Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System
Abstract
:1. Introduction
2. Results
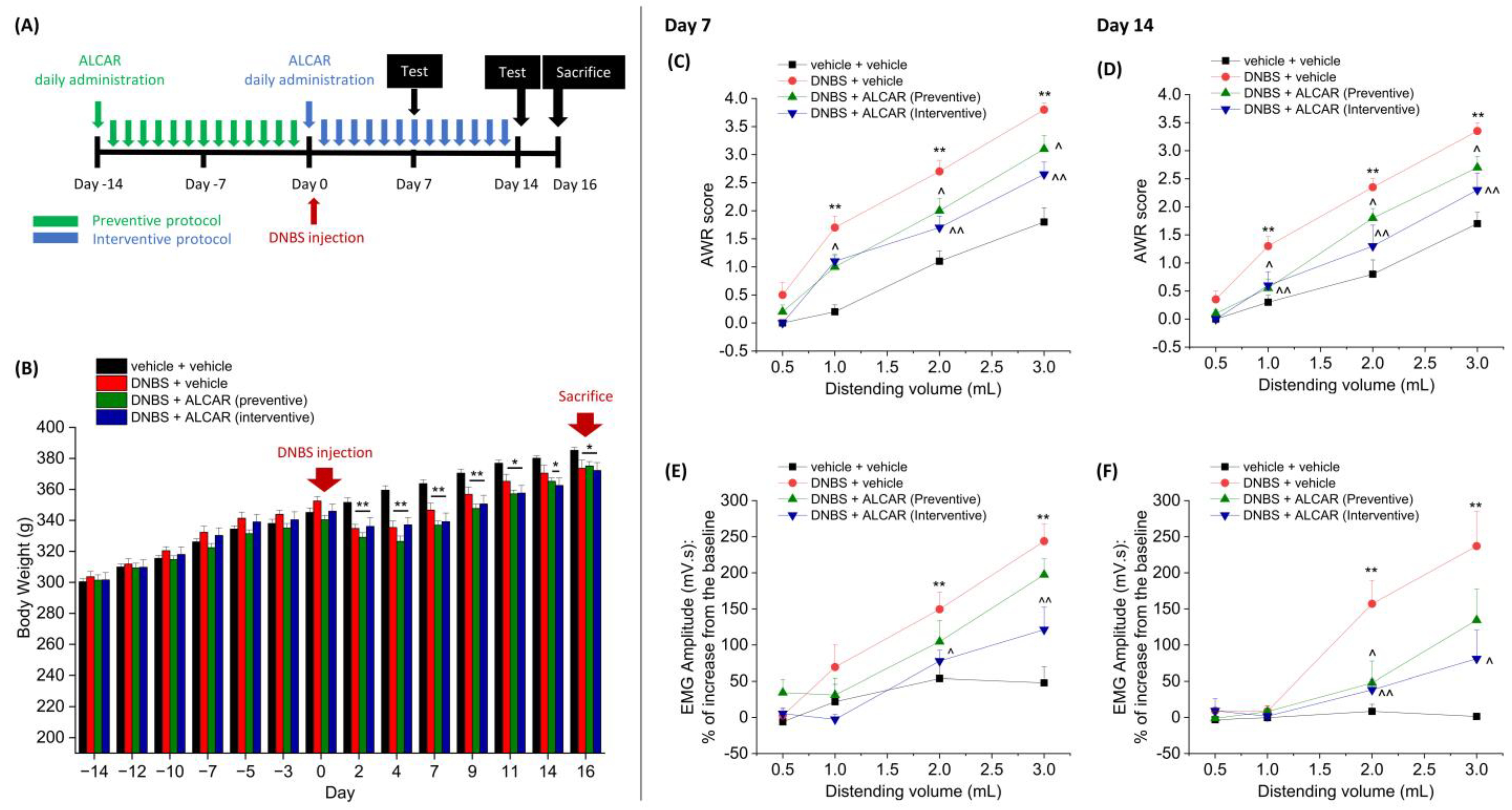
2.1. Anti-Hyperalgesic Efficacy of ALCAR on Visceral Pain Induced by DNBS in Rats
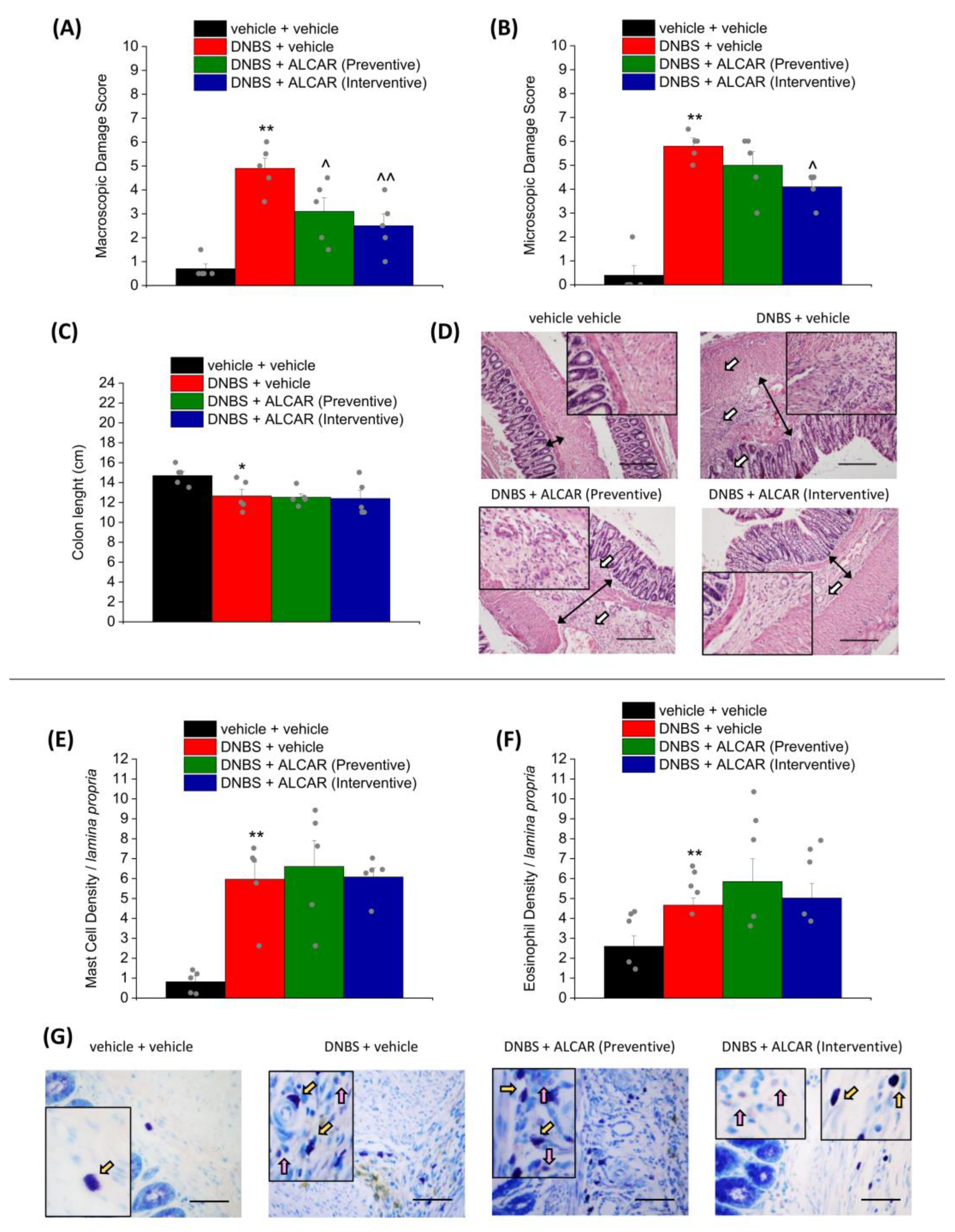
2.2. Effect of ALCAR on Colon Damage and Mast Cell/Eosinophils Infiltration Caused by DNBS Injection
2.3. Neuroprotective Effects of ALCAR on the Enteric Nervous System
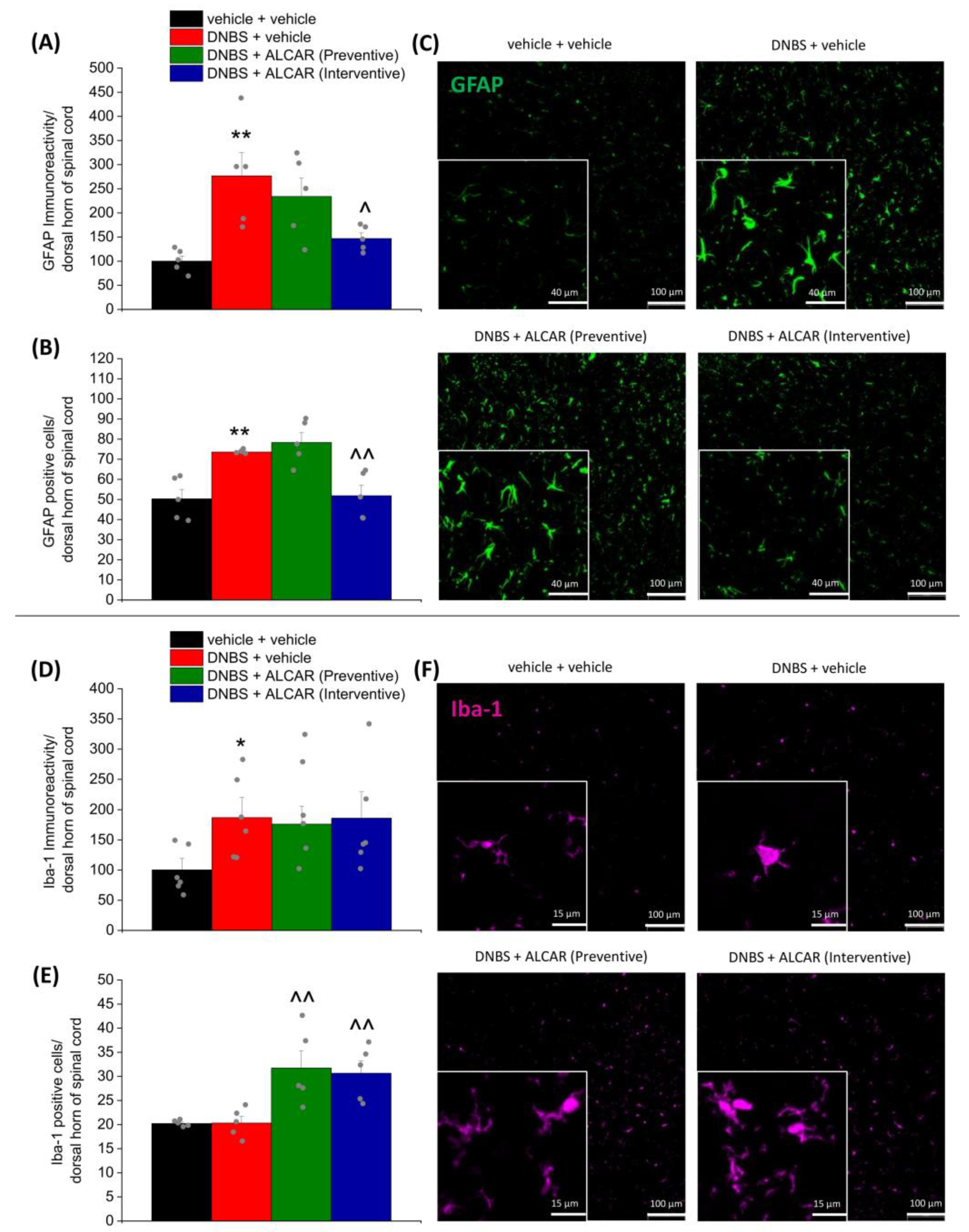
2.4. Neuroprotective Effects of ALCAR on Central Nervous System
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Assessment of Visceral Sensitivity by Visceromotor Response (VMR)
4.4. Assessment of Visceral Sensitivity by Abdominal Withdrawal Reflex (AWR)
4.5. Histological Evaluation of Colon Damage
4.6. Immunofluorescence Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakshi, N.; Hart, A.L.; Lee, M.C.; Williams, A.C.d.C.; Lackner, J.M.; Norton, C.; Croft, P. Chronic pain in patients with inflammatory bowel disease. Pain 2021, 162, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Parisio, C.; Branca, J.J.V.; Segnani, C.; Ippolito, C.; Pellegrini, C.; Antonioli, L.; Fornai, M.; Micheli, L.; Pacini, A.; et al. Deepening the Mechanisms of Visceral Pain Persistence: An Evaluation of the Gut-Spinal Cord Relationship. Cells 2020, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. Assessing the antioxidant and metabolic effect of an alpha-lipoic acid and acetyl-L-carnitine nutraceutical. Curr. Res. Food Sci. 2021, 4, 336–344. [Google Scholar] [CrossRef]
- Maldonado, C.; Vázquez, M.; Fagiolino, P. Potential Therapeutic Role of Carnitine and Acetylcarnitine in Neurological Disorders. Curr. Pharm. Des. 2020, 26, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Copani, A.; Gereau, R.W.; Nicoletti, F. Acetyl-L-carnitine in neuropathic pain: Experimental data. CNS Drugs 2007, 21 (Suppl. 1), 31–38, discussion 45–36. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.; Bulbuloglu, E.; Kantarceken, B.; Ciralik, H.; Kurutas, E.B.; Buyukbese, M.A.; Gumusalan, Y. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig. Dis. Sci. 2006, 51, 488–494. [Google Scholar] [CrossRef]
- Scioli, M.G.; Stasi, M.A.; Passeri, D.; Doldo, E.; Costanza, G.; Camerini, R.; Fociani, P.; Arcuri, G.; Lombardo, K.; Pace, S.; et al. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis through its Action on the Immune Function and Microvasculature. Clin. Transl. Gastroenterol. 2014, 5, e55. [Google Scholar] [CrossRef]
- Imperato, A.; Ramacci, M.T.; Angelucci, L. Acetyl-L-carnitine enhances acetylcholine release in the striatum and hippocampus of awake freely moving rats. Neurosci. Lett. 1989, 107, 251–255. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Calvani, M.; Mosconi, L.; Nicolai, R.; Bartolini, A. Acetyl-l-carnitine induces muscarinic antinocieption in mice and rats. Neuropharmacology 2002, 43, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- White, H.L.; Scates, P.W. Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem. Res. 1990, 15, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Yu, B.P. Upregulation of the high-affinity choline transporter in colon relieves stress-induced hyperalgesia. J. Pain Res. 2018, 11, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Hu, G.; Wang, Z.; Yu, B.; Tan, W. Activation of the High-Affinity Choline Transporter 1 in the Spinal Cord Relieves Stress-Induced Hyperalgesia. Dig. Dis. Sci. 2023, 68, 2414–2426. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, M.; Pan, Y.; Yu, B. Blockage of high-affinity choline transporter increases visceral hypersensitivity in rats with chronic stress. Gastroenterol. Res. Pract. 2018, 2018, 9252984. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Goverse, G.; Stakenborg, M.; Matteoli, G. The intestinal cholinergic anti-inflammatory pathway. J. Physiol. 2016, 594, 5771–5780. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagal tone: Effects on sensitivity, motility, and inflammation. Neurogastroenterol. Motil. 2016, 28, 455–462. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Ghelardini, C.; Calvani, M.; Nicolai, R.; Mosconi, L.; Toscano, A.; Pacini, A.; Bartolini, A. Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: A role for the nicotinic receptor. J. Neurosci. Res. 2009, 87, 200–207. [Google Scholar] [CrossRef]
- Tomassoni, D.; Di Cesare Mannelli, L.; Bramanti, V.; Ghelardini, C.; Amenta, F.; Pacini, A. Treatment with acetyl-L-carnitine exerts a neuroprotective effect in the sciatic nerve following loose ligation: A functional and microanatomical study. Neural Regen. Res. 2018, 13, 692–698. [Google Scholar] [CrossRef]
- Galeotti, N.; Bartolini, A.; Calvani, M.; Nicolai, R.; Ghelardini, C. Acetyl-L-carnitine requires phospholipase C-IP3 pathway activation to induce antinociception. Neuropharmacology 2004, 47, 286–294. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Ghelardini, C.; Calvani, M.; Nicolai, R.; Mosconi, L.; Vivoli, E.; Pacini, A.; Bartolini, A. Protective effect of acetyl-L-carnitine on the apoptotic pathway of peripheral neuropathy. Eur. J. Neurosci. 2007, 26, 820–827. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Ghelardini, C.; Toscano, A.; Pacini, A.; Bartolini, A. The neuropathy-protective agent acetyl-L-carnitine activates protein kinase C-gamma and MAPKs in a rat model of neuropathic pain. Neuroscience 2010, 165, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Vivoli, E.; Di Cesare Mannelli, L.; Salvicchi, A.; Bartolini, A.; Koverech, A.; Nicolai, R.; Benatti, P.; Ghelardini, C. Acetyl-L-carnitine increases artemin level and prevents neurotrophic factor alterations during neuropathy. Neuroscience 2010, 167, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Mannelli, L.D.C.; Menicacci, C.; Lorenzoni, P.; Aglianò, M.; Ghelardini, C. Prophylactic role of acetyl-l-carnitine on knee lesions and associated pain in a rat model of osteoarthritis. Life Sci. 2014, 106, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Shukla, S. ALCAR Exerts Neuroprotective and Pro-Neurogenic Effects by Inhibition of Glial Activation and Oxidative Stress via Activation of the Wnt/β-Catenin Signaling in Parkinsonian Rats. Mol. Neurobiol. 2016, 53, 4286–4301. [Google Scholar] [CrossRef]
- Scafidi, S.; Fiskum, G.; Lindauer, S.L.; Bamford, P.; Shi, D.; Hopkins, I.; McKenna, M.C. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J. Neurochem. 2010, 114, 820–831. [Google Scholar] [CrossRef]
- Hadera, M.G.; McDonald, T.; Smeland, O.B.; Meisingset, T.W.; Eloqayli, H.; Jaradat, S.; Borges, K.; Sonnewald, U. Modification of Astrocyte Metabolism as an Approach to the Treatment of Epilepsy: Triheptanoin and Acetyl-L-Carnitine. Neurochem. Res. 2016, 41, 86–95. [Google Scholar] [CrossRef]
- Calabrese, V.; Ravagna, A.; Colombrita, C.; Scapagnini, G.; Guagliano, E.; Calvani, M.; Butterfield, D.A.; Giuffrida Stella, A.M. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: Involvement of the transcription factor Nrf2. J. Neurosci. Res. 2005, 79, 509–521. [Google Scholar] [CrossRef]
- Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671. [Google Scholar] [CrossRef]
- Dodds, K.N.; Beckett, E.A.; Evans, S.F.; Grace, P.M.; Watkins, L.R.; Hutchinson, M.R. Glial contributions to visceral pain: Implications for disease etiology and the female predominance of persistent pain. Transl. Psychiatry 2016, 6, e888. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.E. The emerging role of mast cells in irritable bowel syndrome. Gastroenterol. Hepatol. 2018, 14, 250. [Google Scholar]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L.; et al. Beneficial Effects of Eruca sativa Defatted Seed Meal on Visceral Pain and Intestinal Damage Resulting from Colitis in Rats. Foods 2022, 11, 580. [Google Scholar] [CrossRef]
- Ippolito, C.; Segnani, C.; Errede, M.; Virgintino, D.; Colucci, R.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Dolfi, A.; Bernardini, N. An integrated assessment of histopathological changes of the enteric neuromuscular compartment in experimental colitis. J. Cell. Mol. Med. 2015, 19, 485–500. [Google Scholar] [CrossRef]
- Holland, A.M.; Bon-Frauches, A.C.; Keszthelyi, D.; Melotte, V.; Boesmans, W. The enteric nervous system in gastrointestinal disease etiology. Cell. Mol. Life Sci. 2021, 78, 4713–4733. [Google Scholar] [CrossRef]
- De Giorgio, R.; Guerrini, S.; Barbara, G.; Stanghellini, V.; De Ponti, F.; Corinaldesi, R.; Moses, P.L.; Sharkey, K.A.; Mawe, G.M. Inflammatory neuropathies of the enteric nervous system. Gastroenterology 2004, 126, 1872–1883. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. 1), S10–S28. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- McIlwrath, S.L.; Starr, M.E.; High, A.E.; Saito, H.; Westlund, K.N. Effect of acetyl-L-carnitine on hypersensitivity in acute recurrent caerulein-induced pancreatitis and microglial activation along the brain’s pain circuitry. World J. Gastroenterol. 2021, 27, 794. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Di Lascio, S.; Fornasari, D. Acetyl-L-carnitine in chronic pain: A narrative review. Pharmacol. Res. 2021, 173, 105874. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Nicoletti, F. Metabotropic glutamate receptors and the control of chronic pain. Curr. Opin. Pharmacol. 2012, 12, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Copani, A.; Zammataro, M.; Battaglia, G.; Gereau, R.W., IV; Nicoletti, F. Transcriptional regulation of type-2 metabotropic glutamate receptors: An epigenetic path to novel treatments for chronic pain. Trends Pharmacol. Sci. 2010, 31, 153–160. [Google Scholar] [CrossRef]
- Chiechio, S.; Copani, A.; De Petris, L.; Morales, M.E.P.; Nicoletti, F.; Gereau, R.W. Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-κB pathway in primary dorsal root ganglia neurons: A possible mechanism for the analgesic effect of L-acetylcarnitine. Mol. Pain 2006, 2, 1–9. [Google Scholar] [CrossRef]
- Formaggio, F.; Rimondini, R.; Delprete, C.; Scalia, L.; Merlo Pich, E.; Liguori, R.; Nicoletti, F.; Caprini, M. L-Acetylcarnitine causes analgesia in mice modeling Fabry disease by up-regulating type-2 metabotropic glutamate receptors. Mol. Pain 2022, 18, 17448069221087033. [Google Scholar] [CrossRef]
- Burand, A.J., Jr.; Stucky, C.L. Fabry disease pain: Patient and preclinical parallels. Pain 2021, 162, 1305–1321. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Fabry disease—A multisystemic disease with gastrointestinal manifestations. Gut Microbes 2022, 14, 2027852. [Google Scholar] [CrossRef]
- Johnson, M.; Kelly, G.; Chamberlain, M. Blockade of pilocarpine-induced cerebellar phosphoinositide hydrolysis with metabotropic glutamate antagonists: Evidence for an indirect control of granule cell glutamate release by muscarinic agonists. Neurosci. Lett. 2000, 285, 71–75. [Google Scholar] [CrossRef]
- Smeland, O.B.; Meisingset, T.W.; Borges, K.; Sonnewald, U. Chronic acetyl-L-carnitine alters brain energy metabolism and increases noradrenaline and serotonin content in healthy mice. Neurochem. Int. 2012, 61, 100–107. [Google Scholar] [CrossRef]
- Gülcin, İ. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Meisingset, T.W.; Sonnewald, U. Dietary supplementation with acetyl-l-carnitine in seizure treatment of pentylenetetrazole kindled mice. Neurochem. Int. 2012, 61, 444–454. [Google Scholar] [CrossRef]
- Hack, A.; Busch, V.; Pascher, B.; Busch, R.; Bieger, I.; Gempel, K.; Baumeister, F.A. Monitoring of ketogenic diet for carnitine metabolites by subcutaneous microdialysis. Pediatr. Res. 2006, 60, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.; Liu, S.; Ruenes, G.; Kajander, K.; Brewer, K. Excitotoxic spinal cord injury: Behavioral and morphological characteristics of a central pain model. Pain 1998, 75, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Inquimbert, P.; Moll, M.; Latremoliere, A.; Tong, C.-K.; Whang, J.; Sheehan, G.F.; Smith, B.M.; Korb, E.; Athié, M.C.; Babaniyi, O. NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep. 2018, 23, 2678–2689. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Motta, E.M.; Manjavachi, M.N.; Cola, M.; Calixto, J.B. Activation of the alpha-7 nicotinic acetylcholine receptor (α7 nAchR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice. Neuropharmacology 2012, 63, 798–805. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Matera, C.; Zanardelli, M.; Mello, T.; De Amici, M.; Dallanoce, C.; Ghelardini, C. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: Effects of selective activation. Neuropharmacology 2014, 79, 37–48. [Google Scholar] [CrossRef]
- Gill, E.L.; Raman, S.; Yost, R.A.; Garrett, T.J.; Vedam-Mai, V. l-Carnitine Inhibits Lipopolysaccharide-Induced Nitric Oxide Production of SIM-A9 Microglia Cells. ACS Chem. Neurosci. 2018, 9, 901–905. [Google Scholar] [CrossRef]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Rawji, K.S.; Yong, V.W. The Benefits and Detriments of Macrophages/Microglia in Models of Multiple Sclerosis. Clin. Dev. Immunol. 2013, 2013, 948976. [Google Scholar] [CrossRef] [PubMed]
- Waltl, I.; Kalinke, U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 2022, 45, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Iryo, Y.; Matsuoka, M.; Igisu, H.; Ikeda, M. Suppression of pentylenetetrazol-induced seizures by carnitine in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 1997, 355, 545–549. [Google Scholar] [CrossRef]
- Rose, J.; Brian, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Mitochondrial Metabolism in Astrocytes Regulates Brain Bioenergetics, Neurotransmission and Redox Balance. Front. Neurosci. 2020, 14, 536682. [Google Scholar] [CrossRef] [PubMed]
- Karalija, A.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Novikov, L.N. The effects of N-acetyl-cysteine and acetyl-L-carnitine on neural survival, neuroinflammation and regeneration following spinal cord injury. Neuroscience 2014, 269, 143–151. [Google Scholar] [CrossRef]
- Morales-Soto, W.; Gulbransen, B.D. Enteric Glia: A New Player in Abdominal Pain. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 433–445. [Google Scholar] [CrossRef]
- Meira de-Faria, F.; Casado-Bedmar, M.; Mårten Lindqvist, C.; Jones, M.P.; Walter, S.A.; Keita, Å.V. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 33, e14130. [Google Scholar] [CrossRef]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef]
- Hald, A. Spinal Astrogliosis in Pain Models: Cause and Effects. Cell. Mol. Neurobiol. 2009, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Lucarini, E.; Lambertucci, C.; Fornai, M.; Pellegrini, C.; Benvenuti, L.; Di Cesare Mannelli, L.; Spinaci, A.; Marucci, G.; Blandizzi, C.; et al. The Anti-Inflammatory and Pain-Relieving Effects of AR170, an Adenosine A(3) Receptor Agonist, in a Rat Model of Colitis. Cells 2020, 9, 1509. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, E.; Micheli, L.; Toti, A.; Ciampi, C.; Margiotta, F.; Di Cesare Mannelli, L.; Ghelardini, C. Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System. Int. J. Mol. Sci. 2023, 24, 14841. https://doi.org/10.3390/ijms241914841
Lucarini E, Micheli L, Toti A, Ciampi C, Margiotta F, Di Cesare Mannelli L, Ghelardini C. Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System. International Journal of Molecular Sciences. 2023; 24(19):14841. https://doi.org/10.3390/ijms241914841
Chicago/Turabian StyleLucarini, Elena, Laura Micheli, Alessandra Toti, Clara Ciampi, Francesco Margiotta, Lorenzo Di Cesare Mannelli, and Carla Ghelardini. 2023. "Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System" International Journal of Molecular Sciences 24, no. 19: 14841. https://doi.org/10.3390/ijms241914841
APA StyleLucarini, E., Micheli, L., Toti, A., Ciampi, C., Margiotta, F., Di Cesare Mannelli, L., & Ghelardini, C. (2023). Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System. International Journal of Molecular Sciences, 24(19), 14841. https://doi.org/10.3390/ijms241914841







