Abstract
FAD (fatty acid desaturase) and SAD (stearoyl-ACP desaturase) genes play key roles in the synthesis of fatty acids (FA) and determination of oil composition in flax (Linum usitatissimum L.). We searched for FAD and SAD genes in the most widely used flax genome of the variety CDC Bethune and three available long-read assembled flax genomes—YY5, 3896, and Atlant. We identified fifteen FAD2, six FAD3, and four SAD genes. Of all the identified genes, 24 were present in duplicated pairs. In most cases, two genes from a pair differed by a significant number of gene-specific SNPs (single nucleotide polymorphisms) or even InDels (insertions/deletions), except for FAD2a-1 and FAD2a-2, where only seven SNPs distinguished these genes. Errors were detected in the FAD2a-1, FAD2a-2, FAD3c-1, and FAD3d-2 sequences in the CDC Bethune genome assembly but not in the long-read genome assemblies. Expression analysis of the available transcriptomic data for different flax organs/tissues revealed that FAD2a-1, FAD2a-2, FAD3a, FAD3b, SAD3-1, and SAD3-2 were specifically expressed in embryos/seeds/capsules and could play a crucial role in the synthesis of FA in flax seeds. In contrast, FAD2b-1, FAD2b-2, SAD2-1, and SAD2-2 were highly expressed in all analyzed organs/tissues and could be involved in FA synthesis in whole flax plants. FAD2c-2, FAD2d-1, FAD3c-1, FAD3c-2, FAD3d-1, FAD3d-2, SAD3-1, and SAD3-2 showed differential expression under stress conditions—Fusarium oxysporum infection and drought. The obtained results are essential for research on molecular mechanisms of fatty acid synthesis, FAD and SAD editing, and marker-assisted and genomic selection for breeding flax varieties with a determined fatty acid composition of oil.
1. Introduction
Stearoyl-ACP (acyl carrier protein) desaturases (SADs) and fatty acid desaturases (FADs) regulate the biosynthesis of unsaturated fatty acids (FAs) in plants [1]. SADs introduce a double bond at the ∆9 position of hydrocarbon chains of C18-carbon saturated FAs (C18:0) and form monounsaturated FAs (C18:1) [2]. FAD2 enzymes introduce a double bond at the ∆12 position of monounsaturated FAs (C18:1) and, thus, convert them to polyunsaturated FAs (PUFAs) with two double bonds (C18:2) [3]. FAD3 enzymes create one more double bond at the ∆15 position of C18:2 FAs, yielding PUFAs with three double bonds (C18:3) [4].
Genes encoding SADs and FADs were identified and characterized in Arabidopsis and other plant species, including canola, olive, sunflower, sesame, corn, rice, wheat, soybean, cotton, chickpea, barrelclover, Perilla, Cyperus esculentus, peanut, chia, Brassica, oil tea, and poplar [3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. SAD and FAD genes in flax (Linum usitatissimum L.) have attracted special attention, as its seeds are among the richest plant sources of polyunsaturated linolenic acid (C18:3). Thus, they are traditionally used in food, pharmaceuticals, feed, and polymer production [31,32,33,34,35,36,37]. The following flax SAD and FAD genes were identified first: SAD genes (SAD1 and SAD2), which convert stearic acid (STE, C18:0) into oleic acid (OLE, C18:1) [38,39,40]; FAD2 genes (FAD2-2 and FAD2), which convert OLE (C18:1) into linoleic acid (LIO, C18:2) [41,42]; and FAD3 genes (FAD3A, FAD3B, and FAD3C), which convert LIO (C18:2) into α-linolenic acid (LIN, C18:3) [43,44].
The sequencing of the whole genome of the linseed variety CDC Bethune [45] allowed You et al. to identify new SAD and FAD genes. Thus, 25 genes were revealed, 24 of which were duplicated and formed 12 pairs: four SAD genes—SAD2-1 (SAD1 according to [40])/SAD2-2 (SAD2 according to [40]) and SAD3-1/SAD3-2; fifteen FAD2 genes—FAD2a-1/FAD2a-2 (FAD2 according to [42]), FAD2b-1/FAD2b-2 (FAD2-2 according to [41]), FAD2c-1/FAD2c-2, FAD2d-1/FAD2d-2, FAD2e-1/FAD2e-2, FAD2f-1/FAD2f-2, FAD2g-1/FAD2g-2, and FAD2h; and six FAD3 genes—FAD3a/FAD3b, FAD3c-1/FAD3c-2, and FAD3d-1/FAD3d-2. Errors were detected in the FAD2a-1 and FAD2a-2 gene sequences in the CDC Bethune genome assembly (https://phytozome-next.jgi.doe.gov/info/Lusitatissimum_v1_0, accessed on 1 August 2023) [46]. The inaccurate genome sequence complicated molecular genetic studies and editing of the FAD2 genes.
For SAD and FAD, You et al. [46] examined the large-scale generation of expressed sequence tags (ESTs) [47] and assessed expression in embryo, endosperm, seed coat, seedlings, stem, leaf, flower, and boll. Due to the high similarity between the gene sequences of duplicated pairs (FAD2a-1/FAD2a-2, FAD2b-1/FAD2b-2, etc.), expression was evaluated only for gene pairs instead of individual genes, except for FAD3a/FAD3b. Among all studied FAD2 genes, FAD2b-1/FAD2b-2 had the highest number of EST hits. Fewer ESTs were identified for FAD2a-1/FAD2a-2. The smallest number of ESTs were revealed for other FAD2 genes. FAD2b-1/FAD2b-2 and FAD2a-1/FAD2a-2 were predominantly expressed in mature embryos. Among the FAD3 genes, FAD3a/FAD3b had the highest number of EST hits, predominantly in mature embryos. For the SAD2-1/SAD2-2 and SAD3-1/SAD3-2 genes, some EST hits were revealed in different organs/tissues and at various developmental stages of flax plants. You et al. suggested that FAD2a-1/FAD2a-2, FAD2b-1/FAD2b-2, and FAD3a/FAD3b play a crucial role in the synthesis of linseed oil FAs [46]. The same role of these genes was also demonstrated in other studies [44,48,49,50].
In the work by Thambugala et al., SAD and FAD loci were compared between the high-LIN cultivar CDC Bethune and the low-LIN line M5791 using bacterial artificial chromosome clones. This allowed researchers to gain insight into the structural organization and diversity of these regions of the flax genome [51].
In the past few years, the genome assembly of CDC Bethune has been significantly improved with different techniques, including optical mapping [52]. Moreover, several genome assemblies of other flax genotypes were obtained from the third-generation sequencing and/or Hi-C data [53,54,55,56]. Long reads allow reliable assembly of highly homologous contiguous loci and enable the evaluation of the differences between pairs of duplicated genes [57,58]. The obtained flax genomes opened up new opportunities for the identification and comparison of the SAD, FAD2, and FAD3 genes of different flax genotypes. In addition to whole-genome sequencing, a significant number of transcriptomes have been obtained for flax plants in recent years [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. Such studies enabled extensive analysis of SAD, FAD2, and FAD3 expression in different organs/tissues and during various developmental stages and under various stress conditions. The obtained data are necessary to determine the role of SAD and FAD in FA synthesis and other processes in flax plants. Our work aimed at the genome-wide identification of FAD2, FAD3, and SAD genes in the most frequently used CDC Bethune genome and three flax genomes sequenced on third-generation platforms. We also analyzed the expression of these genes in different flax organs/tissues and under different stress conditions.
2. Results
2.1. Identification of FAD2 Genes in Flax Genome Assemblies
Using sequences of the FAD2 genes from the study of You et al. [46], we identified the location of the FAD2 genes in four analyzed genomes: fiber flax YY5 (Yiya No. 5; from PacBio HiFi and Hi-C data [54]); linseed line 3896 (from Nanopore data [56]); fiber flax Atlant (from Nanopore data [55]); and linseed CDC Bethune (from Illumina and optical mapping data [52]). Gene coordinates are presented in Table 1.

Table 1.
Coordinates of FAD2 genes in genomes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
Fifteen FAD2 genes were identified in each studied genome, except for that of cultivar Atlant. In this genotype, additional FAD2b-2* and FAD2c-1* genes were revealed. These copies were localized in one contig (JACHUY010001688.1). Several additional SNPs (single nucleotide polymorphisms) and short InDels (insertions/deletions) were revealed in FAD2c-1* compared to other FAD2c genes identified in the studied flax varieties. The Atlant genome assembly is less contiguous than two other long-read flax genome assemblies (N50 = 0.4 Mb for Atlant, N50 = 6.2 Mb for 3896, and N50 = 9.6 Mb for YY5) [54,55,56]. Therefore, the presence of two additional FAD2 genes is unlikely to be the Atlant genotype feature but could be associated with errors in the genome assembly. To avoid inaccuracies, we excluded the FAD2b-2* and FAD2c-1* genes of Atlant from further analysis.
In the genomes of the four studied flax varieties, the FAD2 genes were located similarly. FAD2a-1 and FAD2a-2 were on two different chromosomes/contigs, and the other thirteen FAD2 genes were organized into two clusters. FAD2b-1, FAD2c-1, FAD2d-1, FAD2e-1, FAD2f-1, FAD2g-1, and FAD2h were located on the same chromosome/contig in a 31-32-kb region, whereas FAD2b-2, FAD2c-2, FAD2d-2, FAD2e-2, FAD2f-2, and FAD2g-2 were in about a 28-kb region on another chromosome/contig. However, the analyzed flax varieties slightly differed in the distances between the clustered FAD2 genes (Table 2).

Table 2.
Clusters of FAD2 genes in genomes of flax varieties YY5, 3896, Atlant, and CDC Bethune: (1) from FAD2b-1 to FAD2h and (2) from FAD2b-2 to FAD2g-2.
2.2. Phylogenetic Analysis of FAD2 Genes of Different Flax Varieties
We analyzed sequences of 60 FAD2 genes that we identified in four flax varieties (15 genes per variety) (sequences are presented in Supplementary Table S1). A dendrogram for the FAD2 gene sequences is presented in Figure 1. The sequences of the same FAD2 genes of different varieties clustered with each other and formed subclusters. However, two genes were exceptions: FAD2a-1 and FAD2a-2 of CDC Bethune. For these genes, You et al. showed the presence of mis-assemblies in the previous version of the CDC Bethune genome (https://phytozome-next.jgi.doe.gov/info/Lusitatissimum_v1_0, accessed on 1 August 2023) [46]. Probably, the current version of the CDC Bethune genome assembly (NCBI GenBank, GCA_000224295.2) can still have errors in the FAD2a-1 and FAD2a-2 sequences. Indeed, we found a long deletion in FAD2a-1 and an insertion in FAD2a-2 of CDC Bethune compared to these genes of varieties YY5, 3896, and Atlant. In the YY5, 3896, and Atlant genomes, the FAD2a-1 gene sequences were identical; the same was true for the FAD2a-2 gene. FAD2a-1 differed from FAD2a-2 by only seven SNPs in all three varieties. Thus, FAD2a-1 and FAD2a-2 are very conserved in flax and can be distinguished one from another by seven SNPs. Notably, one part of the FAD2a-1 gene of CDC Bethune was similar to the FAD2a-2 gene of YY5, 3896, and Atlant, but not FAD2a-1 of these varieties. This also indicated an error in the FAD2a-1 sequence of CDC Bethune.
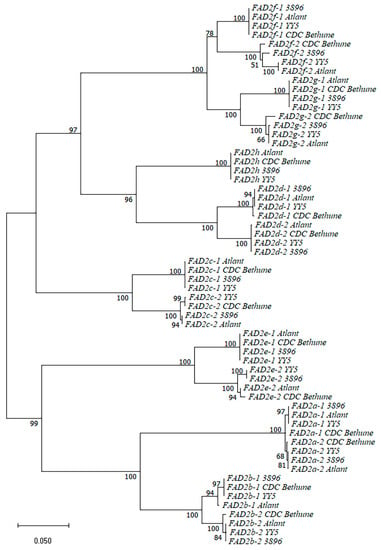
Figure 1.
Phylogenetic analysis of FAD2 genes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
Generally, all pairs of the duplicated FAD2 genes differed from each other by a large number of SNPs and several InDels. The analysis of the FAD2 sequences of the four flax varieties allowed us to separate polymorphisms that were specific to a particular FAD2 gene from those specific to a genotype. In most pairs of the duplicated FAD2 genes (FAD2b-1/FAD2b-2, FAD2c-1/FAD2c-2, FAD2d-1/FAD2d-2, FAD2e-1/FAD2e-2, FAD2f-1/FAD2f-2, FAD2g-1/FAD2g-2), we revealed a significant number of SNPs that were present in all studied flax varieties and allowed distinguishing between two genes from a pair. The FAD2a-1/FAD2a-2 pair was an exception, where FAD2a-1 differed from FAD2a-2 by only seven SNPs.
2.3. Identification and Phylogenetic Analysis of FAD3 Genes in Genomes of Different Flax Varieties
Using the sequences of the FAD3a, FAD3b, FAD3c-1, FAD3c-2, FAD3d-1, and FAD3d-2 flax genes described by You et al. [46], we identified the FAD3 genes in the genomes of the flax varieties YY5, 3896, Atlant, and CDC Bethune. Gene coordinates are presented in Table 3.

Table 3.
Coordinates of FAD3 genes in genomes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
We analyzed the sequences of the FAD3a, FAD3b, FAD3c-1, FAD3c-2, FAD3d-1, and FAD3d-2 genes of the four flax varieties (sequences are presented in Supplementary Table S1). A dendrogram for the FAD3 gene sequences is presented in Figure 2. The sequences of the same FAD3 genes of different flax varieties clustered with each other and formed subclusters. Subclusters of the duplicated genes grouped together: FAD3a and FAD3b, FAD3c-1 and FAD3c-2, FAD3d-1 and FAD3d-2. Pairs of the duplicated FAD3 genes differed from each other by a significant number of SNPs and InDels. For each pair of the duplicated FAD3 genes (FAD3a/FAD3b, FAD3c-1/FAD3c-2, and FAD3d-1/FAD3d-2), we revealed a significant number of SNPs and several short InDels that were present in all studied flax varieties and allowed distinguishing between two genes from a pair. We observed the similarity between the sequences of the same FAD3 genes of the studied genotypes: few SNPs/InDels were revealed for FAD3c-2, FAD3d-1, FAD3a, and FAD3b. The FAD3d-2 gene had no polymorphisms in the YY5, 3896, or Atlant genomes. However, this gene of CDC Bethune had about a 240-bp deletion compared to FAD3d-2 of the other three varieties. The sequence before the deletion corresponded to FAD3d-1 of YY5, 3896, and Atlant, while the sequence after the deletion corresponded to FAD3d-2 of these varieties. We suggested that this was rather an error in the CDC Bethune genome assembly than a genotype feature. A similar picture was observed for FAD3c-1—one region of several dozens of nucleotides in length contained SNPs and InDels and was present only in FAD3c-1 of CDC Bethune. In the same region of FAD3c-1 in the three long-read flax genome assemblies, there was a slight difference in the length of homopolymers. However, this could be an assembly error. Similar to the analysis of the FAD2 genes, the analysis of the FAD3 sequences of the four flax varieties allowed us to distinguish polymorphisms that were specific to a particular FAD3 gene from those specific to a genotype.
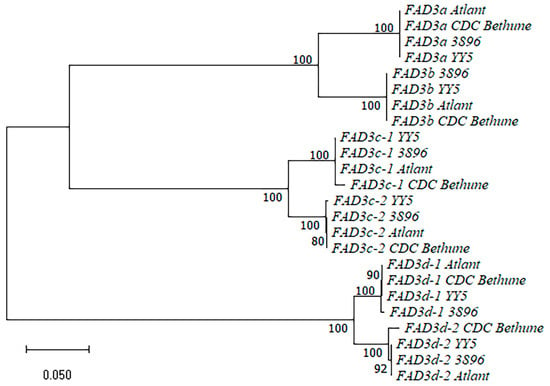
Figure 2.
Phylogenetic analysis of FAD3 genes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
2.4. Identification and Phylogenetic Analysis of SAD Genes in Genomes of Different Flax Varieties
Using the sequences of the SAD2-1, SAD2-2, SAD3-1, and SAD3-2 flax genes described by You et al. [46], we identified the SAD genes in the genomes of flax varieties YY5, 3896, Atlant, and CDC Bethune. Gene coordinates are presented in Table 4.

Table 4.
Coordinates of SAD genes in genomes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
We compared the SAD2-1, SAD2-2, SAD3-1, and SAD3-2 gene sequences of the four flax varieties (sequences are presented in Supplementary Table S1). A dendrogram for the SAD gene sequences is presented in Figure 3. The sequences of the same SAD genes of different flax varieties clustered with each other and formed subclusters. SAD2-1 and SAD2-2 subclusters, as well as SAD3-1 and SAD3-2 subclusters, grouped together. The SAD2-1/SAD2-2 pair differed from the SAD3-1/SAD3-2 pair by a significant number of SNPs and InDels. For each pair of the duplicated SAD genes, we revealed a significant number of SNPs and several short InDels that were present in all the studied flax varieties and allowed distinguishing between two genes from a pair. The sequences of the same SAD genes were similar enough in the studied genotypes and mostly differed by single SNPs. However, a 20-30-nucleotide InDel and several 1-5-nucleotide InDels were identified in the SAD3-1 gene. Two one-nucleotide InDels were revealed in the SAD3-2 gene within the studied varieties. Thus, the analysis of the SAD sequences of the four flax varieties enabled distinguishing between polymorphisms that were specific to a particular SAD gene and those specific to a genotype.
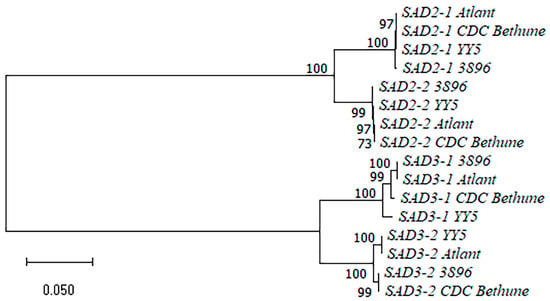
Figure 3.
Phylogenetic analysis of SAD genes of flax varieties YY5, 3896, Atlant, and CDC Bethune.
2.5. Expression Analysis of FAD2 Genes
We analyzed the expression of the FAD2 genes in different flax organs/tissues and under various biotic/abiotic stress conditions (Figure 4 and Figure 5, Supplementary Table S2). The highest expression levels were observed for FAD2b-1 and FAD2b-2 (Figure 4a). These genes were expressed at high levels in all the analyzed samples. For the other FAD2 genes, expression was more organ-/tissue-specific. Notably, FAD2a-1 and FAD2a-2 were predominantly expressed in embryos, endosperm, seeds, and capsules, suggesting their significant role in the synthesis of flax oil FAs (Figure 4b). Increased expression of FAD2a-1 was also observed in leaves in one of the used transcriptomic datasets (Figure 4b). Among the FAD2 genes, FAD2b-1 and FAD2b-2 had the highest expression levels in embryos, endosperm, seeds, and capsules (Figure 4b). However, the expression of FAD2b-1 and FAD2b-2 was also high in other studied organs/tissues (Figure 4b). Therefore, these genes could play a key role in common processes in all flax organs and tissues, as well as the synthesis of flax oil FAs. Pronounced organ-/tissue-specific expression was observed for the FAD2c-1, FAD2d-1, and FAD2f-1 genes. FAD2c-1 had an increased expression level in the xylem part of stem, compared to phloem fibers, the apical part, and the parenchyma of stem (Figure 4b). Probably, this gene is involved in the functioning of stem xylem tissues. For FAD2d-1 and FAD2f-1, high expression levels were specific to root samples (Figure 4b and Figure 5b). Therefore, these genes could have a significant role in root cells.
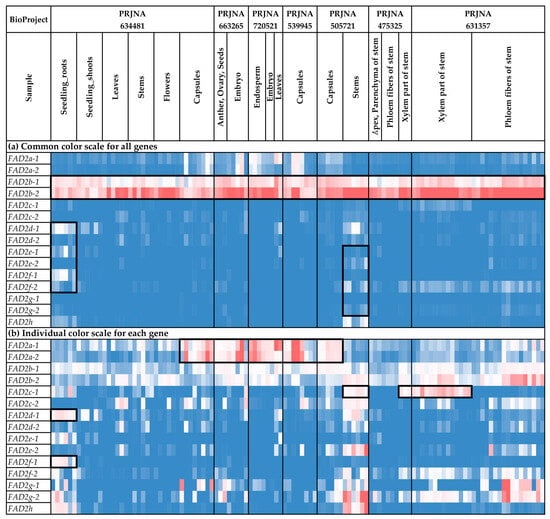
Figure 4.
Expression of FAD2 genes in different flax organs/tissues. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 4 and Figure 5 is common. Noted gene expression patterns of interest are highlighted in boxes.
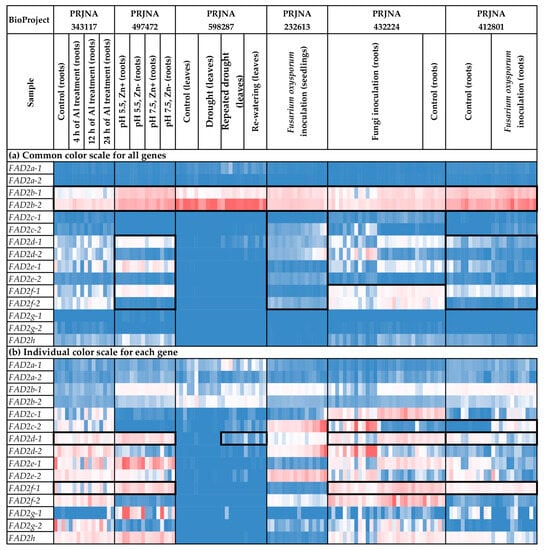
Figure 5.
Expression of FAD2 genes in flax plants under different stress conditions. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 4 and Figure 5 is common. Noted gene expression patterns of interest are highlighted in boxes.
Along with organ-/tissue-specific expression, we discovered the effects of different stressors on FAD2 expression in the analyzed flax samples. As such, Fusarium oxysporum inoculation increased FAD2c-2 expression in roots (Figure 5b). This effect was most prominent in the data from the study of the early response to the fungus (NCBI BioProject, PRJNA412801). The other experimental data (PRJNA432224) demonstrated that the increased expression of FAD2c-2 was more associated with F. oxysporum than other fungal species (Supplementary Table S2). For FAD2d-1, differential expression was detected in the samples from the study of drought influence on flax. The increased expression was characteristic of samples under repeated drought stress and re-watering conditions, compared to control and drought conditions (Figure 5b). However, no FAD2 genes were activated in response to the other analyzed abiotic stresses (different soil pH, increased concentration of aluminum (Al), and zinc (Zn) deficiency) (Figure 5b).
We revealed regularities in the expression profiles for the pairs of duplicated FAD2 genes. The profiles were quite similar between the FAD2a-1 and FAD2a-2 genes, as well as between FAD2b-1 and FAD2b-2 (Figure 5a). In contrast, the expression profiles were significantly different between the genes from a pair for the FAD2c-1/FAD2c-2, FAD2d-1/FAD2d-2, FAD2e-1/FAD2e-2, and FAD2f-1/FAD2f-1 pairs (Figure 5a). However, the profiles of the FAD2c-1, FAD2d-1, FAD2e-1, and FAD2f-1 genes located in a cluster were similar enough for a number of organs/tissues and conditions. The same effect was observed for FAD2c-2, FAD2d-2, FAD2e-2, and FAD2f-2 located in another cluster (Figure 5a).
2.6. Expression Analysis of FAD3 Genes
FAD3 expression was analyzed for different flax organs/tissues and biotic/abiotic stress conditions (Figure 6 and Figure 7, Supplementary Table S2). FAD3a and FAD3b were expressed at high levels in flowers, capsules, and seeds (Figure 6a), with the most remarkable expression increase at the torpedo and cotyledon stages of embryo development (Supplementary Table S2). However, the expression was either low or zero in the majority of other tissues (Figure 6a). FAD3c-1 and FAD3c-2 had increased expression in most root tissues (PRJNA634481, PRJNA497472, PRJNA432224, PRJNA412801); however, they were also expressed in other organs/tissues (Figure 6b and Figure 7b). Meanwhile, decreased expression of FAD3c-1 and FAD3c-2 was observed for samples inoculated with F. oxysporum (PRJNA412801) (Figure 7b). Therefore, these genes could be involved in the response to the pathogen. In contrast to FAD3c-1 and FAD3c-2, inoculation with F. oxysporum increased the expression of FAD3d-1 and FAD3d-2 (PRJNA412801) (Figure 7b). However, the expression of these genes decreased in flax samples under repeated drought conditions in the study of flax response to drought (Figure 7b).
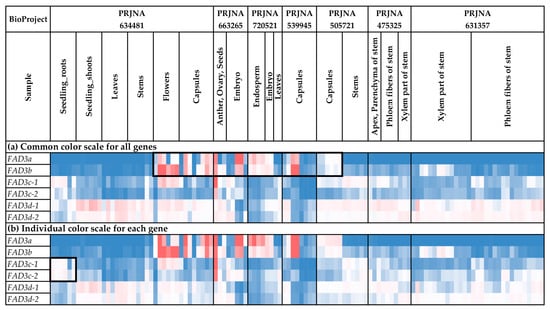
Figure 6.
Expression of FAD3 genes in different flax organs/tissues. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 6 and Figure 7 is common. Noted gene expression patterns of interest are highlighted in boxes.
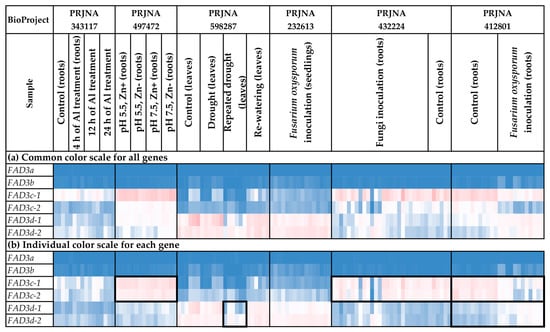
Figure 7.
Expression of FAD3 genes in flax plants under different stress conditions. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 6 and Figure 7 is common. Noted gene expression patterns of interest are highlighted in boxes.
2.7. Expression Analysis of SAD Genes
We analyzed the expression of the SAD genes in different flax organs/tissues and under various biotic/abiotic stress conditions (Figure 8 and Figure 9, Supplementary Table S2). SAD2-1 had expression profiles close to those of SAD2-2. Similarly, the SAD3-1 expression profiles resembled those of SAD3-2 (Figure 8 and Figure 9).
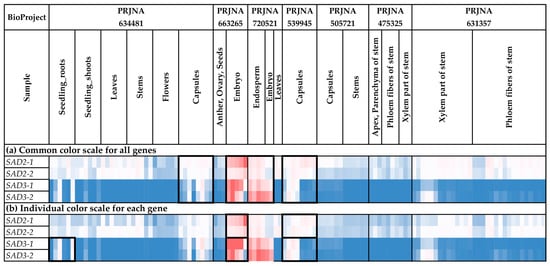
Figure 8.
Expression of SAD genes in different flax organs/tissues. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 8 and Figure 9 is common. Noted gene expression patterns of interest are highlighted in boxes.
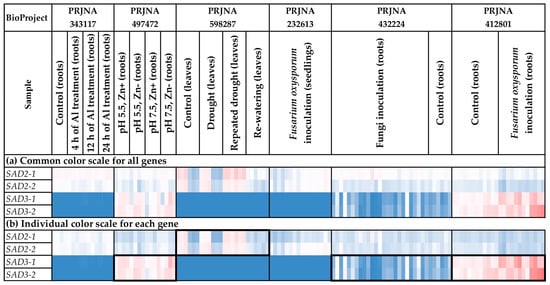
Figure 9.
Expression of SAD genes in flax plants under different stress conditions. (a) Common color scale for all genes. (b) Individual color scale for each gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale is used. The expression data for organs/tissues and stress conditions were analyzed together (as in Supplementary Table S2), so the color scale in Figure 8 and Figure 9 is common. Noted gene expression patterns of interest are highlighted in boxes.
For all SAD genes (SAD2-1, SAD2-2, SAD3-1, and SAD3-2), increased expression was characteristic of embryos, endosperm, and capsules (Figure 8a). However, SAD3-1 and SAD3-2 had an increased expression level in capsules only at the early development stages (PRJNA539945) (Supplementary Table S2). For SAD2-1 and SAD2-2, there were no significant differences between the various stages of capsule development. Notably, the difference between the expression levels of SAD2 and SAD3 was observed in the tissues of the developing flax embryo. While SAD2-1 and SAD2-2 had high expression levels in mature embryos, high SAD3-1 and SAD3-2 expression levels were characteristic of the early stages of embryo development (Supplementary Table S2). Therefore, SAD3-1 and SAD3-2 could be more important for the early stages of seed development. Meanwhile, SAD2-1 and SAD2-2 could also function at later stages of seed development.
SAD2-1 and SAD2-2 were expressed in most analyzed flax organs/tissues (Figure 8a). Unlike SAD2-1 and SAD2-2, differences in the expression levels of SAD3-1 and SAD3-2 between organs/tissues were more pronounced (Figure 8a). In addition to embryos, endosperm, and capsules, SAD3-1 and SAD3-2 had increased expression levels in roots in most studied experiments (all samples of PRJNA497472 and PRJNA412801 and most samples of PRJNA432224 and PRJNA634481) (Figure 8b). In the majority of the other organs/tissues, SAD3-1 and SAD3-2 were unexpressed or expressed at very low levels (Figure 8b). Possibly, the SAD3-1 and SAD3-2 genes are involved in some processes in roots but not in common processes in all flax organs/tissues.
In addition, we revealed trends in the changes of SAD expression in flax under stress conditions. In the study of drought effects, flax genotypes under control and drought conditions significantly differed in SAD2-1 and SAD2-2 expression (Figure 9b, Supplementary Table S2). However, genotype differences almost vanished under repeated drought stress and re-watering conditions (Figure 9b, Supplementary Table S2). For SAD3-1 and SAD3-2, increased expression was revealed in flax samples inoculated with F. oxysporum (Figure 9b).
3. Discussion
Genome assemblies of four flax genotypes allowed us to compare sequences of the FAD2 (FAD2a-1/FAD2a-2, FAD2b-1/FAD2b-2, FAD2c-1/FAD2c-2, FAD2d-1/FAD2d-2, FAD2e-1/FAD2e-2, FAD2f-1/FAD2f-2, FAD2g-1/FAD2g-2, and FAD2h), FAD3 (FAD3a/FAD3b, FAD3c-1/FAD3c-2, and FAD3d-1/FAD3d-2), and SAD (SAD2-1/SAD2-2 and SAD3-1/SAD3-2) genes. We determined gene-specific polymorphisms, which are unrelated to a genotype but distinguish one gene from others. Our study demonstrated that even the latest CDC Bethune assembly contains errors in the FAD2a-1 and FAD2a-2 sequences—the same as in the previous assembly version (according to You et al.) [46]. Initially, the CDC Bethune genome was assembled from short Illumina reads [45]. Then, the assembly was improved to the chromosome level with optical mapping, BAC-clone libraries, and genetic maps [52]. However, the similarity between the FAD2a-1 and FAD2a-2 sequences (differing only by seven SNPs) could have led to errors in the assembly. The other three used genomes (YY5, 3896, and Atlant) were assembled with the use of long third-generation-sequencing reads [54,55,56]. Most likely, this allowed obtaining the correct FAD2a-1 and FAD2a-2 sequences. In the CDC Bethune genome, FAD3c-1 and FAD3d-2 were drastically different from the same genes in the three long-read flax assemblies. Likely, these sequences in the short-read assembly also had errors. The CDC Bethune assembly is often used as a reference in different molecular genetic studies of flax [61,63,75,76,77,78,79]. However, the errors in FAD sequences demonstrate that even a chromosome-level assembly can have inaccuracies in highly homologous regions. Therefore, the use of such sequences in experimental design and analysis can distort the results. Meanwhile, FADs are extensively studied because of their importance for oil crops.
FAD2 genes play a critical role in the biosynthesis of FAs and the determination of OLE and LIO content in oilseed crops [80]. Manipulating the FAD2 genes was used for the suppression of their expression and development of high-OLE plants, including canola, soybeans, Camelina sativa, rice, safflower, and peanut [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. OLE is more resistant to oxidation than LIO and LIN. Therefore, a high content of OLE is preferable for premium-quality oil, and the development of oilseed crops with a high OLE content in their oil is desirable.
However, the effective application of genome editing and RNA interference (RNAi) for the suppression of gene expression requires knowledge of the number and sequences of FAD2. The correct choice of genome editing targets also needs information on the differences between the FAD2 sequences and their expression levels in different tissues. In flax seeds/capsules, the genes of the OLE-LIO transformation are the most promising targets for creating flax varieties with high OLE content. Our analysis established that FAD2a-1 and FAD2a-2 were predominantly expressed in seeds/capsules, but FAD2b-1 and FAD2b-2 had higher expression in the same tissues. Moreover, FAD2b-1 and FAD2b-2 were expressed at high levels in all the studied flax organs/tissues. Another study showed that FAD2b had greater desaturase activity compared to that of FAD2a [48]. Thus, creating flax varieties with high OLE content can be challenging. Probably, all four FAD genes (FAD2a-1/FAD2a-2 and FAD2b-1/FAD2b-2) should be inactivated. Meanwhile, the expression of FAD2b-1 and FAD2b-2 in all the studied flax organs/tissues could point out their vital role in the synthesis of FAs not only in plant seeds, where Fas serve as energy reserves, but also in all other organs/tissues, taking part in plant metabolism. Therefore, FAD2b-1 and FAD2b-2 inactivation could lead to a reduced capacity for flax survival. For example, manipulating the FAD2 genes could result in poor agronomic characteristics of the obtained high-OLE plants [1]. However, RNAi-mediated silencing of the FAD2 genes in flax (the experiment was conducted to suppress FAD2a and FAD2b expression regardless of the gene duplicates) allowed obtaining high-OLE plants with a normal phenotype [33]. In light of such studies, further development of high-OLE flax varieties is promising.
You et al. [46] analyzed the expression of the duplicated FAD and SAD genes only in pairs (except for FAD3a and FAD3b, which were treated individually) and in a small number of tissues. We demonstrated that genes from each pair differed by a significant number of SNPs and InDels (except for the FAD2a-1/FAD2a-2 pair, where only seven SNPs distinguished these genes). Due to these differences, we could analyze the expression of individual genes. Moreover, sequencing a great number of transcriptomes of different organs/tissues of flax plants (including those under biotic/abiotic stress conditions) enabled the analysis of FAD and SAD expression for a representative set of samples [59,60,61,62,63,64,65,66,67,68,69,70].
In this study, we revealed seed-/capsule-specific expression of FAD2a-1, FAD2a-2, FAD3a, FAD3b, SAD3-1, and SAD3-2. Therefore, these genes could substantially contribute to the determination of the FA composition of flax oil. Since oil composition determines the further application of flax seeds, it is one of the most important characteristics of linseed. FAD2b-1, FAD2b-2, SAD2-1, and SAD2-2 had high expression in embryos/seeds/capsules, as well as other tissues. Hence, these genes could be responsible for the synthesis of FAs in seeds and other flax organs/tissues. Organ-/tissue-specific expression was observed for FAD2c-1 (high expression in stem xylem), and FAD2d-1, FAD2f-1, FAD3c-1, and FAD3c-2 (high expression in roots). FAD2 plays an important role in the synthesis of PUFAs in non-photosynthetic tissues, including roots [101,102]. In flax roots, the FAD2d-1 and FAD2f-1 genes seemed to be especially important for FA synthesis. Thus, our study significantly improved the understanding of which FAD and SAD genes play key roles in fatty acid synthesis in various organs and tissues of flax.
We demonstrated that four SAD genes had the highest expression in embryos, endosperm, and capsules. SAD2-1 and SAD2-2 were also expressed in the majority of the analyzed organs/tissues but at a lower level than in capsules. The differences in SAD3-1 and SAD3-2 expression between various flax organs/tissues were more distinct, with extremely low/zero expression in certain samples. In most articles on flax, the authors analyzed the SAD2-1 and SAD2-2 genes [51,79,103,104] (according to the classification of You et al. [46]; called SAD1 and SAD2 in the earlier study [40]). However, SAD3-1 and SAD3-2 remained out of scope in their research. Nevertheless, poorly studied SAD3 could significantly contribute to the synthesis of oil FAs because of the high expression of these genes in flax seeds. Thus, the SAD3 genes deserve to be studied in detail.
FAD and SAD are known to be involved in the response to stressors [80,105,106,107]. We discovered the differential expression of FAD and SAD in flax plants in response to biotic and abiotic stress conditions. FAD2c-2, FAD2d-1, FAD3c-1, FAD3c-2, FAD3d-1, FAD3d-2, SAD3-1, and SAD3-2 changed expression levels on inoculation with the Fusarium species, while the FAD2d-1 and FAD3d-1 expression changed in response to drought. Although the difference between expression in susceptible and resistant flax genotypes was insignificant, these genes could be targeted in research on the stress response of flax plants.
We observed similar expression profiles for duplicated genes from a pair in the FAD3 and SAD families (FAD3a/FAD3b, FAD3c-1/FAD3c-2, FAD3d-1/FAD3d-2, SAD2-1/SAD2-2, and SAD3-1/SAD3-2). The same was revealed for FAD2a-1/FAD2a-2 and FAD2b-1/FAD2b-2. Likely, the genes from a pair co-express and have analogous functions in flax. Previous research showed co-expression and additive effects in LIN synthesis for FAD3a/FAD3b [43,44,46]. Meanwhile, expression profiles were markedly different in the FAD2c-1/FAD2c-2, FAD2d-1/FAD2d-2, FAD2e-1/FAD2e-2, FAD2f-1/FAD2f-2, and FAD2g-1/FAD2g-2 pairs. Probably, duplicated genes from these pairs have different functions in flax plants. However, expression profiles were similar for the clustered FAD2 genes. FAD2c-1, FAD2d-1, FAD2e-1, and FAD2f-1 formed the first cluster, and FAD2c-2, FAD2d-2, FAD2e-2, and FAD2f-2 formed the second one. Coregulation of clustered plant genes was reviewed by Tohge and Fernie [108]. In flax, FAD2 from the same cluster could have common regulatory mechanisms determining their expression profiles.
Thus, the role of certain FAD2, FAD3, and SAD genes in the development of key flax plant characteristics could be elucidated from the analysis of their expression in various organs/tissues, at different developmental stages, and under different growth conditions. For instance, such research could establish the contribution of FAD2, FAD3, and SAD to the FA composition of flax oil. The information collected in the present study is the basis for developing effective flax genome editing procedures, as well as marker-assisted and genomic selection. These technologies are essential for the creation of flax varieties with a determined oil composition and require a solid theoretical background. In addition, recent research into different plant species aimed at FAD and SAD identification [10,12,13,14,16,109,110,111]. Based on the analysis of gene sequences of several genotypes and further expression evaluation in a representative set of transcriptomic data, our approach can be effective in a broad range of studies on the FAD and SAD genes.
4. Materials and Methods
4.1. Phylogenetic Analysis
FAD2, FAD3, and SAD sequences (accessions: Lus10012007 + Lus10012008, Lus10029283 + Lus10029284, Lus10021051, Lus10004175, Lus10021050, Lus10004176, Lus10021049, Lus10004177, Lus10021048, Lus10004178, Lus10021047, Lus10004180, Lus10021046, Lus10004181, Lus10021045, Lus10038321, Lus10036184, Lus10040660, Lus10018245, Lus10027809, Lus10005039, Lus10027486, Lus10039241, Lus10018926, Lus10028627) were downloaded from the phytozome database (https://phytozome-next.jgi.doe.gov/, accessed on 1 August 2023) and blasted (default parameters) against four L. usitatissimum genomes: YY5 (https://zenodo.org/record/4872894#.Y0_723ZBxaQ, accessed on 1 August 2023), Atlant (NCBI GenBank, GCA_014858635.1), line 3896 (GCA_030674075.2), and CDC Bethune (GCA_000224295.2). The selected hits of the chosen identity (>90.0%) and length (>400 bp) were extracted into separate fasta files using a custom script employing bedtools (the bedtools getfasta tool with default parameters) [112]. As the genes of the same family shared a high degree of similarity, duplicated sequences were filtered out manually. Exon–intron junctions in FAD2, FAD3, and SAD genes were determined based on transcript sequences from the phytozome database listed above.
For the extracted sequences, phylogenetic analysis was carried out in MEGA X [113]. Multiple alignment of the extracted sequences was performed using the MUSCLE algorithm with default parameters. Then, phylogenetic trees were constructed using the maximum likelihood method (default parameters, 1000 bootstrap replicates).
4.2. Expression Analysis
Raw fastq reads were downloaded from NCBI SRA using SRA Toolkit 3.0.0 (NCBI BioProject, PRJNA475325, PRJNA631357, PRJNA720521, PRJNA663265, PRJNA634481, PRJNA505721, PRJNA539945, PRJNA598287, PRJNA232613, PRJNA497472, PRJNA432224, PRJNA412801, PRJNA343117). Data were obtained for the following tissues: seedling roots, seedling shoots, leaves, capsules, seeds, embryos, endosperm, stems, the xylem and phloem parts of a stem, the apical part and the parenchyma of a stem. In addition, data for flax tissues under diverse stresses were analyzed, including Al treatment, Zn deficiency, drought, and fungi inoculation. The complete list of BioProjects and samples examined is presented in Supplementary Table S2. Sequencing runs were prefetched (prefetch SRRXXXXXX). The fasterq-dump tool was used to extract fastq files (fasterq-dump SRRXXXXXX). The downloaded reads were checked for integrity using cleanFastq (https://github.com/davidvi/cleanFastq, accessed on 1 August 2023). Paired reads were merged into single files.
Expression analysis was conducted using PPline [114]. Fastq reads were aligned to the YY5 v2.0 genome, and CPM (counts per million) values were calculated for 100-bp intervals covering the FAD2, FAD3, and SAD genes. The following specific options were set: ‘-crop 75′ (trim reads by 75 bases from the end), ‘--coverage-profiles--report-mode--for-intervals tmm_cpm raw_counts’ (output raw counts and calculate CPM values, use TMM normalization for the samples), ‘--coverage-profiles--split-regions--for-intervals--max-chunk-length 100’ (perform analysis for ~100-bp intervals), ‘--star-outFilterMultimapNmax 2’ (or ‘--star-outFilterMultimapNmax 1’), ‘--star-outFilterMismatchNmax 0’, ‘--star-outFilterScoreMinOverLread 0.8’, ‘--star-outFilterMatchNminOverLread 0.8’, ‘--coverage-profiles--count-duplicated-reads--for-intervals yes’, ‘--create-coverage-profiles-for-bed-intervals yes’, ‘--create-coverage-profiles-for-bed-intervals yes’. The FAD2a-1 and FAD2a-2 sequences were very similar (only 7 SNPs distinguish them), so the results of expression analysis for these genes could be biased in some samples. Further analysis and heatmap construction were performed in Microsoft Excel. The gene expression level was calculated as an average CPM for 100-bp intervals covering a gene. Blue (0.01 × Average)-White (Average)-Red (10 × Average) color scale was used, where Average—average expression level of all studied genes (common color scale, Figure 4a, Figure 5a, Figure 6a, Figure 7a, Figure 8a and Figure 9a) or individual genes (individual color scale, Figure 4b, Figure 5b, Figure 6b, Figure 7b, Figure 8b and Figure 9b) in all analyzed samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241914885/s1.
Author Contributions
Conceptualization, A.A.D. and N.V.M.; data analysis, E.M.D., O.L.Z., E.N.P., D.A.Z., T.A.R., L.V.P., R.O.N., E.A.S., A.A.T., E.V.B., G.S.K., C.R., A.A.D. and N.V.M.; writing, E.M.D., A.A.D. and N.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation, grant 21-16-00111.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. Accession numbers can be found in the Section 4.
Acknowledgments
We thank the Center for Precision Genome Editing and Genetic Technologies for Biomedicine, EIMB RAS for providing the computing power and techniques for the data analysis. This work was performed using the equipment of EIMB RAS “Genome” center (http://www.eimb.ru/ru1/ckp/ccu_genome_ce.php, accessed on 1 August 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kazaz, S.; Miray, R.; Lepiniec, L.; Baud, S. Plant monounsaturated fatty acids: Diversity, biosynthesis, functions and uses. Prog. Lipid Res. 2022, 85, 101138. [Google Scholar] [CrossRef] [PubMed]
- Shanklin, J.; Somerville, C. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc. Natl. Acad. Sci. USA 1991, 88, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Okuley, J.; Lightner, J.; Feldmann, K.; Yadav, N.; Lark, E.; Browse, J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 1994, 6, 147–158. [Google Scholar] [CrossRef]
- Arondel, V.; Lemieux, B.; Hwang, I.; Gibson, S.; Goodman, H.M.; Somerville, C.R. Map-based cloning of a gene controlling omega-3 fatty acid desaturation in Arabidopsis. Science 1992, 258, 1353–1355. [Google Scholar] [CrossRef]
- Beisson, F.; Koo, A.J.; Ruuska, S.; Schwender, J.; Pollard, M.; Thelen, J.J.; Paddock, T.; Salas, J.J.; Savage, L.; Milcamps, A.; et al. Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol. 2003, 132, 681–697. [Google Scholar] [CrossRef]
- Pirtle, I.L.; Kongcharoensuntorn, W.; Nampaisansuk, M.; Knesek, J.E.; Chapman, K.D.; Pirtle, R.M. Molecular cloning and functional expression of the gene for a cotton Delta-12 fatty acid desaturase (FAD2). Biochim. Biophys. Acta 2001, 1522, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.L.; Mancha, M.; Martinez-Rivas, J.M. Molecular cloning and characterization of genes encoding two microsomal oleate desaturases (FAD2) from olive. Phytochemistry 2005, 66, 1417–1426. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Gai, J.; Yu, D. Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. J. Plant Physiol. 2007, 164, 1516–1526. [Google Scholar] [CrossRef]
- Kargiotidou, A.; Deli, D.; Galanopoulou, D.; Tsaftaris, A.; Farmaki, T. Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum). J. Exp. Bot. 2008, 59, 2043–2056. [Google Scholar] [CrossRef]
- Contreras, C.; Mariotti, R.; Mousavi, S.; Baldoni, L.; Guerrero, C.; Roka, L.; Cultrera, N.; Pierantozzi, P.; Maestri, D.; Gentili, L.; et al. Characterization and validation of olive FAD and SAD gene families: Expression analysis in different tissues and during fruit development. Mol. Biol. Rep. 2020, 47, 4345–4355. [Google Scholar] [CrossRef]
- E, Z.; Chen, C.; Yang, J.; Tong, H.; Li, T.; Wang, L.; Chen, H. Genome-wide analysis of fatty acid desaturase genes in rice (Oryza sativa L.). Sci. Rep. 2019, 9, 19445. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, S. Genome-wide identification, characterization and in-silico profiling of genes encoding FAD (fatty acid desaturase) proteins in chickpea (Cicer arietinum L.). Plant Gene 2019, 18, 100180. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, S.; Zhang, Y.; Liu, G.; Aghaei-Dargiri, S.; Ghaderi Zefrehei, M.; Zhu, S.; Yu, C.; Chen, Y.; et al. Genome-Wide Characterization and Expression Analysis of Fatty acid Desaturase Gene Family in Poplar. Int. J. Mol. Sci. 2022, 23, 11109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, X.; Liu, W.; Min, X.; Jin, X.; Ndayambaza, B.; Wang, Y. Genome-wide identification and expression analysis of the fatty acid desaturase genes in Medicago truncatula. Biochem. Biophys. Res. Commun. 2018, 499, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, J.; He, L.; Zhang, Y.; Zhao, Y.; Xu, X.; Wei, Y.; Ge, S.; Ding, D.; Liu, M.; et al. Identification of Fatty Acid Desaturases in Maize and Their Differential Responses to Low and High Temperature. Genes 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, S.; Wang, S.; Wang, H.; Zhang, Z. Identification and analysis of the FAD gene family in walnuts (Juglans regia L.) based on transcriptome data. BMC Genom. 2020, 21, 299. [Google Scholar] [CrossRef]
- Niu, E.; Gao, S.; Hu, W.; Zhang, C.; Liu, D.; Shen, G.; Zhu, S. Genome-Wide Identification and Functional Differentiation of Fatty Acid Desaturase Genes in Olea europaea L. Plants 2022, 11, 1415. [Google Scholar] [CrossRef]
- Hajiahmadi, Z.; Abedi, A.; Wei, H.; Sun, W.; Ruan, H.; Zhuge, Q.; Movahedi, A. Identification, evolution, expression, and docking studies of fatty acid desaturase genes in wheat (Triticum aestivum L.). BMC Genom. 2020, 21, 778. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Xue, J.; Mao, X.; Jia, X.; Li, R. Stearoyl-ACP Delta(9) Desaturase 6 and 8 (GhA-SAD6 and GhD-SAD8) Are Responsible for Biosynthesis of Palmitoleic Acid Specifically in Developing Endosperm of Upland Cotton Seeds. Front. Plant Sci. 2019, 10, 703. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Yin, Y.; Chen, K.; He, J.; Yu, L.; Li, M. In Silico Analysis of Fatty Acid Desaturases Structures in Camelina sativa, and Functional Evaluation of Csafad7 and Csafad8 on Seed Oil Formation and Seed Morphology. Int. J. Mol. Sci. 2021, 22, 10857. [Google Scholar] [CrossRef]
- Li, J.; Liu, A.; Najeeb, U.; Zhou, W.; Liu, H.; Yan, G.; Gill, R.A.; Yun, X.; Bai, Q.; Xu, L. Genome-wide investigation and expression analysis of membrane-bound fatty acid desaturase genes under different biotic and abiotic stresses in sunflower (Helianthus annuus L.). Int. J. Biol. Macromol. 2021, 175, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, Y.; Dong, Y.; Xie, T.; Sun, W.; Yu, S. Natural Variation of Fatty Acid Desaturase Gene Affects Linolenic Acid Content and Starch Pasting Viscosity in Rice Grains. Int. J. Mol. Sci. 2022, 23, 12055. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, Y.; Chen, Y.; Gao, Y.; Gao, H.; Liu, B.; Xue, J.; Li, R.; Jia, X. Characterisation of two novel genes encoding Delta(9) fatty acid desaturases (CeSADs) for oleic acid accumulation in the oil-rich tuber of Cyperus esculentus. Plant Sci. Int. J. Exp. Plant Biol. 2022, 319, 111243. [Google Scholar] [CrossRef]
- Bae, S.H.; Zoclanclounon, Y.A.B.; Kumar, T.S.; Oh, J.H.; Lee, J.; Kim, T.H.; Park, K.Y. Advances in Understanding the Genetic Basis of Fatty Acids Biosynthesis in Perilla: An Update. Plants 2022, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Marsh, J.; Tirnaz, S.; Nguyen, H.T.; Batley, J.; Bayer, P.E.; Edwards, D. Diversity of fatty acid biosynthesis genes across the soybean pangenome. Plant Genome 2023, 16, e20334. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, H.; Yu, B.; Huang, L.; Liu, N.; Chen, W.; Liao, B.; Lei, Y.; Huai, D.; Guo, P.; et al. Genetic dissection of fatty acid components in the Chinese peanut (Arachis hypogaea L.) mini-core collection under multi-environments. PLoS ONE 2022, 17, e0279650. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, F.; Chen, R.; Wang, X.; Tseke Inkabanga, A.; Huang, L.; Qin, S.; Zhang, M.; Chai, Y. Genome-wide analysis of fatty acid desaturase genes in chia (Salvia hispanica) reveals their crucial roles in cold response and seed oil formation. Plant Physiol. Biochem. PPB 2023, 199, 107737. [Google Scholar] [CrossRef]
- Shaheen, N.; Khan, U.M.; Farooq, A.; Zafar, U.B.; Khan, S.H.; Ahmad, S.; Azhar, M.T.; Atif, R.M.; Rana, I.A.; Seo, H. Comparative transcriptomic and evolutionary analysis of FAD-like genes of Brassica species revealed their role in fatty acid biosynthesis and stress tolerance. BMC Plant Biol. 2023, 23, 250. [Google Scholar] [CrossRef]
- Gai, W.; Sun, H.; Hu, Y.; Liu, C.; Zhang, Y.; Gai, S.; Yuan, Y. Genome-Wide Identification of Membrane-Bound Fatty Acid Desaturase Genes in Three Peanut Species and Their Expression in Arachis hypogaea during Drought Stress. Genes 2022, 13, 1718. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Manan, S.; Li, P.; Liu, C.; She, G.; Zhao, S.; Zhao, J. Critical metabolic pathways and SAD/FADs, WRI1s, and DGATs cooperate for high-oleic acid oil production in developing oil tea (Camellia oleifera) seeds. Hortic. Res. 2022, 9, uhac087. [Google Scholar] [CrossRef]
- Muir, A.D.; Westcott, N.D. Flax: The genus Linum; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X.-R.; Zhang, Z.-J.; Dribnenki, P.; Singh, S.; Green, A. Development of high oleic oil crop platform in flax through RNAi-mediated multiple FAD2 gene silencing. Plant Cell Rep. 2015, 34, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Brutch, N.; Porokhoviniva, E.; Shelenga, T. Perspectives of the creation of oil flax varieties for the specialized purpose. Agrar. Report. South-East 2016, 1, 50–52. [Google Scholar]
- Cullis, C.A. Genetics and Genomics of Linum; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Fombuena, V.; Petrucci, R.; Dominici, F.; Jorda-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. [Google Scholar] [CrossRef]
- Ben-Shahar, M.; Rosenblatt, E.; Green, J.; Cohen, I. Malignant thymoma associated with progressive systemic sclerosis. Am. J. Med. Sci. 1987, 294, 262–267. [Google Scholar] [CrossRef]
- Jain, R.K.; Thompson, R.G.; Taylor, D.C.; MacKenzie, S.L.; McHughen, A.; Rowland, G.G.; Tenaschuk, D.; Coffey, M. Isolation and characterization of two promoters from linseed for genetic engineering. Crop Sci. 1999, 39, 1696–1701. [Google Scholar] [CrossRef]
- Singh, S.; McKinney, S.; Green, A. Sequence of a cDNA from Linum usitatissimum encoding the stearoyl-acyl carrier protein desaturase. Plant Physiol. 1994, 104, 1075. [Google Scholar] [CrossRef][Green Version]
- Fofana, B.; Duguid, S.; Cloutier, S. Cloning of fatty acid biosynthetic genes β-ketoacyl CoA synthase, fatty acid elongase, stearoyl-ACP desaturase, and fatty acid desaturase and analysis of expression in the early developmental stages of flax (Linum usitatissimum L.) seeds. Plant Sci. 2004, 166, 1487–1496. [Google Scholar] [CrossRef]
- Khadake, R.M.; Ranjekar, P.K.; Harsulkar, A.M. Cloning of a novel omega-6 desaturase from flax (Linum usitatissimum L.) and its functional analysis in Saccharomyces cerevisiae. Mol. Biotechnol. 2009, 42, 168–174. [Google Scholar] [CrossRef]
- Krasowska, A.; Dziadkowiec, D.; Polinceusz, A.; Plonka, A.; Łukaszewicz, M. Cloning of flax oleic fatty acid desaturase and its expression in yeast. J. Am. Oil Chem. Soc. 2007, 84, 809–816. [Google Scholar] [CrossRef]
- Vrinten, P.; Hu, Z.; Munchinsky, M.A.; Rowland, G.; Qiu, X. Two FAD3 desaturase genes control the level of linolenic acid in flax seed. Plant Physiol. 2005, 139, 79–87. [Google Scholar] [CrossRef]
- Banik, M.; Duguid, S.; Cloutier, S. Transcript profiling and gene characterization of three fatty acid desaturase genes in high, moderate, and low linolenic acid genotypes of flax (Linum usitatissimum L.) and their role in linolenic acid accumulation. Genome 2011, 54, 471–483. [Google Scholar] [CrossRef]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. Cell Mol. Biol. 2012, 72, 461–473. [Google Scholar] [CrossRef] [PubMed]
- You, F.M.; Li, P.; Kumar, S.; Ragupathy, R.; Li, Z.; Fu, Y.-B.; Cloutier, S. Genome-wide identification and characterization of the gene families controlling fatty acid biosynthesis in flax (Linum usitatissimum L.). J Proteom. Bioinform 2014, 7, 310–326. [Google Scholar]
- Venglat, P.; Xiang, D.; Qiu, S.; Stone, S.L.; Tibiche, C.; Cram, D.; Alting-Mees, M.; Nowak, J.; Cloutier, S.; Deyholos, M.; et al. Gene expression analysis of flax seed development. BMC Plant Biol. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, N.; Thambugala, D.; Duguid, S.; Loewen, E.; Cloutier, S. Functional characterization of flax fatty acid desaturase FAD2 and FAD3 isoforms expressed in yeast reveals a broad diversity in activity. Mol. Biotechnol. 2014, 56, 609–620. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Wang, Y.; Guo, X. Biosynthesis and profiles of fatty acids, vitamin E and carotenoids during flax (Linum usitatissimum L.) seed capsule maturation. Int. J. Food Sci. Technol. 2021, 56, 4108–4118. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, Z.; Li, Z.; Yi, L.; Yao, L.; Wang, X. Gene screening for fatty acid synthesis of flax based on transcriptome sequencing. Food Sci. Technol. 2022, 42, e93721. [Google Scholar] [CrossRef]
- Thambugala, D.; Ragupathy, R.; Cloutier, S. Structural organization of fatty acid desaturase loci in linseed lines with contrasting linolenic acid contents. Funct. Integr. Genom. 2016, 16, 429–439. [Google Scholar] [CrossRef]
- You, F.M.; Xiao, J.; Li, P.; Yao, Z.; Jia, G.; He, L.; Zhu, T.; Luo, M.C.; Wang, X.; Deyholos, M.K.; et al. Chromosome-scale pseudomolecules refined by optical, physical and genetic maps in flax. Plant J. Cell Mol. Biol. 2018, 95, 371–384. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Wang, L.; Wang, L.; Yan, X.; Dang, Z.; Li, W.; Zhao, W.; Pei, X.; Li, X.; et al. Genomic Comparison and Population Diversity Analysis Provide Insights into the Domestication and Improvement of Flax. iScience 2020, 23, 100967. [Google Scholar] [CrossRef]
- Sa, R.; Yi, L.; Siqin, B.; An, M.; Bao, H.; Song, X.; Wang, S.; Li, Z.; Zhang, Z.; Hazaisi, H.; et al. Chromosome-Level Genome Assembly and Annotation of the Fiber Flax (Linum usitatissimum) Genome. Front. Genet. 2021, 12, 735690. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.A.; Pushkova, E.N.; Novakovskiy, R.O.; Beniaminov, A.D.; Rozhmina, T.A.; Zhuchenko, A.A.; Bolsheva, N.L.; Muravenko, O.V.; Povkhova, L.V.; Dvorianinova, E.M.; et al. Genome Sequencing of Fiber Flax Cultivar Atlant Using Oxford Nanopore and Illumina Platforms. Front. Genet. 2020, 11, 590282. [Google Scholar] [CrossRef] [PubMed]
- Dvorianinova, E.M.; Bolsheva, N.L.; Pushkova, E.N.; Rozhmina, T.A.; Zhuchenko, A.A.; Novakovskiy, R.O.; Povkhova, L.V.; Sigova, E.A.; Zhernova, D.A.; Borkhert, E.V.; et al. Isolating Linum usitatissimum L. Nuclear DNA Enabled Assembling High-Quality Genome. Int. J. Mol. Sci. 2022, 23, 13244. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.A.; Pushkova, E.N.; Melnikova, N.V. Plant Genome Sequencing: Modern Technologies and Novel Opportunities for Breeding. Mol. Biol. 2022, 56, 531–545. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, J.; Gao, W.; Jia, Y.; Wei, Y.; Wang, G. Complex genome assembly based on long-read sequencing. Brief. Bioinform. 2022, 23, bbac305. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Mahato, A.K.; Gaikwad, K.; Singh, N.K. Transcriptome Landscape at Different Developmental Stages of a Drought Tolerant Cultivar of Flax (Linum usitatissimum). Front. Chem. 2017, 5, 82. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Kudryavtseva, A.V.; Krasnov, G.S.; Koroban, N.V.; Speranskaya, A.S.; Krinitsina, A.A.; Belenikin, M.S.; Snezhkina, A.V.; Sadritdinova, A.F.; Kishlyan, N.V.; et al. Gene expression profiling of flax (Linum usitatissimum L.) under edaphic stress. BMC Plant Biol. 2016, 16, 237. [Google Scholar] [CrossRef]
- Galindo-Gonzalez, L.; Deyholos, M.K. RNA-seq Transcriptome Response of Flax (Linum usitatissimum L.) to the Pathogenic Fungus Fusarium oxysporum f. sp. lini. Front. Plant Sci. 2016, 7, 1766. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Novakovskiy, R.O.; Snezhkina, A.V.; Fedorova, M.S.; Yurkevich, O.Y.; Muravenko, O.V.; Bolsheva, N.L.; Kudryavtseva, A.V.; et al. Differential gene expression in response to Fusarium oxysporum infection in resistant and susceptible genotypes of flax (Linum usitatissimum L.). BMC Plant Biol. 2017, 17, 253. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Novakovskiy, R.O.; Pushkova, E.N.; Rozhmina, T.A.; Zhuchenko, A.A.; Bolsheva, N.L.; Beniaminov, A.D.; Mitkevich, V.A.; Povkhova, L.V.; Dvorianinova, E.M.; et al. Transcriptomes of Different Tissues of Flax (Linum usitatissimum L.) Cultivars With Diverse Characteristics. Front. Genet. 2020, 11, 565146. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Zyablitsin, A.V.; Snezhkina, A.V.; Fedorova, M.S.; Pushkova, E.N.; Kezimana, P.; Novakovskiy, R.O.; Povkhova, L.V.; et al. Flax (Linum usitatissimum L.) response to non-optimal soil acidity and zinc deficiency. BMC Plant Biol. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.; Kozlova, L.; Gorshkov, O.; Nazipova, A.; Ageeva, M.; Gorshkova, T. Cell Wall Layer Induced in Xylem Fibers of Flax Upon Gravistimulation Is Similar to Constitutively Formed Cell Walls of Bast Fibers. Front. Plant Sci. 2021, 12, 660375. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, O.; Chernova, T.; Mokshina, N.; Gogoleva, N.; Suslov, D.; Tkachenko, A.; Gorshkova, T. Intrusive Growth of Phloem Fibers in Flax Stem: Integrated Analysis of miRNA and mRNA Expression Profiles. Plants 2019, 8, 47. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Wu, G.; Yuan, H.; Ma, Y.; Lin, H.; Pan, L.; Li, S.; Sun, D. Comprehensive Analysis of Differentially Expressed Unigenes under NaCl Stress in Flax (Linum usitatissimum L.) Using RNA-Seq. Int. J. Mol. Sci. 2019, 20, 369. [Google Scholar] [CrossRef]
- Gorshkova, T.; Chernova, T.; Mokshina, N.; Gorshkov, V.; Kozlova, L.; Gorshkov, O. Transcriptome Analysis of Intrusively Growing Flax Fibers Isolated by Laser Microdissection. Sci. Rep. 2018, 8, 14570. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, O.; Mokshina, N.; Ibragimova, N.; Ageeva, M.; Gogoleva, N.; Gorshkova, T. Phloem fibres as motors of gravitropic behaviour of flax plants: Level of transcriptome. Funct. Plant Biol. FPB 2018, 45, 203–214. [Google Scholar] [CrossRef]
- Gorshkov, O.; Mokshina, N.; Gorshkov, V.; Chemikosova, S.; Gogolev, Y.; Gorshkova, T. Transcriptome portrait of cellulose-enriched flax fibres at advanced stage of specialization. Plant Mol. Biol. 2017, 93, 431–449. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, D.; Liu, Y.; Zhu, L.; Xie, F.; Xie, L. RNA-Seq combined with population-level analysis reveals important candidate genes related to seed size in flax (Linum usitatissimum L.). Front. Plant Sci. 2022, 13, 1015399. [Google Scholar] [CrossRef]
- Danaeipour, Z.; Garoosi, G.; Tohidfar, M.; Bakhtiarizadeh, M.R.; Mirjalili, M.H. Comprehensive RNA-Seq-based study and metabolite profiling to identify genes involved in podophyllotoxin biosynthesis in Linum album Kotschy ex Boiss. (Linaceae). Sci. Rep. 2023, 13, 9219. [Google Scholar] [CrossRef]
- House, M.A.; Young, L.W.; Robinson, S.J.; Booker, H.M. Transcriptomic Analysis of Early Flowering Signals in ‘Royal’ Flax. Plants 2022, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Kostyn, K.; Boba, A.; Kozak, B.; Sztafrowski, D.; Widula, J.; Szopa, J.; Preisner, M. Transcriptome profiling of flax plants exposed to a low-frequency alternating electromagnetic field. Front. Genet. 2023, 14, 1205469. [Google Scholar] [CrossRef]
- Gao, P.; Qiu, S.; Ma, X.; Parkin, I.A.P.; Xiang, D.; Datla, R. Spatiotemporal Transcriptomic Atlas of Developing Embryos and Vegetative Tissues in Flax. Plants 2022, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Speck, A.; Trouve, J.P.; Enjalbert, J.; Geffroy, V.; Joets, J.; Moreau, L. Genetic Architecture of Powdery Mildew Resistance Revealed by a Genome-Wide Association Study of a Worldwide Collection of Flax (Linum usitatissimum L.). Front. Plant Sci. 2022, 13, 871633. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lin, Y.; Qi, F.; Xiaoyang, C.; Peng, Z.; Yu, Y.; Liu, Y.; Zhang, J.; Qi, X.; Deyholos, M.; et al. Comprehensive Analysis of Differentially Expressed Genes and Epigenetic Modification-Related Expression Variation Induced by Saline Stress at Seedling Stage in Fiber and Oil Flax, Linum usitatissimum L. Plants 2022, 11, 2053. [Google Scholar] [CrossRef]
- Povkhova, L.V.; Melnikova, N.V.; Rozhmina, T.A.; Novakovskiy, R.O.; Pushkova, E.N.; Dvorianinova, E.M.; Zhuchenko, A.A.; Kamionskaya, A.M.; Krasnov, G.S.; Dmitriev, A.A. Genes Associated with the Flax Plant Type (Oil or Fiber) Identified Based on Genome and Transcriptome Sequencing Data. Plants 2021, 10, 2616. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Kezimana, P.; Rozhmina, T.A.; Zhuchenko, A.A.; Povkhova, L.V.; Pushkova, E.N.; Novakovskiy, R.O.; Pavelek, M.; Vladimirov, G.N.; Nikolaev, E.N.; et al. Genetic diversity of SAD and FAD genes responsible for the fatty acid composition in flax cultivars and lines. BMC Plant Biol. 2020, 20, 301. [Google Scholar] [CrossRef]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef]
- Spasibionek, S.; Mikolajczyk, K.; Cwiek-Kupczynska, H.; Pietka, T.; Krotka, K.; Matuszczak, M.; Nowakowska, J.; Michalski, K.; Bartkowiak-Broda, I. Marker assisted selection of new high oleic and low linolenic winter oilseed rape (Brassica napus L.) inbred lines revealing good agricultural value. PLoS ONE 2020, 15, e0233959. [Google Scholar] [CrossRef]
- Pham, A.T.; Lee, J.D.; Shannon, J.G.; Bilyeu, K.D. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010, 10, 195. [Google Scholar] [CrossRef]
- Bai, S.; Engelen, S.; Denolf, P.; Wallis, J.G.; Lynch, K.; Bengtsson, J.D.; Van Thournout, M.; Haesendonckx, B.; Browse, J. Identification, characterization and field testing of Brassica napus mutants producing high-oleic oils. Plant J. Cell Mol. Biol. 2019, 98, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogue, F.; Faure, J.D. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Araki, E.; Suzuki, Y.; Toki, S.; Saika, H. Production of high oleic/low linoleic rice by genome editing. Plant Physiol. Biochem. PPB 2018, 131, 58–62. [Google Scholar] [CrossRef]
- Okuzaki, A.; Ogawa, T.; Koizuka, C.; Kaneko, K.; Inaba, M.; Imamura, J.; Koizuka, N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol. Biochem. PPB 2018, 131, 63–69. [Google Scholar] [CrossRef]
- Do, P.T.; Nguyen, C.X.; Bui, H.T.; Tran, L.T.N.; Stacey, G.; Gillman, J.D.; Zhang, Z.J.; Stacey, M.G. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and alpha-linolenic acid phenotype in soybean. BMC Plant Biol. 2019, 19, 311. [Google Scholar] [CrossRef]
- Huang, H.; Cui, T.; Zhang, L.; Yang, Q.; Yang, Y.; Xie, K.; Fan, C.; Zhou, Y. Modifications of fatty acid profile through targeted mutation at BnaFAD2 gene with CRISPR/Cas9-mediated gene editing in Brassica napus. TAG Theor. Appl. Genetics. Theor. Und Angew. Genet. 2020, 133, 2401–2411. [Google Scholar] [CrossRef]
- Lee, K.R.; Jeon, I.; Yu, H.; Kim, S.G.; Kim, H.S.; Ahn, S.J.; Lee, J.; Lee, S.K.; Kim, H.U. Increasing Monounsaturated Fatty Acid Contents in Hexaploid Camelina sativa Seed Oil by FAD2 Gene Knockout Using CRISPR-Cas9. Front. Plant Sci. 2021, 12, 702930. [Google Scholar] [CrossRef]
- Mroczka, A.; Roberts, P.D.; Fillatti, J.J.; Wiggins, B.E.; Ulmasov, T.; Voelker, T. An intron sense suppression construct targeting soybean FAD2-1 requires a double-stranded RNA-producing inverted repeat T-DNA insert. Plant Physiol. 2010, 153, 882–891. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, H.; Go, Y.S.; Lee, S.B.; Hur, C.G.; Kim, H.U.; Suh, M.C. Identification of functional BrFAD2-1 gene encoding microsomal delta-12 fatty acid desaturase from Brassica rapa and development of Brassica napus containing high oleic acid contents. Plant Cell Rep. 2011, 30, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.D.; Zhang, Y.Y.; Yang, J.; Qi, G.X.; Guo, D.Q.; Xing, G.J.; Yao, Y.; Xu, W.J.; Li, H.Y.; et al. Changes in oleic Acid content of transgenic soybeans by antisense RNA mediated posttranscriptional gene silencing. Int. J. Genom. 2014, 2014, 921950. [Google Scholar] [CrossRef]
- Zaplin, E.S.; Liu, Q.; Li, Z.; Butardo, V.M.; Blanchard, C.L.; Rahman, S. Production of high oleic rice grains by suppressing the expression of the OsFAD2-1 gene. Funct. Plant Biol. FPB 2013, 40, 996–1004. [Google Scholar] [CrossRef]
- Wood, C.C.; Okada, S.; Taylor, M.C.; Menon, A.; Mathew, A.; Cullerne, D.; Stephen, S.J.; Allen, R.S.; Zhou, X.R.; Liu, Q.; et al. Seed-specific RNAi in safflower generates a superhigh oleic oil with extended oxidative stability. Plant Biotechnol. J. 2018, 16, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xing, G.; Niu, L.; He, H.; Guo, D.; Du, Q.; Qian, X.; Yao, Y.; Li, H.; Zhong, X.; et al. Improved oil quality in transgenic soybean seeds by RNAi-mediated knockdown of GmFAD2-1B. Transgenic Res. 2018, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Shannon, J.G.; Bilyeu, K.D. Combinations of mutant FAD2 and FAD3 genes to produce high oleic acid and low linolenic acid soybean oil. TAG Theor. Appl. Genetics. Theor. Und Angew. Genet. 2012, 125, 503–515. [Google Scholar] [CrossRef]
- Demorest, Z.L.; Coffman, A.; Baltes, N.J.; Stoddard, T.J.; Clasen, B.M.; Luo, S.; Retterath, A.; Yabandith, A.; Gamo, M.E.; Bissen, J.; et al. Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol. 2016, 16, 225. [Google Scholar] [CrossRef]
- Jarvis, B.A.; Romsdahl, T.B.; McGinn, M.G.; Nazarenus, T.J.; Cahoon, E.B.; Chapman, K.D.; Sedbrook, J.C. CRISPR/Cas9-Induced fad2 and rod1 Mutations Stacked With fae1 Confer High Oleic Acid Seed Oil in Pennycress (Thlaspi arvense L.). Front. Plant Sci. 2021, 12, 652319. [Google Scholar] [CrossRef]
- Miquel, M.; Browse, J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J. Biol. Chem. 1992, 267, 1502–1509. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, J.; Li, B.; Zhu, Q.; Chen, S.; Zhang, H. Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE 2012, 7, e30355. [Google Scholar] [CrossRef]
- Rajwade, A.V.; Kadoo, N.Y.; Borikar, S.P.; Harsulkar, A.M.; Ghorpade, P.B.; Gupta, V.S. Differential transcriptional activity of SAD, FAD2 and FAD3 desaturase genes in developing seeds of linseed contributes to varietal variation in alpha-linolenic acid content. Phytochemistry 2014, 98, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Fofana, B.; Cloutier, S.; Duguid, S.; Ching, J.; Rampitsch, C. Gene expression of stearoyl-ACP desaturase and delta12 fatty acid desaturase 2 is modulated during seed development of flax (Linum usitatissimum). Lipids 2006, 41, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zou, Y.; Guo, X.; Li, H.; Lu, H. Fatty acid desaturases (FADs) modulate multiple lipid metabolism pathways to improve plant resistance. Mol. Biol. Rep. 2022, 49, 9997–10011. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, S.; Miray, R.; Baud, S. Acyl-Acyl Carrier Protein Desaturases and Plant Biotic Interactions. Cells 2021, 10, 674. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Co-Regulation of Clustered and Neo-Functionalized Genes in Plant-Specialized Metabolism. Plants 2020, 9, 622. [Google Scholar] [CrossRef]
- Duan, W.; Shi-Mei, Y.; Zhi-Wei, S.; Jing, X.; De-Gang, Z.; Hong-Bin, W.; Qi, S. Genome-Wide Analysis of the Fatty Acid Desaturase Gene Family Reveals the Key Role of PfFAD3 in alpha-Linolenic Acid Biosynthesis in Perilla Seeds. Front. Genet. 2021, 12, 735862. [Google Scholar] [CrossRef]
- Feng, J.; Dong, Y.; Liu, W.; He, Q.; Daud, M.K.; Chen, J.; Zhu, S. Genome-wide identification of membrane-bound fatty acid desaturase genes in Gossypium hirsutum and their expressions during abiotic stress. Sci. Rep. 2017, 7, 45711. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Rezaee, S.; Heidari, P. Genome-wide characterization and expression analysis of fatty acid desaturase gene family in Camelina sativa. Gene Rep. 2020, 21, 100894. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, G.S.; Dmitriev, A.A.; Kudryavtseva, A.V.; Shargunov, A.V.; Karpov, D.S.; Uroshlev, L.A.; Melnikova, N.V.; Blinov, V.M.; Poverennaya, E.V.; Archakov, A.I.; et al. PPLine: An Automated Pipeline for SNP, SAP, and Splice Variant Detection in the Context of Proteogenomics. J. Proteome Res. 2015, 14, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).