The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca splendens)
Abstract
1. Introduction
2. Results
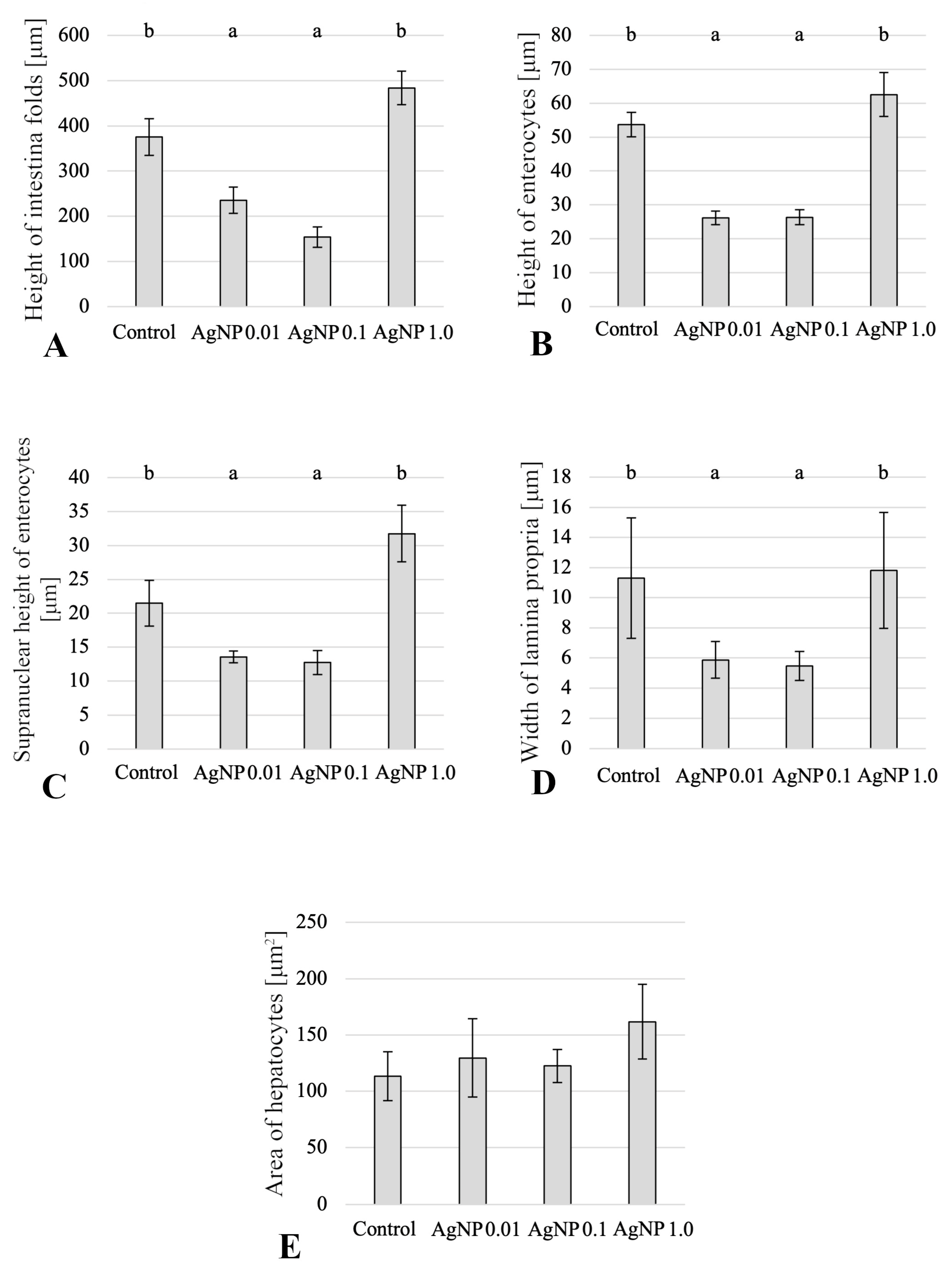
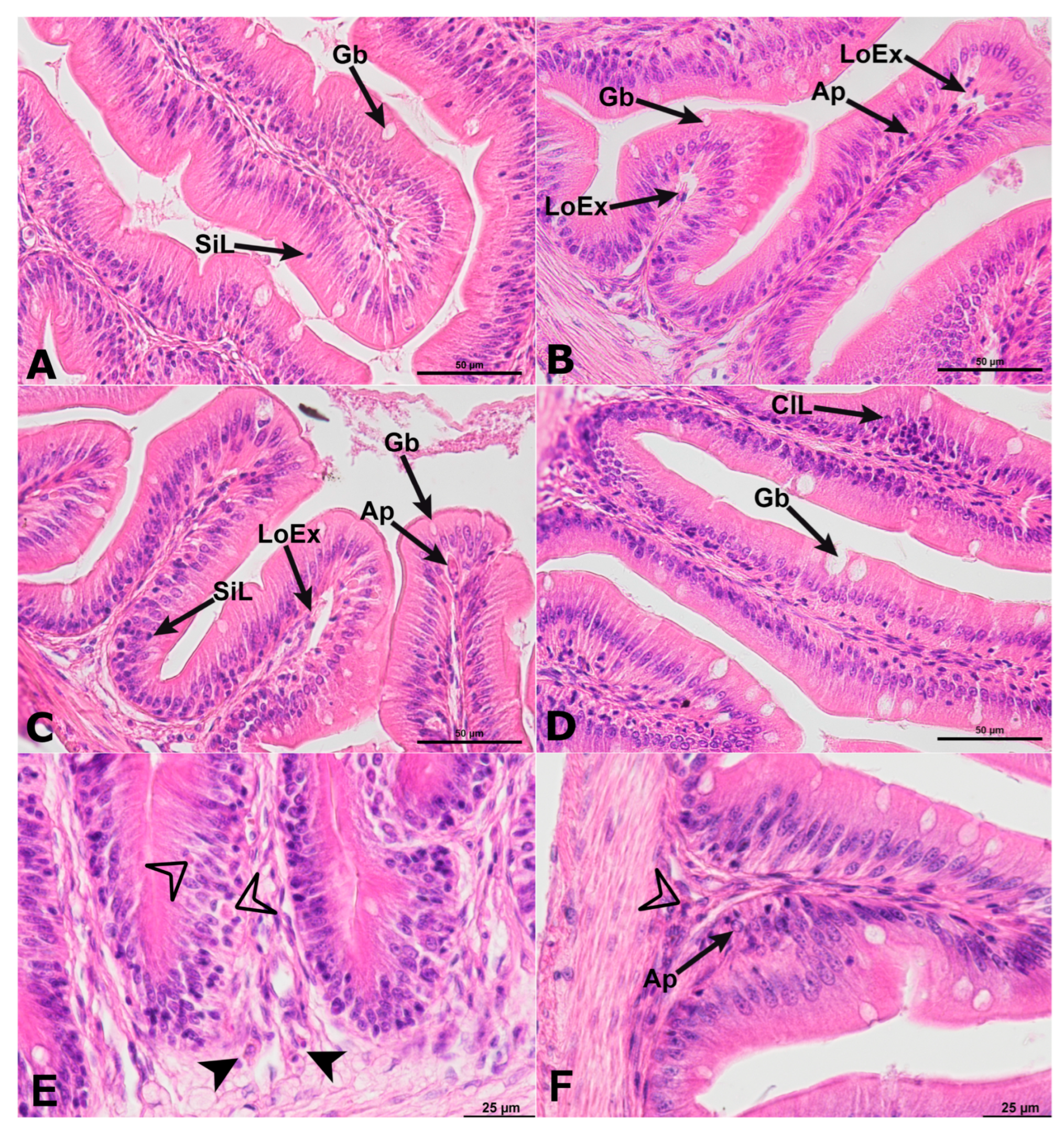
2.1. Histology of Anterior Intestine
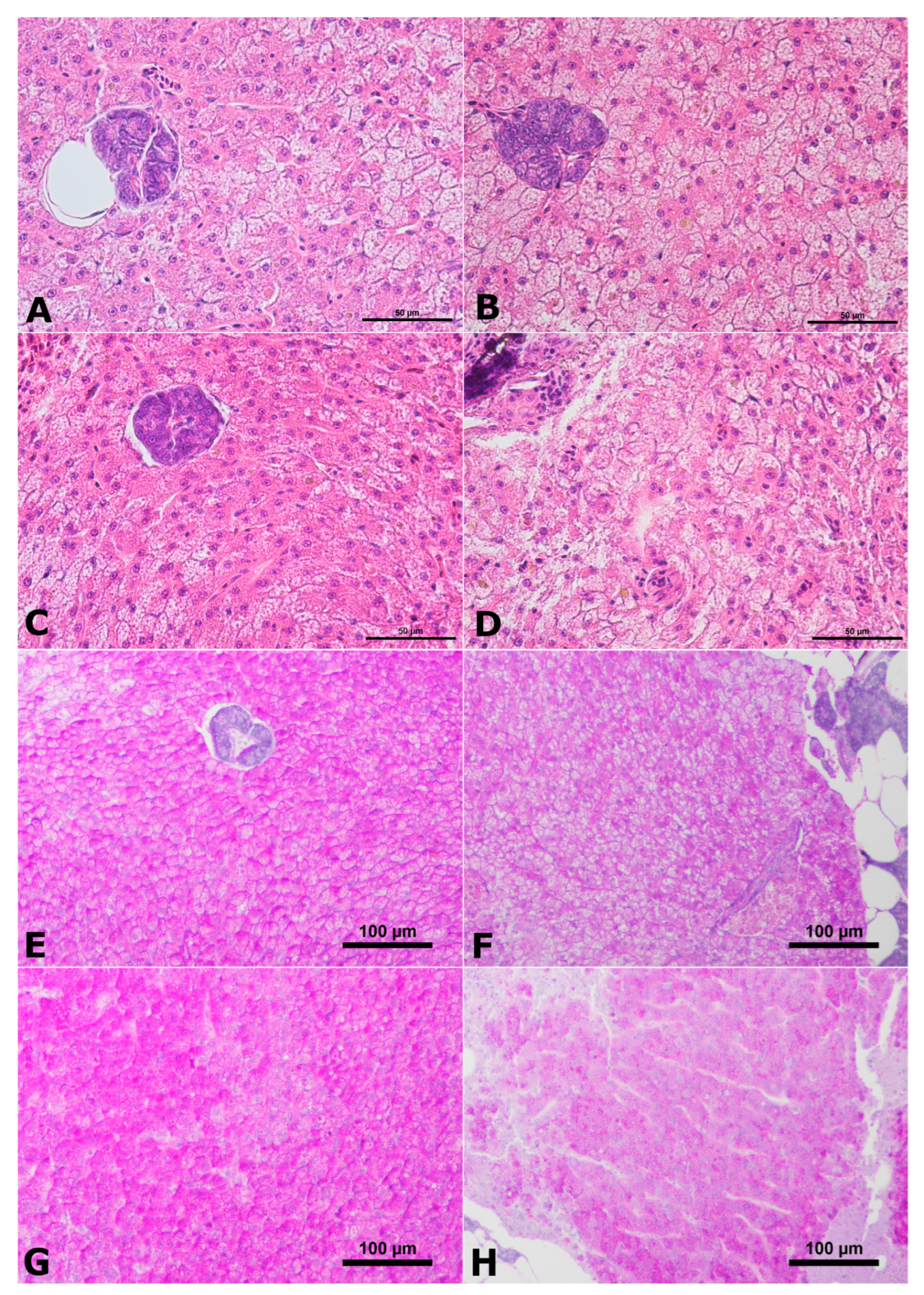
2.2. Histology of Liver
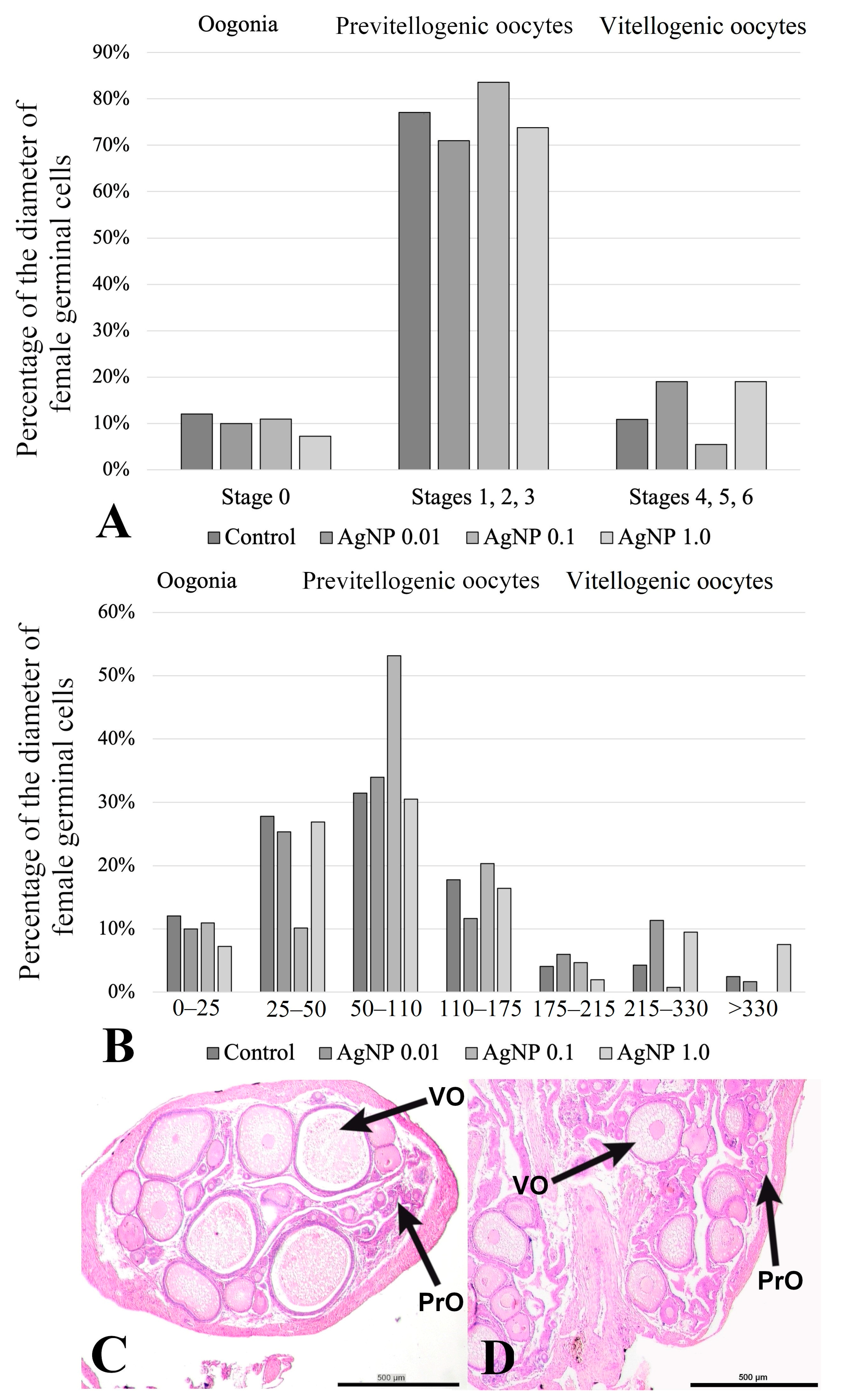
2.3. Histology of the Ovaries
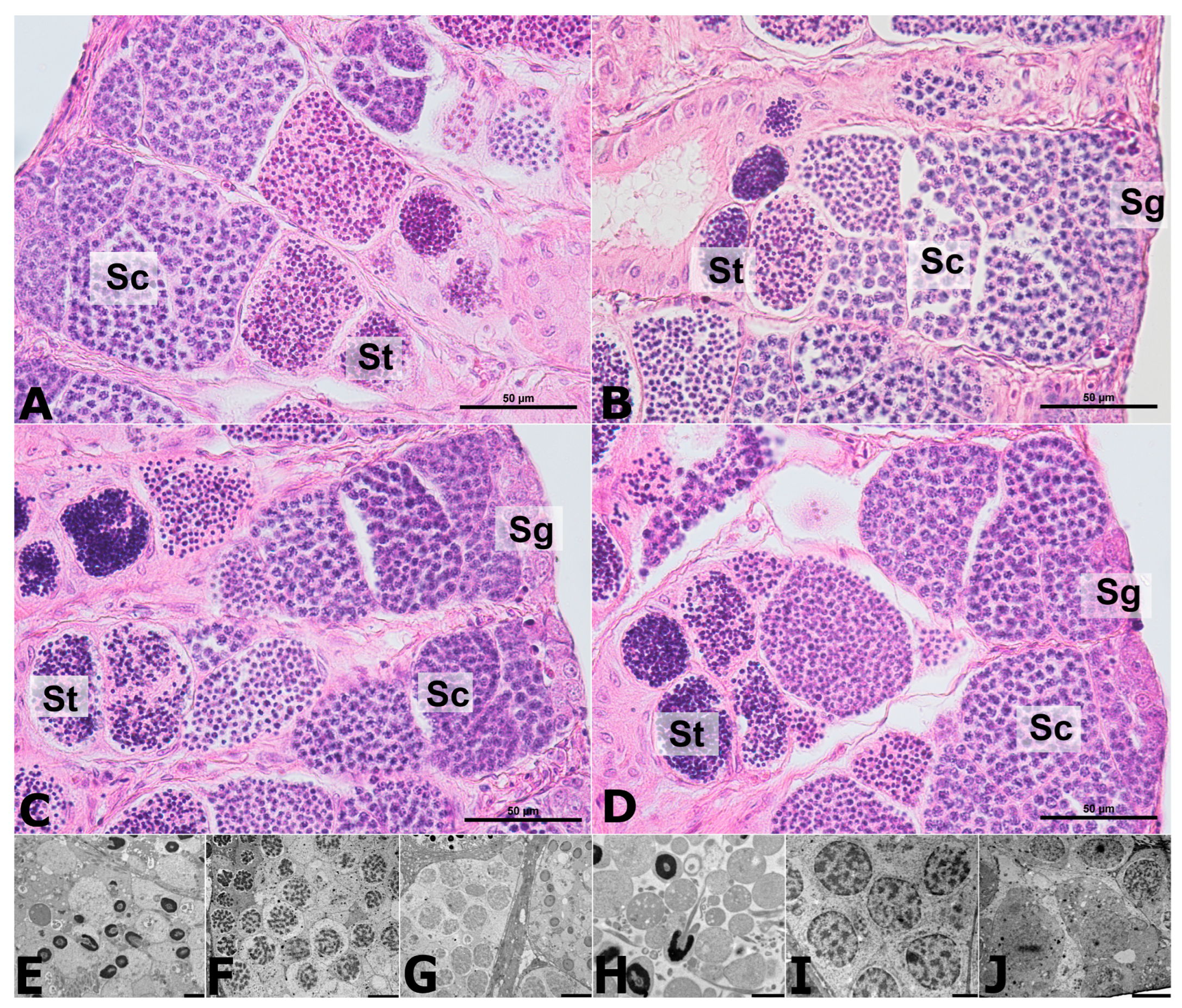
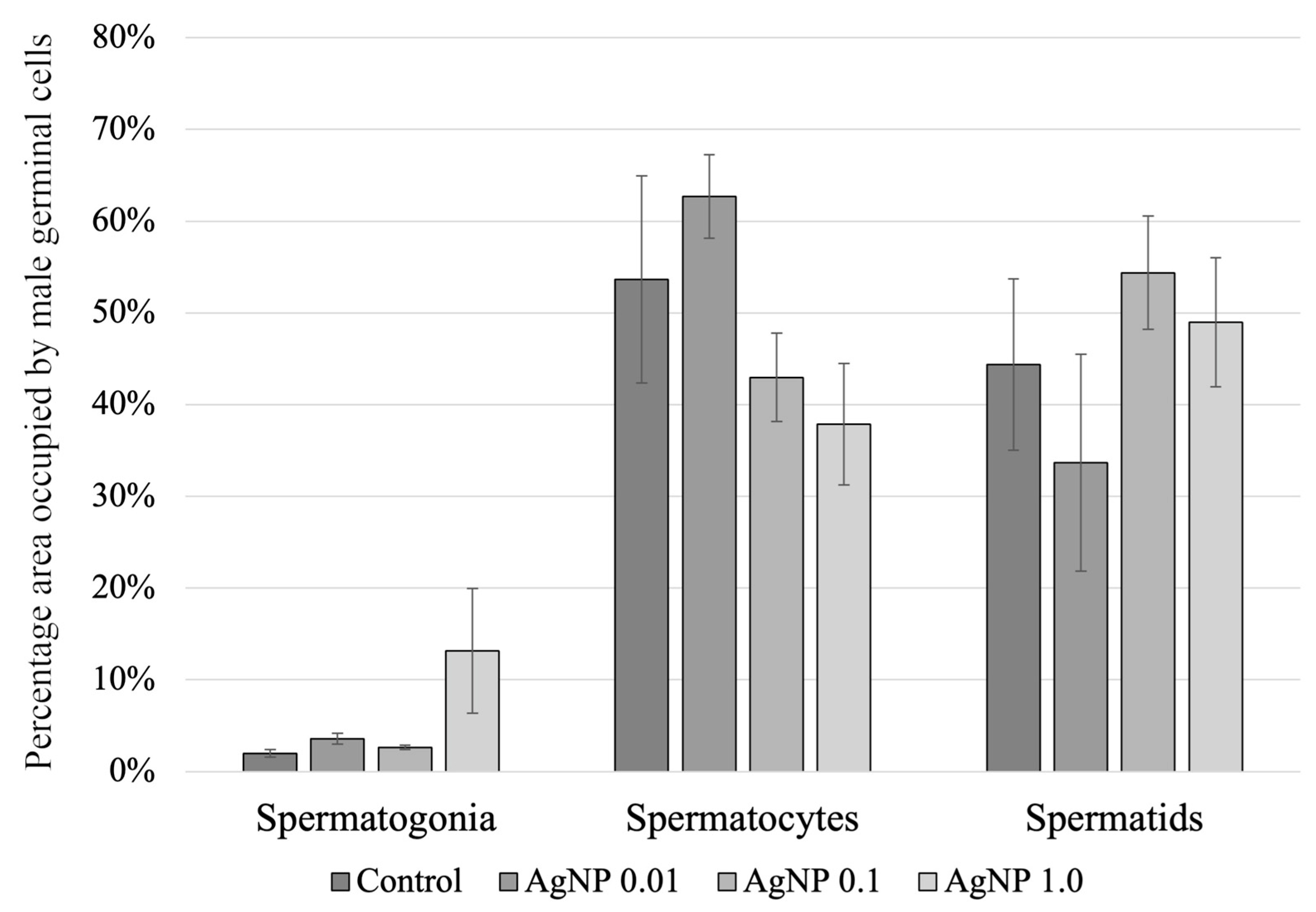
2.4. Histology of the Testes
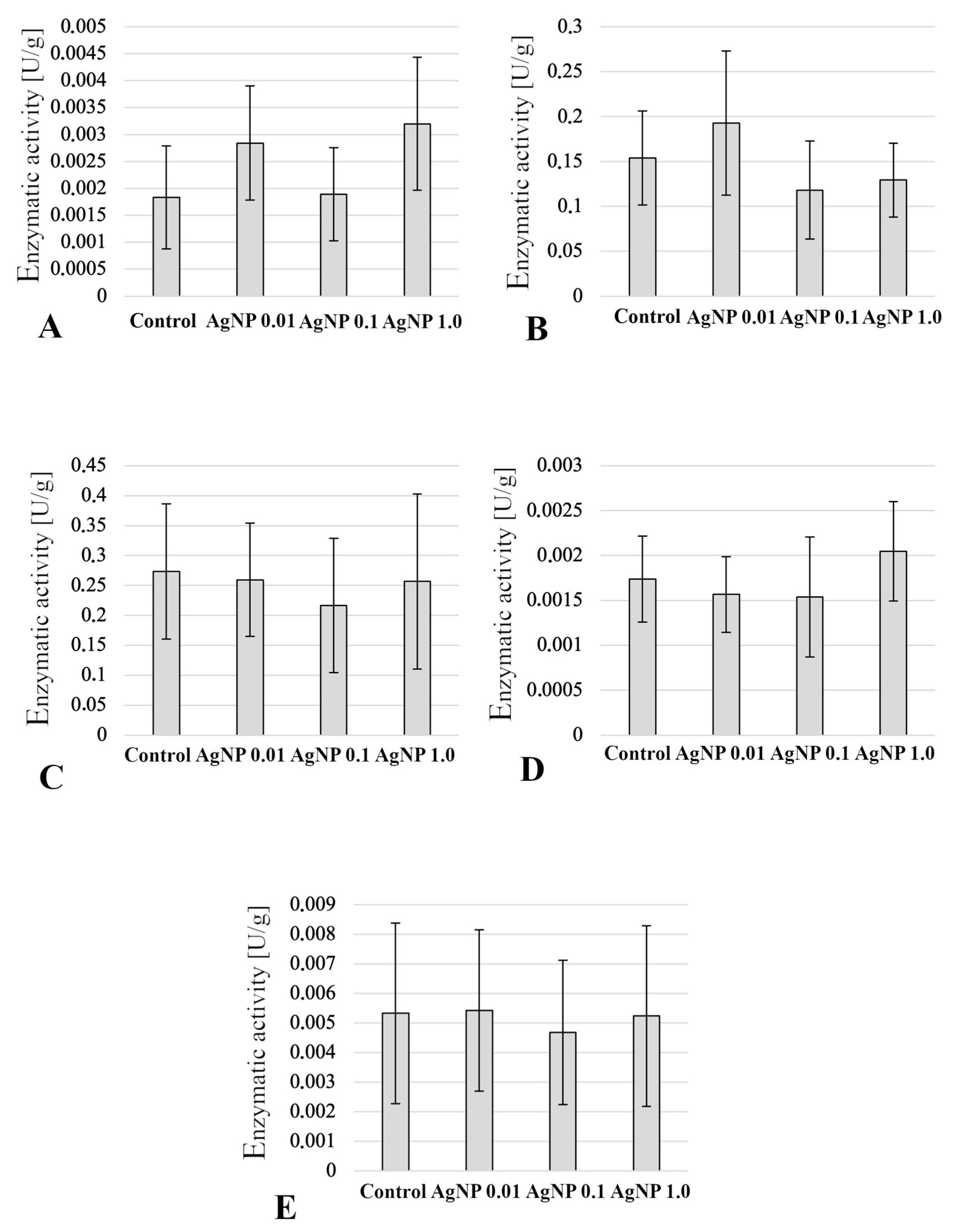
2.5. Enzymatic Activity in Anterior Intestine
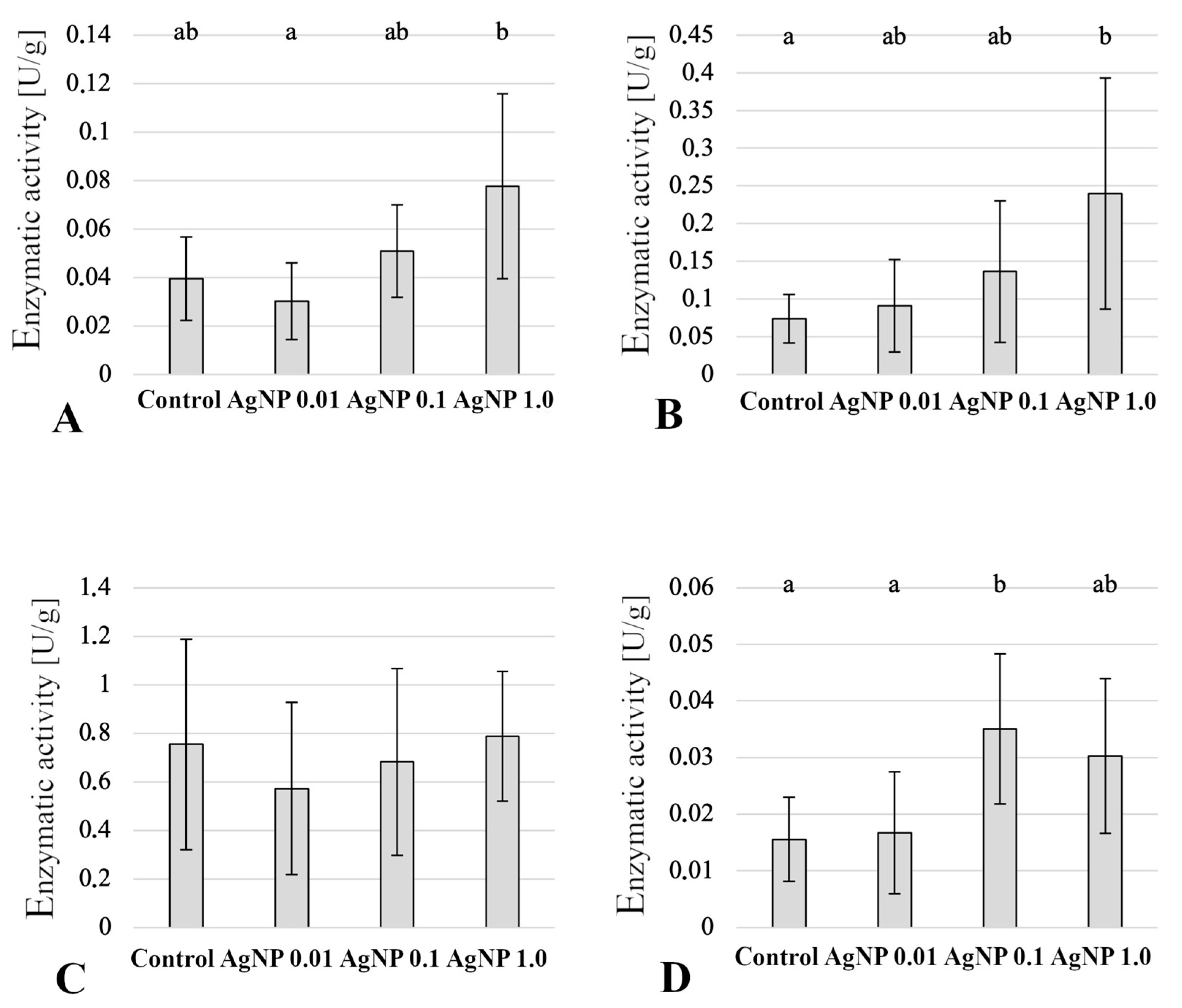
2.6. Enzymatic Activity in Liver
3. Discussion
4. Materials and Methods
4.1. Scheme of the Experiment
- m—body weight (g),
- Si—body length (mm).
4.2. Characterization of Nanoparticles
4.3. Histological Analyses
4.4. Detection of Enzymatic Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, T.; El Badawy, A.; Genaidy, A.; Abdelraheem, W.; Sequeira, R. Analysis of metallic and metal oxide nanomaterial environmental emissions. J. Clean. Prod. 2017, 143, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Zuin, S.; Gaiani, M.; Ferrari, A.; Golanski, L. Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. J. Nanoparticle Res. 2013, 16, 1–17. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Wichser, A.; Vonbank, R.; Brunner, S.; Ulrich, A.; Zuin, S.; Arroyo, Y.; Golanski, L.; Nowack, B. Characterization of materials released into water from paint containing nano-SiO2. Chemosphere 2015, 119, 1314–1321. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef]
- Degger, N.; Anna, C.K.; Wu, R.S. Silver nanoparticles disrupt regulation of steroidogenesis in fish ovarian cells. Aquat. Toxicol. 2015, 169, 143–151. [Google Scholar] [CrossRef]
- Dayal, N.; Thakur, M.; Patil, P.; Singh, D.; Vanage, G.; Joshi, D.S. Histological and genotoxic evaluation of gold nanoparticles in ovarian cells of zebrafish (Danio rerio). J. Nanoparticle Res. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Chen, S.X.; Yang, X.Z.; Deng, Y.; Huang, J.; Li, Y.; Sun, Q.; Yu, C.; Zhu, Y.; Hong, W.S. Silver nanoparticles induce oocyte maturation in zebrafish (Danio rerio). Chemosphere 2017, 170, 51–60. [Google Scholar] [CrossRef]
- Szudrowicz, H.; Kamaszewski, M.; Adamski, A.; Skrobisz, M.; Frankowska-Łukawska, J.; Wójcik, M.; Bochenek, J.; Kawalski, K.; Martynow, J.; Bujarski, P.; et al. The Effects of Seven-Day Exposure to Silver Nanoparticles on Fertility and Homeostasis of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 11239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Si, Y.; Zhou, D.; Dang, F. Differential bioaccumulation patterns of nanosized and dissolved silver in a land snail Achatina fulica. Environ. Pollut. 2017, 222, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.G. Classification of the reproductive patterns of amniotes. Herpetol. Monogr. 2000, 14, 371–377. [Google Scholar] [CrossRef]
- Tinguely, S.M. Xenotoca eiseni (Cyprinodontiformes, Goodeidae) as a Potential New Model for Studies on Maternal Transfer of Environmental Contaminants. Ph.D. Thesis, University of Exeter, Exeter, UK, 2015. [Google Scholar]
- Latoszek, E.; Kamaszewski, M.; Milczarek, K.; Puppel, K.; Szudrowicz, H.; Adamski, A.; Bury-Burzymski, P.; Ostaszewska, T. Histochemical characteristics of macrophages of butterfly splitfin (Ameca splendens). Folia Biol. 2019, 67, 53–60. [Google Scholar] [CrossRef]
- Rackham, B.D.; Woodhead, D.S. Effects of chronic γ-irradiation on the gonads of adult Ameca splendens (Osteichthyes: Teleostei). Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1984, 45, 645–656. [Google Scholar] [CrossRef]
- Tejeda-Vera, R.; López-López, E.; Sedeño-Díaz, J.E. Biomarkers and bioindicators of the health condition of Ameca splendens and Goodea atripinnis (Pisces: Goodeaidae) in the Ameca River, Mexico. Environ. Int. 2007, 33, 521–531. [Google Scholar] [CrossRef]
- Miller, R.R.; Fitzsimons, J.M. Ameca splendens, a new genus and species of goodeid fish from western Mexico, with remarks on the classification of the Goodeidae. Copeia 1971, 1, 13. [Google Scholar] [CrossRef]
- Lombardi, J.; Wourms, J.P. The trophotaenial placenta of a viviparous goodeid fish. II. Ultrastructure of trophotaeniae, the embryonic component. J. Morphol. 1985, 184, 293–309. [Google Scholar] [CrossRef]
- Schindler, J.F.; Hamlett, W.C. Maternal–embryonic relations in viviparous teleosts. J. Exp. Zool. 1993, 266, 378–393. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Śliwiński, J.; Kamaszewski, M.; Sysa, P.; Chojnacki, M. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environ. Sci. Pollut. Res. 2018, 25, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Canli, E.G.; Canli, M. Effects of aluminum, copper and titanium nanoparticles on the liver antioxidant enzymes of the Nile fish (Oreochromis niloticus). Energy Rep. 2020, 6, 62–67. [Google Scholar] [CrossRef]
- Naguib, M.; Mahmoud, U.M.; Mekkawy, I.A.; Sayed, A.E.D.H. Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; Biochemical, histopathological, and histochemical studies. Toxicol. Rep. 2020, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, R.L.D.; de Brito-Gitirana, L. Effects of titanium dioxide nanoparticles on the intestine, liver, and kidney of Danio rerio. Ecotoxicol. Environ. Saf. 2020, 203, 111032. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio). Environ. Sci. Eur. 2021, 33, 1–11. [Google Scholar]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.A.; El-Shafai, N.M.; Dawood, M.A.; Abdel-Latif, H.M. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Wang, T.; Long, X.; Cheng, Y.; Liu, Z.; Yan, S. A comparison effect of copper nanoparticles versus copper sulphate on juvenile Epinephelus coioides: Growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int. J. Genom. 2015, 2015, 783021. [Google Scholar]
- Ostaszewska, T.; Chojnacki, M.; Kamaszewski, M.; Sawosz-Chwalibóg, E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ. Sci. Pollut. Res. 2016, 23, 1621–1633. [Google Scholar] [CrossRef]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G.; et al. Evaluation of chronic nanosilver toxicity to adult zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Qiang, L.; Arabeyyat, Z.H.; Xin, Q.; Paunov, V.N.; Dale, I.J.; Lloyd Mills, R.I.; Rotchell, J.M.; Cheng, J. Silver nanoparticles in Zebrafish (Danio rerio) embryos: Uptake, growth and molecular responses. Int. J. Mol. Sci. 2020, 21, 1876. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Grün, A.L.; Straskraba, S.; Schulz, S.; Schloter, M.; Emmerling, C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. J. Environ. Sci. 2018, 69, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.A.; Lima Neto, M.C.; Léo, P.; Ortolan, B.D.; Barbieri, E.; De Souza, A.O. Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698. [Google Scholar] [CrossRef]
- Mohsenpour, R.; Mousavi-Sabet, H.; Hedayati, A.; Rezaei, A.; Yalsuyi, A.M.; Faggio, C. In vitro effects of silver nanoparticles on gills morphology of female Guppy (Poecilia reticulate) after a short-term exposure. Microsc. Res. Technol. 2020, 83, 1552–1557. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Y.; Zhu, P.; Bao, X.; Su, P. Polystyrene nanoplastics mediated the toxicity of silver nanoparticles in zebrafish embryos. Front. Mar. Sci. 2023, 10, 1195125. [Google Scholar] [CrossRef]
- Deng, Y.S.; Chen, Q.J. Affects of nano-selenium on the growth of nile tilapia (Oreochromis niloticus). Inland Aquat Prod 2003, 6, 28–30. [Google Scholar]
- Faiz, H.; Zuberi, A.; Nazir, S.; Rauf, M.; Younus, N. Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: Comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol. 2015, 17, 568–574. [Google Scholar] [CrossRef]
- Ramsden, C.S.; Smith, T.J.; Shaw, B.J.; Handy, R.D. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): No effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology 2009, 18, 939–951. [Google Scholar] [CrossRef]
- Forouhar Vajargah, M.; Imanpoor, M.R.; Shabani, A.; Hedayati, A.; Faggio, C. Effect of long-term exposure of silver nanoparticles on growth indices, hematological and biochemical parameters and gonad histology of male goldfish (Carassius auratus gibelio). Microsc. Res. Tech. 2019, 82, 1224–1230. [Google Scholar] [CrossRef]
- Shahare, B.; Yashpal, M.; Gajendra. Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicol. Mech. Methods 2013, 23, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.; Antonopulos, A.; Diendorf, J.; Dringen, R.; Epple, M.; Flöck, R.; Zellner, R. PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J. Nanotechnol. 2014, 5, 1944–1965. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, Z.; Moghanlou, K.S.; Imani, A.; Behrouzi, S.; Policar, T.; Rahimnejad, S. Interactive Effects of Curcumin and Silver Nanoparticles on Growth, Hemato-Biochemical Parameters, Digestive Enzymes Activity and Histology of Common Carp (Cyprinus carpio). Research Square 2021. [Google Scholar] [CrossRef]
- Mwaanga, P.; Carraway, E.R.; van den Hurk, P. The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquat. Toxicol. 2014, 150, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Qureshi, N.A.; Jabeen, F.; Shakeel, M.; Asghar, M.S. Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in Labeo rohita by histological and hematological profiles. Biol. Trace Elem. Res. 2018, 182, 130–139. [Google Scholar] [CrossRef]
- López-Barrera, E.A.; Grötzner, S.R.; Esquivel, L.; Voigt, C.L.; Campos, S.X.; Oliveira Ribeiro, C.A. Histopathological effects of silver nanoparticles in Rhamdia quelen after oral exposure. Ecotoxicol. Environ. Contam. 2021, 16, 83–89. [Google Scholar] [CrossRef]
- Rajkumar, K.S.; Kanipandian, N.; Thirumurugan, R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl. Nanosci. 2016, 6, 19–29. [Google Scholar] [CrossRef]
- Bacchetta, C.; Ale, A.; Simoniello, M.F.; Gervasio, S.; Davico, C.; Rossi, A.S.; Desimone, M.F.; Poletta, G.; López, G.; Monserrat, J.M.; et al. Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol. Indic. 2017, 76, 230–239. [Google Scholar] [CrossRef]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Valerio-García, R.C.; Carbajal-Hernández, A.L.; Martínez-Ruíz, E.B.; Jarquín-Díaz, V.H.; Haro-Pérez, C.; Martínez-Jerónimo, F. Exposure to silver nanoparticles produces oxidative stress and affects macromolecular and metabolic biomarkers in the goodeid fish Chapalichthys pardalis. Sci. Total Environ. 2017, 583, 308–318. [Google Scholar] [CrossRef]
- Orbea, A.; González-Soto, N.; Lacave, J.M.; Barrio, I.; Cajaraville, M.P. Developmental and reproductive toxicity of PVP/PEI-coated silver nanoparticles to zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Ruggeri, S.; Mattioli, S.; Bernardini, G.; Macchioni, L.; Moretti, E.; Collodel, G. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Syst. Biol. Reprod. Med. 2014, 60, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Huang, Y.; Du, Q.; Sun, Y. Silver nanoparticles (AgNPs) induced changes of reproductive parameters and gene expression was involved in apoptosis in the murine male testis. Fertil. Steril. 2016, 106, e283–e284. [Google Scholar] [CrossRef][Green Version]
- Thakur, M.; Gupta, H.; Singh, D.; Mohanty, I.R.; Maheswari, U.; Vanage, G.; Joshi, D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J. Nanobiotechnology 2014, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Hoseinipanah, S.M.; Alizadeh, Z.; Assari, M.J.; Moghimbeigi, A.; Mortazavi, M.; Hosseini, M.H.; Bahmanzadeh, M. The effect of silver nanoparticles on the reproductive system of adult male rats: A morphological, histological and DNA integrity study. Adv. Clin. Exp. Med. 2019, 28, 299–305. [Google Scholar] [CrossRef]
- Olugbodi, J.O.; David, O.; Oketa, E.N.; Lawal, B.; Okoli, B.J.; Mtunzi, F. Silver nanoparticles stimulates spermatogenesis impairments and hematological alterations in testis and epididymis of male rats. Molecules 2020, 25, 1063. [Google Scholar] [CrossRef]
- Božić, G.; Rašković, B.; Stanković, M.; Poleksić, V.; Marković, Z. Effects of different feeds on growth performance parameters, histology of liver, distal intestine, and erythrocytes morphology of common carp (Cyprinus carpio L.). Biologia 2021, 76, 3769–3779. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Palińska-Żarska, K.; Woźny, M.; Kamaszewski, M.; Szudrowicz, H.; Brzuzan, P.; Żarski, D. Domestication process modifies digestion ability in larvae of Eurasian perch (Perca fluviatilis), a freshwater Teleostei. Sci. Rep. 2020, 10, 2211. [Google Scholar] [CrossRef]
- Wiszniewski, G.; Jarmołowicz, S.; Hassaan, M.S.; Kamaszewski, M.; Szudrowicz, H.; Terech-Majewska, E.; Kawalski, K.; Martynow, J.; Szczepański, A.; Siwicki, A.K. Dietary effect of actinidin enzyme on growth, digestive enzymes activity, immunity, liver and intestine histology of juvenile sterlet sturgeon (Acipenser ruthenus). Aquac. Rep. 2022, 25, 101196. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Hydrodynamic diameter range (DLS analysis) | min. 39 nm; max. 93 nm |
| Diameter of agglomerates (DLS analysis) | >202 nm |
| Diameter range (TEM analysis) | min. 6.14 nm; max. 108.79 nm |
| Diameter of agglomerates (TEM analysis) | >200 nm |
| Mean of zeta potential (in 25 °C) | −23.4 mV |
| Stage | Name | Diameter (µm) | |
|---|---|---|---|
| Oogonia | 0 | Oogonia | 0–25 |
| Primary growth (previtellogenic) | 1 | Early primary growth | 25–50 |
| 2 | Mid primary growth | 50–110 | |
| 3 | Late primary growth | 110–175 | |
| Secondary growth (vitellogenic) | 4 | Early secondary growth | 175–215 |
| 5 | Late secondary growth | 215–330 | |
| 6 | Fully growth oocytes | above 330 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaszewski, M.; Kawalski, K.; Wiechetek, W.; Szudrowicz, H.; Martynow, J.; Adamek-Urbańska, D.; Łosiewicz, B.; Szczepański, A.; Bujarski, P.; Frankowska-Łukawska, J.; et al. The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca splendens). Int. J. Mol. Sci. 2023, 24, 14598. https://doi.org/10.3390/ijms241914598
Kamaszewski M, Kawalski K, Wiechetek W, Szudrowicz H, Martynow J, Adamek-Urbańska D, Łosiewicz B, Szczepański A, Bujarski P, Frankowska-Łukawska J, et al. The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca splendens). International Journal of Molecular Sciences. 2023; 24(19):14598. https://doi.org/10.3390/ijms241914598
Chicago/Turabian StyleKamaszewski, Maciej, Kacper Kawalski, Wiktoria Wiechetek, Hubert Szudrowicz, Jakub Martynow, Dobrochna Adamek-Urbańska, Bogumił Łosiewicz, Adrian Szczepański, Patryk Bujarski, Justyna Frankowska-Łukawska, and et al. 2023. "The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca splendens)" International Journal of Molecular Sciences 24, no. 19: 14598. https://doi.org/10.3390/ijms241914598
APA StyleKamaszewski, M., Kawalski, K., Wiechetek, W., Szudrowicz, H., Martynow, J., Adamek-Urbańska, D., Łosiewicz, B., Szczepański, A., Bujarski, P., Frankowska-Łukawska, J., Chwaściński, A., & Aksakal, E. (2023). The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca splendens). International Journal of Molecular Sciences, 24(19), 14598. https://doi.org/10.3390/ijms241914598






