A Neutral Polysaccharide from Spores of Ophiocordyceps gracilis Regulates Oxidative Stress via NRF2/FNIP1 Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Weight and Chemical Composition of GSP-1a
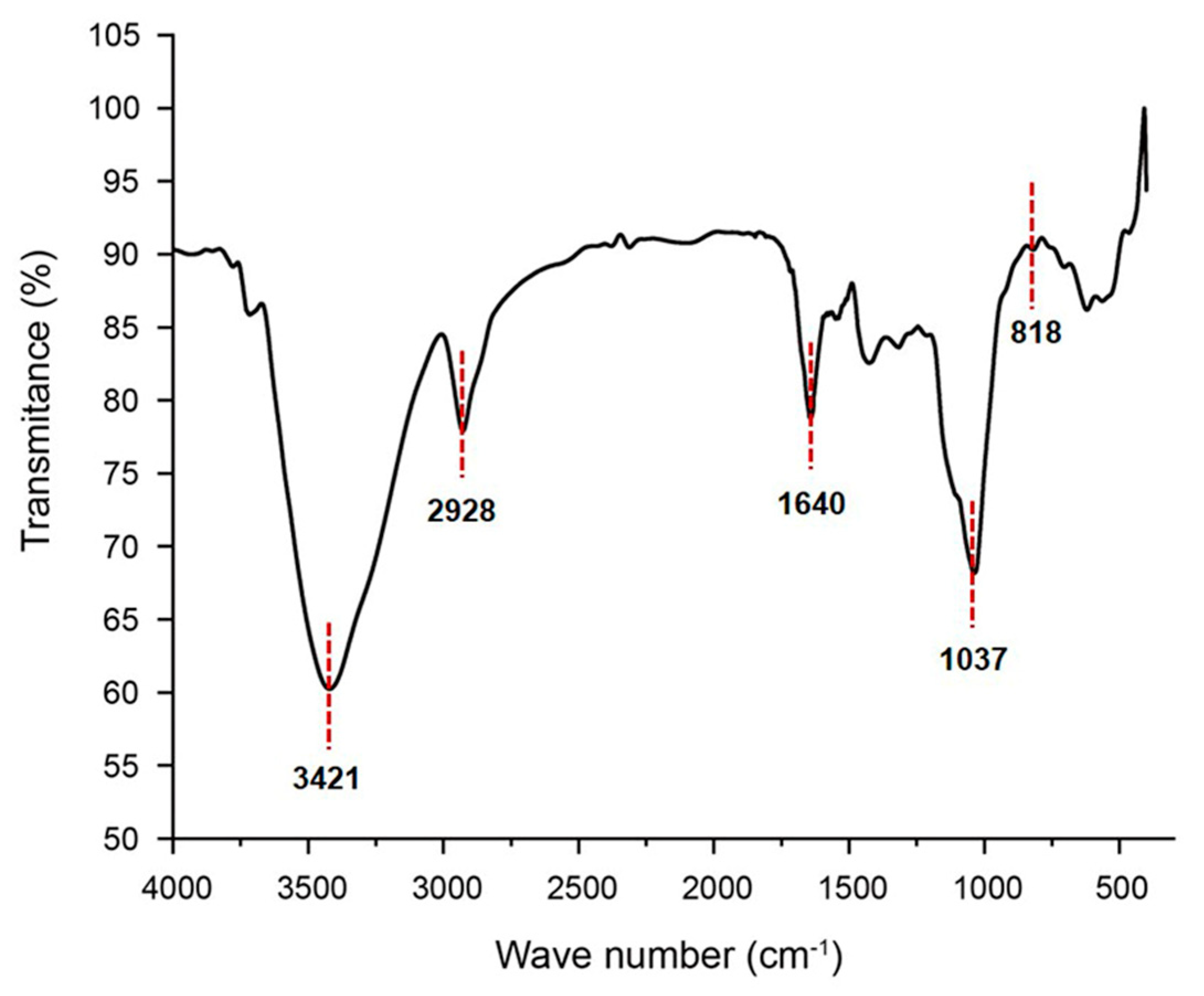
2.2. FT–IR Analysis of GSP-1a
2.3. Methylation Analysis of GSP-1a
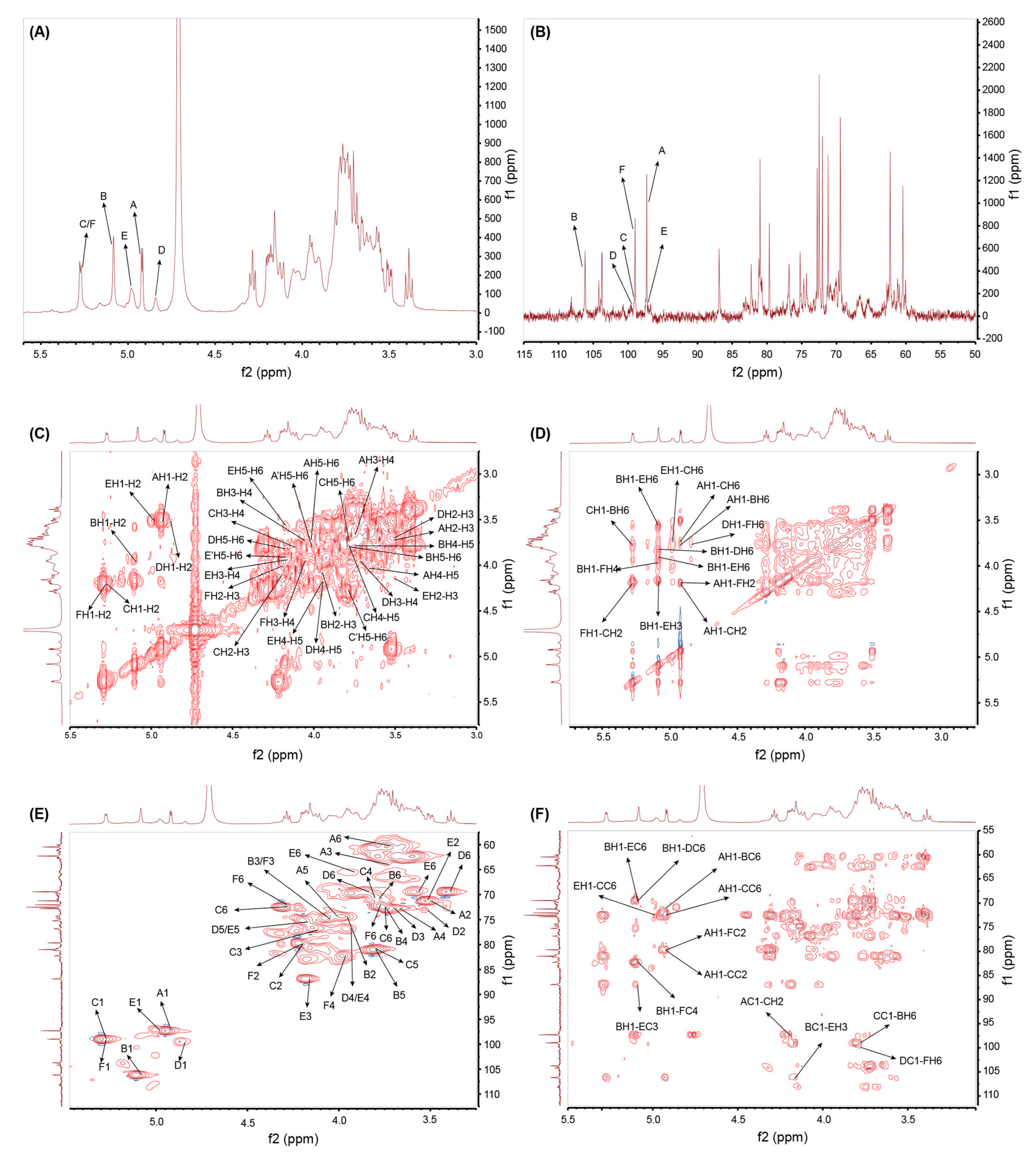
2.4. NMR Spectroscopy Analysis of GSP-1a
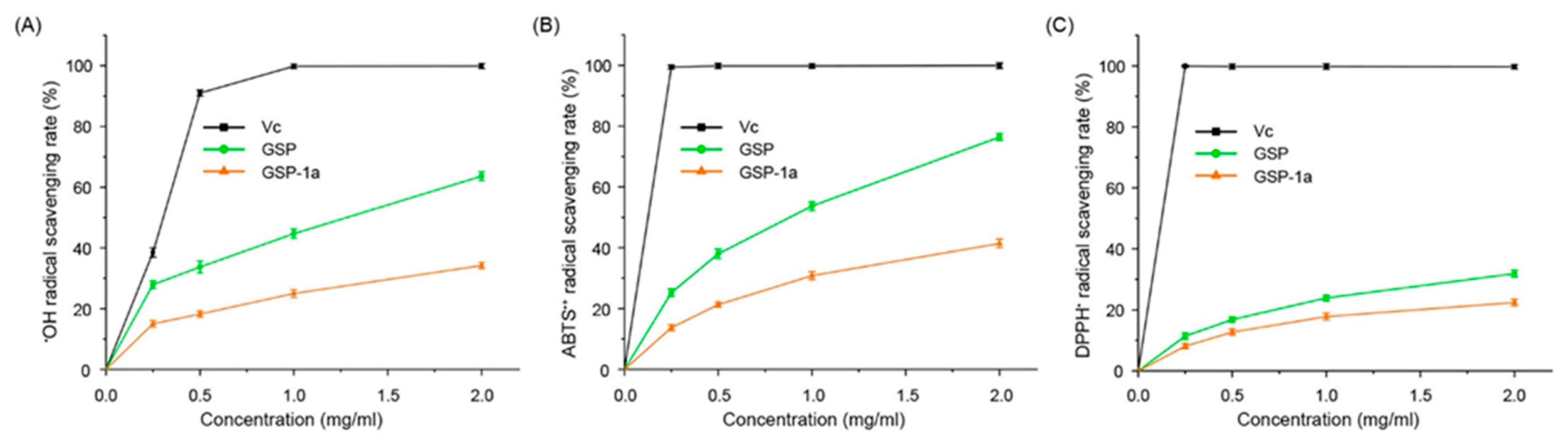
2.5. Antioxidant Activities In Vitro of GSP-1a
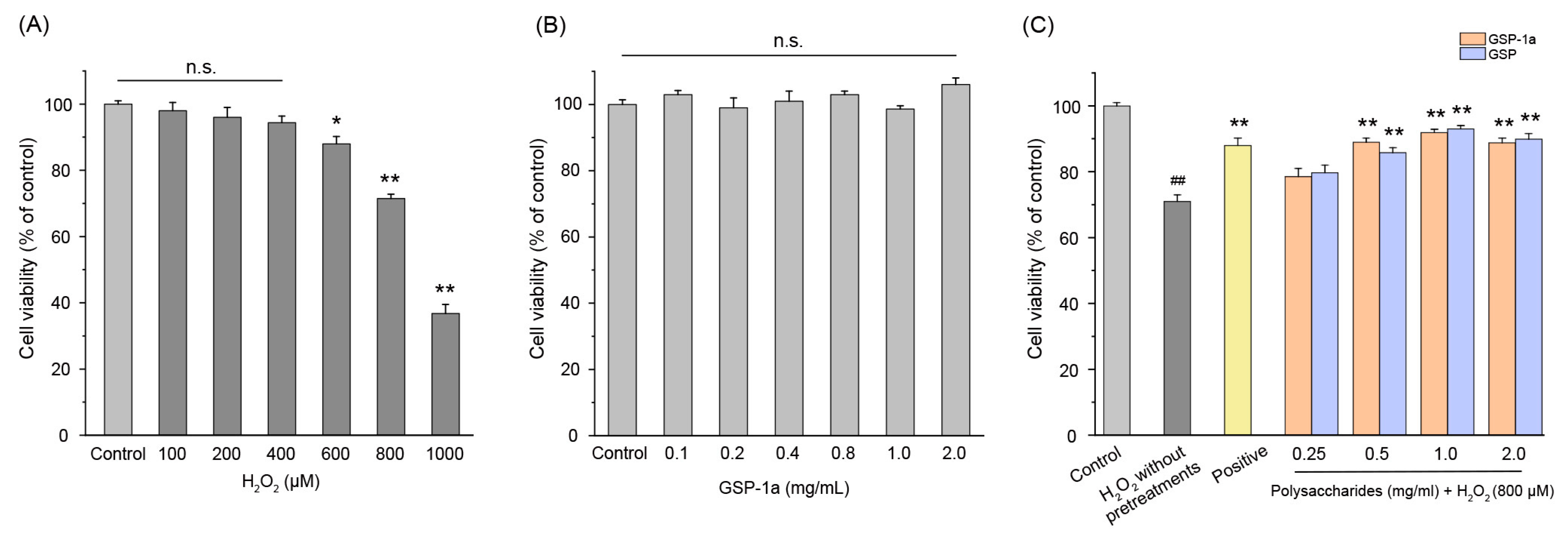
2.6. Effect of GSP-1a on H2O2-Induced Oxidative Stress
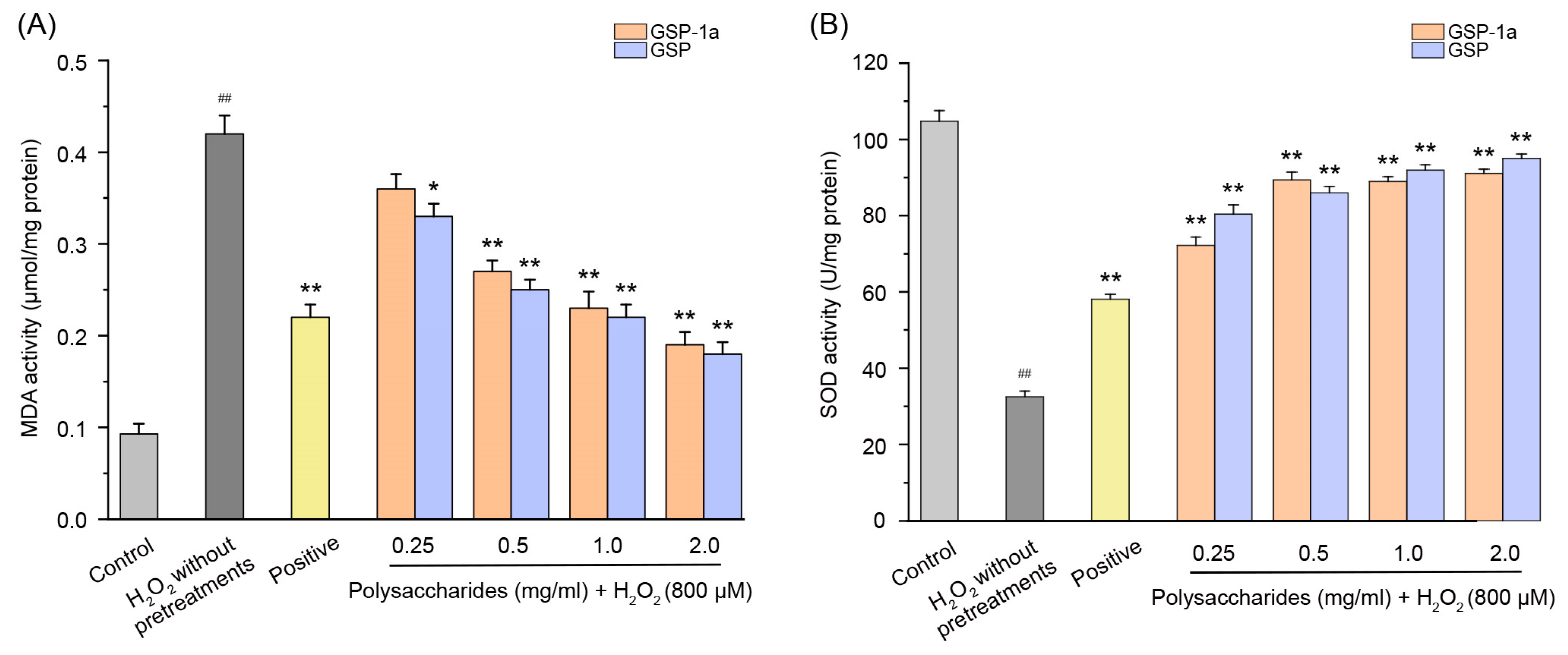
2.6.1. Effect of GSP-1a on the Change of ROS, MDA Content, and SOD Activities in H2O2-Treated HepG2 Cells
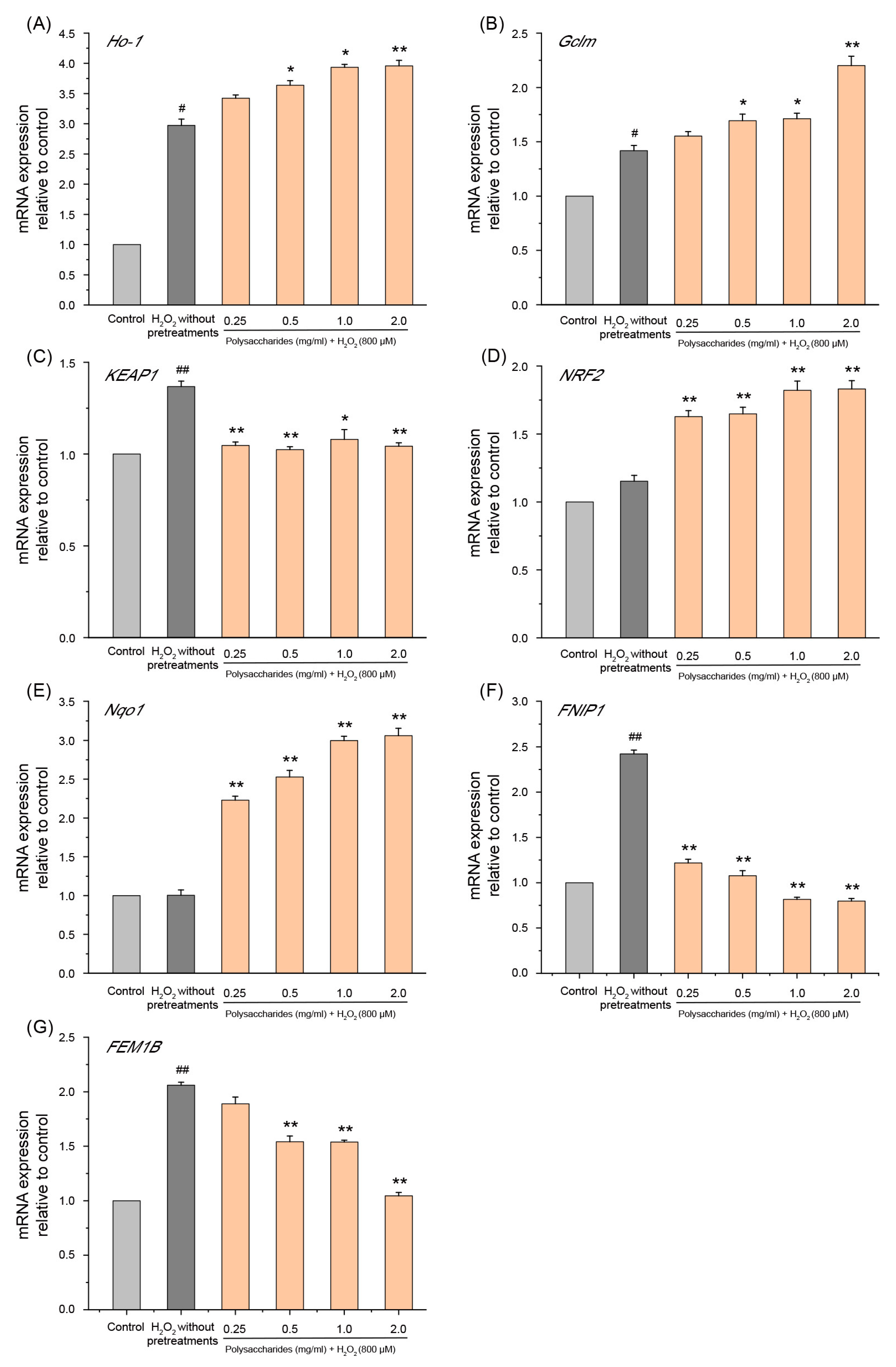
2.6.2. Effect of GSP-1a on mRNA Expression of NRF2 and FNIP1 Pathway
3. Materials and Methods
3.1. Materials
3.2. Polysaccharides Extraction of O. gracilis Spores
3.2.1. Preparation of Crude Polysaccharides
3.2.2. Isolation and Purification of Polysaccharides
3.3. Chemical Composition, UV Spectroscopy, and Molecular Weight Analysis
3.4. Structural Characterization of GSP-1a
3.4.1. FT–IR Analysis
3.4.2. Monosaccharide Composition Analysis
3.4.3. Methylation Analysis
3.4.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.5. Assay of Antioxidant Activity In Vitro
3.6. Cellular Antioxidant Activity
3.6.1. Cell Culture
3.6.2. Oxidative Stress Model Induced by H2O2
3.6.3. Treatment of Cells and Cell Viability Assay
3.6.4. Determination of ROS, MDA Content and SOD Activity
3.6.5. Total RNA Extraction and qRT-PCR
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, V.; Singh, A.; Arya, A.; Sindhu, S.C.; Sindhu, A.; Singh, A. Enhanced production of cordycepin in Ophiocordyceps sinensis using growth supplements under submerged conditions. Biotechnol. Rep. 2020, 28, e00557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef]
- Li, L.F.; But, G.W.C.; Zhang, Q.W.; Liu, M.; Chen, M.M.; Wen, X.; Wu, H.Y.; Cheng, H.Y.; Puno, P.T.; Zhang, J.X.; et al. A specific and bioactive polysaccharide marker for Cordyceps. Carbohydr. Polym. 2021, 269, 118343. [Google Scholar] [CrossRef]
- Perez-Villamares, J.C.; Burrola-Aguilar, C.; Aguilar-Miguel, X.; Sanjuan, T.; Jimenez-Sanchez, E. New records of entomopathogenous fungi of the genus Cordyceps s. l. (Ascomycota: Hypocreales) from the State of Mexico. Rev. Mex. Biodivers. 2017, 88, 773–783. [Google Scholar] [CrossRef]
- Tong, L.L.; Wang, Y.; Yuan, L.; Liu, M.Z.; Du, Y.H.; Mu, X.Y.; Yang, Q.H.; Wei, S.X.; Li, J.Y.; Wang, M.A.; et al. Enhancement of polysaccharides production using microparticle enhanced technology by Paraisaria dubia. Microb. Cell Factories 2022, 21, 12. [Google Scholar] [CrossRef]
- Tong, L.L.; Wang, Y.; Du, Y.H.; Yuan, L.; Liu, M.Z.; Mu, X.Y.; Chen, Z.L.; Zhang, Y.D.; He, S.J.; Li, X.J.; et al. Transcriptomic Analysis of Morphology Regulatory Mechanisms of Microparticles to Paraisaria dubia in Submerged Fermentation. Appl. Biochem. Biotechnol. 2022, 194, 4333–4347. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Lau, C.B.S.; Fung, K.P.; Leung, P.C.; Ko, W.H. Effects of Cordyceps sinensis, Cordyceps militaris and their isolated compounds on ion transport in Calu-3 human airway epithelial cells. J. Ethnopharmacol. 2008, 117, 92–101. [Google Scholar] [CrossRef]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; El-Shazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Wen, C.T.; Duan, Y.Q.; Zhang, H.H.; Ma, H.L. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.S.; Cui, X.L.; Chen, Z.Y.; Huang, L.J.; Song, L.Y.; Liu, T.; Lv, W.J.; Yu, R.M. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014, 102, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Yu, X.F.; Ge, Q.; Li, J.; Wang, D.J.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Y.; Cui, Y.S.; Liu, H.M.; Dong, C.X.; Sun, Y.X. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020, 235, 115969. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Yang, X.; Zhang, H. Extracellular polysaccharide biosynthesis in Cordyceps. Crit. Rev. Microbiol. 2020, 46, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef]
- Wen, X.; Wu, J.M.; Wang, F.T.; Liu, B.; Huang, C.H.; Wei, Y.Q. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 2013, 65, 402–410. [Google Scholar] [CrossRef]
- Milisav, I.; Ribaric, S.; Poljsak, B. Antioxidant Vitamins and Ageing. Subcell. Biochem. 2018, 90, 1–23. [Google Scholar] [CrossRef]
- Wu, D.T.; Lv, G.P.; Zheng, J.; Li, Q.; Ma, S.C.; Li, S.P.; Zhao, J. Cordyceps collected from Bhutan, an appropriate alternative of Cordyceps sinensis. Sci. Rep. 2016, 6, 37668. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cheong, K.L.; Wu, D.T.; Meng, L.Z.; Zhao, J.; Li, S.P. Fermentation optimization for the production of bioactive polysaccharides from Cordyceps sinensis fungus UM01. Int. J. Biol. Macromol. 2015, 79, 180–185. [Google Scholar] [CrossRef]
- Zhao, S.; Rong, C.B.; Gao, Y.; Wu, L.F.; Luo, X.H.; Song, S.; Liu, Y.; Wong, J.H.; Wang, H.X.; Yi, L.T.; et al. Antidepressant-like effect of Ganoderma lucidum spore polysaccharide-peptide mediated by upregulation of prefrontal cortex brain-derived neurotrophic factor. Appl. Microbiol. Biotechnol. 2021, 105, 8675–8688. [Google Scholar] [CrossRef]
- Wen, L.R.; Sheng, Z.L.; Wang, J.P.; Jiang, Y.M.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.Y.; Li, C.; Shao, Y.; Chen, A.H. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, T.T.; Li, H.; Wang, Y.D.; Wang, J.H.; Yuan, H.B. Structural Characterization and Hypoglycemic Function of Polysaccharides from Cordyceps cicadae. Molecules 2023, 28, 526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, L.L.; Yuan, L.; Liu, M.Z.; Du, Y.H.; Yang, L.H.; Ren, B.; Guo, D.S. Integration of Physiological, Transcriptomic and Metabolomic Reveals Molecular Mechanism of Paraisaria dubia Response to Zn2+ Stress. J. Fungi 2023, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wei, S.; Huang, H. Recent advances in Grifola frondosa polysaccharides: Production, properties, and bioactivities. Curr. Opin. Food Sci. 2022, 49, 100946. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhong, R.F.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.M.; Linhardt, R.J. Structural and immunological studies on the polysaccharide from spores of a medicinal entomogenous fungus Paecilomyces cicadae. Carbohydr. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.L.; Liang, L.; Huang, L.L.; Yu, G.Y.; Li, Q.H. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Wang, S.N.; Zhao, L.L.; Li, Q.H.; Liu, C.; Han, J.L.; Zhu, L.J.; Zhu, D.S.; He, Y.T.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocolloid 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jimenez-Escrig, A.; Ruperez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef]
- Li, F.S.; Hu, X.; Qin, L.Y.; Li, H.S.; Yang, Y.; Zhang, X.L.; Lu, J.R.; Li, Y.M.; Bao, M.T. Characterization and protective effect against ultraviolet radiation of a novel exopolysaccharide from Bacillus marcorestinctum QDR3-1. Int. J. Biol. Macromol. 2022, 221, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.H.; Yin, Z.H.; Liu, X.P.; Ma, C.Y.; Wang, J.M.; Zhang, Y.; Kang, W.Y. A glucomannogalactan from Pleurotus geesteranus: Structural characterization, chain conformation and immunological effect. Carbohydr. Polym. 2022, 287, 119346. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Qiu, Z.C.; Li, L.Y.; Vidyarthi, S.K.; Zheng, Z.J.; Zhang, R.T. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.H.; Song, M.M.; Yin, Z.H.; Wang, G.S.; Wang, J.M.; Liu, L.J.; Kang, W.Y. Structural characterization and anticoagulant activity of homogalacturonan from durian peel. J. Mol. Struct. 2022, 1248, 131467. [Google Scholar] [CrossRef]
- Tian, J.J.; Zhang, C.P.; Wang, X.M.; Rui, X.; Zhang, Q.Q.; Chen, X.H.; Dong, M.S.; Li, W. Structural characterization and immunomodulatory activity of intracellular polysaccharide from the mycelium of Paecilomyces cicadae TJJ1213. Food Res. Int. 2021, 147, 110515. [Google Scholar] [CrossRef]
- Liu, Z.J.; Jiao, Y.C.; Lu, H.Y.; Shu, X.L.; Chen, Q.H. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wu, J.H.; Huang, M.Q.; Zhao, M.M.; Sun, W.Z.; Sun, X.T.; Zheng, F.P. Structural characterization and immuno-stimulating activities of a novel polysaccharide from Huangshui, a byproduct of Chinese Baijiu. Food Res. Int. 2020, 136, 109493. [Google Scholar] [CrossRef]
- Hu, J.H.; Yao, W.Z.; Chang, S.Y.; You, L.J.; Zhao, M.M.; Cheung, P.C.K.; Hileuskaya, K. Structural characterization and anti-photoaging activity of a polysaccharide from Sargassum fusiforme. Food Res. Int. 2022, 157, 111267. [Google Scholar] [CrossRef]
- Shi, Q.; Yan, J.Y.; Jiang, B.; Chi, X.J.; Wang, J.H.; Liang, X.M.; Ai, X.J. A general strategy for the structural determination of carbohydrates by multi-dimensional NMR spectroscopies. Carbohydr. Polym. 2021, 267, 118218. [Google Scholar] [CrossRef]
- Wang, Y.D.; Han, W.W.; Song, L.L.; Zhao, X. Compositional analysis and structural characterization of raffinose family oligosaccharides from Eupatorium. J. Food Compos. Anal. 2019, 84, 103298. [Google Scholar] [CrossRef]
- Tang, N.Y.; Wang, X.M.; Yang, R.; Liu, Z.M.; Liu, Y.X.; Tian, J.J.; Xiao, L.Y.; Li, W. Extraction, isolation, structural characterization and prebiotic activity of cell wall polysaccharide from Kluyveromyces marxianus. Carbohydr. Polym. 2022, 289, 119457. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.X.; Zhao, L.Y.; Li, Z.H.; Harqin, C.; Peng, Y.F.; Liu, J.K. Physicochemical analysis, structural elucidation and bioactivities of a high-molecular-weight polysaccharide from Phellinus igniarius mycelia. Int. J. Biol. Macromol. 2018, 120, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hou, G.H.; Li, J.L.; Surhio, M.M.; Ye, M. Structure characterization, modification through carboxymethylation and sulfation, and in vitro antioxidant and hypoglycemic activities of a polysaccharide from Lachnum sp. Process Biochem. 2018, 72, 177–187. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wu, J.H.; Zhao, M.M.; Sun, W.Z.; Sun, J.Y.; Li, H.H.; Huang, M.Q. Immunomodulatory activity of a novel polysaccharide extracted from Huangshui on THP-1 cells through NO production and increased IL-6 and TNF-alpha expression. Food Chem. 2020, 330, 127257. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Jun, L.; Wan-Rong, B.; Chung-Hang, L.; Dik-Lung, M.; Ge, Z.; Ai-Ping, L.; Shun-Chun, W.; Quan-Bin, H.J.M. Chemical Structure and Immunomodulating Activities of an α-Glucan Purified from Lobelia chinensis Lour. Molecules 2016, 21, 779. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Qiu, Y.J.; Duan, Y.Q.; He, Y.Q.; Xiang, H.; Sun, W.X.; Zhang, H.H.; Ma, H.L. Characterization, antioxidant, antineoplastic and immune activities of selenium modified Sagittaria sagittifolia L. polysaccharides. Food Res. Int. 2022, 153, 110913. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Y.X.; Chen, X.F.; Cui, D.D.; Wang, M.R.; Yang, W.J.; Chen, F.X. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.M.; Zhang, Y.B.; Zhang, H.; Chen, Y.B.; Cui, Y.L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Li, T.; Liu, Y.H.; Zhang, Z.J.; Zhang, Y.N. Purification, characterization and in vitro antioxidant activity of a polysaccharide AAP-3-1 from Auricularia auricula. Int. J. Biol. Macromol. 2020, 162, 1453–1464. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Song, X.Y.; Jiang, Y.Z.; Yao, J.R.; Jiang, Y.; Li, Z.; Dai, F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris Radix et Rhizoma. Carbohydr. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, G.; Zhang, X.F.; Wang, Y.Q.; Qiang, Y.; Wang, B.L.; Zou, J.B.; Niu, J.F.; Wang, Z.Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.P.; Li, J.W.; Deng, K.Q.; Ai, L.Z. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Kan, L.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Xie, M. Comparison of structural features and antioxidant activity of polysaccharides from natural and cultured Cordyceps sinensis. Food Sci. Biotechnol. 2017, 26, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Sun, S.; Li, R.; Shen, Z.; Wang, P.; Jiang, X. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr. Polym. 2015, 117, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, W.; Chen, Y.; Feng, S.; Qin, Y.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Extraction, Purification, Physicochemical Properties, and Activity of a New Polysaccharide From Cordyceps cicadae. Front. Nutr. 2022, 9, 911310. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M.; Muneer, G.; Zhou, Y.; Sun, L. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2019, 123, 945–956. [Google Scholar] [CrossRef]
- Li, G.Q.; Chen, P.F.; Zhao, Y.T.; Zeng, Q.H.; Ou, S.Y.; Zhang, Y.H.; Wang, P.C.; Chen, N.H.; Ou, J.Y. Isolation, structural characterization and anti-oxidant activity of a novel polysaccharide from garlic bolt. Carbohydr. Polym. 2021, 267, 118194. [Google Scholar] [CrossRef]
- Alia, M.; Ramos, S.; Mateos, R.; Bravo, L.; Goya, L. Response of the antioxidant Defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J. Biochem. Mol. Toxicol. 2005, 19, 119–128. [Google Scholar] [CrossRef]
- Guzman, T.J.; Dufer, M.; Wiedemann, M.; Olguin-Alor, R.; Soldevila, G.; Gurrola-Diaz, C.M. Lupin gamma-conglutin protects against cell death induced by oxidative stress and lipotoxicity, but transiently inhibits in vitro insulin secretion by increasing K-ATP channel currents. Int. J. Biol. Macromol. 2021, 187, 76–90. [Google Scholar] [CrossRef]
- Sun, S.Q.; Zhang, C.X.; Gao, J.H.; Qin, Q.Y.; Zhang, Y.Y.; Zhu, H.; Yang, X.J.; Yang, D.R.; Yan, H.T. Benzoquinone induces ROS-dependent mitochondria-mediated apoptosis in HL-60 cells. Toxicol. Ind. Health 2018, 34, 270–281. [Google Scholar] [CrossRef]
- Wu, J.H.; Huo, J.Y.; Huang, M.Q.; Zhao, M.M.; Luo, X.L.; Sun, B.G. Structural Characterization of a Tetrapeptide from Sesame Flavor-Type Baijiu and Its Preventive Effects against AAPH-Induced Oxidative Stress in HepG2 Cells. J. Agric. Food Chem. 2017, 65, 10495–10504. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Liu, Q.; Wang, W.; Li, X.B.; Zhang, J. A polysaccharide isolated from Cynomorium songaricum Rupr. protects PC12 cells against H2O2-induced injury. Int. J. Biol. Macromol. 2016, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.E.; Witte, M.; Hondius, D.; Rozermuller, A.J.M.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant Protection: A Promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Leng, B.; Wang, G. Pharmacology, Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can. J. Physiol. Pharmacol. 2019, 97, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Y.; Wang, G.; Ge, S.; Liu, H.J.O.M.; Longevity, C. Dendrobium officinale polysaccharides protect against MNNG-induced PLGC in rats via activating the NRF2 and antioxidant enzymes HO-1 and NQO-1. Oxid. Med. Cell. Longev. 2019, 2019, 9310245. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ko, R.S.; Goshima, H.; Koike, A.; Shibano, M.; Fujimori, K. Formononetin attenuates H2O2-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology 2021, 85, 186–200. [Google Scholar] [CrossRef]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. H2O2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res. Ther. 2020, 11, 335. [Google Scholar] [CrossRef]
- Henning, N.J.; Manford, A.G.; Spradlin, J.N.; Brittain, S.M.; Zhang, E.; McKenna, J.M.; Tallarico, J.A.; Schirle, M.; Rape, M.; Nomura, D.K. Discovery of a covalent FEM1B recruiter for targeted protein degradation applications. J. Am. Chem. Soc. 2022, 144, 701–708. [Google Scholar] [CrossRef]
- Manford, A.G.; Rodriguez-Perez, F.; Shih, K.Y.; Shi, Z.; Berdan, C.A.; Choe, M.Y.; Titov, D.V.; Nomura, D.K.; Rape, M. A Cellular Mechanism to Detect and Alleviate Reductive Stress. Cell 2020, 183, 46–61. [Google Scholar] [CrossRef]
- Dai, H.; Han, X.Q.; Gong, F.Y.; Dong, H.; Tu, P.F.; Gao, X.M. Structure elucidation and immunological function analysis of a novel β-glucan from the fruit bodies of Polyporus umbellatus (Pers.) Fries. Glycobiology 2012, 22, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.K.; Yang, T.X.; Wang, J.R. Polysaccharides from Polyporus umbellatus: A review on their extraction, modification, structure, and bioactivities. Int. J. Biol. Macromol. 2021, 189, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.H.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.Y.; Yang, Y.N. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Chen, J.C.; Yuan, Y.; Zhang, X.; Li, L.; Zhai, Y.Z.; Gong, X.; Li, B. Structural characterization, antioxidant, and anti-inflammatory activity of polysaccharides from Plumula Nelumbinis. Int. J. Biol. Macromol. 2022, 212, 111–122. [Google Scholar] [CrossRef]

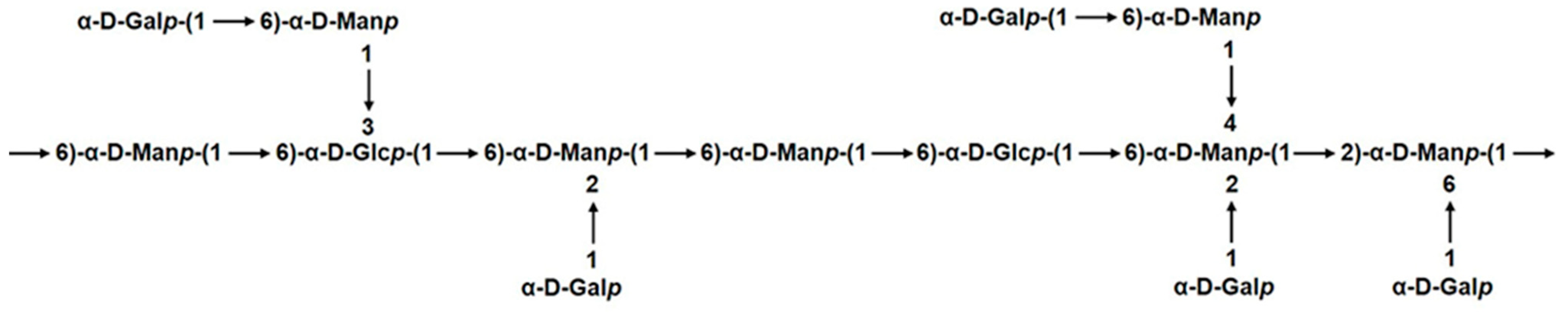

| Samples | GSP-1a |
|---|---|
| Yield/% | 33.5 ± 0.64 |
| Total carbohydrate (%) | 82 ± 1.5 |
| Protein (%) | ND |
| Nucleic acid (%) | ND |
| Mw (kDa) | 72.8 |
| Mw/Mn | 1.1 |
| Monosaccharide composition (molar ratio) | |
| Man | 42.28% |
| Gal | 35.7% |
| Glc | 22.02% |
| Retention Time (min) | Type of Linkages | PMAAs | Relative Molar Ratio (%) | Mass Fragments (m/z) |
|---|---|---|---|---|
| 8.477 | t-Gal(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 34.36 | 60, 71, 87, 102, 118, 145, 162, 205 |
| 11.847 | 2-Man(p) | 1,2,5-tri-O-acetyl-3,4,6-tri-O-methyl mannitol | 1.95 | 60, 71, 88, 118, 129, 145, 161, 190 |
| 13.074 | 6-Man(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl mannitol | 19.99 | 71, 87, 99, 102, 118, 129, 162, 173.1, 189 |
| 13.153 | 6-Glc(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl glucitol | 9.87 | 60, 87, 102, 118, 129, 137, 162, 173, 189 |
| 13.508 | 4-Glc(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 3.68 | 60, 87, 102, 118, 129, 137, 162, 191 |
| 17.316 | 3,6-Glc(p) | 1,3,5,6-tetra-O-acetyl-2,4-di-O-methyl glucitol | 9.60 | 71, 87, 101, 118, 129, 137, 49, 174, 189, 202, 234, 245, 299 |
| 17.610 | 2,6-Man(p) | 1,2,5,6-tetra-O-acetyl-3,4-di-O-methyl mannitol | 15.46 | 71, 87, 99, 118, 129, 149, 167, 189, 202, 218, 261, 299, 338 |
| 18.292 | 2,4,6-Man(p) | 1,2,4,5,6-penta-O-acetyl-3-O-methyl mannitol | 5.09 | 60, 74, 88, 117, 130, 159, 190, 202, 218, 233.1, 260, 299, 338 |
| Code | Glycosyl Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| A | α-D-Galp-(1→ | H | 4.92 | 3.50 | 3.74 | 3.66 | 4.01 | 3.71, 3.8 |
| C | 97.29 | 71.24 | 72.09 | 72.68 | 74.37 | 60.39 | ||
| B | →6)-α-D-Manp-(1→ | H | 5.08 | 3.95 | 4.05 | 3.75 | 3.78 | 3.77 |
| C | 106.28 | 74.37 | 74.72 | 72.34 | 80.94 | 70.65 | ||
| C | →2,6)-α-D-Manp-(1→ | H | 5.27 | 4.19 | 4.11 | 3.79 | 3.78 | 3.75, 4.28 |
| C | 98.97 | 79.72 | 76.88 | 70.65 | 80.94 | 72.36 | ||
| D | →6)-α-D-Glcp-(1→ | H | 4.84 | 3.50 | 3.71 | 3.94 | 4.17 | 3.39, 3.83 |
| C | 99.56 | 73.06 | 72.81 | 76.75 | 75.31 | 69.46 | ||
| E | →3,6)-α-D-Glcp-(1→ | H | 4.98 | 3.51 | 4.16 | 3.94 | 4.17 | 3.91, 3.56 |
| C | 97.24 | 71.03 | 86.89 | 76.75 | 75.31 | 69.59 | ||
| F | →2,4,6)-α-D-Manp-(1→ | H | 5.27 | 4.19 | 4.05 | 3.96 | ND | 3.76, 4.28 |
| C | 99.09 | 79.72 | 74.72 | 82.23 | ND | 72.09 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, S.; Lian, H.; Tong, L.; Yang, L.; Ren, B.; Guo, D.; Huang, H. A Neutral Polysaccharide from Spores of Ophiocordyceps gracilis Regulates Oxidative Stress via NRF2/FNIP1 Pathway. Int. J. Mol. Sci. 2023, 24, 14721. https://doi.org/10.3390/ijms241914721
Wang Y, Wei S, Lian H, Tong L, Yang L, Ren B, Guo D, Huang H. A Neutral Polysaccharide from Spores of Ophiocordyceps gracilis Regulates Oxidative Stress via NRF2/FNIP1 Pathway. International Journal of Molecular Sciences. 2023; 24(19):14721. https://doi.org/10.3390/ijms241914721
Chicago/Turabian StyleWang, Yue, Shixiang Wei, Hui Lian, Lingling Tong, Linhui Yang, Bo Ren, Dongsheng Guo, and He Huang. 2023. "A Neutral Polysaccharide from Spores of Ophiocordyceps gracilis Regulates Oxidative Stress via NRF2/FNIP1 Pathway" International Journal of Molecular Sciences 24, no. 19: 14721. https://doi.org/10.3390/ijms241914721
APA StyleWang, Y., Wei, S., Lian, H., Tong, L., Yang, L., Ren, B., Guo, D., & Huang, H. (2023). A Neutral Polysaccharide from Spores of Ophiocordyceps gracilis Regulates Oxidative Stress via NRF2/FNIP1 Pathway. International Journal of Molecular Sciences, 24(19), 14721. https://doi.org/10.3390/ijms241914721







