Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership
Abstract
:1. Introduction
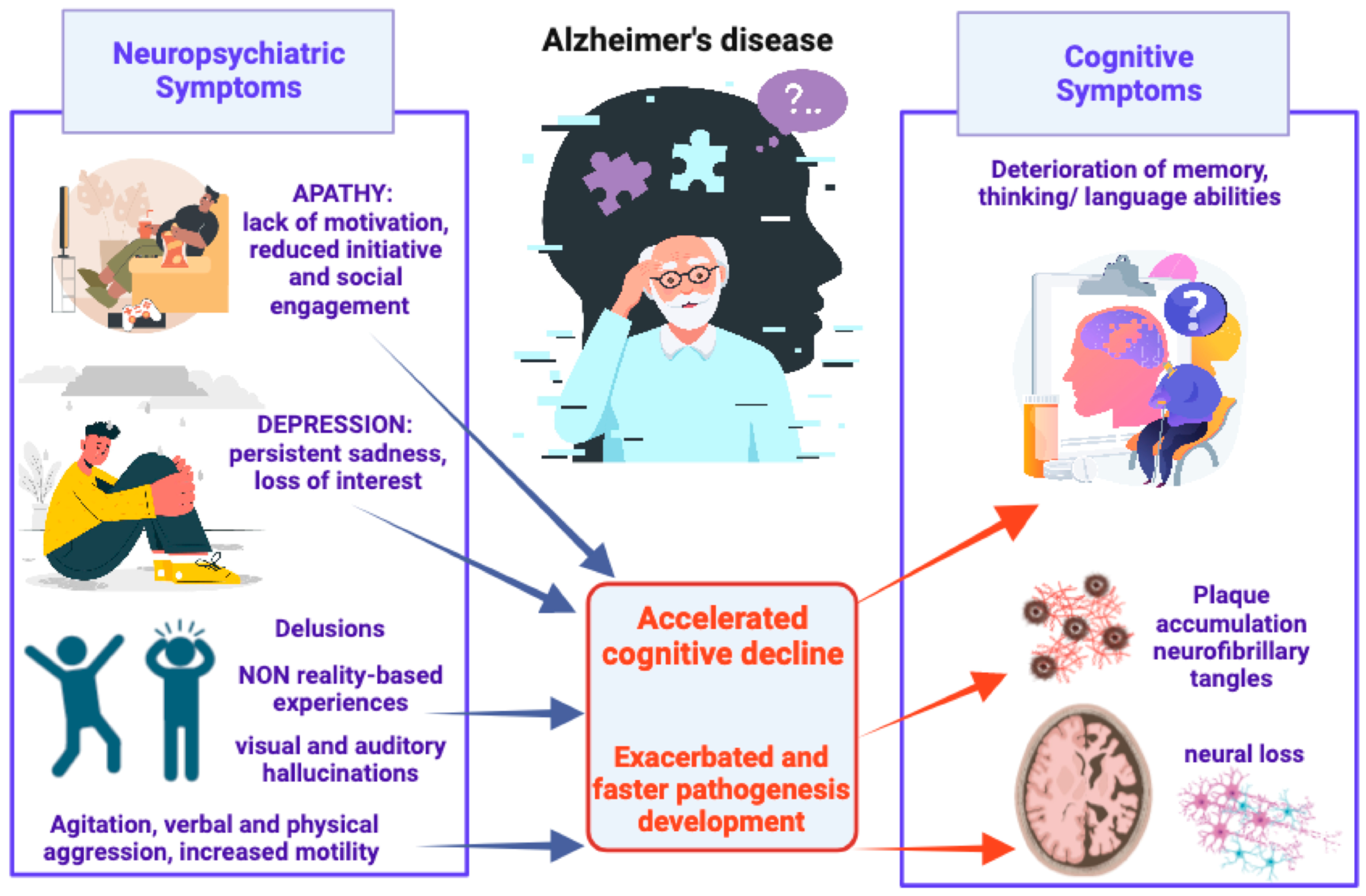
2. Alzheimer’s Disease and Neuropsychiatric Symptoms
3. Physical Exercise, Gut Microbiota, and Alzheimer’s Disease Risk
3.1. The Role of Physical Exercise for Brain Health and Alzheimer’s Disease Prevention
3.2. Exploring the Impact of Gut Microbiota Dysbiosis and Neuroinflammation in Alzheimer’s Disease Pathogenesis
3.3. A Complex Connection: Neurotransmitter Deregulation, Gut–Brain Axis, and Neurological Disorders
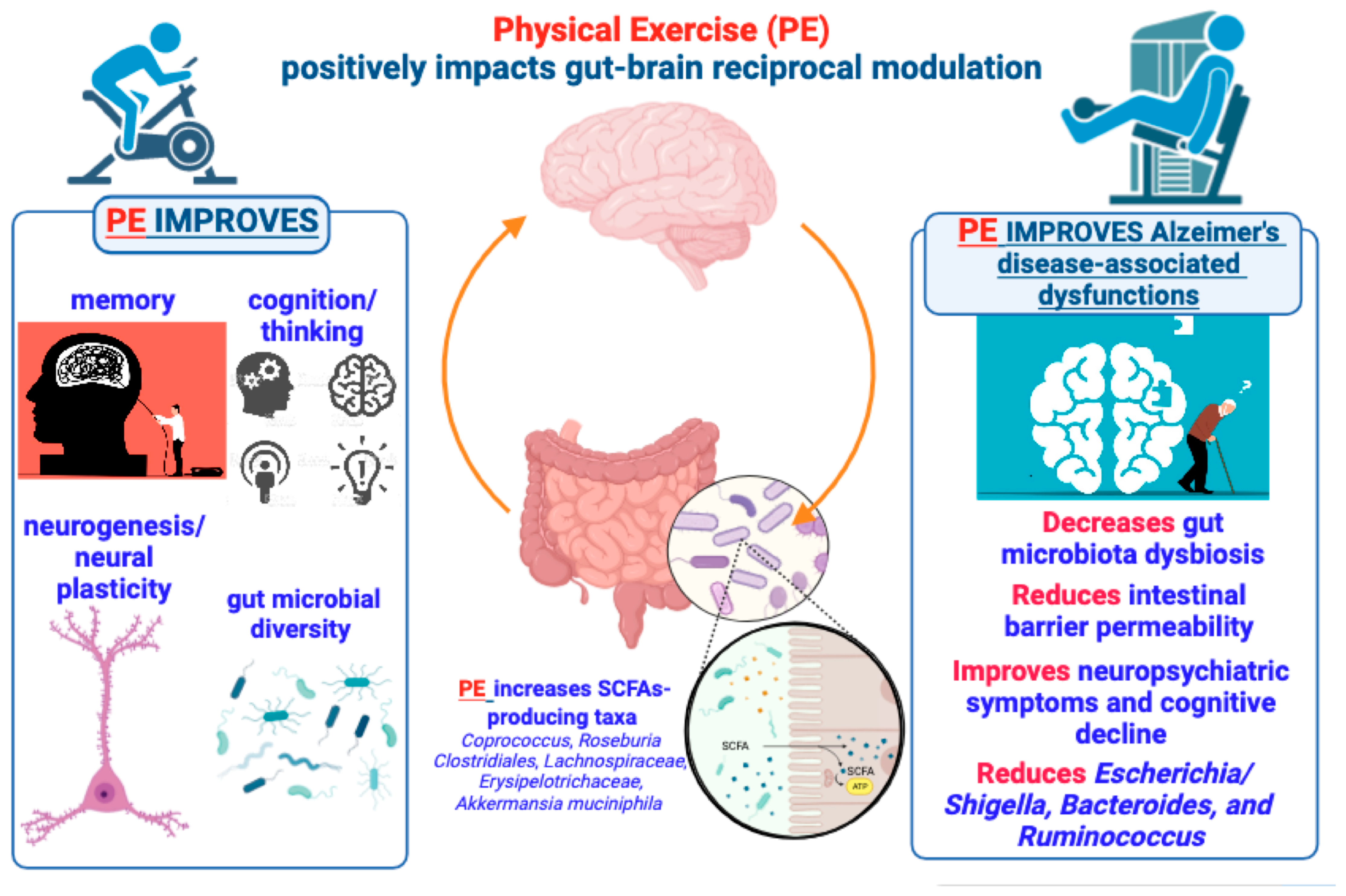
3.4. Impact of Physical Exercise on the Gut–Brain Axis and Neurological Disorders: An Overlooked Role
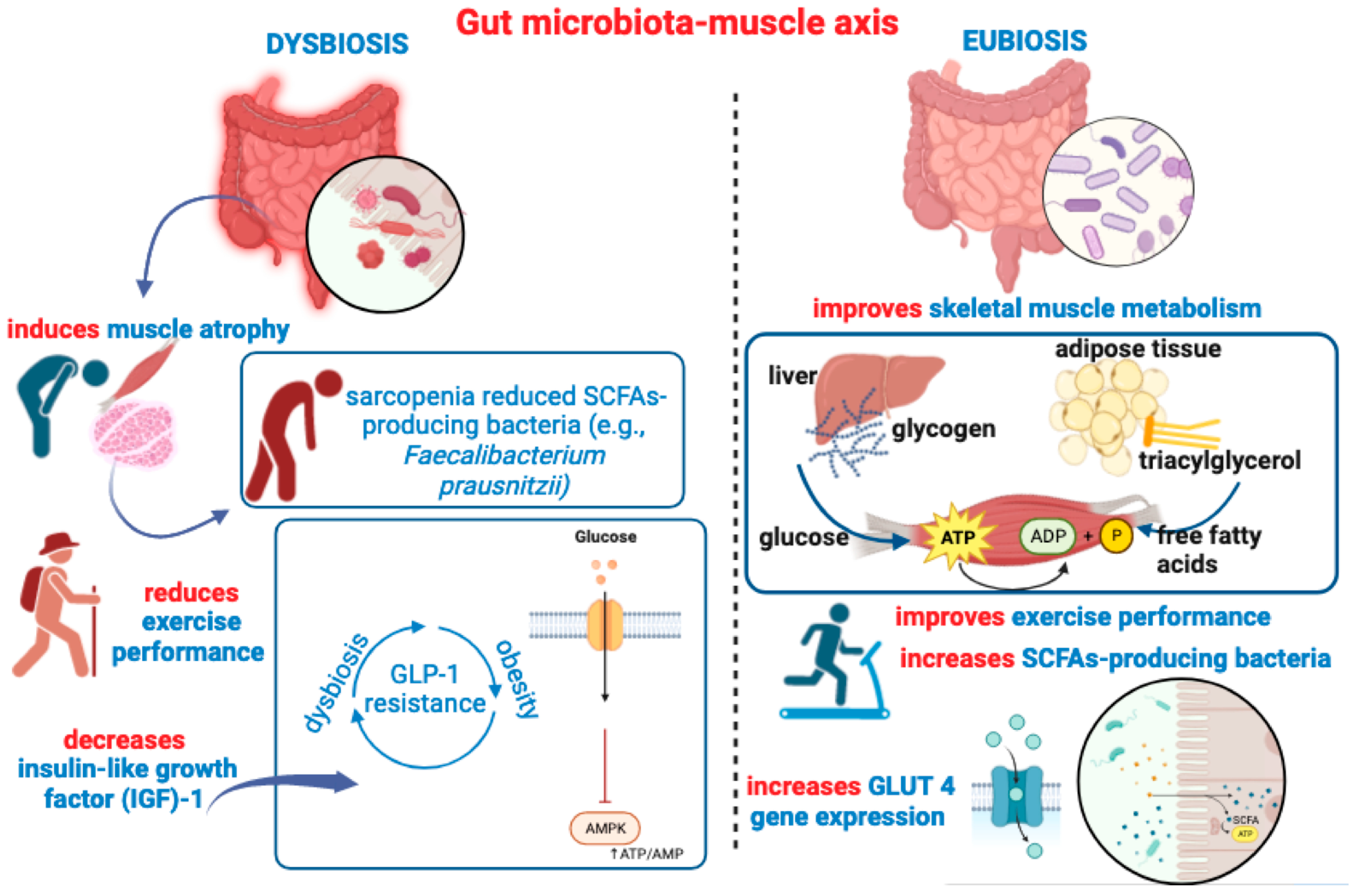
4. Gut Microbiota-to-Skeletal Muscle Axis
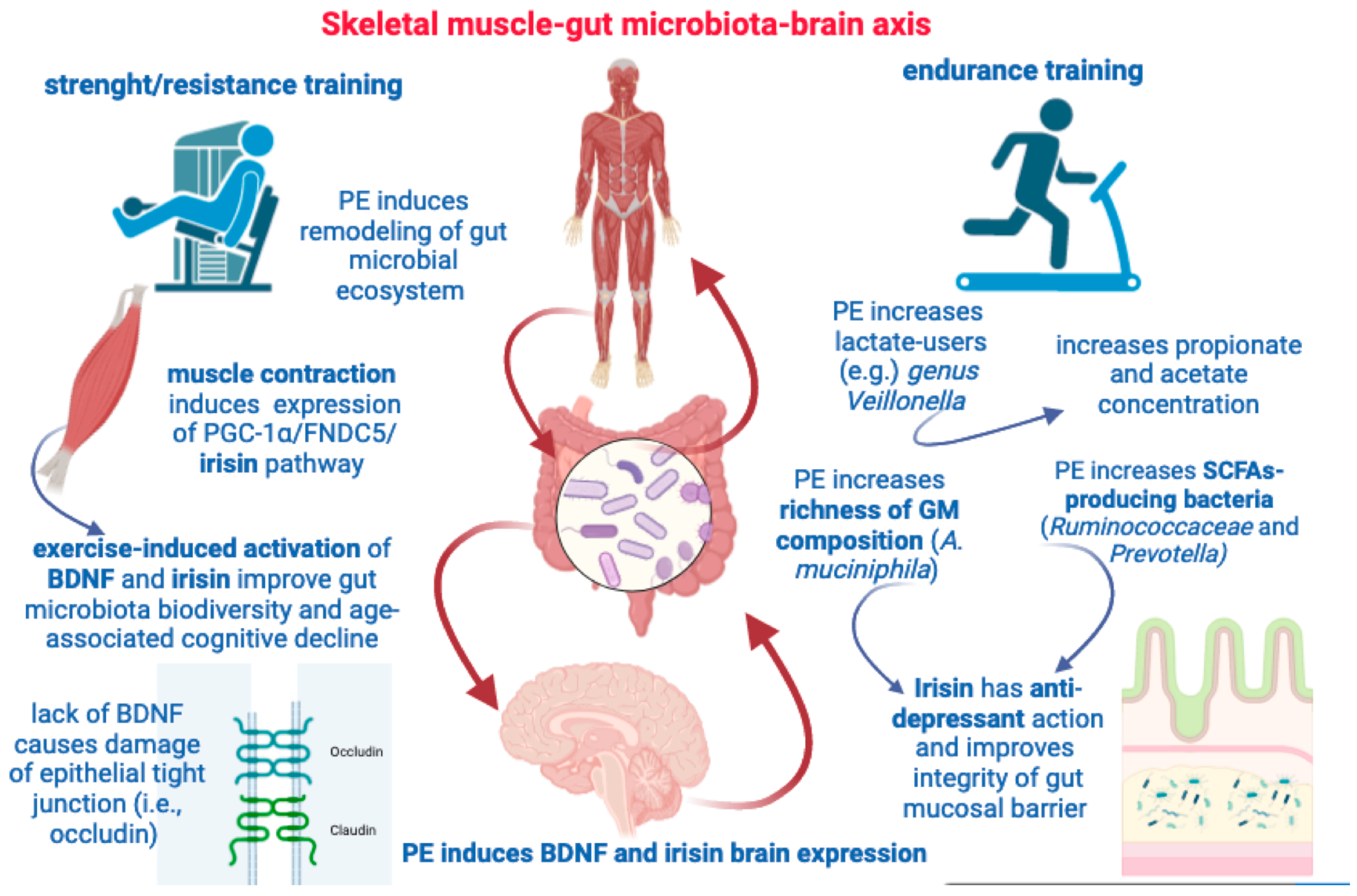
5. Skeletal Muscle-to-Gut Microbiota Axis
5.1. Irisin and BDNF Are Paradigmatic Myokines Linking Physical Activity to the Shaping of Gut Microbiota
5.2. Irisin and BDNF as “Ideal” Players Connecting Physical Activity and Risk of Developing AD
5.3. Irisin, BDNF, and the Anxiety–Depression Spectrum: Muscle–Gut–Brain Axis and Non-Cognitive Symptoms in Alzheimer’s Disease
6. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Morres, I.D.; Hatzigeorgiadis, A.; Stathi, A.; Comoutos, N.; Arpin-Cribbie, C.; Krommidas, C.; Theodorakis, Y. Aerobic Exercise for Adult Patients with Major Depressive Disorder in Mental Health Services: A Systematic Review and Meta-Analysis. Depress. Anxiety 2019, 36, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a Treatment for Depression: A Meta-Analysis Adjusting for Publication Bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Brundin, L.; Erhardt, S.; Hållmarker, U.; James, S.; Deierborg, T. Physical Activity Is Associated With Lower Long-Term Incidence of Anxiety in a Population-Based, Large-Scale Study. Front. Psychiatry 2021, 12, 714014. [Google Scholar] [CrossRef] [PubMed]
- Dasso, N.A. How Is Exercise Different from Physical Activity? A Concept Analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef]
- Murri, M.B.; Ekkekakis, P.; Magagnoli, M.; Zampogna, D.; Cattedra, S.; Capobianco, L.; Serafini, G.; Calcagno, P.; Zanetidou, S.; Amore, M. Physical Exercise in Major Depression: Reducing the Mortality Gap While Improving Clinical Outcomes. Front. Psychiatry 2019, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the Risk of Cognitive Decline and Dementia: WHO Recommendations. Front. Neurol. 2022, 12, 765584. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical Activity Can Improve Cognition in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Scheltens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The Effect of Physical Activity on Cognitive Function in Patients with Dementia: A Meta-Analysis of Randomized Control Trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Girdler, S.J.; Confino, J.E.; Woesner, M.E. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol. Bull. 2019, 49, 56–69. [Google Scholar]
- Zhou, S.; Chen, S.; Liu, X.; Zhang, Y.; Zhao, M.; Li, W. Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 1216. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric Symptoms in Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.E.; Schwartz, S.; Han, D.; Rabins, P.V.; Steinberg, M.; Tschanz, J.T.; Lyketsos, C.G. Neuropsychiatric Symptoms as Predictors of Progression to Severe Alzheimer’s Dementia and Death: The Cache County Dementia Progression Study. Am. J. Psychiatry 2015, 172, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.J.; Raman, R.; He, F.; Salmon, D.P.; Ferris, S.; Aisen, P.; Cummings, J. The Alzheimer’s Disease Cooperative Study Prevention Instrument Project: Longitudinal Outcome of Behavioral Measures as Predictors of Cognitive Decline. Dement. Geriatr. Cogn. Disord. Extra 2014, 4, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Roberto, N.; Portella, M.J.; Marquié, M.; Alegret, M.; Hernández, I.; Mauleón, A.; Rosende-Roca, M.; Abdelnour, C.; Esteban de Antonio, E.; Tartari, J.P.; et al. Neuropsychiatric Profile as a Predictor of Cognitive Decline in Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 718949. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, V.; Ismail, Z.; Nikolai, T.; Markova, H.; Cechova, K.; Nedelska, Z.; Laczó, J.; Wang, M.; Hort, J.; Vyhnalek, M. Mild Behavioral Impairment Is Associated With Atrophy of Entorhinal Cortex and Hippocampus in a Memory Clinic Cohort. Front. Aging Neurosci. 2021, 13, 643271. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Breteler, M.M.B.; Dekosky, S.T.; Dorenlot, P.; Fratiglioni, L.; Hock, C.; Kenigsberg, P.A.; Scheltens, P.; De Strooper, B. The World of Dementia beyond 2020. J. Am. Geriatr. Soc. 2011, 59, 923–927. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Liu, K.Y.; Villain, N.; Ayton, S.; Ackley, S.F.; Planche, V.; Howard, R.; Thambisetty, M. Key Questions for the Evaluation of Anti-Amyloid Immunotherapies for Alzheimer’s Disease. Brain Commun. 2023, 5, fcad175. [Google Scholar] [CrossRef]
- Van Dyck, C.H. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol. Psychiatry 2018, 83, 311–319. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Rojiani, A.; Rosenthal, A.; Levkowitz, G.; Subbarao, S.; Alamed, J.; Wilson, D.; Wilson, N.; Freeman, M.J.; Gordon, M.N.; et al. Passive Amyloid Immunotherapy Clears Amyloid and Transiently Activates Microglia in a Transgenic Mouse Model of Amyloid Deposition. J. Neurosci. 2004, 24, 6144–6151. [Google Scholar] [CrossRef] [PubMed]
- Latina, V.; Giacovazzo, G.; Cordella, F.; Balzamino, B.O.; Micera, A.; Varano, M.; Marchetti, C.; Malerba, F.; Florio, R.; Ercole, B.B.; et al. Systemic Delivery of a Specific Antibody Targeting the Pathological N-Terminal Truncated Tau Peptide Reduces Retinal Degeneration in a Mouse Model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Borreca, A.; Latina, V.; Giacovazzo, G.; Pignataro, A.; Krashia, P.; Natale, F.; Cocco, S.; Rinaudo, M.; Malerba, F.; et al. Passive Immunotherapy for N-Truncated Tau Ameliorates the Cognitive Deficits in Two Mouse Alzheimer’s Disease Models. Brain Commun. 2020, 2, fcaa039. [Google Scholar] [CrossRef] [PubMed]
- Latina, V.; Giacovazzo, G.; Calissano, P.; Atlante, A.; La Regina, F.; Malerba, F.; Dell’aquila, M.; Stigliano, E.; Balzamino, B.O.; Micera, A.; et al. Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer’s Disease (sAD) Mouse Model. Int. J. Mol. Sci. 2021, 22, 12158. [Google Scholar] [CrossRef] [PubMed]
- Yadollahikhales, G.; Rojas, J.C. Anti-Amyloid Immunotherapies for Alzheimer’s Disease: A 2023 Clinical Update. Neurotherapeutics 2023, 20, 914–931. [Google Scholar] [CrossRef]
- Iwatsubo, T. Clinical Implementation of Lecanemab: Challenges, Questions and Solutions. J. Prev. Alzheimer’s Dis. 2023, 10, 353–355. [Google Scholar] [CrossRef]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Theleritis, C.; Politis, A.; Siarkos, K.; Lyketsos, C.G. A Review of Neuroimaging Findings of Apathy in Alzheimer’s Disease. Int. Psychogeriatr. 2014, 26, 195–207. [Google Scholar] [CrossRef]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Assessment and Management of Behavioral and Psychological Symptoms of Dementia. BMJ 2015, 350, h369. [Google Scholar] [CrossRef]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Management of Neuropsychiatric Symptoms of Dementia in Clinical Settings: Recommendations from a Multidisciplinary Expert Panel. J. Am. Geriatr. Soc. 2014, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Majer, R.; Adeyi, O.; Bagoly, Z.; Simon, V.; Csiba, L.; Kardos, L.; Hortobágyi, T.; Frecska, E. Neuropsychiatric Symptoms, Quality of Life and Caregivers’ Burden in Dementia. Open Med. 2020, 15, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Coccurello, R.; Moles, A. Potential Mechanisms of Atypical Antipsychotic-Induced Metabolic Derangement: Clues for Understanding Obesity and Novel Drug Design. Pharmacol. Ther. 2010, 127, 210–251. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, E.; Secnik, J.; Garcia-Ptacek, S.; Johansson, B.; Nagga, K.; Eriksdotter, M.; Winblad, B.; Religa, D. Antipsychotic Treatment Associated With Increased Mortality Risk in Patients With Dementia. A Registry-Based Observational Cohort Study. J. Am. Med. Dir. Assoc. 2019, 20, 323–329.e2. [Google Scholar] [CrossRef]
- Zhao, Q.-F.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Xu, W.; Li, J.-Q.; Wang, J.; Lai, T.-J.; et al. The Prevalence of Neuropsychiatric Symptoms in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and Psychological Symptoms of Dementia. Front. Neurol. 2012, 3, 23573. [Google Scholar] [CrossRef]
- van der Linde, R.M.; Dening, T.; Stephan, B.C.M.; Prina, A.M.; Evans, E.; Brayne, C. Longitudinal Course of Behavioural and Psychological Symptoms of Dementia: Systematic Review. Br. J. Psychiatry 2016, 209, 366–377. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kim, S.; Anstey, K.J. Dementia Risk Estimates Associated with Measures of Depression: A Systematic Review and Meta-Analysis. BMJ Open 2015, 5, e008853. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of Depressive Symptoms Before Diagnosis of Dementia: A 28-Year Follow-up Study. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef]
- Boccia, M.; Acierno, M.; Piccardi, L. Neuroanatomy of Alzheimer’s Disease and Late-Life Depression: A Coordinate-Based Meta-Analysis of MRI Studies. J. Alzheimer’s Dis. 2015, 46, 963–970. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and Risk of Developing Dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Corbett, A. Agitation and Aggression in People with Alzheimer’s Disease. Curr. Opin. Psychiatry 2013, 26, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.R.; Gill, S.; Hu, S.; Foster, E.D.; Ruthirakuhan, M.T.; Sellek, A.F.; Mortby, M.E.; Matušková, V.; Ng, K.P.; Tarawneh, R.M.; et al. Agitation and Impulsivity in Mid and Late Life as Possible Risk Markers for Incident Dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12016. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric Symptoms in Alzheimer’s Disease: What Might Be Associated Brain Circuits? Mol. Asp. Med. 2015, 43–44, 25–37. [Google Scholar] [CrossRef]
- Nowrangi, M.A.; Marano, C.; Oishi, K.; Mori, S.; Sair, H.I.; Outen, J.; Leoutsakos, J.; Lyketsos, C.; Rosenberg, P.B. The Association of Neuropsychiatric Symptoms with Regional Brain Volumes from Patients in a Tertiary Multi-Disciplinary Memory Clinic. Int. Psychogeriatr. 2021, 33, 233–244. [Google Scholar] [CrossRef]
- Grupe, D.W.; Nitschke, J.B. Uncertainty and Anticipation in Anxiety: An Integrated Neurobiological and Psychological Perspective. Nat. Rev. Neurosci. 2013, 14, 488–501. [Google Scholar] [CrossRef]
- Rafii, M.S.; Taylor, C.S.; Kim, H.T.; Desikan, R.S.; Fleisher, A.S.; Katibian, D.; Brewer, J.B.; Dale, A.M.; Aisen, P.S. Neuropsychiatric Symptoms and Regional Neocortical Atrophy in Mild Cognitive Impairment and Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 159–165. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Mielke, M.M.; Appleby, B.S.; Oh, E.S.; Geda, Y.E.; Lyketsos, C.G. The Association of Neuropsychiatric Symptoms in MCI with Incident Dementia and Alzheimer Disease. Am. J. Geriatr. Psychiatry 2013, 21, 685–695. [Google Scholar] [CrossRef]
- Kosel, F.; Pelley, J.M.S.; Franklin, T.B. Behavioural and Psychological Symptoms of Dementia in Mouse Models of Alzheimer’s Disease-Related Pathology. Neurosci. Biobehav. Rev. 2020, 112, 634–647. [Google Scholar] [CrossRef]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of Exercise Training on Chronic Inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Qiu, P.; Xia, R.; Lin, H.; Ye, B.; Tao, J.; Chen, L. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Aging Neurosci. 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Mok, A.; Khaw, K.T.; Luben, R.; Wareham, N.; Brage, S. Physical Activity Trajectories and Mortality: Population Based Cohort Study. BMJ 2019, 365, l2323. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.-X.; Liang, J.-H.; Xu, Y.; Wang, Y.-Q. Effects of Physical Activity and Exercise on the Cognitive Function of Patients with Alzheimer Disease: A Meta-Analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical Exercise in the Prevention and Treatment of Alzheimer’s Disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Ebrahimi, K.; Jourkesh, M.; Sadigh-Eteghad, S.; Stannard, S.R.; Earnest, C.P.; Ramsbottom, R.; Antonio, J.; Navin, K.H. Effects of Physical Activity on Brain Energy Biomarkers in Alzheimer’s Diseases. Diseases 2020, 8, 18. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, Y.; Li, J.; Chang, J.; Jia, Q. Effect of Physical Exercise on Cognitive Function of Alzheimer’s Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Psychiatry 2022, 13, 927128. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of Aerobic Exercise on Hippocampal Volume in Humans: A Systematic Review and Meta-Analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, J.P.D.; Mensink, R.P.; Ivanov, D.; Adam, J.J.; Uludağ, K.; Joris, P.J. Aerobic Exercise Training Improves Cerebral Blood Flow and Executive Function: A Randomized, Controlled Cross-Over Trial in Sedentary Older Men. Front. Aging Neurosci. 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise Induces Cerebral VEGF and Angiogenesis via the Lactate Receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Endres, M.; Dimeo, F.; Jungehulsing, G.J. Train the Vessel, Gain the Brain: Physical Activity and Vessel Function and the Impact on Stroke Prevention and Outcome in Cerebrovascular Disease. Cerebrovasc. Dis. 2013, 35, 303–312. [Google Scholar] [CrossRef]
- Kurdi, F.N.; Flora, R. The Impact of Physical Exercise on Brain-Derived Neurotrophic Factor (BDNF) Level in Elderly Population. Open Access Maced. J. Med. Sci. 2019, 7, 1618–1620. [Google Scholar] [CrossRef]
- Farmer, J.; Zhao, X.; Van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of Voluntary Exercise on Synaptic Plasticity and Gene Expression in the Dentate Gyrus of Adult Male Sprague-Dawley Rats in Vivo. Neuroscience 2004, 124, 71–79. [Google Scholar] [CrossRef]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Martín-Hernández, J.; Seisdedos, M.M.; García-López, O.; Toschi, N.; Di Giuliano, F.; Garaci, F.; Mercuri, N.B.; et al. Physical Exercise and Alzheimer’s Disease: Effects on Pathophysiological Molecular Pathways of the Disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef]
- Malin, S.K.; Stewart, N.R.; Ude, A.A.; Alderman, B.L. Brain Insulin Resistance and Cognitive Function: Influence of Exercise. J. Appl. Physiol. 2022, 133, 1368–1380. [Google Scholar] [CrossRef]
- Scarfò, G.; Piccarducci, R.; Daniele, S.; Franzoni, F.; Martini, C. Exploring the Role of Lipid-Binding Proteins and Oxidative Stress in Neurodegenerative Disorders: A Focus on the Neuroprotective Effects of Nutraceutical Supplementation and Physical Exercise. Antioxidants 2022, 11, 2116. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain Modulation by the Gut Microbiota: From Disease to Therapy. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Giacovazzo, G.; Decandia, D.; Coccurello, R. Alzheimer’s Disease and Depression in the Elderly: A Trajectory Linking Gut Microbiota and Serotonin Signaling. Front. Psychiatry 2022, 13, 1010169. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Guzmán-Martínez, L.; Cerda-Troncoso, C.; Farías, G.A.; Maccioni, R.B. Neuroinflammation in the Pathogenesis of Alzheimer’s Disease. A Rational Framework for the Search of Novel Therapeutic Approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of Microglia and Astrocytes: A Roadway to Neuroinflammation and Alzheimer’s Disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Yetirajam, R.; Kanneganti, T.D. Innate Immune Cell Death in Neuroinflammation and Alzheimer’s Disease. Cells 2022, 11, 1885. [Google Scholar]
- Le Page, A.; Dupuis, G.; Frost, E.H.; Larbi, A.; Pawelec, G.; Witkowski, J.M.; Fulop, T. Role of the Peripheral Innate Immune System in the Development of Alzheimer’s Disease. Exp. Gerontol. 2018, 107, 59–66. [Google Scholar] [CrossRef]
- Park, J.-C.; Han, S.-H.; Mook-Jung, I. Peripheral Inflammatory Biomarkers in Alzheimer’s Disease: A Brief Review. BMB Rep. 2020, 53, 10–19. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, S.; Manichanh, C.; Nielsen, T.; Pons, N.; Yamada, T.; Mende, D.R.; et al. A Human Gut Microbial Gene Catalog Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2015, 65, 1812–1821. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of Gut Microbiota and Brain via Immune and Neuroendocrine Signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Nankova, B.B.; Agarwal, R.; MacFabe, D.F.; La Gamma, E.F. Enteric Bacterial Metabolites Propionic and Butyric Acid Modulate Gene Expression, Including CREB-Dependent Catecholaminergic Neurotransmission, in PC12 Cells—Possible Relevance to Autism Spectrum Disorders. PLoS ONE 2014, 9, e103740. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. In Endotoxemia and Endotoxin Shock: Disease, Diagnosis and Therapy; Contributions to Nephrology; S. Karger AG: Basel, Switzerland, 2010; Volume 167, pp. 14–24. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Molinero, N.; Antón-Fernández, A.; Hernández, F.; Ávila, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gut Microbiota, an Additional Hallmark of Human Aging and Neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schäfer, K.-H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer’s Disease Animal Model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides from Morinda Officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 2017, 9, 403. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2018, 4, 396–403. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. D-Glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2676. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef]
- Qian, X.-H.; Liu, X.-L.; Chen, G.; Chen, S.-D.; Tang, H. dong Injection of Amyloid-β to Lateral Ventricle Induces Gut Microbiota Dysbiosis in Association with Inhibition of Cholinergic Anti-Inflammatory Pathways in Alzheimer’s Disease. J. Neuroinflamm. 2022, 19, 236. [Google Scholar] [CrossRef]
- Maldonado Weng, J.; Parikh, I.; Naqib, A.; York, J.; Green, S.J.; Estus, S.; Ladu, M.J. Synergistic Effects of APOE and Sex on the Gut Microbiome of Young EFAD Transgenic Mice. Mol. Neurodegener. 2019, 14, 47. [Google Scholar] [CrossRef]
- Kaur, H.; Nookala, S.; Singh, S.; Mukundan, S.; Nagamoto-Combs, K.; Combs, C.K. Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 2370. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How Biological Sex of the Host Shapes Its Gut Microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Calvo-Flores Guzmán, B.; Vinnakota, C.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The GABAergic System as a Therapeutic Target for Alzheimer’s Disease. J. Neurochem. 2018, 146, 649–669. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine Neuronal Loss Contributes to Memory and Reward Dysfunction in a Model of Alzheimer’s Disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Ashford, J.W. Treatment of Alzheimer’s Disease: Trazodone, Sleep, Serotonin, Norepinephrine, and Future Directions. J. Alzheimer’s Dis. 2019, 67, 923–930. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2019, 10, 40. [Google Scholar] [CrossRef]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef]
- Nikolaus, S.; Antke, C.; Beu, M.; Müller, H.W.; Nikolaus, S. Cortical GABA, Striatal Dopamine and Midbrain Serotonin as the Key Players in Compulsiveand Anxiety Disorders—Results from in Vivo Imaging Studies. Rev. Neurosci. 2010, 21, 119–140. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Sagud, M.; Nikolac Perkovic, M.; Svob Strac, D.; Konjevod, M.; Tudor, L.; Uzun, S.; Pivac, N. Depression: Biological Markers and Treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110139. [Google Scholar] [CrossRef]
- Bruch, J.D. Intestinal Infection Associated with Future Onset of an Anxiety Disorder: Results of a Nationally Representative Study. Brain Behav. Immun. 2016, 57, 222–226. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2012, 18, 666–673. [Google Scholar] [CrossRef]
- Yang, B.; Wei, J.; Ju, P.; Chen, J. Effects of Regulating Intestinal Microbiota on Anxiety Symptoms: A Systematic Review. Gen. Psychiatry 2019, 32, e100056. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Stower, H. Depression Linked to the Microbiome. Nat. Med. 2019, 25, 358. [Google Scholar] [CrossRef]
- Marrone, M.C.; Coccurello, R. Dietary Fatty Acids and Microbiota-Brain Communication in Neuropsychiatric Diseases. Biomolecules 2020, 10, 12. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative Metaproteomics Analysis Shows Altered Fecal Microbiota Signatures in Patients with Major Depressive Disorder. NeuroReport 2018, 29, 417–425. [Google Scholar] [CrossRef]
- Park, C.; Brietzke, E.; Rosenblat, J.D.; Musial, N.; Zuckerman, H.; Ragguett, R.-M.; Pan, Z.; Rong, C.; Fus, D.; McIntyre, R.S. Probiotics for the Treatment of Depressive Symptoms: An Anti-Inflammatory Mechanism? Brain Behav. Immun. 2018, 73, 115–124. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M.; Geffard, M.; Bosmans, E. In Depression, Bacterial Translocation May Drive Inflammatory Responses, Oxidative and Nitrosative Stress (O&NS), and Autoimmune Responses Directed against O&NS-Damaged Neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef]
- Martín-Hernández, D.; Caso, J.R.; Bris, Á.G.; Maus, S.R.; Madrigal, J.L.M.; García-Bueno, B.; MacDowell, K.S.; Alou, L.; Gómez-Lus, M.L.; Leza, J.C. Bacterial Translocation Affects Intracellular Neuroinflammatory Pathways in a Depression-like Model in Rats. Neuropharmacology 2016, 103, 122–133. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and Probiotics Attenuate the Development of Alzheimer’s Disease in Transgenic Mice: Role of Microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Schlegel, P.; Novotny, M.; Klimova, B.; Valis, M. “Muscle-Gut-Brain Axis”: Can Physical Activity Help Patients with Alzheimer’s Disease Due to Microbiome Modulation? J. Alzheimer’s Dis. 2019, 71, 861–878. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary Running Exercise Alters Microbiota Composition and Increases N-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Van Der Lugt, B.; Van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.C.; De Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia Muciniphila Ameliorates the Age-Related Decline in Colonic Mucus Thickness and Attenuates Immune Activation in Accelerated Aging Ercc1−/Δ7 Mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The Gut Microbiota and Its Relationship to Diet and Obesity. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and Forced Exercise Differentially Alters the Gut Microbiome in C57BL/6J Mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Aas, V.; Tingstad, R.H.; Van Hees, A.; Nikolić, N. Utilization of Lactic Acid in Human Myotubes and Interplay with Glucose and Fatty Acid Metabolism. Sci. Rep. 2018, 8, 9814. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.S.-J.; Kim, S.; Moon, S.; Park, D.-H.; Kang, J.-H. Lactate Overload Inhibits Myogenic Activity in C2C12 Myotubes. Open Life Sci. 2019, 14, 29–37. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Runov, A.L.; Ponomartsev, S.V.; Vonsky, M.S.; Elmuratov, A.U.; Vinogradov, A.E. Long-Term Transcriptomic Changes and Cardiomyocyte Hyperpolyploidy after Lactose Intolerance in Neonatal Rats. Int. J. Mol. Sci. 2023, 24, 7063. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The Gut Microbiota Influences Skeletal Muscle Mass and Function in Mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The Histone Deacetylase Inhibitor Butyrate Improves Metabolism and Reduces Muscle Atrophy during Aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Martín, A.I.; Priego, T.; López-Calderón, A. Hormones and Muscle Atrophy. In Advances in Experimental Medicine and Biology; Xiao, J., Ed.; Springer: Sinagpore, 2018; Volume 1088, pp. 207–233. [Google Scholar]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Huang, W.-C.; Pan, C.-H.; Wei, C.-C.; Huang, H.-Y. Lactobacillus plantarum Ps128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-Chain Fatty Acids as Potential Regulators of Skeletal Muscle Metabolism and Function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Londhe, P.; Guttridge, D.C. Inflammation Induced Loss of Skeletal Muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Atkins, J.L. Muscle Loss and Obesity: The Health Implications of Sarcopenia and Sarcopenic Obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring Specific Lactobacilli Levels Decreases Inflammation and Muscle Atrophy Markers in an Acute Leukemia Mouse Model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.H.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the Gut Microbiota and Sarcopenia: A Systematic Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef]
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut–Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Han, J.-H.; Kim, I.-S.; Jung, S.-H.; Lee, S.-G.; Son, H.-Y.; Myung, C.-S. The Effects of Propionate and Valerate on Insulin Responsiveness for Glucose Uptake in 3T3-L1 Adipocytes and C2C12 Myotubes via G Protein-Coupled Receptor 41. PLoS ONE 2014, 9, e95268. [Google Scholar] [CrossRef]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.-H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic Acid Enhances Endurance Capacity of Exercise-Trained Mice by Increasing Skeletal Muscle Oxidative Properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome Potentiates Endurance Exercise through Intestinal Acetate Production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Villa, S.R.; Fuller, M.; Wicksteed, B.; Mackay, C.R.; Alquier, T.; Poitout, V.; Mancebo, H.; Mirmira, R.G.; Gilchrist, A.; et al. An Acetate-Specific GPCR, FFAR2, Regulates Insulin Secretion. Mol. Endocrinol. 2015, 29, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, D.; Dedeo, D.; Milligan, G. Metabolic and Inflammatory Functions of Short-Chain Fatty Acid Receptors. Curr. Opin. Endocr. Metab. Res. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- McIntosh, C.H.S.; Widenmaier, S.; Kim, S.-J. Pleiotropic Actions of the Incretin Hormones. Vitam. Horm. 2010, 84, 21–79. [Google Scholar] [CrossRef]
- Green, C.J.; Henriksen, T.I.; Pedersen, B.K.; Solomon, T.P.J. Glucagon Like Peptide-1-Induced Glucose Metabolism in Differentiated Human Muscle Satellite Cells Is Attenuated by Hyperglycemia. PLoS ONE 2012, 7, e44284. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of Muscle Wasting by Glucagon-like Peptide-1 Receptor Agonist in Muscle Atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Li, T.; Dong, N.; Yi, L.; Zhang, Q.; Mi, M. GLP-1 Regulates Exercise Endurance and Skeletal Muscle Remodeling via GLP-1R/AMPK Pathway. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119300. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 25, 1075–1090.e5. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. Exercise and Glucagon-like Peptide-1: Does Exercise Potentiate the Effect of Treatment? World J. Diabetes 2018, 9, 138–140. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, S.; Du, G. Effects of Exercise Frequency on the Gut Microbiota in Elderly Individuals. Microbiologyopen 2020, 9, e1053. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- O’Donovan, C.M.; Madigan, S.M.; Garcia-Perez, I.; Rankin, A.; O’ Sullivan, O.; Cotter, P.D. Distinct Microbiome Composition and Metabolome Exists across Subgroups of Elite Irish Athletes. J. Sci. Med. Sport 2020, 23, 63–68. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, P.; Zhou, D.; Huang, L.; Zhang, L.; Gao, X.; Maitiabula, G.; Wang, S.; Wang, X. Multi-Omics Analyses Characterize the Gut Microbiome and Metabolome Signatures of Soldiers Under Sustained Military Training. Front. Microbiol. 2022, 13, 827071. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine Cells-Sensory Sentinels of the Intestinal Environment and Orchestrators of Mucosal Immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Packer, N.; Guan, J. Freewheel Training Decreases Pro- and Increases Anti-Inflammatory Cytokine Expression in Mouse Intestinal Lymphocytes. Brain Behav. Immun. 2010, 24, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Kelly, M.; Gauthier, M.-S.; Saha, A.K.; Ruderman, N.B. Activation of AMP-Activated Protein Kinase by Interleukin-6 in Rat Skeletal Muscle: Association with Changes in CAMP, Energy State, and Endogenous Fuel Mobilization. Diabetes 2009, 58, 1953–1960. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-like Peptide-1 Secretion from L Cells and Alpha Cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Handschin, C. Regulation of Skeletal Muscle Cell Plasticity by the Peroxisome Proliferator-Activated Receptor γ Coactivator 1α. J. Recept. Signal Transduct. 2010, 30, 376–384. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Khan, I.U.; Polo, I.R.; Zubair, H.; Drummer, C.; Shahab, M.; Behr, R. Irisin in the Primate Hypothalamus and Its Effect on GnRH in Vitro. J. Endocrinol. 2019, 241, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. In Handbook of Experimental Pharmacology; Lewin, G., Carter, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 220, pp. 223–250. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Gomes da Silva, S.; Unsain, N.; Mascó, D.H.; Toscano-Silva, M.; De Amorim, H.A.; Silva Araújo, B.H.; Simões, P.S.R.; Da Graça Naffah-Mazzacoratti, M.; Mortara, R.A.; Scorza, F.A.; et al. Early Exercise Promotes Positive Hippocampal Plasticity and Improves Spatial Memory in the Adult Life of Rats. Hippocampus 2012, 22, 347–358. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wu, H.; Li, X.; Ke, J.; Zhang, Z.; Zhao, Q. Effects of Irisin on the Dysfunction of Blood–Brain Barrier in Rats after Focal Cerebral Ischemia/Reperfusion. Brain Behav. 2019, 9, e01425. [Google Scholar] [CrossRef]
- Jin, Z.; Guo, P.; Li, X.; Ke, J.; Wang, Y.; Wu, H. Neuroprotective Effects of Irisin against Cerebral Ischemia/Reperfusion Injury via Notch Signaling Pathway. Biomed. Pharmacother. 2019, 120, 109452. [Google Scholar] [CrossRef]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar] [CrossRef]
- Huangfu, L.; Cai, X.; Yang, J.; Wang, H.; Li, Y.; Dai, Z.; Yang, R.; Lin, X. Irisin Attenuates Inflammation in a Mouse Model of Ulcerative Colitis by Altering the Intestinal Microbiota. Exp. Ther. Med. 2021, 22, 1433. [Google Scholar] [CrossRef]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) Family Cytokines: Role in Immune Pathogenesis and Treatment of CNS Autoimmune Disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef]
- Toussirot, É. The IL23/Th17 Pathway as a Therapeutic Target in Chronic Inflammatory Diseases. Inflamm. Allergy Drug Targets 2012, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, Y.; Li, G.; Guo, T.; Jin, M.; Xi, D.; Wang, S.; Liu, X.; Guo, S.; Liu, H.; et al. Irisin Ameliorates Myocardial Ischemia-Reperfusion Injury through Modulation of Gut Microbiota and Intestinal Permeability in Rats. PLoS ONE 2023, 18, e0291022. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-F.; Wang, M.-Z.; Bi, J.-B.; Zhang, J.; Zhang, L.; Liu, W.-M.; Wei, S.-S.; Lv, Y.; Wu, Z.; Wu, R.-Q. Irisin Attenuates Intestinal Injury, Oxidative and Endoplasmic Reticulum Stress in Mice with L-Arginine-Induced Acute Pancreatitis. World J. Gastroenterol. 2019, 25, 6653–6667. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, Y.-Y.; Yan, Z.-X. Brain-Derived Neurotrophic Factor Preserves Intestinal Mucosal Barrier Function and Alters Gut Microbiota in Mice. Kaohsiung J. Med. Sci. 2018, 34, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Su, Z.; Qu, C.; Dong, Y. Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front. Physiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Jandova, T.; Buendía-Romero, A.; Polanska, H.; Hola, V.; Rihova, M.; Vetrovsky, T.; Courel-Ibáñez, J.; Steffl, M. Long-Term Effect of Exercise on Irisin Blood Levels—Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1438. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; De Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.-H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Belviranlı, M.; Okudan, N. Exercise Training Protects Against Aging-Induced Cognitive Dysfunction via Activation of the Hippocampal PGC-1α/FNDC5/BDNF Pathway. Neuromolecular Med. 2018, 20, 386–400. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Teixeira, A.L.; Radanovic, M.; Talib, L.L.; Rocha, N.P.; Gattaz, W.F. Lower Cerebrospinal Fluid Concentration of Brain-Derived Neurotrophic Factor Predicts Progression from Mild Cognitive Impairment to Alzheimer’s Disease. Neuromolecular Med. 2015, 17, 326–332. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined Adult Neurogenesis and BDNF Mimic Exercise Effects on Cognition in an Alzheimer’s Mouse Model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.; Santos, A.D.S.A.; Afonso, M.B.; Rodrigues, P.M.; Sá Santos, S.; Castro, R.E.; Rodrigues, C.M.P.; Solá, S. Diet-Dependent Gut Microbiota Impacts on Adult Neurogenesis through Mitochondrial Stress Modulation. Brain Commun. 2020, 2, fcaa165. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric Short-Chain Fatty Acids Promote Proliferation of Human Neural Progenitor Cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum Short Chain Fatty Acids Mediate Hippocampal BDNF and Correlate with Decreasing Neuroinflammation Following High Pectin Fiber Diet in Mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, O.F.; Ogbonnaya, E.S.; Felice, D.; Levone, B.R.; Conroy, L.C.; Fitzgerald, P.; Bravo, J.A.; Forsythe, P.; Bienenstock, J.; Dinan, T.G.; et al. The Vagus Nerve Modulates BDNF Expression and Neurogenesis in the Hippocampus. Eur. Neuropsychopharmacol. 2018, 28, 307–316. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Haidar, E.A.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Xia, D.Y.; Huang, X.; Bi, C.F.; Mao, L.L.; Peng, L.J.; Qian, H.R. PGC-1α or FNDC5 Is Involved in Modulating the Effects of Aβ1-42 Oligomers on Suppressing the Expression of BDNF, a Beneficial Factor for Inhibiting Neuronal Apoptosis, Aβ Deposition and Cognitive Decline of APP/PS1 Tg Mice. Front. Aging Neurosci. 2017, 9, 65. [Google Scholar] [CrossRef]
- Mahdieh, M.S.; Maryam, J.; Bita, B.; Neda, F.; Motahare, M.; Mahboobeh, B.; LeBris, S.Q.; Kalani Behrooz, S. A Pilot Study on the Relationship between Lactobacillus, Bifidibactrium Counts and Inflammatory Factors Following Exercise Training. Arch. Physiol. Biochem. 2021, 129, 778–787. [Google Scholar] [CrossRef]
- Shamsipour, S.; Sharifi, G.; Taghian, F. Impact of Interval Training with Probiotic (L. plantarum/Bifidobacterium bifidum) on Passive Avoidance Test, ChAT and BDNF in the Hippocampus of Rats with Alzheimer’s Disease. Neurosci. Lett. 2021, 756, 135949. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, Y.-K. Reduced Platelet BDNF Level in Patients with Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 849–853. [Google Scholar] [CrossRef]
- Tu, W.-J.; Qiu, H.-C.; Liu, Q.; Li, X.; Zhao, J.-Z.; Zeng, X. Decreased Level of Irisin, a Skeletal Muscle Cell-Derived Myokine, Is Associated with Post-Stroke Depression in the Ischemic Stroke Population. J. Neuroinflamm. 2018, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Siteneski, A.; Cunha, M.P.; Lieberknecht, V.; Pazini, F.L.; Gruhn, K.; Brocardo, P.S.; Rodrigues, A.L.S. Central Irisin Administration Affords Antidepressant-like Effect and Modulates Neuroplasticity-Related Genes in the Hippocampus and Prefrontal Cortex of Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.R.; McDowell, C.P.; Hallgren, M.; Meyer, J.D.; Lyons, M.; Herring, M.P. Association of Efficacy of Resistance Exercise Training With Depressive Symptoms: Meta-Analysis and Meta-Regression Analysis of Randomized Clinical Trials. JAMA Psychiatry 2018, 75, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, Z.; Sun, L.; Zhou, L.; Wang, G.; Xiao, L.; Wang, H. The Effects and Mechanisms of Exercise on the Treatment of Depression. Front. Psychiatry 2021, 12, 705559. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.-C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression—Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Y.; Lu, W.; Zhang, R.; Shao, R.; Yau, S.-Y.; Stubbs, B.; McIntyre, R.S.; Su, K.-P.; Xu, G.; et al. Exercise Effect on the Gut Microbiota in Young Adolescents with Subthreshold Depression: A Randomized Psychoeducation-Controlled Trial. Psychiatry Res. 2023, 319, 115005. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ueyama, J.; Kashihara, K.; Ito, M.; Hamaguchi, T.; Maeda, T.; Tsuboi, Y.; Katsuno, M.; Hirayama, M.; Ohno, K. Gut Microbiota in Dementia with Lewy Bodies. npj Park. Dis. 2022, 8, 169. [Google Scholar] [CrossRef]
- D’Argenio, V.; Veneruso, I.; Gong, C.; Cecarini, V.; Bonfili, L.; Eleuteri, A.M. Gut Microbiome and Mycobiome Alterations in an In Vivo Model of Alzheimer’s Disease. Genes 2022, 13, 1564. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between Gut Microbiota and Alzheimer’s Disease, Major Depressive Disorder, and Schizophrenia. J. Neuroinflamm. 2020, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, J.A.; Reddy, P.; Davis-Lameloise, N.; Philpot, B.; Laatikainen, T.; Kilkkinen, A.; Bunker, S.J.; Best, J.D.; Vartiainen, E.; Lo, S.K.; et al. Depression: An Important Comorbidity With Metabolic Syndrome in a General Population. Diabetes Care 2008, 31, 2368–2373. [Google Scholar] [CrossRef]
- Wang, S.; Pan, J. Irisin Ameliorates Depressive-like Behaviors in Rats by Regulating Energy Metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef]
- Watson, K.; Nasca, C.; Aasly, L.; McEwen, B.; Rasgon, N. Insulin Resistance, an Unmasked Culprit in Depressive Disorders: Promises for Interventions. Neuropharmacology 2018, 136, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Cho, Y.H.; Kim, M.; Jeong, C.-W.; Cha, J.M.; Won, G.H.; Noh, J.S.; Son, S.J.; Park, R.W. Association between Impaired Glucose Metabolism and Long-Term Prognosis at the Time of Diagnosis of Depression: Impaired Glucose Metabolism as a Promising Biomarker Proposed through a Machine-Learning Approach. Eur. Psychiatry 2023, 66, e21. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Rostamkhani, F.; Meftahi, G.H.; Shirvani, H. Comparative Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Soleus Muscle Fibronectin Type III Domain-Containing Protein 5, Myonectin and Glucose Transporter Type 4 Gene Expressions: A Study on the Diabetic Rat Model. Mol. Biol. Rep. 2021, 48, 6123–6129. [Google Scholar] [CrossRef]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The Human Gut Microbiota and Glucose Metabolism: A Scoping Review of Key Bacteria and the Potential Role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef]
- Xuan, W.; Ou, Y.; Chen, W.; Huang, L.; Wen, C.; Huang, G.; Tang, W.; Zeng, D.; Huang, S.; Xiao, L.; et al. Faecalibacterium Prausnitzii Improves Lipid Metabolism Disorder and Insulin Resistance in Type 2 Diabetic Mice. Br. J. Biomed. Sci. 2023, 80, 10794. [Google Scholar] [CrossRef]
- Marinelli, S.; Coccurello, R. From the Gender Gap to Neuroactive Steroids: Exploring Multiple Cases to Further Understand Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 8577. [Google Scholar] [CrossRef]
- De Angelis, F.; Vacca, V.; Tofanicchio, J.; Strimpakos, G.; Giacovazzo, G.; Pavone, F.; Coccurello, R.; Marinelli, S. Sex Differences in Neuropathy: The Paradigmatic Case of MetFormin. Int. J. Mol. Sci. 2022, 23, 14503. [Google Scholar] [CrossRef]
- Vacca, V.; Marinelli, S.; De Angelis, F.; Angelini, D.F.; Piras, E.; Battistini, L.; Pavone, F.; Coccurello, R. Sexually Dimorphic Immune and Neuroimmune Changes Following Peripheral Nerve Injury in Mice: Novel Insights for Gender Medicine. Int. J. Mol. Sci. 2021, 22, 4397. [Google Scholar] [CrossRef]
- Rosenberg, A.; Mangialasche, F.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J. Prev. Alzheimer’s Dis. 2020, 7, 29–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. https://doi.org/10.3390/ijms241914686
Cutuli D, Decandia D, Giacovazzo G, Coccurello R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership. International Journal of Molecular Sciences. 2023; 24(19):14686. https://doi.org/10.3390/ijms241914686
Chicago/Turabian StyleCutuli, Debora, Davide Decandia, Giacomo Giacovazzo, and Roberto Coccurello. 2023. "Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership" International Journal of Molecular Sciences 24, no. 19: 14686. https://doi.org/10.3390/ijms241914686
APA StyleCutuli, D., Decandia, D., Giacovazzo, G., & Coccurello, R. (2023). Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership. International Journal of Molecular Sciences, 24(19), 14686. https://doi.org/10.3390/ijms241914686




