; Lian, G.; Chen, Y.; Li, J.; Su, Y.; Li, J.; Yang, K.; et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics 2018, 8, 3074–3086. [Google Scholar] [CrossRef]
- Wang, C.; Ma, H.-X.; Jin, M.-S.; Zou, Y.-B.; Teng, Y.-L.; Tian, Z.; Wang, H.-Y.; Wang, Y.-P.; Duan, X.-M. Association of Matrix Metalloproteinase (MMP)-2 and -9 Expression with Extra-gastrointestinal Stromal Tumor Metastasis. Asian Pac. J. Cancer Prev. 2014, 15, 4187–4192. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef]
- Kwon, M.J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front. Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp: Armonk, NY, USA, 2019.
- Liu, S.; Wang, Z.; Zhu, R.; Wang, F.; Cheng, Y.; Liu, Y. Three Differential Expression Analysis Methods for RNA Sequencing: Limma, EdgeR, DESeq2. J. Vis. Exp. 2021, 175, e62528. [Google Scholar] [CrossRef]
- McDermaid, A.; Monier, B.; Zhao, J.; Liu, B.; Ma, Q. Interpretation of differential gene expression results of RNA-seq data: Review and integration. Brief. Bioinform. 2018, 20, 2044–2054. [Google Scholar] [CrossRef]
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 29 June 2020).
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Sun, D.; Wang, J.; Han, Y.; Dong, X.; Ge, J.; Zheng, R.; Shi, X.; Wang, B.; Li, Z.; Ren, P.; et al. TISCH: A comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 2021, 49, D1420–D1430. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef]
- Larsen, S.J.; do Canto, L.M.; Rogatto, S.R.; Baumbach, J. CoNVaQ: A web tool for copy number variation-based association studies. BMC Genom. 2018, 19, 369. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weusthof, C.; Burkart, S.; Semmelmayer, K.; Stögbauer, F.; Feng, B.; Khorani, K.; Bode, S.; Plinkert, P.; Plath, K.; Hess, J. Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 8938. https://doi.org/10.3390/ijms24108938
Weusthof C, Burkart S, Semmelmayer K, Stögbauer F, Feng B, Khorani K, Bode S, Plinkert P, Plath K, Hess J. Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(10):8938. https://doi.org/10.3390/ijms24108938
Chicago/Turabian StyleWeusthof, Christopher, Sebastian Burkart, Karl Semmelmayer, Fabian Stögbauer, Bohai Feng, Karam Khorani, Sebastian Bode, Peter Plinkert, Karim Plath, and Jochen Hess. 2023. "Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 24, no. 10: 8938. https://doi.org/10.3390/ijms24108938
APA StyleWeusthof, C., Burkart, S., Semmelmayer, K., Stögbauer, F., Feng, B., Khorani, K., Bode, S., Plinkert, P., Plath, K., & Hess, J. (2023). Establishment of a Machine Learning Model for the Risk Assessment of Perineural Invasion in Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences, 24(10), 8938. https://doi.org/10.3390/ijms24108938






