Effects of SKCPT on Osteoarthritis in Beagle Meniscectomy and Cranial Cruciate Ligament Transection Models
Abstract
1. Introduction
2. Results
2.1. The Significance of the Tablets Was Confirmed through Dissolution Test
2.2. Identification of Consistency for Comparison between Groups
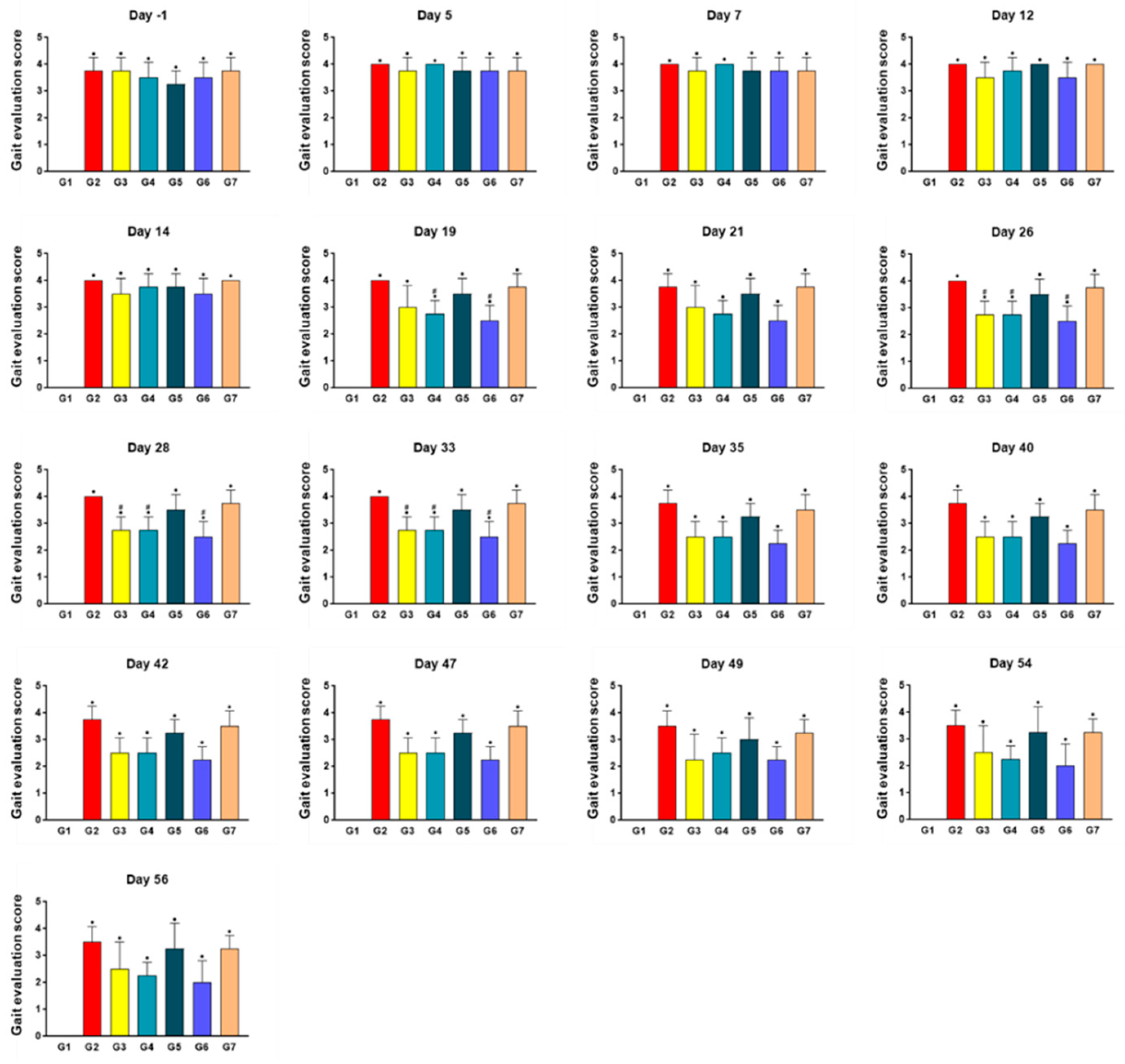
2.3. SKCPT Treatment Improved the Most Kinetics Assessment
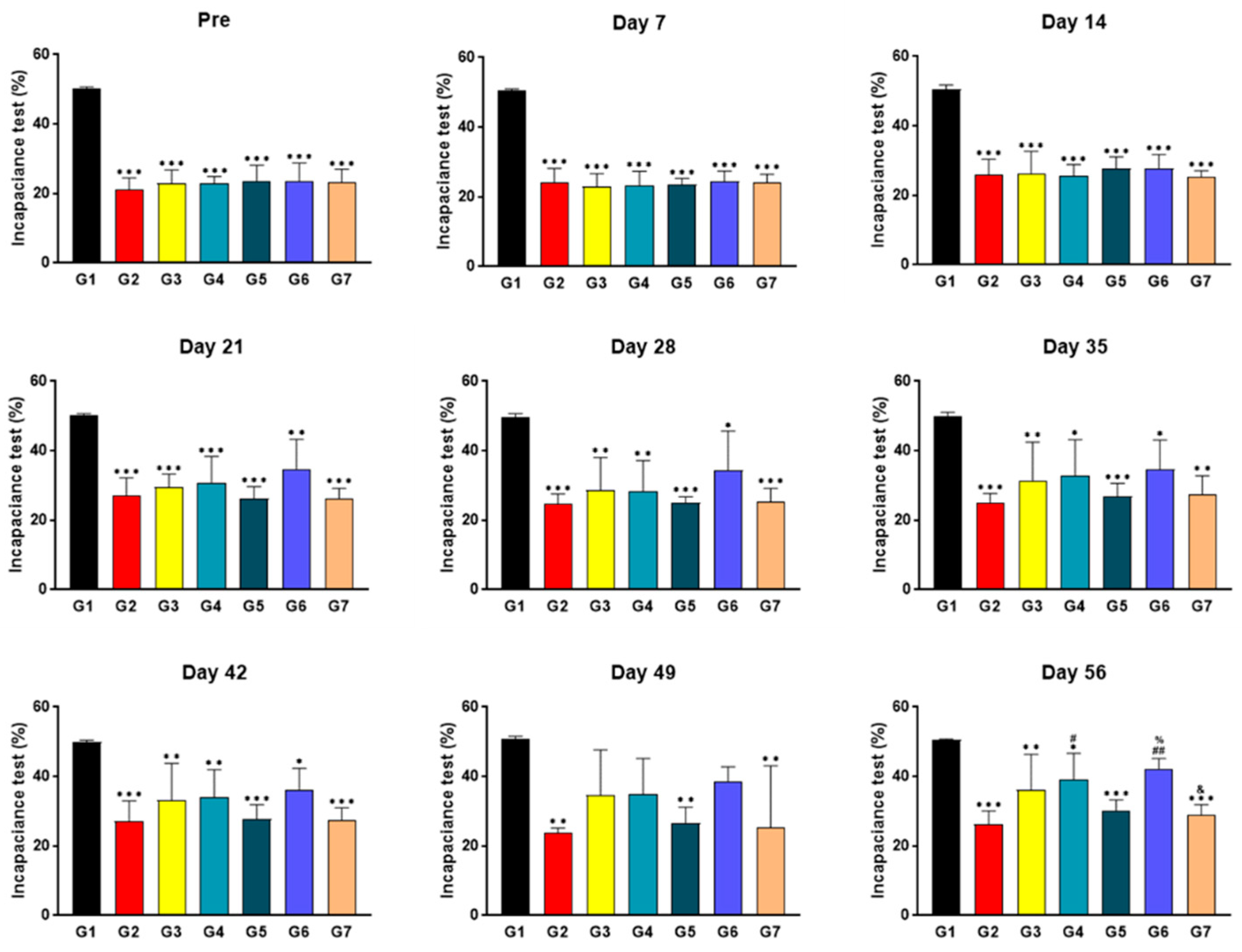
2.4. SKCPT Treatment Significantly Improved the Right Hind Limb Weight-Bearing Capacity
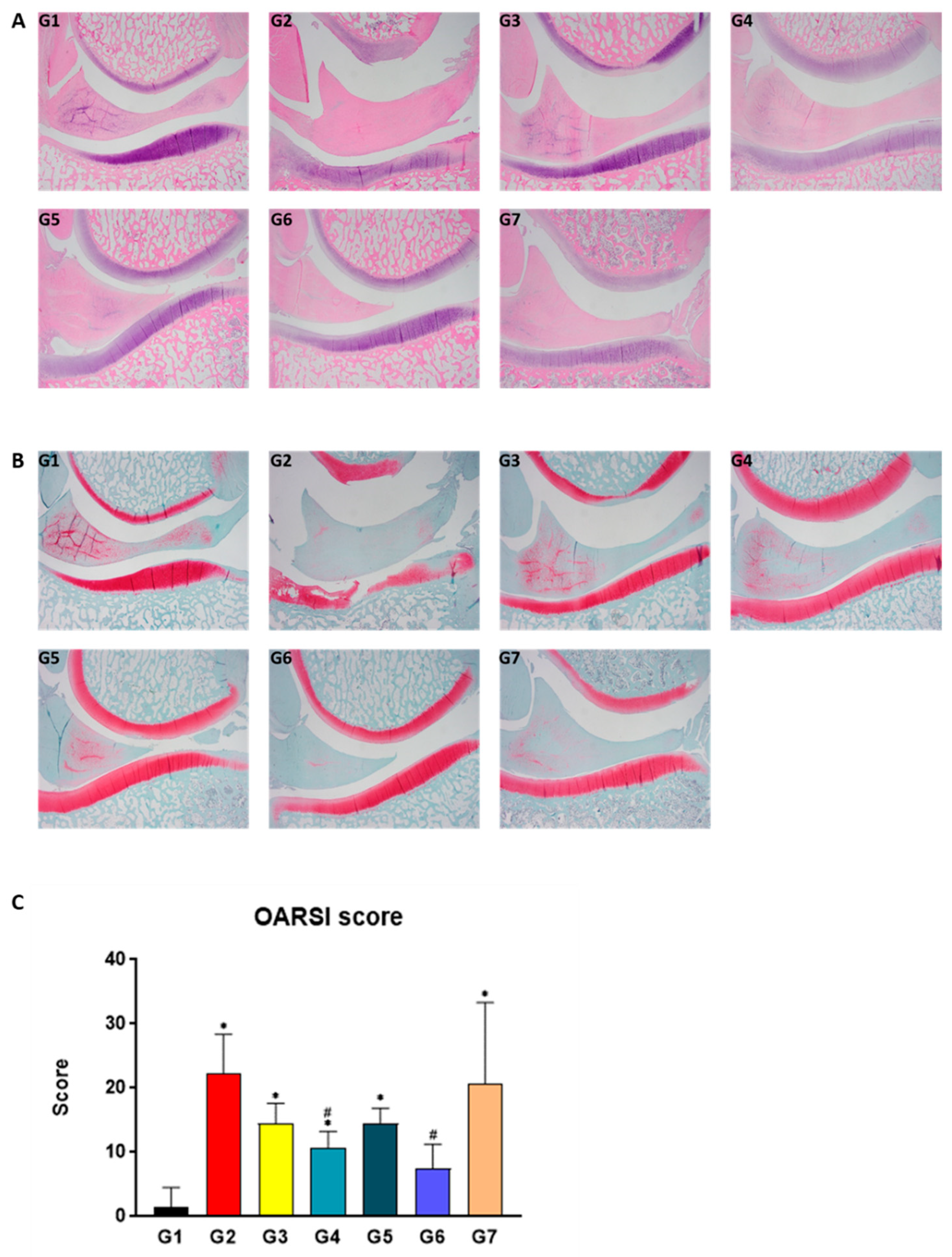
2.5. SKCPT Alleviated the Images of OA Severity and OARSI Scores
2.6. SKCPT Reduced the Expression of OA-Related Proteins
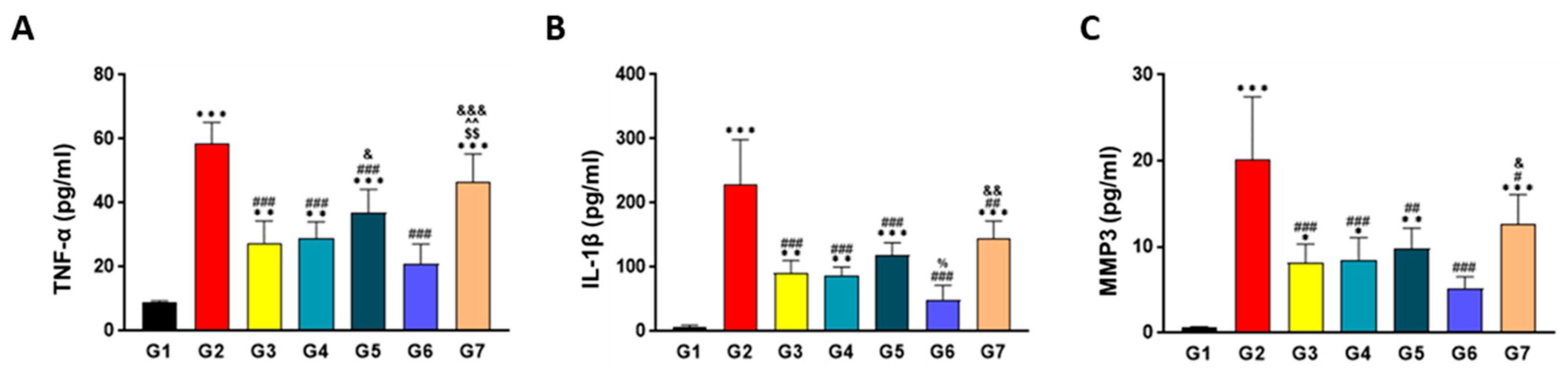
2.7. SKCPT Significantly Reduced the OA-Related Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Meniscectomy and Cranial Cruciate Ligament Resection (CCLx)
4.3. Sample Preparation and Evaluation of Drug Efficacy According to Dissolution Pattern
4.4. Sample Administration
4.5. Measurement of Stifle Circumference
4.6. Gait Evaluation
4.7. Incapacitance Test
4.8. Histological and Histopathological Examination
4.9. ELISA Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henrotin, Y. Osteoarthritis in year 2021: Biochemical markers. Osteoarthr. Cartil. 2022, 30, 237–248. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Van den Bosch, M.H.J. Osteoarthritis year in review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar]
- Maqbool, M.; Fekadu, G.; Jiang, X.; Bekele, F.; Tolossa, T.; Turi, E.; Fetensa, G.; Fanta, K. An up to date on clinical prospects and management of osteoarthritis. Ann. Med. Surg. 2021, 72, 103077. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, K.H.; Jung, K.W.; Han, C.K.; Kwak, W.J.; Cho, Y.B. Effects of SKI306X on arachidonate metabolism and other inflammatory mediators. Biol. Pharm. Bull. 2005, 28, 1615–1620. [Google Scholar] [CrossRef][Green Version]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. Jama 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Mao, L.; Wu, W.; Wang, M.; Guo, J.; Li, H.; Zhang, S.; Xu, J.; Zou, J. Targeted treatment for osteoarthritis: Drugs and delivery system. Drug Deliv. 2021, 28, 1861–1876. [Google Scholar] [CrossRef]
- Grosser, T.; Ricciotti, E.; FitzGerald, G.A. The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol. Sci. 2017, 38, 733–748. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.O.; Farquhar, C.; Proctor, M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD001751. [Google Scholar] [CrossRef]
- Smedslund, G.; Kjeken, I.; Musial, F.; Sexton, J.; Osteras, N. Interventions for osteoarthritis pain: A systematic review with network meta-analysis of existing Cochrane reviews. Osteoarthr. Cartil. Open 2022, 4, 100242. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, T.H.; Sung, Y.K.; Choi, C.B.; Na, Y.I.; Yoo, H.; Jun, J.B. SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes. Korean J. Intern. Med. 2014, 29, 647–655. [Google Scholar] [CrossRef]
- Lee, S.W.; Chung, W.T.; Choi, S.M.; Kim, K.T.; Yoo, K.S.; Yoo, Y.H. Clematis mandshurica protected to apoptosis of rat chondrocytes. J. Ethnopharmacol. 2005, 101, 294–298. [Google Scholar] [CrossRef]
- Hartog, A.; Hougee, S.; Faber, J.; Sanders, A.; Zuurman, C.; Smit, H.F.; van der Kraan, P.M.; Hoijer, M.A.; Garssen, J. The multicomponent phytopharmaceutical SKI306X inhibits in vitro cartilage degradation and the production of inflammatory mediators. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Rhee, H.I.; Jung, I.H.; Ryu, K.; Jung, K.; Han, C.K.; Kwak, W.J.; Cho, Y.B.; Joo, H.J. SKI306X, an oriental herbal mixture, suppresses gastric leukotriene B4 synthesis without causing mucosal injury and the diclofenac-induced gastric lesions. Life Sci. 2005, 77, 1181–1193. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, K.H.; Jung, K.W.; Han, C.K.; Kwak, W.J.; Cho, Y.B. SKI306X suppresses cartilage destruction and inhibits the production of matrix metalloproteinase in rabbit joint cartilage explant culture. J. Pharmacol. Sci. 2005, 98, 298–306. [Google Scholar] [CrossRef]
- Jung, Y.B.; Roh, K.J.; Jung, J.A.; Jung, K.; Yoo, H.; Cho, Y.B.; Kwak, W.J.; Kim, D.K.; Kim, K.H.; Han, C.K. Effect of SKI 306X, a new herbal anti-arthritic agent, in patients with osteoarthritis of the knee: A double-blind placebo controlled study. Am. J. Chin. Med. 2001, 29, 485–491. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, J.H.; Kim, D.Y.; Yoon, J.H.; Youn, H.Y.; Yi, J.B.; Rhee, H.I.; Ryu, K.H.; Jung, K.; Han, C.K.; et al. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthr. Cartil. 2002, 10, 471–478. [Google Scholar] [CrossRef]
- Lee, M.I.; Kim, J.H.; Kwak, H.H.; Woo, H.M.; Han, J.H.; Yayon, A.; Jung, Y.C.; Cho, J.M.; Kang, B.J. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J. Orthop. Surg. Res. 2019, 14, 314. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Ryu, J.; Brittberg, M.; Nam, B.; Chae, J.; Kim, M.; Colon Iban, Y.; Magneli, M.; Takahashi, E.; Khurana, B.; Bragdon, C.R. Evaluation of Three-Dimensional Bioprinted Human Cartilage Powder Combined with Micronized Subcutaneous Adipose Tissues for the Repair of Osteochondral Defects in Beagle Dogs. Int. J. Mol. Sci. 2022, 23, 2743. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K., Jr.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Cook, J.L.; Kuroki, K.; Visco, D.; Pelletier, J.P.; Schulz, L.; Lafeber, F.P. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S66–S79. [Google Scholar] [CrossRef]
- Sabanci, S.S.; Ocal, M.K. Comparison of goniometric measurements of the stifle joint in seven breeds of normal dogs. Vet. Comp. Orthop. Traumatol. 2016, 29, 214–219. [Google Scholar]
- Mazdarani, P.; Pedram, M.S.; Miles, J.E. Effect of center of rotation of angulation-based leveling osteotomy on ex vivo stifle joint stability following cranial cruciate ligament transection and medial meniscal release with and without a hamstring load. Vet. Surg. 2022, 51, 940–951. [Google Scholar] [CrossRef]
- Jeong, J.; Jeong, S.M.; Kim, S.E.; Lewis, D.D.; Lee, H. Subsequent meniscal tears following tibial tuberosity advancement and tibial plateau leveling osteotomy in dogs with cranial cruciate ligament deficiency: An in vivo experimental study. Vet. Surg. 2021, 50, 966–974. [Google Scholar] [CrossRef]
- Luo, H.; Kong, W.; Hu, Y.; Chen, P.; Wu, X.; Wan, L.; Yang, M. Quality evaluation of Salvia miltiorrhiza Bge. by ultra high performance liquid chromatography with photodiode array detection and chemical fingerprinting coupled with chemometric analysis. J. Sep. Sci. 2015, 38, 1544–1551. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Luo, C.; Zhan, Y.; Zhong, B. Design, cyclization, and optimization of MMP13-TIMP1 interaction-derived self-inhibitory peptides against chondrocyte senescence in osteoarthritis. Int. J. Biol. Macromol. 2019, 121, 921–929. [Google Scholar] [CrossRef]
- Salerno, A.; Brady, K.; Rikkers, M.; Li, C.; Caamano-Gutierrez, E.; Falciani, F.; Blom, A.W.; Whitehouse, M.R.; Hollander, A.P. MMP13 and TIMP1 are functional markers for two different potential modes of action by mesenchymal stem/stromal cells when treating osteoarthritis. Stem Cells 2020, 38, 1438–1453. [Google Scholar] [CrossRef]
- Bakilan, F.; Armagan, O.; Ozgen, M.; Tascioglu, F.; Bolluk, O.; Alatas, O. Effects of Native Type II Collagen Treatment on Knee Osteoarthritis: A Randomized Controlled Trial. Eurasian J. Med. 2016, 48, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.; Dhollander, A.; Malone, A.; Price, J.; Helliwell, J. Pharmacokinetic Profile of Intra-articular Fluticasone Propionate Microparticles in Beagle Dog Knees. Cartilage 2019, 10, 139–147. [Google Scholar] [CrossRef]
- Salman, A.; Shabana, A.I.; El-Ghazouly, D.E.; Maha, E. Protective effect of glucosamine and risedronate (alone or in combination) against osteoarthritic changes in rat experimental model of immobilized knee. Anat. Cell Biol. 2019, 52, 498–510. [Google Scholar] [CrossRef]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthr. Cartil. 2022, 30, 32–41. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1beta signaling in osteoarthritis—Chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Lung, Y.B.; Seong, S.C.; Lee, M.C.; Shin, Y.U.; Kim, D.H.; Kim, J.M.; Jung, Y.K.; Ahn, J.H.; Seo, J.G.; Park, Y.S.; et al. A four-week, randomized, double-blind trial of the efficacy and safety of SKI306X: A herbal anti-arthritic agent versus diclofenac in osteoarthritis of the knee. Am. J. Chin. Med. 2004, 32, 291–301. [Google Scholar]
- Huang, H.; Lou, Z.; Zheng, S.; Wu, J.; Yao, Q.; Chen, R.; Kou, L.; Chen, D. Intra-articular drug delivery systems for osteoarthritis therapy: Shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022, 29, 767–791. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Xiong, L.; Feng, W.; Williams, R.O., 3rd. Bioavailability Improvement of Carbamazepine via Oral Administration of Modified-Release Amorphous Solid Dispersions in Rats. Pharmaceutics 2020, 12, 1023. [Google Scholar] [CrossRef]
- Bruno, M.C.; Cristiano, M.C.; Celia, C.; d’Avanzo, N.; Mancuso, A.; Paolino, D.; Wolfram, J.; Fresta, M. Injectable Drug Delivery Systems for Osteoarthritis and Rheumatoid Arthritis. ACS Nano 2022, 16, 19665–19690. [Google Scholar] [CrossRef]



| G4 | G5 | G6 | G7 | |
|---|---|---|---|---|
| SKI306X | 200 | 300 | 300 | 300 |
| Hydrophobic collodial silica (Aerosil R972) | 10 | 15 | 20 | 20 |
| Corn starch | 50 | |||
| Povidon K30 | 5 | |||
| Ethylcellulose | 4 | 4 | ||
| Microcrystalline cellulose | 113 | 85 | 115 | 115 |
| Kollidon SR | 50 | |||
| Sodium starch glycolate | 25 | |||
| Crospovidone | 15 | |||
| Croscarmellose sodium | 25 | 10 | 10 | |
| Magnesium stearate | 2 | 5 | ||
| Sodium stearyl fumarate | 5 | 5 | ||
| Opadry | 30 | 27 | 29 | 30 |
| Total weight | 430 | 477 | 483 | 534 |
| ID | Sex | Number of Animals | Animal Number | Meniscectomy | Administrated Substances | Number of Administrations | Dosage (mg) | Dosage (Tablet) |
|---|---|---|---|---|---|---|---|---|
| G1 | M | 4 | 1–4 | N | ||||
| G2 | M | 4 | 5–8 | Y | Vehicle | b.i.d | N/A | 1T |
| G3 | M | 4 | 9–12 | Y | Celecoxib | q.d. | 200 | 1T |
| G4 | M | 4 | 13–16 | Y | IR | t.i.d | 200 | 1T |
| G5 | M | 4 | 17–20 | Y | IR | b.i.d | 300 | 1T |
| G6 | M | 4 | 21–24 | Y | MR | b.i.d | 300 | 1T |
| G7 | M | 4 | 25–28 | Y | CR-1 | b.i.d | 300 | 1T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Kang, M.; Jung, Y.-S.; Lee, Y.-J.; Choi, W.; Yoo, H.; Kim, J.; An, H.-J. Effects of SKCPT on Osteoarthritis in Beagle Meniscectomy and Cranial Cruciate Ligament Transection Models. Int. J. Mol. Sci. 2023, 24, 14972. https://doi.org/10.3390/ijms241914972
Kim H-M, Kang M, Jung Y-S, Lee Y-J, Choi W, Yoo H, Kim J, An H-J. Effects of SKCPT on Osteoarthritis in Beagle Meniscectomy and Cranial Cruciate Ligament Transection Models. International Journal of Molecular Sciences. 2023; 24(19):14972. https://doi.org/10.3390/ijms241914972
Chicago/Turabian StyleKim, Hye-Min, Minseok Kang, Yoon-Seok Jung, Yoon-Jung Lee, Wonjae Choi, Hunseung Yoo, JeongHoon Kim, and Hyo-Jin An. 2023. "Effects of SKCPT on Osteoarthritis in Beagle Meniscectomy and Cranial Cruciate Ligament Transection Models" International Journal of Molecular Sciences 24, no. 19: 14972. https://doi.org/10.3390/ijms241914972
APA StyleKim, H.-M., Kang, M., Jung, Y.-S., Lee, Y.-J., Choi, W., Yoo, H., Kim, J., & An, H.-J. (2023). Effects of SKCPT on Osteoarthritis in Beagle Meniscectomy and Cranial Cruciate Ligament Transection Models. International Journal of Molecular Sciences, 24(19), 14972. https://doi.org/10.3390/ijms241914972







