Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease
Abstract
:1. Introduction
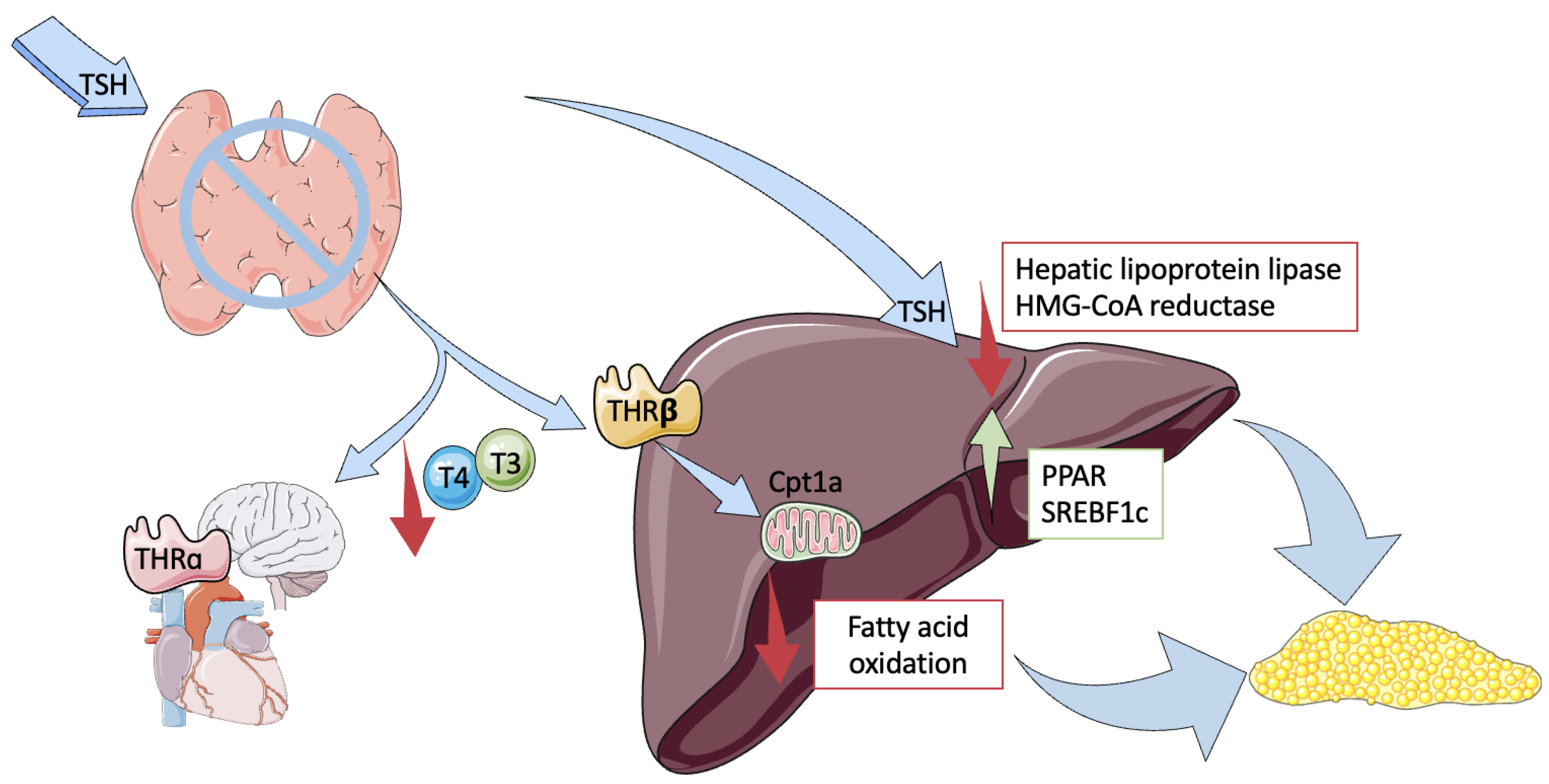
2. Pathophysiology
- High TSH Levels
- Low Thyroid Hormones
- Chronic Inflammation and Hormone Interactions
3. Epidemiological Evidence
4. Effects of Hypothyroidism Treatment in NAFLD
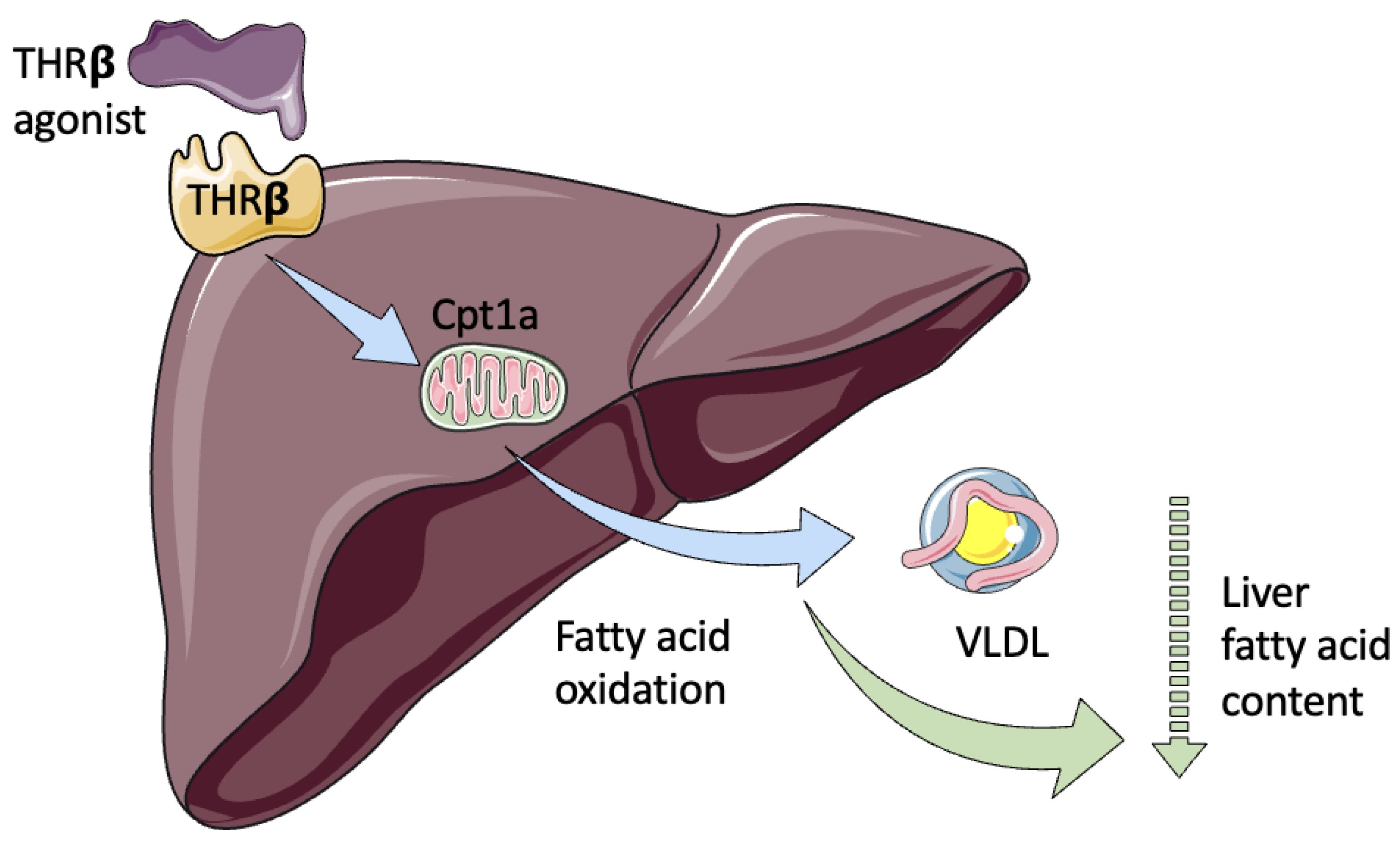
5. Thyroid Hormone Receptor Agonists and Metabolites
6. Resmetirom
7. Hyperthyroidism and Nafld
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almomani, A.; Hitawala, A.A.; Kumar, P.; Alqaisi, S.; Alshaikh, D.; Alkhayyat, M.; Asaad, I. Prevalence of hypothyroidism and effect of thyroid hormone replacement therapy in patients with non-alcoholic fatty liver disease: A population-based study. World J. Hepatol. 2022, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Phadke, A.; Sawant, P. Prevalence of hypothyroidism in nonalcoholic fatty liver disease in patients attending a tertiary hospital in western India. Indian J. Gastroenterol. 2015, 34, 169–173. [Google Scholar] [CrossRef]
- Ludwig, U.; Holzner, D.; Denzer, C.; Greinert, A.; Haenle, M.M.; Oeztuerk, S.; Koenig, W.; Boehm, B.O.; Mason, R.A.; Kratzer, W.; et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: A cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr. Disord. 2015, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Madariaga, A.G.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Jarvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023; in press. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Targher, G. Pathogenesis of hypothyroidism-induced NAFLD: Evidence for a distinct disease entity? Dig. Liver Dis. 2019, 51, 462–470. [Google Scholar] [CrossRef]
- Gor, R.; Siddiqui, N.A.; Wijeratne Fernando, R.; Sreekantan Nair, A.; Illango, J.; Malik, M.; Hamid, P. Unraveling the Role of Hypothyroidism in Non-alcoholic Fatty Liver Disease Pathogenesis: Correlations, Conflicts, and the Current Stand. Cureus 2021, 13, e14858. [Google Scholar] [CrossRef]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of nash in endocrine diseases. Endocr. Connect. 2021, 10, R52–R65. [Google Scholar] [CrossRef] [PubMed]
- Mavromati, M.; Jornayvaz, F.R. Review hypothyroidism-associated dyslipidemia: Potential molecular mechanisms leading to NAFLD. Int. J. Mol. Sci. 2021, 22, 12797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Zhou, L.; Song, Y.; Ma, S.; Yu, C.; Zhao, J.; Xu, C.; Gao, L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol. Cell. Endocrinol. 2017, 446, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Escudé, A.; Pera, G.; Costa-Garrido, A.; Rodríguez, L.; Arteaga, I.; Expósito-Martínez, C.; Torán-Monserrat, P.; Caballería, L. TSH levels as an independent risk factor for NAFLD and liver fibrosis in the general population. J. Clin. Med. 2021, 10, 2907. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-induced nonalcoholic fatty liver disease (Hin): Mechanisms and emerging therapeutic options. Int. J. Mol. Sci. 2020, 21, 5927. [Google Scholar] [CrossRef]
- Krause, C.; Grohs, M.; El Gammal, A.T.; Wolter, S.; Lehnert, H.; Mann, O.; Mittag, J.; Kirchner, H. Reduced expression of thyroid hormone receptor β in human nonalcoholic steatohepatitis. Endocr. Connect. 2018, 7, 1448–1456. [Google Scholar] [CrossRef]
- Cody, V.; Davis, P.J.; Davis, F.B. Molecular modeling of the thyroid hormone interactions with αvβ3 integrin. Steroids 2007, 72, 165–170. [Google Scholar] [CrossRef]
- Furuya, F.; Hanover, J.A.; Cheng, S.Y. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 1780–1785. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Zhou, J.; Xie, S.Y.; You, S.H.; Gauthier, K.; Yen, P.M. FoxO1 deacetylation regulates thyroid hormone-induced transcription of key hepatic gluconeogenic genes. J. Biol. Chem. 2013, 288, 30365–30372. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Gnoni, A.; Siculella, L. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: Differences in metabolic effects and molecular mechanisms. Int. J. Mol. Sci. 2017, 18, 744. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Thyroid Hormones and Thyromimetics: A New Approach to Nonalcoholic Steatohepatitis? Hepatology 2020, 72, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Abisambra Socarrás, J.F.; Bedi, M.; Ness, G.C. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2007, 1771, 1216–1225. [Google Scholar] [CrossRef]
- Bonde, Y.; Breuer, O.; Lütjohann, D.; Sjöberg, S.; Angelin, B.; Rudling, M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 2014, 55, 2408–2415. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ishida, E.; Matsumoto, S.; Okada, S.; Yamada, M.; Satoh, T.; Monden, T.; Mori, M. Carbohydrate response element binding protein gene expression is positively regulated by thyroid hormone. Endocrinology 2009, 150, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Muraca, E.; Ciardullo, S.; Oltolini, A.; Zerbini, F.; Bianconi, E.; Perra, S.; Villa, M.; Cannistraci, R.; Castoldi, G.; Pizzi, P.; et al. Resting energy expenditure in obese women with primary hypothyroidism and appropriate levothyroxine replacement therapy. J. Clin. Endocrinol. Metab. 2020, 105, E1741–E1748. [Google Scholar] [CrossRef]
- Abdel-moez, F.A.B.; Mohamed, G.A.; Abbas, W.A.; Abozaid, M.A.A.; Mohammed, S. Thyroid dysfunction in obese adults in relation to nonalcoholic fatty liver disease. Egypt. J. Intern. Med. 2020, 31, 629–634. [Google Scholar] [CrossRef]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.A. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid J. 2017, 6, 208–215. [Google Scholar] [CrossRef]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef]
- Qiu, S.; Cao, P.; Guo, Y.; Lu, H.; Hu, Y. Exploring the Causality Between Hypothyroidism and Non-alcoholic Fatty Liver: A Mendelian Randomization Study. Front. Cell Dev. Biol. 2021, 9, 643582. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.A.; Leow, M.K.S.; et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J. Clin. Endocrinol. Metab. 2018, 103, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.A.; Columbano, A.; Perra, A. Thyroid hormones, thyromimetics and their metabolites in the treatment of liver disease. Front. Endocrinol. 2018, 9, 382. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Zhao, M.; Zheng, D.; Zhang, X.; Guan, Q.; Xu, C.; Gao, L.; Zhao, J.; Zhang, H. Benefits of levothyroxine replacement therapy on nonalcoholic fatty liver disease in subclinical hypothyroidism patients. Int. J. Endocrinol. 2017, 2017, 5753039. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.J.; Egan, D.M.; Sleph, P.G.; Beehler, B.C.; Chiellini, G.; Nguyen, N.-H.; Baxter, J.D.; Scanlan, T.S. Effects of the Thyroid Hormone Receptor Agonist GC-1 on Metabolic Rate and Cholesterol in Rats and Primates: Selective Actions Relative to 3,5,3′-Triiodo-L-Thyronine. Endocrinology 2004, 145, 1656–1661. Available online: https://academic.oup.com/endo/article-lookup/doi/10.1210/en.2003-0973 (accessed on 1 June 2023). [CrossRef]
- Villicev, C.M.; Freitas, F.R.S.; Aoki, M.S.; Taffarel, C.; Scanlan, T.S.; Moriscot, A.S.; Ribeiro, M.O.; Bianco, A.C.; Gouveia, C.H.A. Thyroid hormone receptor β-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J. Endocrinol. 2007, 193, 21–29. Available online: https://joe.bioscientifica.com/view/journals/joe/193/1/1930021.xml (accessed on 1 June 2023). [CrossRef]
- Vatner, D.F.; Weismann, D.; Beddow, S.A.; Kumashiro, N.; Erion, D.M.; Liao, X.-H.; Grover, G.J.; Webb, P.; Phillips, K.J.; Weiss, R.E.; et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiol. Metab. 2013, 305, E89–E100. Available online: https://www.physiology.org/doi/10.1152/ajpendo.00573.2012 (accessed on 1 June 2023). [CrossRef]
- Sjouke, B.; Langslet, G.; Ceska, R.; Nicholls, S.J.; Nissen, S.E.; Öhlander, M.; Ladenson, P.W.; Olsson, A.G.; Hovingh, G.K.; Kastelein, J.J.P. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): A randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2014, 2, 455–463. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2213858714700063 (accessed on 8 June 2023). [CrossRef]
- Angelin, B.; Kristensen, J.D.; Eriksson, M.; Carlsson, B.; Klein, I.; Olsson, A.G.; Chester Ridgway, E.; Ladenson, P.W. Reductions in serum levels of LDL cholesterol, apolipoprotein B, triglycerides and lipoprotein(a) in hypercholesterolaemic patients treated with the liver-selective thyroid hormone receptor agonist eprotirome. J. Intern. Med. 2015, 277, 331–342. Available online: https://onlinelibrary.wiley.com/doi/10.1111/joim.12261 (accessed on 8 June 2023). [CrossRef]
- van der Valk, F.; Hassing, C.; Visser, M.; Thakkar, P.; Mohanan, A.; Pathak, K.; Dutt, C.; Chauthaiwale, V.; Ackermans, M.; Nederveen, A.; et al. The Effect of a Diiodothyronine Mimetic on Insulin Sensitivity in Male Cardiometabolic Patients: A Double-Blind Randomized Controlled Trial. PLoS ONE 2014, 9, e86890. Available online: https://dx.plos.org/10.1371/journal.pone.0086890 (accessed on 8 June 2023). [CrossRef] [PubMed]
- Ladenson, P.W.; McCarren, M.; Morkin, E.; Edson, R.G.; Shih, M.-C.; Warren, S.R.; Barnhill, J.G.; Churby, L.; Thai, H.; O’Brien, T.; et al. Effects of the Thyromimetic Agent Diiodothyropropionic Acid on Body Weight, Body Mass Index, and Serum Lipoproteins: A Pilot Prospective, Randomized, Controlled Study. J. Clin. Endocrinol. Metab. 2010, 95, 1349–1354. Available online: https://academic.oup.com/jcem/article/95/3/1349/2597364 (accessed on 8 June 2023). [CrossRef] [PubMed]
- Sherman, S.I.; Ringel, M.D.; Smith, M.J.; Kopelen, H.A.; Zoghbi, W.A.; Ladenson, P.W. Augmented Hepatic and Skeletal Thyromimetic Effects of Tiratricol in Comparison with Levothyroxine 1. J. Clin. Endocrinol. Metab. 1997, 82, 2153–2158. Available online: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jcem.82.7.4054 (accessed on 8 June 2023). [CrossRef] [PubMed]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.-E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.-S.; et al. Discovery of 2-[3,5-Dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor β Agonist in Clinical Trials for the Treatment of Dys. J. Med. Chem. 2014, 57, 3912–3923. [Google Scholar] [CrossRef] [PubMed]
- Taub, R.; Chiang, E.; Chabot-Blanchet, M.; Kelly, M.J.; Reeves, R.A.; Guertin, M.C.; Tardif, J.C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis 2013, 230, 373–380. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; McCarty, K.; Pablo Frias, J.; Taub, R.; Alkhouri, N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatol. Commun. 2021, 5, 573–588. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Taub, R.A.; Barbone, J.M.; Harrison, S.A. Hepatic Fat Reduction Due to Resmetirom in Patients with Nonalcoholic Steatohepatitis Is Associated With Improvement of Quality of Life. Clin. Gastroenterol. Hepatol. 2022, 20, 1354–1361.e7. [Google Scholar] [CrossRef]
- Alkhouri, N. Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: Rationale for the development of resmetirom (MGL-3196). Expert Opin. Investig. Drugs 2020, 29, 99–101. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Yang, Y.; Xu, J.; Hong, M.; Xia, M.; Li, X.; Gao, X. Thyroid function and non-alcoholic fatty liver disease in hyperthyroidism patients. BMC Endocr. Disord. 2021, 21, 27. [Google Scholar] [CrossRef]
- Labenz, C.; Kostev, K.; Armandi, A.; Galle, P.R.; Schattenberg, J.M. Impact of thyroid disorders on the incidence of non-alcoholic fatty liver disease in Germany. United Eur. Gastroenterol. J. 2021, 9, 829–836. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. Tg68, a novel thyroid hormone receptor-β agonist for the treatment of nafld. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef] [PubMed]
| Compound | Study | Study Participants | Effects on Lipids | Effects on Liver | Side Effects |
|---|---|---|---|---|---|
| THR-β receptor agonists | |||||
| Sobetirome | Grover et al. [37] Villicev et al. [38] Vatner et al. [39] | Murine models. | ↓ LDL | ↓ Liver fat | Stopped after phase 1. ↑ hyperglycaemia and insulin resistance. |
| Eprotirome | Sjouke et al. [40] Angelin et al. [41] | Patients with familial hypercholesterolaemia + statin treatment. Patients with primary hypercholesterolaemia. | ↓ LDL ↓ TAG ↓ LDL ↓ Apo B = HDL | ↑ ALT ↑ AST ↑ GGT ↑ ALT | Potential liver injury. |
| TH Metabolites | |||||
| Omzotirome | van der Valk et al. [42] | Patients with metabolic syndrome. | = TAG | = ALT = AST = GGT = Liver Fat | ↑ FT4. |
| DITPA | Landenson et al. [43] | Stable congestive heart failure. | ↓ LDL ↓ TAG | Not studied. | ↓ TT4, TSH ↑ Heart rate Poorly tolerated. |
| Triac | Sherman et al. [44] | Patients with thyroidectomy and radioiodine ablation. | ↓ LDL ↓ TAG = HDL | Not studied. | Increased skeletal turnover. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Cevallos, P.; Murúa-Beltrán Gall, S.; Uribe, M.; Chávez-Tapia, N.C. Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease. Int. J. Mol. Sci. 2023, 24, 14605. https://doi.org/10.3390/ijms241914605
Vidal-Cevallos P, Murúa-Beltrán Gall S, Uribe M, Chávez-Tapia NC. Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease. International Journal of Molecular Sciences. 2023; 24(19):14605. https://doi.org/10.3390/ijms241914605
Chicago/Turabian StyleVidal-Cevallos, Paulina, Sofía Murúa-Beltrán Gall, Misael Uribe, and Norberto C. Chávez-Tapia. 2023. "Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease" International Journal of Molecular Sciences 24, no. 19: 14605. https://doi.org/10.3390/ijms241914605
APA StyleVidal-Cevallos, P., Murúa-Beltrán Gall, S., Uribe, M., & Chávez-Tapia, N. C. (2023). Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease. International Journal of Molecular Sciences, 24(19), 14605. https://doi.org/10.3390/ijms241914605







